Abstract
Acetylcholine (ACh), acting at muscarinic ACh receptors (mAChRs), modulates the excitability and synaptic connectivity of hippocampal pyramidal neurons. CA1 pyramidal neurons respond to transient (“phasic”) mAChR activation with biphasic responses in which inhibition is followed by excitation, whereas prolonged (“tonic”) mAChR activation increases CA1 neuron excitability. Both phasic and tonic mAChR activation excites pyramidal neurons in the CA3 region, yet ACh suppresses glutamate release at the CA3-to-CA1 synapse (the Schaffer–collateral pathway). Using mice genetically lacking specific mAChRs (mAChR knockout mice), we identified the mAChR subtypes responsible for cholinergic modulation of hippocampal pyramidal neuron excitability and synaptic transmission. Knockout of M1 receptors significantly reduced, or eliminated, most phasic and tonic cholinergic responses in CA1 and CA3 pyramidal neurons. On the other hand, in the absence of other Gq-linked mAChRs (M3 and M5), M1 receptors proved sufficient for all postsynaptic cholinergic effects on CA1 and CA3 pyramidal neuron excitability. M3 receptors were able to participate in tonic depolarization of CA1 neurons, but otherwise contributed little to cholinergic responses. At the Schaffer–collateral synapse, bath application of the cholinergic agonist carbachol suppressed stratum radiatum–evoked excitatory postsynaptic potentials (EPSPs) in wild-type CA1 neurons and in CA1 neurons from mice lacking M1 or M2 receptors. However, Schaffer–collateral EPSPs were not significantly suppressed by carbachol in neurons lacking M4 receptors. We therefore conclude that M1 and M4 receptors are the major mAChR subtypes responsible for direct cholinergic modulation of the excitatory hippocampal circuit.
INTRODUCTION
Acetylcholine (ACh) activates nicotinic and muscarinic ACh receptors (mAChRs) to modulate neuronal excitability, synaptic transmission, and synaptic plasticity in the hippocampus (Cobb and Davies 2005). The main excitatory hippocampal pathway is comprised of CA3 and CA1 pyramidal neurons, both of which are modulated by muscarinic, rather than nicotinic, receptors (Frazier et al. 1998; Jones and Yakel 1997; McQuiston and Madison 1999). Transient (“phasic”) mAChR activation generates biphasic responses in CA1 pyramidal neurons in which inhibition is followed by excitation (Gulledge and Kawaguchi 2007; Segal 1982). In contrast, phasic mAChR activation generally evokes only excitatory responses in CA3 pyramidal neurons (Gulledge and Kawaguchi 2007), although transient inhibition is observed with very high (>1 mM) concentrations of ACh (Segal 1982). Pyramidal neurons in both hippocampal regions are excited by prolonged (“tonic”) mAChR stimulation, which, in CA1 neurons, induces membrane depolarization, a decrease in the afterhyperpolarization (AHP) that follows brief periods of activity, and the generation of an afterdepolarization (ADP) that can promote additional action potential generation (Benardo and Prince 1981, 1982; Benson et al. 1988; Colino and Halliwell 1993; Fraser and MacVicar 1996; Haas 1982; Madison and Nicoll 1984; Madison et al. 1987; Muller et al. 1988; Storm 1989). These actions of ACh rely on the activation of Gq-linked (M1, M3, and M5) mAChRs (Gulledge and Kawaguchi 2007; Young et al. 2005), but the relative contribution of these specific “M1-like” mAChR subtypes is unknown.
In addition to modulating pyramidal neuron excitability directly, ACh also modulates synaptic transmission between CA3 and CA1 pyramidal neurons. Bath-applied cholinergic agonists suppress glutamate release at the Schaffer–collateral synapses linking CA3 pyramidal neurons to those in CA1 (Fernandez de Sevilla and Buno 2003; Fernandez de Sevilla et al. 2002; Hasselmo and Schnell 1994; Hounsgaard 1978; Scanziani et al. 1995; Segal 1982; Valentino and Dingledine 1981). Cholinergic suppression of synaptic transmission is mediated by mAChRs, but the available data are inconclusive as to the receptor subtype(s) involved. At the Schaffer–collateral synapse, data exist implicating M1 (Kremin et al. 2006; Leung and Péloquin 2010; Sheridan and Sutor 1990; Shinoe et al. 2005), M2 (Dutar and Nicoll 1988; Seeger et al. 2004; Segal 1989), and M4 (Shirey et al. 2008) receptors.
Since all five mAChR subtypes (M1–M5) are expressed in the hippocampus (Flynn et al. 1995; Levey et al. 1995; Reever et al. 1997), it is possible that multiple mAChRs are involved in generating the diverse cholinergic responses observed in hippocampal pyramidal neurons. Identification of the mAChR subtypes involved will enhance our understanding of how disrupted cholinergic signaling contributes to the cognitive deficits associated with disease states and age-related dementia (Bartus et al. 1982; Felder et al. 2001; Perry et al. 1999). Here we use mice genetically lacking specific mAChRs (mAChR “knockout mice”) to determine the relative contribution of mAChR subtypes in modulating the excitability of, and synaptic transmission between, hippocampal CA3 and CA1 pyramidal neurons. Our results identify two specific mAChR subtypes responsible for the majority of direct cholinergic modulation of the excitatory hippocampal circuit.
METHODS
Animals
Most experiments were performed on wild-type C57BL/6 mice or mice genetically modified to lack specific mAChRs (“mAChR knockout” mice, backcrossed to C57BL/6 for ≥10 generations), according to methods approved by the Institutional Animal Care and Use Committee of Dartmouth College. The generation and characterization of mAChR knockout mice have been described previously (Matsui et al. 2000; Nakamura et al. 2004; Ohno-Shosaku et al. 2003). For most experiments, animals used were between 6 and 8 wk of age. Mice lacking both M1 and M3 receptors (M1/M3 double-knockout mice) were less viable beyond 5 wk of age and therefore we used neurons from these animals at 4 to 5 wk of age. Experiments examining cholinergic modulation of synaptic transmission in wild-type and mAChR knockout mice also used neurons from mice between 4 and 5 wk of age. In some experiments, recordings were made from CA1 pyramidal neurons in hippocampal slices from 4-wk-old Sprague–Dawley rats.
Electrophysiology
Recordings were made from visually identified pyramidal neurons in coronal brain slices (250 μm thick) containing the dorsal hippocampus. Following isoflurane anesthesia, animals were decapitated and brains removed into an ice-cold cutting solution containing (in mM): 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 6 MgCl2, and 25 glucose (bubbled with 95% O2-5% CO2). Slices were stored and perfused with a similar solution (artificial cerebrospinal fluid [aCSF]) containing 2 mM CaCl2 and 1 mM MgCl2 and continuously bubbled with 95% O2-5% CO2. Whole cell recording pipettes (5–8 MΩ) contained (in mM): 135 K-gluconate, 2 NaCl, 2 MgCl2, 10 HEPES, 3 Na2ATP, and 0.3 NaGTP (pH 7.2 with KOH). Data were acquired with AxographX software (AxographX, Sydney, Australia) using a BVC-700 amplifier (Dagan, Minneapolis, MN) and an ITC-18 digitizer (HEKA Instruments, Bellmore, NY). Data were filtered at 5 kHz and digitized at 25 or 40 kHz. Whole cell series resistance (between 10 and 30 MΩ) was maximally compensated. Experiments were carried out at 35°C and membrane potentials were corrected for the liquid junction potential (12 mV). Input resistance (RN) was determined from the slope of voltage–current relationship established by measuring the magnitude at steady state of voltage deflections generated by a series of somatic current injections (−50 to +50 pA). Magnitudes of “sag” potentials (see results) were quantified using hyperpolarizing current injections sufficient to generate a 20 mV peak hyperpolarization (relative to the resting membrane potential [RMP]). Sag was measured as the percentage return to RMP (from peak hyperpolarization) occurring at the steady-state potential generated by the hyperpolarizing current step.
Phasic and tonic cholinergic stimulation
Phasic cholinergic responses were induced with focal ACh applications using a pneumatic drug-application device (Toohey, Fairfield, NJ). ACh (100 μM) was dissolved in aCSF and loaded into a patch pipette positioned about 20 μm from the soma on the stratum oriens side of the neuron studied. ACh was applied for 40 ms at 10 lb/in2. Prior to ACh applications at RMPs, cells were “primed” using 5 s of current-induced action potential generation to maximize phasic cholinergic responses (Gulledge et al. 2007). Focal applications of ACh were typically made at 20 s intervals and 3.5 s following priming current steps. ACh-induced inhibition was measured as the peak amplitude of hyperpolarizing responses from RMPs. For neurons in which inhibitory cholinergic signaling was absent (such as CA3 pyramidal neurons or neurons lacking specific mAChR subunits), phasic ACh “responses” were measured as the relative membrane potential 700 ms following ACh application, a time point corresponding to peak hyperpolarization in neurons exhibiting cholinergic inhibition.
Phasic cholinergic excitation was measured as the percentage change in mean instantaneous spike frequency (ISF) following focal ACh application to neurons undergoing current-evoked sustained action potential generation. In wild-type CA1 neurons, ACh generates a transient inhibition of action potential generation (∼1 s) that is followed by a higher frequency of action potential generation than observed in baseline conditions before ACh application. The mean ISF observed during the 2 s before ACh application was compared with the mean ISF occurring during the initial 2 s of activity following the resumption of action potential generation (in most CA1 neurons, since SK inhibition has variable duration), or beginning 500 ms after ACh application (in CA3 neurons and certain mAChR-lacking CA1 neurons in which ACh-induced SK responses do not occur). Cholinergic excitation was calculated as the percentage change in mean ISF over the two time periods (Gulledge et al. 2009).
