Abstract
Topographically organized neurons represent multiple stimuli within complex visual scenes and compete for subsequent processing in higher visual centers. The underlying neural mechanisms of this process have long been elusive. We investigate an experimentally constrained model of a midbrain structure: the optic tectum and the reciprocally connected nucleus isthmi. We show that a recurrent antitopographic inhibition mediates the competitive stimulus selection between distant sensory inputs in this visual pathway. This recurrent antitopographic inhibition is fundamentally different from surround inhibition in that it projects on all locations of its input layer, except to the locus from which it receives input. At a larger scale, the model shows how a focal top-down input from a forebrain region, the arcopallial gaze field, biases the competitive stimulus selection via the combined activation of a local excitation and the recurrent antitopographic inhibition. Our findings reveal circuit mechanisms of competitive stimulus selection and should motivate a search for anatomical implementations of these mechanisms in a range of vertebrate attentional systems.
INTRODUCTION
The process of vision as the competitive interaction in a dynamical neural system is poorly understood (Rabinovich et al. 2008). Spike trains from topographically organized neurons in early visual pathways represent the occurrence of a stimulus in the retinal image. When multiple visual stimuli appear at different locations (Fig. 1), the neural populations compete for dominance, and only the winning representations propagate to higher visual centers for further processing (Desimone and Duncan 1995; Kastner and Ungerleider 2000). Such competitive neural interaction is thought to mediate the selection of the most salient stimulus in a given parameter space from complex visual scenes (Itti and Koch 2001; Knudsen 2007).
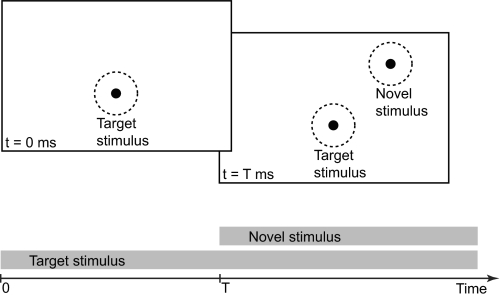
Fig. 1.
Presentation of multiple visual stimuli. The presentation of a target (black dot) at t = 0 ms within a neuron's receptive field (dashed circle), followed by the presentation of a novel stimulus (black dot) at a later time, t = T ms, at a distant location within another neuron's receptive field (dashed circle). The timings of the 2 stimuli are indicated by the gray bars.
Understanding competitive neural interaction in terms of the neurobiological components and the systems dynamics is a major goal in neuroscience. A prime candidate mechanism for competition among inputs is mutual inhibition (Mao and Massaquoi 2007; Sum et al. 1999), which is present in phenomenological models of shifting attention aimed at selecting salient stimuli from visual scenes (Koch and Ullman 1985; Lee et al. 1999; Olshausen et al. 1993; Reynolds et al. 1999; Usher and Niebur 1996). Here we present a comprehensive microcircuit-level investigation of competitive input selection for time-varying stimuli (Fig. 1) within a concrete neural circuit, the superior colliculus, a midbrain structure that receives sensory information and directs the animal's gaze and attention (Bisley 2010; Hall and Moschovakis 2004; Mulckhuyse and Theeuwes 2010; Stein and Meredith 1993).
The superior colliculus represents the locations of stimuli as a topographic map of space and participates in stimulus selection when competing visual stimuli are present (Ignashchenkova et al. 2004; Li and Basso 2005; Lovejoy and Krauzlis 2010; McPeek and Keller 2004; Müller et al. 2005). Similarly, the avian optic tectum (homolog of the superior colliculus in mammals) and its satellite, the nucleus isthmi (homolog of the parabigeminal nucleus in mammals) participate in stimulus competition (Asadollahi et al. 2010; Marin et al. 2007; Mysore et al. 2010), which is further modulated by top-down inputs from a forebrain region (Winkowski and Knudsen 2008). Within the avian isthmotectal circuitry (Wang et al. 2004, 2006), we consider the tectal layer 10 neurons (L10), the parvocellular (Ipc), and two types of magnocellular (Imc) isthmic neurons (Fig. 2A). In this circuit, the GABAergic Imc projection to the optic tectum displays a heterotopic organization (Wang et al. 2004), i.e., a given Imc neuron does not project back to the locus in the optic tectum from which it receives L10 input, but projects on all other locations in that layer. In this sense, the Imc projection to the optic tectum can be termed antitopographic.