Tonic cholinergic effects were induced via bath application of the cholinergic agonist carbachol (10 μM; 5 min). In CA1 cells, three aspects of membrane excitability were monitored during carbachol application: changes in the RMP, changes in the ACh-sensitive “medium” AHP (mAHP; see Storm 1989) that normally occurs after spike trains, and the appearance of ACh-generated ADPs immediately following spike trains. To monitor mAHP and ADP amplitudes, periodic (0.05 Hz) current injections (∼450 pA, 250 ms) were used to evoke brief trains of action potentials. Amplitudes of mAHPs and ADPs were measured relative to the membrane potential occurring just before current injections. Peak mAHPs were measured as the maximum hyperpolarization occurring within the first 500 ms following current injections (mean time-to-peak mAHP hyperpolarization in wild-type neurons was 58 ± 4 ms; range = 34 to 92 ms; n = 13), whereas ADPs were measured as the peak depolarization occurring within the first 1.5 s following current injections. In some experiments we also tested cholinergic modulation of longer-lasting “slow” AHPs (sAHPs) generated by trains of action potentials evoked by short (2 ms), high-amplitude (3 nA), somatic current injections. Amplitudes of the “early” and “late” sAHPs (see Schwindt et al. 1988) were measured at 1 and 5 s, respectively, following the last action potential of the spike train.
CA3 pyramidal neurons did not consistently have AHPs under baseline conditions and never exhibited CA1-like ADPs in the presence of carbachol. Instead, carbachol generated “shoulder” potentials occurring immediately after the cessation of current steps, during which the membrane potential slowly returned to the RMP over a period of hundreds of milliseconds to several seconds. Therefore to measure tonic cholinergic effects in CA3 pyramidal neurons we monitored carbachol-induced changes in the RMP and in the duration of shoulder potentials. Due to potential changes in RMP and action potential height during the experiment, shoulder potential widths were measured at an arbitrary fixed amplitude defined as 5 mV above the RMP.
Synaptic stimulation of Schaffer–collateral EPSPs
Synaptic responses were evoked with a bipolar glass electrode (θ-glass) filled with aCSF placed in the stratum radiatum of CA1. Stimuli (50 μs) were delivered at 15-s intervals using an isolated stimulator (Iso-flex; AMPI, Jerusalem, Israel). Stimulation intensity was adjusted to evoke excitatory postsynaptic potentials (EPSPs) of about 4–6 mV. In some experiments picrotoxin (100 μM) was included in the aCSF to block γ-aminobutyric acid type A (GABAA) receptors. After 5 min of stable baseline recording, carbachol (10 μM) was bath-applied for 5 min before the initiation of a washout period lasting ≥15 min. To control for carbachol-induced changes in RMPs and the resulting changes in EPSP driving force, somatic current injection was used to keep membrane potentials at −82 mV during synaptic experiments. To compare EPSPs before and after carbachol application, and between genotypes, data from four consecutive EPSPs occurring just before, at the end of 5 min of carbachol, or 15 min after starting the washout of carbachol were averaged together.
Drugs and statistical analysis
All drugs were purchased from Sigma-Aldrich. Data are presented as means ± SE. Statistical analysis for unpaired samples used either a Student's t-test (two-tailed) or a one-way ANOVA with Tukey–Kramer post-tests. Comparisons of paired data used a repeated-measures ANOVA with Tukey–Kramer multiple-comparison post-tests or a Student's t-test for paired samples (two-tailed).
RESULTS
Phasic cholinergic responses in hippocampal pyramidal neurons
Whole cell recordings were made from CA1 and CA3 pyramidal neurons from the dorsal hippocampus of wild-type mice (n = 12 for each cell type). These two neuron populations were physiologically distinct (Table 1), with CA1 neurons being more depolarized (P < 0.0001; unpaired t-test) and exhibiting a more pronounced depolarizing “sag” potential (P < 0.0001; see also Gulledge and Kawaguchi 2007) during prolonged hyperpolarizing current injections (indicative of the presence of hyperpolarization-activated nonspecific cation channels). Pyramidal neurons in both hippocampal regions had similar input resistances (RN; see Table 1).
Table 1.
Comparison of physiological properties and phasic cholinergic responsiveness in hippocampal pyramidal neurons from different genetic backgrounds
| Genotype | Layer | n | RMP, mV | RN, MΩ | Sag, % | SK Response, mV, | Spike Acceleration, % |
|---|---|---|---|---|---|---|---|
| Wild-type (WT) | CA1 | 12 | −79 ± 1 | 114 ± 10 | 18 ± 1 | −2.8 ± 0.1 | +131 ± 18 |
| WT in atropine | CA1 | 4 | −79 ± 1 | 110 ± 13 | 21 ± 2 | −0.1 ± 0.1* | −6 ± 1* |
| M3/M5 DKO | CA1 | 12 | −79 ± 1 | 134 ± 12 | 18 ± 1 | −3.5 ± 0.4 | +208 ± 21* |
| M1 KO | CA1 | 12 | −79 ± 1 | 111 ± 7 | 20 ± 1 | +0.2 ± 0.0* | +20 ± 6* |
| M1/M3 DKO | CA1 | 7 | −75 ± 1* | 113 ± 9 | 22 ± 2 | 0.0 ± 0.0* | +5 ± 4* |
| Wild-type (WT) | CA3 | 12 | −86 ± 1† | 128 ± 17 | 4 ± 1† | +0.4 ± 0.4† | +175 ± 41 |
| WT in atropine | CA3 | 5 | −84 ± 2 | 217 ± 10* | 3 ± 1 | +0.3 ± 0.1 | +3 ± 3* |
| M3/M5 DKO | CA3 | 10 | −87 ± 1 | 140 ± 12 | 3 ± 1 | +0.8 ± 0.2 | +148 ± 32 |
| M1 KO | CA3 | 12 | −86 ± 1 | 259 ± 33* | 5 ± 1 | +0.1 ± 0.1 | +27 ± 8* |
| M1/M3 DKO | CA3 | 12 | −81 ± 1* | 294 ± 47* | 5 ± 1 | −0.1 ± 0.2 | +4 ± 4* |
Values are means ± SE. Comparison of physiological characteristics and phasic cholinergic responses at resting membrane potentials (“SK response”) or during current-evoked action potential firing (“spike acceleration”). Numbers in boldface type indicate the presence of significant (P < 0.05) acetylcholine-induced “SK” responses and/or spike acceleration (paired t-test). Asterisks indicate P < 0.01 when values were compared with those in wild-type cells in the same hippocampal region (one-way ANOVA with post-tests). Daggers indicate P < 0.01 when values in wild-type CA3 neurons are compared with those in wild-type CA1 neurons (unpaired t-test). M1 KO, genetic knockout for M1 receptor; DKO, double-knockout for indicated mAChR subtypes; RMP, resting membrane potential; RN, input resistance; sag, percentage voltage rectification at steady state relative to peak potential reached during negative current injections.
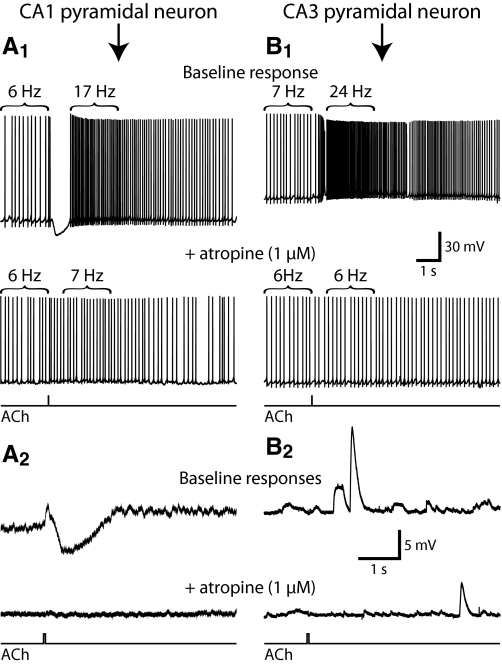
CA1 and CA3 pyramidal neurons differed in their responses to cholinergic stimulation. Focal applications of ACh to CA1 pyramidal neurons generated hyperpolarizing responses from RMPs (mean response was −2.8 ± 0.4 mV, with a duration at half-amplitude of 654 ± 88 ms) and transiently suppressed action potential generation when applied to cells experiencing suprathreshold depolarizing somatic current injection (Fig. 1 and Table 1). Cholinergic inhibition of action potential generation in CA1 neurons was followed by significant spike acceleration (see methods) of 131 ± 18% (P < 0.0001; paired t-test). As previously found in rat CA1 neurons (Gulledge and Kawaguchi 2007), phasic cholinergic responses in mouse CA1 neurons were atropine sensitive (Fig. 1; n = 4) and cholinergic inhibition was blocked by apamin, a selective blocker of SK-type calcium-activated potassium channels (n = 3; data not shown). At RMPs, focal ACh applications failed to modulate membrane potentials in CA3 neurons (n = 12, P = 0.35; paired t-test). However, when applied during spike trains, ACh produced a 175 ± 41% increase in instantaneous firing rate (Fig. 1 and Table 1). Spike acceleration in CA3 neurons was also atropine sensitive (Fig. 1; n = 5), indicating that phasic cholinergic responses are mediated by mAChRs in both CA1 and CA3 pyramidal neurons.
Fig. 1.
Phasic muscarinic receptor–mediated modulation of hippocampal pyramidal neurons. A and B: somatic recordings of phasic responses to focal acetylcholine (ACh) application in CA1 (A) and CA3 (B) pyramidal neurons. When applied during action potential generation (A1 and B1), CA1 pyramidal neurons exhibited transient inhibition followed by spike acceleration, whereas CA3 neurons exhibited only spike acceleration (top traces). Cholinergic responses were eliminated after muscarinic acetylcholine receptors (mAChRs) were antagonized with 1 μM atropine (bottom traces in A1 and B1). At resting membrane potentials (RMPs; A2 and B2), ACh hyperpolarized CA1 pyramidal neurons, but had no effect on pyramidal neurons in CA3 (top traces). Atropine blocked phasic cholinergic responses at RMPs (bottom traces). ACh timing traces at the bottom of panels apply to all voltage traces above them.
Muscarinic receptor subtypes mediating phasic cholinergic responses
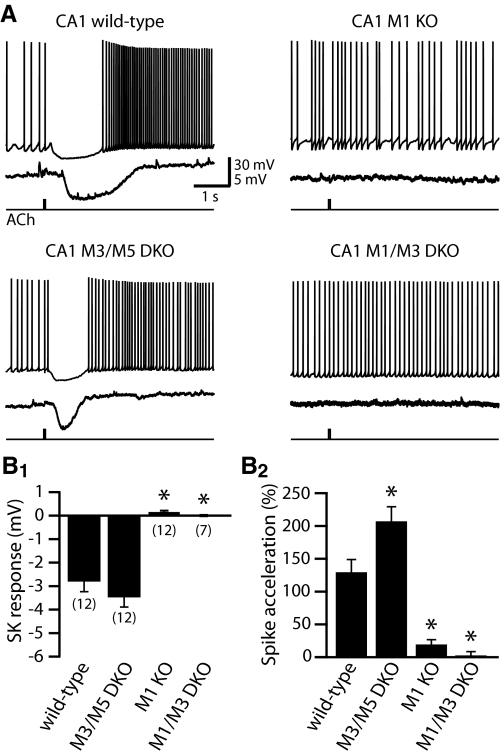
M1-like mAChRs are implicated in mediating phasic cholinergic responses in hippocampal pyramidal neurons (Gulledge and Kawaguchi 2007). To determine which M1-like mAChR subtypes contribute to phasic cholinergic signaling in CA1 pyramidal neurons, we compared responses in neurons from wild-type mice and mice genetically lacking M1 (n = 12), M3, and M5 (n = 12) or M1 and M3 receptors (n = 7) (Fig. 2 and Table 1). CA1 neurons lacking M1 or M3 and M5 receptors had RMPs similar to those of wild-type neurons, whereas the RMPs of neurons from mice lacking both M1 and M3 receptors were depolarized when compared with neurons from all other genetic groups (P < 0.01; one-way ANOVA with Tukey–Kramer post-tests; see Table 1). No significant differences in RN or sag potentials were found among CA1 neurons from animals with different genotypes. When focally applied to CA1 neurons expressing only the M1 subtype of M1-like mAChRs (i.e., from M3/M5 double-knockout mice), ACh generated hyperpolarizing responses from RMPs (−3.5 ± 0.4 mV, with a duration at half-amplitude of 743 ± 107 ms) that were similar to responses measured in wild-type neurons (unpaired t-test; P = 0.29 and 0.53, respectively, for amplitude and half-width of inhibitory responses from RMPs). Focally applied ACh also induced spike acceleration (+208 ± 21%) in M3/M5-lacking neurons that was significantly larger than spike acceleration in wild-type neurons (P < 0.01 unpaired t-test; see also ANOVA in Table 1). On the other hand, CA1 pyramidal neurons lacking M1 receptors (i.e., neurons from M1-knockout and M1/M3 double-knockout mice) were not responsive to focal ACh at RMPs and exhibited significantly less ACh-induced spike acceleration than did wild-type neurons (Fig. 2 and Table 1). Neurons from M1-knockout animals exhibited a small (+20 ± 6%), yet significant (P = 0.01, paired t-test), spike acceleration that was absent in neurons lacking both M1 and M3 receptors (mean spike acceleration = +5 ± 4%; P = 0.42). These results demonstrate that M1 receptors play a critical role in phasic cholinergic modulation of CA1 pyramidal neurons.
Fig. 2.
M1 receptors are necessary and sufficient for phasic cholinergic modulation of CA1 pyramidal neuron excitability. A: traces of responses to focal ACh application (40 ms) during sustained activity (top traces), or at resting membrane potentials (bottom traces), in CA1 pyramidal neurons from wild-type mice or mice lacking specific mAChR subtypes. In wild-type neurons, and those lacking M3 and M5 receptors, focal ACh application generates hyperpolarizing responses from RMPs and inhibits action potential generation during periods of current-induced spike trains. B: summary histograms comparing the magnitude of inhibitory (B1) and excitatory (B2) responses to phasic cholinergic stimulation in CA1 pyramidal neurons. The number of cells tested is shown in parentheses for each genotype in B1. Inhibitory and excitatory responses were absent or greatly reduced in neurons lacking M1 receptors. Asterisks indicate P < 0.001 when compared with wild-type neurons (one-way ANOVA with post-tests).
We next tested the roles of M1-like mAChR subtypes in generating phasic cholinergic responses in CA3 pyramidal neurons (Fig. 3 and Table 1). Similar to CA1 neurons, we observed few differences in baseline membrane properties in CA3 neurons from the various genotypes (Table 1), with the exception of a more depolarized RMP in CA3 neurons from M1/M3 double-knockout animals, and a significantly higher RN in CA3 neurons lacking M1 receptors. The increased RN of M1-lacking CA3 neurons was replicated in wild-type neurons in the presence of atropine (Table 1), suggesting that the membrane properties of pyramidal neurons in the CA3 region, which receive somewhat greater cholinergic afferent input than do neurons in CA1 (Aznavour et al. 2005), are under tonic modulation by ambient endogenous ACh.
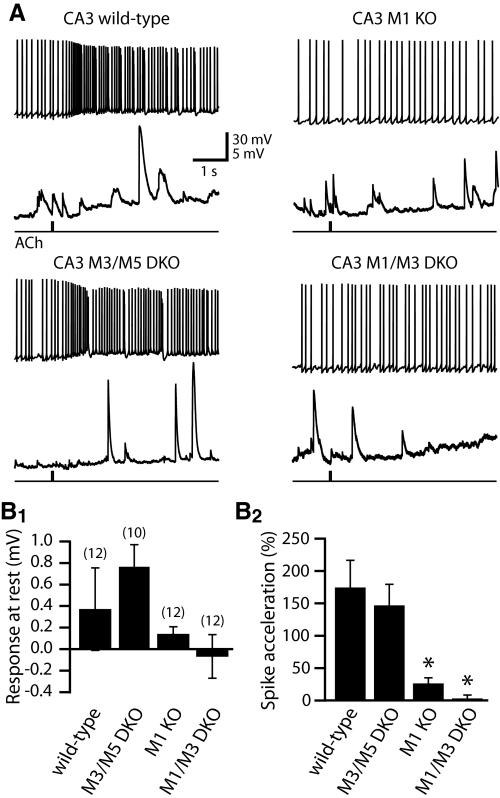
Fig. 3.
M1 receptors mediate phasic cholinergic modulation of CA3 pyramidal neurons. A: responses to focal ACh application (40 ms) during sustained activity (top traces) or at RMPs (bottom traces) in CA3 pyramidal neurons from mice expressing different combinations of mAChR subtypes. Notice the large spontaneous excitatory postsynaptic potentials (EPSPs) typically observed in CA3 (but not CA1) pyramidal neurons (see Gulledge and Kawaguchi 2007). B: summary histograms comparing phasic cholinergic responses in CA3 neurons at RMPs (B1) and during current-induced spike trains (B2). ACh failed to inhibit CA3 neurons regardless of which mAChRs were expressed. Although M1 receptors are sufficient for cholinergic excitation of CA3 neurons, responses were only fully blocked in neurons lacking both M1 and M3 receptors. Asterisks indicate P < 0.01 when compared with wild-type neurons (one-way ANOVA with post-tests).
Regardless of their mAChR expression, CA3 pyramidal neurons were unresponsive to focal ACh applications at RMPs. However, when delivered during periods of current-evoked activity, CA3 pyramidal neurons from M3/M5 double-knockout mice (n = 10) exhibited significant (P < 0.001, paired t-test) spike acceleration of +148 ± 32%, an amount similar to that found in wild-type CA3 neurons (P = 0.61, unpaired t-test; Fig. 3 and Table 1). On the other hand, CA3 neurons from M1-knockout mice (n = 12) exhibited only weak spike acceleration (+27 ± 8%; P < 0.01, paired t-test) that was significantly less that that observed in wild-type or M3/M5 double-knockout neurons (P < 0.01 for each, ANOVA with post-tests). Residual spike acceleration in M1-knockout neurons was absent in neurons lacking both M1 and M3 receptors (mean change in spike frequency was +4 ± 4%; P = 0.70, paired t-test) (Fig. 3). These data demonstrate that M1 receptors are the primary mediators of phasic cholinergic modulation of CA1 and CA3 pyramidal neurons, with only minor additional participation of M3 receptors.
Tonic cholinergic signaling in CA1 pyramidal neurons
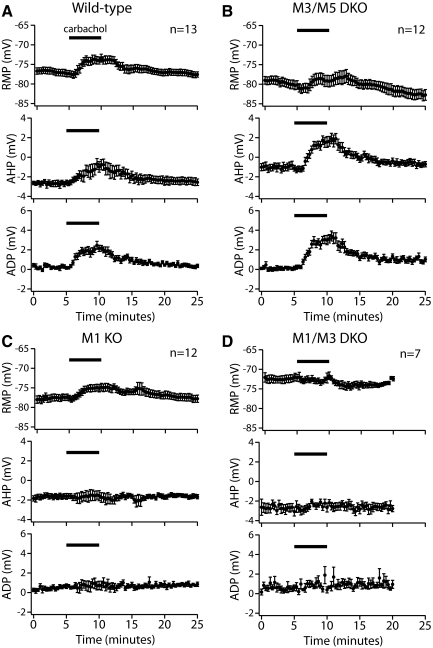
To measure tonic cholinergic responses, we used periodic (0.05 Hz) somatic current injections (∼450 pA, 250 ms) to evoke short spike trains in CA1 and CA3 pyramidal neurons. When the cholinergic agonist carbachol was bath-applied for a period of 5 min, clear changes in cellular excitability were evident in wild-type hippocampal pyramidal neurons from both the CA1 and CA3 regions. In wild-type CA1 neurons (n = 13), carbachol reversibly depolarized RMPs by 3.6 ± 0.5 mV, decreased mAHPs by 1.8 ± 0.4 mV (from 2.7 ± 0.2 to 0.9 ± 0.5 mV), and generated ADPs of 1.9 ± 0.2 mV (P < 0.0001 for each, repeated-measures ANOVA with post-tests) (Figs. 4 and 5 and Table 2).
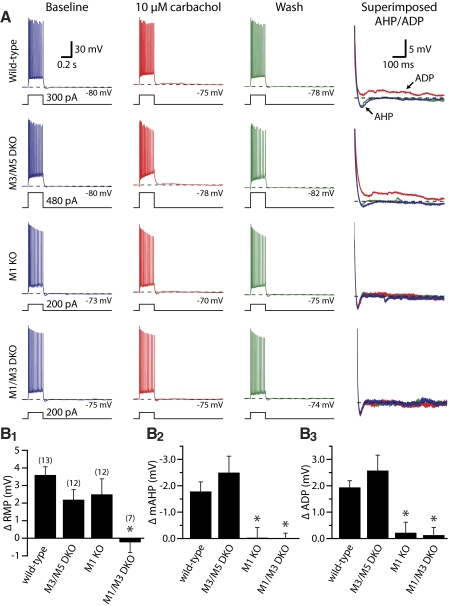
Fig. 4.
M1 and M3 receptors mediate tonic cholinergic modulation of CA1 pyramidal neurons. A: responses of CA1 pyramidal neurons to depolarizing current injection (250 ms) before (blue traces), during (red traces), and 15 min after (green traces) bath application of carbachol (10 μM). At far right, traces are expanded, baselined to RMPs, and superimposed to show the influence of carbachol on medium afterhyperpolarization (mAHPs) and afterdepolarizations (ADPs). B: summary histograms comparing carbachol-induced effects on RMP (B1), mAHP amplitude (B2), and ADP amplitude (B3) for cells containing different combinations of mAChR subtypes. M1 receptors were necessary and sufficient for modulation of mAHPs and ADPs, but carbachol-induced depolarization was absent only in neurons lacking both M1 and M3 receptors. Asterisks indicate P < 0.01 when compared with wild-type neurons (one-way ANOVA with post-tests).
Fig. 5.
Time courses of tonic cholinergic effects in CA1 pyramidal neurons. A–D: plots of RMP (top), mAHP amplitude (middle), and ADP amplitude (bottom) over time in CA1 neurons from wild-type mice (A), M3/M5 double-knockout mice (B), M1 knockout mice (C), and M1/M3 double-knockout mice (D).
Table 2.
Comparison of tonic cholinergic responses in hippocampal CA1 pyramidal neurons expressing different mAChR subtypes
| Genotype | Layer | n | ΔRMP, mV | ΔAHP Amplitude, mV | ΔADP Amplitude, mV |
|---|---|---|---|---|---|
| Wild-type (WT) | CA1 | 13 | +3.6 ± 0.5 | −1.8 ± 0.4 | +1.9 ± 0.2 |
| M3/M5 DKO | CA1 | 12 | +2.2 ± 0.6 | −2.5 ± 0.6 | +2.6 ± 0.6 |
| M1 KO | CA1 | 12 | +2.5 ± 0.9 | 0.0 ± 0.4* | +0.2 ± 0.4* |
| M1/M3 DKO | CA1 | 7 | −0.2 ± 0.6* | 0.0 ± 0.2* | +0.1 ± 0.3* |
Values are means ± SE. Cholinergic responses were evoked by bath-applied carbachol (10 μM for 5 min) in CA1 pyramidal neurons. Listed are changes in RMP and AHP and ADP amplitudes measured at the end of carbachol treatment and relative to values measured in baseline conditions. Numbers in boldface type indicate P < 0.05 for carbachol-induced effects within individual cell types (repeated-measures ANOVA with post-tests: baseline, carbachol, and wash). Asterisks indicate P < 0.01 when compared with results in wild-type neurons (one-way ANOVA with post-tests).
Tonic cholinergic modulation of CA1 pyramidal neuron excitability was not impaired in neurons from M3/M5 double-knockout mice (n = 12), but cholinergic modulation of the mAHP and ADP were eliminated in neurons lacking M1 receptors (n = 12; Figs. 4 and 5 and Table 2). However, carbachol still depolarized CA1 neurons from M1-knockout mice by 2.5 ± 0.9 mV (P < 0.05, repeated-measures ANOVA with post-tests), indicating that although M1 receptor expression is sufficient to induce tonic cholinergic effects in CA1 neurons, it is necessary and sufficient only for modulation of the mAHP and ADP. Consistent with the limited role of M3 receptors observed in phasic cholinergic signaling (described earlier), carbachol-induced depolarization of CA1 pyramidal neurons was absent in neurons from M1/M3 double-knockout mice (n = 7) (Fig. 4 and Table 2). Similarly, wild-type, M1-lacking, and M3/M5-lacking neurons, but not neurons from M1/M3 double-knockout animals, showed small, but significant, increases in spike number following carbachol application, consistent with previous findings showing that mAChR stimulation reduces spike-frequency adaptation during current steps (Morton and Davies 1997). The mean increases in spike number following carbachol application were +1.2 ± 1.6, +1.2 ± 1.8, and +1.9 ± 2.7 spikes for wild-type, M1-lacking, and M3/M5-lacking neurons, respectively (P < 0.05 for each genotype; repeated-measures ANOVA with post-tests). On the other hand, neurons lacking both M1 and M3 receptors showed no significant change in spike number (mean change was +0.1 ± 1.1 spikes; P = 0.46). Plots of the time courses for tonic cholinergic effects in CA1 neurons expressing different mAChR subunits are shown in Fig. 5.
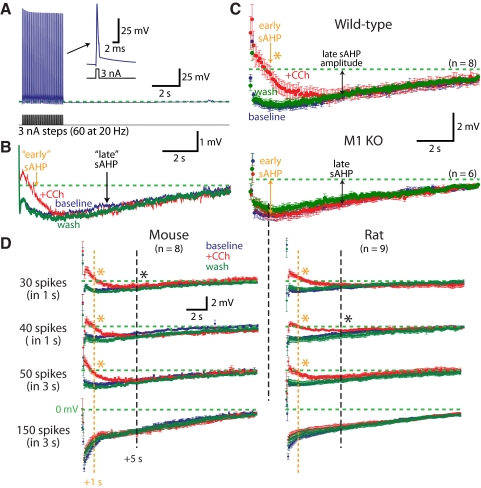
In CA1 neurons we also tested cholinergic modulation of slow AHPs (sAHPs) generated with prolonged spike trains (60 action potentials at 20 Hz) (Fig. 6A). Carbachol reversibly reduced the early components of the sAHP measured 1 s following the spike train, but did not reduce the late sAHP measured at 5 s following the spike train (Fig. 6B). In wild-type neurons, carbachol consistently reduced early sAHPs by 80 ± 20% (P = 0.001; repeated-measures ANOVA with post-tests), but did not reduce the late sAHP (P = 0.91) (Fig. 6C). Cholinergic suppression of the early sAHP was blocked in neurons from M1 knockout animals (mean change in early sAHP was +2 ± 13%; P = 0.50), indicating a central role for M1 receptors in sAHP modulation (Fig. 6C).
Fig. 6.
Carbachol modulates early components of the slow AHP (sAHP) in CA1 pyramidal neurons. A: trains of 60 action potentials evoked at 20 Hz by somatic current injections (3 nA for 2 ms) generate sAHPs. Inset: enlarged trace of the last action potential in the train. B: averages of 10 slow AHPs generated in baseline conditions (blue), in the presence of 10 μM carbachol (red), and after 10 min of wash (green) for the cell shown in A. Early sAHPs and late sAHPs measured at 1 and 5 s, respectively, following spike train. C: averages (±SE) of slow AHPs (resampled at 12 Hz) in 8 wild-type CA1 neurons (top) and 6 M1 KO CA1 neurons (bottom) in baseline conditions (blue), in the presence of carbachol (red), and after wash (green). D: sAHPs (means ± SE) generated by a variety of spike train protocols in baseline conditions (blue), in the presence of carbachol (red), and in wash (green) for neurons from mice (left) or rats (right). Cholinergic stimulation preferentially reduces the early sAHP in a paradigm-dependent manner. Orange asterisks indicate significant reductions of the early component by carbachol (repeated-measures ANOVA, post-tests; P < 0.01). Black asterisks indicate significant differences between carbachol and wash conditions in late components (P < 0.05). Regardless of experimental conditions, no significant differences were found between baseline and carbachol at the 5-s latency.
Previous studies described cholinergic reduction of the sAHP using a variety of induction protocols and animal models (Benardo and Prince 1982; Krause et al. 2002; Madison et al. 1987; Sah and Isaacson 1995). In an effort to explore the interaction of M1 receptor activation and sAHPs generated under different conditions, we varied the frequency and duration of spike trains delivered to CA1 neurons from wild-type mice and 4-wk-old Sprague–Dawley rats (Fig. 6D). In the majority of conditions, bath-applied carbachol (10 μM) preferentially reduced the early sAHP, with the extent of sAHP reduction greatest when sAHPs were induced with fewer spikes (Fig. 6D). Indeed, using a 30-Hz spike train for 1 s, sAHPs were shallower of shorter duration, and were almost fully blocked by carbachol application. However, in the same neurons, induction of longer-lasting sAHPs using longer spike trains revealed little if any cholinergic modulation of the “late” sAHP measured at 5 s following the spike train (Fig. 6D). Although a more comprehensive study will be required to fully define the range of circumstances under which ACh modulates the sAHP, our results indicate M1 receptors are primarily responsible for cholinergic sAHP suppression.
Tonic cholinergic signaling in CA3 pyramidal neurons
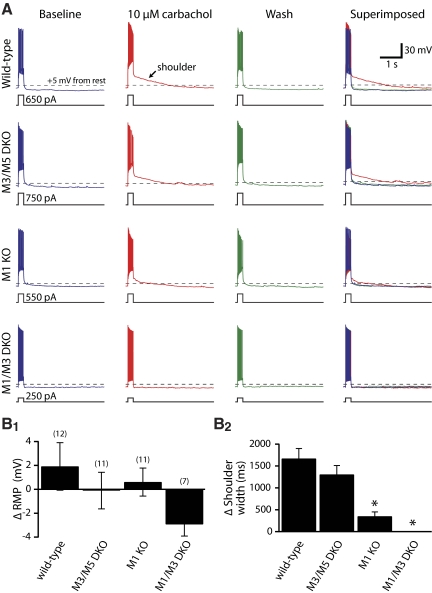
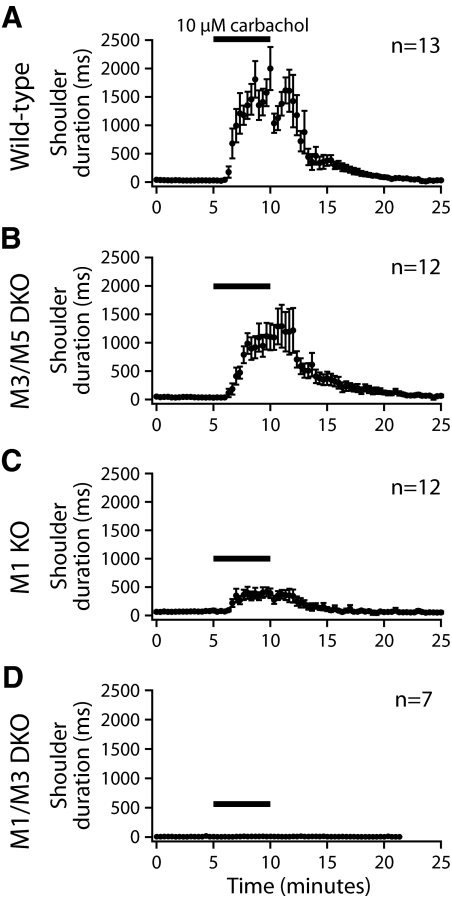
CA3 pyramidal neurons were also excited by tonic mAChR stimulation, although their excitatory responses were different from those observed in CA1 neurons (Figs. 7 and 8 and Table 3). Wild-type CA3 neurons (n = 12) were not depolarized by carbachol (mean change in RMP was +1.9 ± 2.0 mV; P = 0.36; repeated-measures ANOVA with post-tests), did not generally have mAHPs in baseline conditions, and did not exhibit CA1-like ADPs in the presence of carbachol. Instead, carbachol induced “shoulder potentials” following current-evoked spike trains, during which the membrane potential slowly hyperpolarized back to the RMP (Fig. 7). In wild-type neurons, carbachol reversibly induced shoulder potentials lasting 1.6 ± 0.2 s (P < 0.001; repeated-measures ANOVA with post-tests) (Table 3). Broad carbachol-induced shoulder potentials (1.0 ± 0.2 s) were also observed in CA3 neurons from M3/M5 double-knockout mice (n = 11), whereas shoulder potentials were greatly reduced (0.3 ± 0.1 s) or absent (0.0 ± 0.0 s) in M1-lacking neurons (n = 11) and neurons lacking both M1 and M3 receptors (n = 7), respectively. A one-way ANOVA found that, whereas wild-type and M3/M5-lacking neurons had shoulder potentials of similar durations, shoulder potentials in M1-lacking CA3 neurons (i.e., from M1, or M1 and M3, knockout mice) were of significantly shorter duration (P < 0.01 for each of M1-knockout and M1/M3 double-knockout neurons). Only in neurons lacking both M1 and M3 receptors were shoulder potentials absent (P = 0.36, repeated-measures ANOVA). The time course of tonic cholinergic effects in CA3 neurons expressing various M1-like mAChRs is shown in Fig. 8. These results demonstrate that M1 receptors are critical for robust expression of tonic cholinergic excitation of CA3 pyramidal neurons. M3 receptors, although not required for cholinergic signaling, may provide limited excitation, especially in the absence of M1 receptors.
Fig. 7.
M1 and M3 receptors are involved in cholinergic modulation of CA3 pyramidal neurons. A: responses of CA3 pyramidal neurons to depolarizing current steps (250 ms) before (blue traces), during (red traces), and 15 min after (green traces) bath application of carbachol (10 μM). Carbachol did not significantly modulate RMPs, but induced “shoulder potentials” that persisted for up to several seconds following current steps. At far right, individual traces are superimposed to show the carbachol-induced shoulder potential. Dashed lines indicate +5 mV from the RMP, an arbitrary potential used to calculate carbachol-induced shoulder potential duration. B: summary histograms comparing changes in RMPs (B1) and shoulder potential duration (B2) for CA3 neurons expressing different combinations of mAChRs. Expression of M1 receptors was sufficient to generate large shoulder potentials, but a residual response remained in neurons lacking only M1 receptors. Knockout of both M1 and M3 receptors was required to fully eliminate carbachol-induced shoulder potentials in CA3 pyramidal neurons. Asterisks indicate P < 0.05 when compared with wild-type neurons (one-way ANOVA with post-tests).
Fig. 8.
Time courses of enhancement of “shoulder potentials” during tonic carbachol applications in CA3 pyramidal neurons from mice with differential mAChR expression. A–D: plots of mean shoulder potential durations measured at 5 mV above resting potentials in CA3 pyramidal neurons from wild-type mice (A), M3/M5 double-knockout mice (B), M1-knockout mice (C), and M1/M3 double-knockout mice (D).
Table 3.
Comparison of carbachol-induced changes in RMP and shoulder-potential width in hippocampal CA3 pyramidal neurons expressing different mAChR subtypes
| Genotype | Layer | n | ΔRMP, mV | ΔShoulder Width, ms |
|---|---|---|---|---|
| Wild-type (WT) | CA3 | 12 | +1.9 ± 2.0 | +1,600 ± 221 |
| M3/M5 DKO | CA3 | 11 | −0.1 ± 1.5 | +998 ± 194 |
| M1 KO | CA3 | 11 | +0.6 ± 1.2 | +313 ± 88* |
| M1/M3 DKO | CA3 | 7 | −2.9 ± 1.0* | +2 ± 2* |
Values are means ± SE. Cholinergic responses evoked by bath application of carbachol (10 μM for 5 min) in CA3 pyramidal neurons. Boldface numbers indicate P < 0.05 for carbachol-induced effects within individual cell types (repeated-measures ANOVA with post-tests: baseline, carbachol, and wash). Asterisks indicate P < 0.01 when compared with values in wild-type neurons (one-way ANOVA with post-tests).
M4 receptors mediate cholinergic suppression of Schaffer–collateral EPSPs
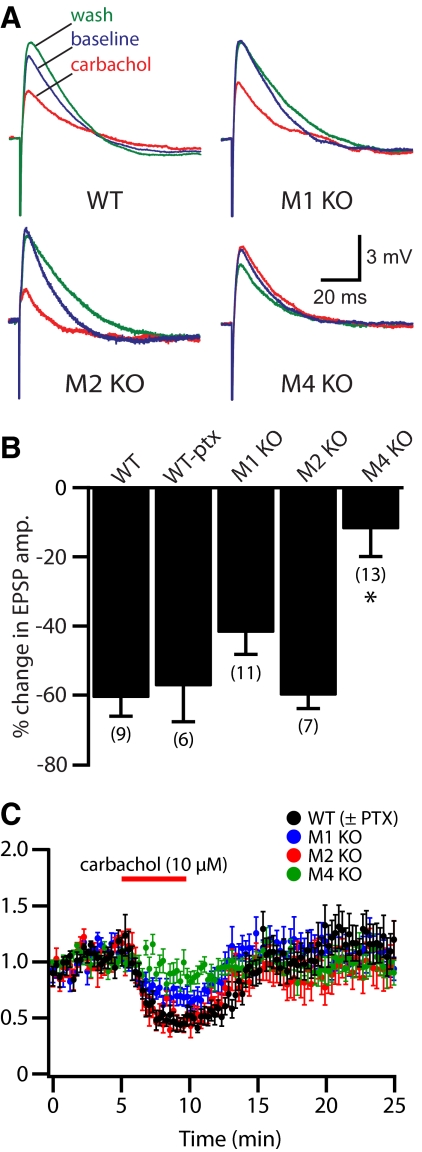
M1, M2, and M4 receptors have each been implicated in suppressing synaptic transmission between CA3 and CA1 pyramidal neurons. We tested the relative contribution of these receptors to synaptic suppression at the Schaffer–collateral synapse in CA1 neurons from wild-type and mAChR knockout mice (Fig. 9 and Table 4). In neurons from wild-type mice (n = 9), bath-applied carbachol (5 min, 10 μM) suppressed EPSP amplitudes by 61 ± 5% (P < 0.05, repeated-measures ANOVA with post-tests). In additional experiments (n = 6), picrotoxin (100 μM) was included in the bathing saline to block GABAA receptors. In the presence of picrotoxin, carbachol suppressed EPSP amplitudes by 57 ± 10% (P < 0.05), an amount similar to that observed without picrotoxin (P = 0.76, t-test; see also ANOVA in Table 4).
Fig. 9.
M4 receptors mediate cholinergic suppression of Schaffer–collateral EPSPs. A: averages of 4 consecutive Schaffer–collateral-evoked EPSPs generated in baseline conditions (blue), after a 5-min exposure to 10 μM carbachol (red), and after 15 min of wash (green). B: summary showing the mean changes in EPSP amplitudes for CA1 neurons expressing different mAChR subunits. Asterisk indicates P < 0.001 when compared with wild-type and P < 0.05 when compared with any other group (one-way ANOVA with post-tests). C: plot of normalized EPSP amplitudes over time for experiments using tissue from wild-type and mAChR knockout animals. Wild-type data include experiments with and without picrotoxin in the bath (n = 15).
Table 4.
Cholinergic suppression of Schaffer–collateral EPSPs in hippocampal CA1 pyramidal neurons expressing different mAChR subunits
| Genotype | n | Baseline EPSP Amplitude, mV | EPSP in Carbachol, mV | EPSP After Wash, mV | Percentage Change in EPSP Amplitude (10 μM CCH) |
|---|---|---|---|---|---|
| Wild-type (WT) | 9 | 4.0 ± 0.8 | 1.5 ± 0.3 | 5.0 ± 1.4 | −61 ± 5% |
| Wild-type (PTX) | 6 | 4.8 ± 1.3 | 1.5 ± 0.2 | 3.6 ± 0.5 | −57 ± 10% |
| M1 KO | 9 | 5.9 ± 0.4 | 3.5 ± 0.5 | 6.1 ± 0.4 | −42 ± 6% |
| M2 KO | 7 | 5.1 ± 0.7 | 2.1 ± 0.4 | 4.6 ± 0.9 | −60 ± 4% |
| M4 KO | 13 | 4.7 ± 0.4 | 4.2 ± 0.5 | 4.8 ± 0.5 | −12 ± 8%* |
Values are means ± SE. Cholinergic suppression of Schaffer–collateral EPSPs by bath-applied carbachol (10 μM for 5 min) in CA1 pyramidal neurons. Changes in EPSP amplitude were measured at the end of carbachol treatment and relative to values measured in baseline conditions. Numbers in boldface type indicate P < 0.05 for carbachol-induced effects within individual cell types (repeated-measures ANOVA). Asterisks indicate P < 0.01 when compared with results in wild-type neurons (one-way ANOVA with post-tests).
Bath application of carbachol also suppressed Schaffer–collateral EPSPs evoked in CA1 neurons lacking either M1 or M2 receptors (Fig. 9 and Table 4). In neurons from M1-knockout animals (n = 9), EPSP amplitudes were reduced by 42 ± 6% (n = 9; P < 0.001, repeated-measures ANOVA with post-tests), whereas EPSPs in M2-knockout neurons (n = 7) were suppressed by 60 ± 4% (P < 0.01). On the other hand, Schaffer–collateral EPSPs recorded in CA1 neurons lacking M4 receptors (n = 13) were not significantly suppressed by carbachol, with EPSPs being only slightly reduced by 12 ± 8% (P = 0.16). When the magnitudes of EPSP suppression for each genotype were compared (one-way ANOVA with post-tests), M4-lacking neurons showed significantly less EPSP suppression than that of all other groups (P < 0.05 for all between-group comparisons with M4 KO neurons), with the level of EPSP suppression among non-M4 KO groups being statistically equivalent (Table 4). These data demonstrate that M4 receptor activation is responsible for cholinergic suppression of Shaffer–collateral EPSPs.
DISCUSSION
Our results identify two mAChR subtypes that are primarily responsible for direct cholinergic modulation of pyramidal neuron excitability and connectivity in the hippocampus. Of the M1-like mAChRs, M1 receptors are both necessary and sufficient for robust phasic cholinergic modulation of pyramidal neuron excitability. M1 receptors are also sufficient, and in most cases necessary, for tonic cholinergic excitation of CA1 and CA3 pyramidal neurons. On the other hand, cholinergic suppression of synaptic transmission between CA3 and CA1 pyramidal neurons requires M4 receptors.
Cholinergic modulation of hippocampal pyramidal neuron excitability
Acetylcholine has historically been considered an excitatory neurotransmitter in the hippocampus (Benardo and Prince 1981, 1982; Benson et al. 1988; Bird and Aghajanian 1976; Biscoe and Straughan 1966; Cole and Nicoll 1983, 1984; Colino and Halliwell 1993; Dodd et al. 1981; Fraser and MacVicar 1996; Haas 1982; Young et al. 2005). Cholinergic excitation of hippocampal pyramidal neurons requires activation of muscarinic, rather than nicotinic, receptors (Frazier et al. 1998; McQuiston and Madison 1999) and M1-like mAChRs have been implicated based on pharmacological (Benson et al. 1988; Dutar and Nicoll 1988; Scuvee-Moreau et al. 1997; Segal and Fisher 1992) and biochemical signaling studies (Bymaster et al. 2003; Porter et al. 2002; Young et al. 2005). Our data demonstrate that M1 receptors are critical for phasic cholinergic modulation of excitability. CA1 neurons lacking M1 receptors did not exhibit phasic cholinergic inhibition and CA1 and CA3 neurons from M1-knockout animals demonstrated only limited ACh-induced spike acceleration (Table 1). Residual spike acceleration in M1-lacking neurons was fully accounted for by M3 receptors because it was absent in neurons from M1/M3 double-knockout mice. Because our experiments used focal ACh applications near the soma, it remains possible that other mAChR subtypes could mediate local responses in distal dendritic compartments. However, previous work in neocortical pyramidal neurons suggests phasic responses preferentially occur proximal to the soma, with responses absent when ACh was applied to dendrites beyond 100 μm from the soma (Gulledge et al. 2009).
M1 receptors also mediate tonic cholinergic effects, yet carbachol-induced depolarization of CA1 neurons was eliminated only in neurons from M1/M3 double-knockout mice. This suggests that M1 and M3 receptors are each independently capable of depolarizing CA1 neurons. Similarly, using M1-knockout mice, Rouse et al. (2000) found no deficit of cholinergic suppression of the potassium conductances thought responsible for cholinergic depolarization. However, in their experiments, knockout of the M1 receptor failed to block reduction of the mAHP by carbachol (Rouse et al. 2000), whereas we found M1 receptors necessary for mAHP reduction. The reasons for the different results are unclear, but could involve varying amounts of compensatory processes occurring in the two M1-knockout mice (but see following text). Our findings that M1 receptors are centrally involved in cholinergic signaling are consistent with their prominent expression in hippocampal pyramidal neurons (Yamasaki et al. 2010). Indeed, M1 receptors make up about 60% of the total mAChR content of the hippocampus, whereas M3 receptors make up <10% of mAChRs (Flynn et al. 1995; Levey et al. 1995), and M5 receptors, which are associated with vascular tissue (Araya et al. 2006), are only sparsely expressed.
We found that M1 receptors also modulate the “early” portion of slow AHPs occurring within the first few seconds after spike trains in CA1 pyramidal neurons. Later components of the sAHP were less sensitive to cholinergic stimulation. Our results are consistent with earlier reports of cholinerigic modulation of short-duration sAHPs lasting up to several seconds (Benardo and Prince 1982; Krause et al. 2002; Madison et al. 1987; Sah and Isaacson 1995). However, the differential impact of carbachol on the early and late sAHP suggests that the sAHP of CA1 neurons may comprise several components relying on distinct ionic mechanisms, as described in neocortical pyramidal neurons (Schwindt et al. 1988). A more comprehensive study, exploring a full range of experimental conditions, will be required to fully characterize cholinergic modulation of the sAHP.
As in all studies using constitutive gene “knockout,” it is possible that compensatory mechanisms activated during development will complicate characterization of the functional role of targeted genes. However, in this study, and in our previous work in neocortex (Gulledge et al. 2009), we found clear and often complete deficits in cholinergic signaling following the elimination of a single gene (the M1 receptor), with little, if any, reduction in responses in the absence of both M3 and M5 receptors. This suggests compensatory regulation of mAChR expression is limited (see also Hamilton et al. 1997) and enhances our confidence that M1 receptors normally mediate most, if not all, postsynaptic cholinergic responses in hippocampal pyramidal neurons.
However, the mechanisms linking M1 receptors to physiological responses in pyramidal neurons have not been fully characterized. ACh-induced depolarization and the reduction of spike-frequency adaptation likely result from Gq- and phospholipase C–dependent inhibition of the M-current (Aiken et al. 1995; Brown and Adams 1980; Suh and Hille 2002), a potassium conductance mediated by KCNQ channels (Shah et al. 2002; Wang et al. 1998). Inhibition of potassium conductances also underlies cholinergic modulation of the mAHP (Storm 1989) and sAHP (Sah and Isaacson 1995), although consensus regarding the ion channels involved in these processes remains elusive. Also elusive is the identity of the nonspecific cation channel(s) mediating the ADPs (in CA1 neurons) and shoulder potentials (in CA3 neurons) and the relative contribution of nonspecific cation channels to phasic cholinergic spike acceleration and AHP suppression. Better understood is the ionic mechanism underlying phasic cholinerigic inhibition, which results from Gq-induced calcium release from internal stores and subsequent activation of SK-type calcium-dependent potassium channels (Gulledge and Stuart 2005).
Cholinergic suppression of excitatory synaptic transmission
In addition to modulating pyramidal neuron excitability, mAChR activation inhibits glutamate release at the CA3-to-CA1 synapse (Fernandez de Sevilla and Buno 2003; Fernandez de Sevilla et al. 2002; Hasselmo and Schnell 1994; Hounsgaard 1978; Scanziani et al. 1995; Segal 1982; Valentino and Dingledine 1981). Previous studies have implicated M1 (Kremin et al. 2006; Leung and Péloquin 2010; Sheridan and Sutor 1990; Shinoe et al. 2005), M2 (Dutar and Nicoll 1988; Segal 1989), and M4 receptors (Shirey et al. 2008) as mediating cholinergic suppression of glutamate release. We found that M4 receptors are required for normal cholinergic suppression of synaptic transmission at the Schaffer–collateral synapse. Our results are consistent with data showing M4 receptors are present at high densities in stratum radiatum (Levey et al. 1995) and that allosteric modulation of M4 receptors enhances carbachol-induced EPSP suppression (Shirey et al. 2008). On the other hand, our data and those of Shirey et al. (2008) contrast sharply with those of Sánchez et al. (2009) who found pharmacological blockade of M4 receptors alone suppresses evoked Shaffer–collateral field potentials, suggesting that ambient endogenous ACh acting at M4 receptors potentiates glutamate release at this synapse. Discrepancies in results likely reflect critical differences in experimental setup, including the inclusion by Sánchez et al. (2009) of the adenosine receptor agonist 2-chloroadenosine in the bath saline (which might occlude muscarinic suppression of EPSPs by activating presynaptic adenosine receptors; see Lupica et al. 1992) and the use by Sánchez and colleagues of organotypic cultures rather than acute hippocampal slice preparations from more mature animals.
Although several studies have implicated M1 receptors in presynaptic inhibition of glutamate release, direct involvement of M1 receptors is unlikely because they are not highly expressed in hippocampal presynaptic boutons (Yamasaki et al. 2010). It is possible that previous reports finding a role for M1 receptors in synaptic suppression overly relied on the specificity of pirenzepine (Leung and Péloquin 2010; Sheridan and Sutor 1990), an antagonist often considered specific to M1 receptors, but that also has a high affinity for M4 receptors (Caulfield and Birdsall 1998). Alternatively, the limited reduction in carbachol-induced synaptic inhibition observed in M1-knockout mice (Kremin et al. 2006; Shinoe et al. 2005) might result from a loss of M1-dependent postsynaptic endocannabinoid release (Colgin et al. 2003; Kim et al. 2002; Ohno-Shosaku et al. 2003; Takahashi and Castillo 2006) or M1 receptor-mediated changes in postsynaptic membrane conductance that may influence the amplitude of somatically recorded EPSPs (Gulledge et al. 2005). Also possible is M1-knockout-induced compensatory down-regulation of EPSP-suppressing M4 mAChRs that would otherwise further decrease excitatory drive (Hamilton et al. 1997). Regardless of the mechanisms involved, we found carbachol-induced EPSP suppression in M1-knockouts to be somewhat less (42% reduction) than that in wild-type neurons (61% reduction). Comparable amounts of cholinergic EPSP suppression were found in wild-type and M1-knockout neurons by Shinoe et al. (2005) and Kremin et al. (2006). However, when the available data are viewed together, M4 receptors are clearly the major obligatory contributor to cholinergic suppression of EPSPs at the CA3-to-CA1 synapse.
Parallels between hippocampus and neocortex
The hippocampus, piriform cortex, and neocortex share many organizational features, including physiological and morphological characteristics of excitatory and inhibitory neuron types, local connectivity patterns, and subcortical neuromodulatory inputs. These cortical areas receive afferents from cholinergic neurons in the basal forebrain (Woolf 1991) and analogous cell types in neocortex and hippocampus exhibit similar physiological responses to ACh (Gulledge and Kawaguchi 2007; Lawrence 2008). We can now show that pyramidal neurons in both the neocortex and hippocampus respond to ACh primarily via M1 receptors, with only minor involvement of M3 receptors (Gulledge et al. 2009). Interestingly, phasic cholinergic inhibition preferentially occurs in pyramidal neurons that project out of the cortex (CA1 and layer 5 neocortical neurons), with pyramidal neurons whose connectivity is largely confined within the cortex (CA3 and layer 2/3 pyramidal neurons) only excited by ACh (Gulledge and Kawaguchi 2007; Gulledge et al. 2007, 2009; but see Segal 1982). Muscarinic receptors also suppress glutamate release at synapses in the hippocampus, piriform cortex, and neocortex (Gil et al. 1997; Hasselmo and Bower 1992; Hsieh et al. 2000; Levy et al. 2006; Vidal and Changeux 1993), further suggesting conservation of cholinergic mechanisms across cortical areas.
However, not all aspects of cholinergic signaling are identical in hippocampal and neocortical pyramidal neurons. For instance, in layer 5 neocortical neurons, cholinergic suppression of the mAHP requires activation of both M1 and M3 receptors (Gulledge et al. 2009), whereas carbachol-induced depolarizations and ADPs require only M1 receptors. We found in CA1 neurons that mAHP suppression and ADP genesis depend solely on M1 receptors, but that carbachol-induced depolarizations can be initiated by activation of either M1 or M3 receptors. In addition, ACh-induced spike acceleration in intrinsically connected CA3 pyramidal neurons was more robust than that observed in their neocortical analogs, layer 2/3 neocortical neurons (Gulledge et al. 2009), and excitatory responses to tonic mAChR stimulation were qualitatively different in these two cell types. In spite of these differences, it is clear that most cholinergic mechanisms in pyramidal neurons in the neocortex and hippocampus are similar and, in the case of postsynaptic effects, rely primarily on activation of M1 receptors.
Functional significance
Our results suggest a surge of acetylcholine release in the hippocampus may selectively enhance the output of CA3 pyramidal neurons receiving suprathreshold excitatory input. On the other hand, CA1 neurons would be transiently hyperpolarized to a common RMP via M1 receptor-dependent SK-channel activation, establishing a “level playing field” from which subsequent synaptic integration of excitatory input can occur. At the same time, ACh acting at presynaptic M4 receptors will inhibit glutamate release at Schaffer–collateral inputs to further limit excitatory drive. Cholinergic synaptic suppression of Schaffer–collateral inputs may tip the balance of afferent input to CA1 neurons, perhaps favoring ACh-resistant medial perforant path inputs to the distal apical dendrites (Hasselmo and Schnell 1994). Additionally, presynaptic suppression of Schaffer–collateral inputs, combined with postsynaptic M1-dependent excitation, may enhance signal-to-noise in CA1 pyramidal neurons by increasing the number of afferent inputs necessary to reach threshold while amplifying the resulting action potential output. Such a mechanism has recently been proposed to explain how endogenous ACh sharpens the place fields of CA1 pyramidal neurons in vivo (Brazhnik et al. 2003, 2004).
GRANTS
This work was supported by Public Health Service/National Institute of Mental Health Grant R01 MH-83806 and the Neuroscience Center at Dartmouth.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Dr. Minoru Matsui for providing the muscarinic receptor knockout mice used in this study and M. Li, D. Avesar, G. St. Germain, and A. Rudkin for technical assistance.
REFERENCES
- Aiken SP, Lampe BJ, Murphy PA, Brown BS. Reduction of spike frequency adaptation and blockade of M-current in rat CA1 pyramidal neurones by linopirdine (DuP 996), a neurotransmitter release enhancer. Br J Pharmacol 115: 1163–1168, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya R, Noguchi T, Yuhki M, Kitamura N, Higuchi M, Saido TC, Seki K, Itohara S, Kawano M, Tanemura K, Takashima A, Yamada K, Kondoh Y, Kanno I, Wess J, Yamada M. Loss of M5 muscarinic acetylcholine receptors leads to cerebrovascular and neuronal abnormalities and cognitive deficits in mice. Neurobiol Dis 24: 334–344, 2006 [DOI] [PubMed] [Google Scholar]
- Aznavour N, Watkins KC, Descarries L. Postnatal development of the cholinergic innervation in the dorsal hippocampus of rat: Quantitative light and electron microscopic immunocytochemical study. J Comp Neurol 486: 61–75, 2005 [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science 217: 408–414, 1982 [DOI] [PubMed] [Google Scholar]
- Benardo LS, Prince DA. Acetylcholine induced modulation of hippocampal pyramidal neurons. Brain Res 211: 227–234, 1981 [DOI] [PubMed] [Google Scholar]
- Benardo LS, Prince DA. Cholinergic excitation of mammalian hippocampal pyramidal cells. Brain Res 249: 315–333, 1982 [DOI] [PubMed] [Google Scholar]
- Benson DM, Blitzer RD, Landau EM. An analysis of the depolarization produced in guinea-pig hippocampus by cholinergic receptor stimulation. J Physiol 404: 479–496, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird SJ, Aghajanian GK. The cholinergic pharmacology of hippocampal pyramidal cells: a microiontophoretic study. Neuropharmacology 15: 273–282, 1976 [DOI] [PubMed] [Google Scholar]
- Biscoe TJ, Straughan DW. Micro-electrophoretic studies of neurones in the cat hippocampus. J Physiol 183: 341–359, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazhnik ES, Borgnis R, Muller RU, Fox SE. The effects on place cells of local scopolamine dialysis are mimicked by a mixture of two specific muscarinic antagonists. J Neurosci 24: 9313–9323, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazhnik ES, Muller RU, Fox SE. Muscarinic blockade slows and degrades the location-specific firing of hippocampal pyramidal cells. J Neurosci 23: 611–621, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature 283: 673–676, 1980 [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Carter PA, Yamada M, Gomeza J, Wess J, Hamilton SE, Nathanson NM, McKinzie DL, Felder CC. Role of specific muscarinic receptor subtypes in cholinergic parasympathomimetic responses, in vivo phosphoinositide hydrolysis, and pilocarpine-induced seizure activity. Eur J Neurosci 17: 1403–1410, 2003 [DOI] [PubMed] [Google Scholar]
- Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50: 279–290, 1998 [PubMed] [Google Scholar]
- Cobb SR, Davies CH. Cholinergic modulation of hippocampal cells and circuits. J Physiol 562: 81–88, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science 221: 1299–1301, 1983 [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol 352: 173–188, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Kramar EA, Gall CM, Lynch G. Septal modulation of excitatory transmission in hippocampus. J Neurophysiol 90: 2358–2366, 2003 [DOI] [PubMed] [Google Scholar]
- Colino A, Halliwell JV. Carbachol potentiates Q current and activates a calcium-dependent non-specific conductance in rat hippocampus in vitro. Eur J Neurosci 5: 1198–1209, 1993 [DOI] [PubMed] [Google Scholar]
- Dodd J, Dingledine R, Kelly JS. The excitatory action of acetylcholine on hippocampal neurones of the guinea pig and rat maintained in vitro. Brain Res 207: 109–127, 1981 [DOI] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. Classification of muscarinic responses in hippocampus in terms of receptor subtypes and second-messenger systems: electrophysiological studies in vitro. J Neurosci 8: 4214–4224, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Porter AC, Skillman TL, Zhang L, Bymaster FP, Nathanson NM, Hamilton SE, Gomeza J, Wess J, McKinzie DL. Elucidating the role of muscarinic receptors in psychosis. Life Sci 68: 2605–2613, 2001 [DOI] [PubMed] [Google Scholar]
- Fernandez de Sevilla D, Buno W. Presynaptic inhibition of Schaffer collateral synapses by stimulation of hippocampal cholinergic afferent fibres. Eur J Neurosci 17: 555–558, 2003 [DOI] [PubMed] [Google Scholar]
- Fernandez de Sevilla D, Cabezas C, de Prada AN, Sanchez-Jimenez A, Buno W. Selective muscarinic regulation of functional glutamatergic Schaffer collateral synapses in rat CA1 pyramidal neurons. J Physiol 545: 51–63, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn DD, Ferrari-DiLeo G, Mash DC, Levey AI. Differential regulation of molecular subtypes of muscarinic receptors in Alzheimer's disease. J Neurochem 64: 1888–1891, 1995 [DOI] [PubMed] [Google Scholar]
- Fraser DD, MacVicar BA. Cholinergic-dependent plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci 16: 4113–4128, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an alpha-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci 18: 1187–1195, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron 19: 679–686, 1997 [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Bucci DJ, Zhang SS, Matsui M, Yeh HH. M1 receptors mediate cholinergic modulation of excitability in neocortical pyramidal neurons. J Neurosci 29: 9888–9902, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Kampa BM, Stuart GJ. Synaptic integration in dendritic trees. J Neurobiol 64: 75–90, 2005 [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Kawaguchi Y. Phasic cholinergic signaling in the hippocampus: functional homology with the neocortex? Hippocampus 17: 327–332, 2007 [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Park SB, Kawaguchi Y, Stuart GJ. Heterogeneity of phasic cholinergic signalling in neocortical neurons. J Neurophysiol 97: 2215–2229, 2007 [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Stuart GJ. Cholinergic inhibition of neocortical pyramidal neurons. J Neurosci 25: 10308–10320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas HL. Cholinergic disinhibition in hippocampal slices of the rat. Brain Res 233: 200–204, 1982 [DOI] [PubMed] [Google Scholar]
- Hamilton SE, Loose MD, Qi M, Levey AI, Hille B, McKnight GS, Idzerda RL, Nathanson NM. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci USA 94: 13311–13316, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Cholinergic suppression specific to intrinsic not afferent fiber synapses in rat piriform (olfactory) cortex. J Neurophysiol 67: 1222–1229, 1992 [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E. Laminar selectivity of the cholinergic suppression of synaptic transmission in rat hippocampal region CA1: computational modeling and brain slice physiology. J Neurosci 14: 3898–3914, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J. Presynaptic inhibitory action of acetylcholine in area CA1 of the hippocampus. Exp Neurol 62: 787–797, 1978 [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Cruikshank SJ, Metherate R. Differential modulation of auditory thalamocortical and intracortical synaptic transmission by cholinergic agonist. Brain Res 880: 51–64, 2000 [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol 504: 603–610, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci 22: 10182–10191, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Offermanns S, Stocker M, Pedarzani P. Functional specificity of G alpha q and G alpha 11 in the cholinergic and glutamatergic modulation of potassium currents and excitability in hippocampal neurons. J Neurosci 22: 666–673, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremin T, Gerber D, Giocomo LM, Huang SY, Tonegawa S, Hasselmo ME. Muscarinic suppression in stratum radiatum of CA1 shows dependence on presynaptic M1 receptors and is not dependent on effects at GABA(B) receptors. Neurobiol Learn Mem 85: 153–163, 2006 [DOI] [PubMed] [Google Scholar]
- Lawrence JJ. Cholinergic control of GABA release: emerging parallels between neocortex and hippocampus. Trends Neurosci 31: 317–327, 2008 [DOI] [PubMed] [Google Scholar]
- Leung LS, Péloquin P. Cholinergic modulation differs between basal and apical dendritic excitation of hippocampal CA1 pyramidal cells. Cereb Cortex 20: 1865–1877, 2010 [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci 15: 4077–4092, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy RB, Reyes AD, Aoki C. Nicotinic and muscarinic reduction of unitary excitatory postsynaptic potentials in sensory cortex; dual intracellular recording in vitro. J Neurophysiol 95: 2155–2166, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Proctor WR, Dunwiddie TV. Presynaptic inhibition of excitatory synaptic transmission by adenosine in rat hippocampus: analysis of unitary EPSP variance measured by whole-cell recording. J Neurosci 12: 3753–3764, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Lancaster B, Nicoll RA. Voltage clamp analysis of cholinergic action in the hippocampus. J Neurosci 7: 733–741, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol 354: 319–331, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M, Motomura D, Karasawa H, Fujikawa T, Jiang J, Komiya Y, Takahashi S, Taketo MM. Multiple functional defects in peripheral autonomic organs in mice lacking muscarinic acetylcholine receptor gene for the M3 subtype. Proc Natl Acad Sci USA 97: 9579–9584, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston AR, Madison DV. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J Neurosci 19: 2887–2896, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton RA, Davies CH. Regulation of muscarinic acetylcholine receptor-mediated synaptic responses by adenosine receptors in the rat hippocampus. J Physiol 502: 75–90, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller W, Misgeld U, Heinemann U. Carbachol effects on hippocampal neurons in vitro: dependence on the rate of rise of carbachol tissue concentration. Exp Brain Res 72: 287–298, 1988 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Matsui M, Uchida K, Futatsugi A, Kusakawa S, Matsumoto N, Nakamura K, Manabe T, Taketo MM, Mikoshiba K. M(3) muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice. J Physiol 558: 561–575, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Matsui M, Fukudome Y, Shosaku J, Tsubokawa H, Taketo MM, Manabe T, Kano M. Postsynaptic M1 and M3 receptors are responsible for the muscarinic enhancement of retrograde endocannabinoid signalling in the hippocampus. Eur J Neurosci 18: 109–116, 2003 [DOI] [PubMed] [Google Scholar]
- Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci 22: 273–280, 1999 [DOI] [PubMed] [Google Scholar]
- Porter AC, Bymaster FP, DeLapp NW, Yamada M, Wess J, Hamilton SE, Nathanson NM, Felder CC. M1 muscarinic receptor signaling in mouse hippocampus and cortex. Brain Res 944: 82–89, 2002 [DOI] [PubMed] [Google Scholar]
- Reever CM, Ferrari-DiLeo G, Flynn DD. The M5 (m5) receptor subtype: fact or fiction? Life Sci 60: 1105–1112, 1997 [DOI] [PubMed] [Google Scholar]
- Rouse ST, Hamilton SE, Potter LT, Nathanson NM, Conn PJ. Muscarinic-induced modulation of potassium conductances is unchanged in mouse hippocampal pyramidal cells that lack functional M1 receptors. Neurosci Lett 278: 61–64, 2000 [DOI] [PubMed] [Google Scholar]
- Sah P, Isaacson JS. Channels underlying the slow afterhyperpolarization in hippocampal pyramidal neurons: neurotransmitters modulate the open probability. Neuron 15: 435–441, 1995 [DOI] [PubMed] [Google Scholar]
- Sánchez G, Alvares Lde O, Oberholzer MV, Genro B, Quillfeldt J, da Costa JC, Cerveñansky C, Jerusalinsky D, Kornisiuk E. M4 muscarinic receptors are involved in modulation of neurotransmission at synapses of Schaffer collaterals on CA1 hippocampal neurons in rats. J Neurosci Res 87: 691–700, 2009 [DOI] [PubMed] [Google Scholar]
- Scanziani M, Gahwiler BH, Thompson SM. Presynaptic inhibition of excitatory synaptic transmission by muscarinic and metabotropic glutamate receptor activation in the hippocampus: are Ca2+ channels involved? Neuropharmacology 34: 1549–1557, 1995 [DOI] [PubMed] [Google Scholar]
- Schwindt PC, Spain WJ, Foehring RC, Chubb MC, Crill WE. Slow conductances in neurons from cat sensorimotor cortex in vitro and their role in slow excitability changes. J Neurophysiol 59: 450–467, 1988 [DOI] [PubMed] [Google Scholar]
- Scuvee-Moreau J, Seutin V, Vrijens B, Pirotte B, De Tullio P, Massotte L, Albert A, Delarge J, Dresse A. Effect of potassium channel openers on the firing rate of hippocampal pyramidal cells and A10 dopaminergic neurons in vitro. Arch Physiol Biochem 105: 421–428, 1997 [DOI] [PubMed] [Google Scholar]
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J. M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci 24: 10117–10127, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Multiple action of acetylcholine at a muscarinic receptor studied in the rat hippocampal slice. Brain Res 246: 77–87, 1982 [DOI] [PubMed] [Google Scholar]
- Segal M. Presynaptic cholinergic inhibition in hippocampal cultures. Synapse 4: 305–312, 1989 [DOI] [PubMed] [Google Scholar]
- Segal M, Fisher A. AF102B, a muscarinic M1 receptor agonist, mimics some effects of acetylcholine on neurons of rat hippocampus slices. Eur J Pharmacol 220: 103–106, 1992 [DOI] [PubMed] [Google Scholar]
- Shah MM, Mistry M, Marsh SJ, Brown DA, Delmas P. Molecular correlates of the M-current in cultured rat hippocampal neurons. J Physiol 544: 29–37, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan RD, Sutor B. Presynaptic M1 muscarinic cholinoceptors mediate inhibition of excitatory synaptic transmission in the hippocampus in vitro. Neurosci Lett 108: 273–278, 1990 [DOI] [PubMed] [Google Scholar]
- Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci 25: 11194–11200, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirey JK, Xiang Z, Orton D, Brady AE, Johnson KA, Williams R, Ayala JE, Rodriguez AL, Wess J, Weaver D, Niswender CM, Conn PJ. An allosteric potentiator of M4 mAChR modulates hippocampal synaptic transmission. Nat Chem Biol 4: 42–50, 2008 [DOI] [PubMed] [Google Scholar]
- Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol 409: 171–190, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron 35: 507–520, 2002 [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Castillo PE. The CB1 cannabinoid receptor mediates glutamatergic synaptic suppression in the hippocampus. Neuroscience 139: 795–802, 2006 [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Dingledine R. Presynaptic inhibitory effect of acetylcholine in the hippocampus. J Neurosci 1: 784–792, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal C, Changeux JP. Nicotinic and muscarinic modulations of excitatory synaptic transmission in the rat prefrontal cortex in vitro. Neuroscience 56: 23–32, 1993 [DOI] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 282: 1890–1893, 1998 [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37: 475–524, 1991 [DOI] [PubMed] [Google Scholar]
- Yamasaki M, Matsui M, Watanabe M. Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J Neurosci 30: 4408–4418, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KW, Billups D, Nelson CP, Johnston N, Willets JM, Schell MJ, Challiss RA, Nahorski SR. Muscarinic acetylcholine receptor activation enhances hippocampal neuron excitability and potentiates synaptically evoked Ca(2+) signals via phosphatidylinositol 4,5-bisphosphate depletion. Mol Cell Neurosci 30: 48–57, 2005 [DOI] [PubMed] [Google Scholar]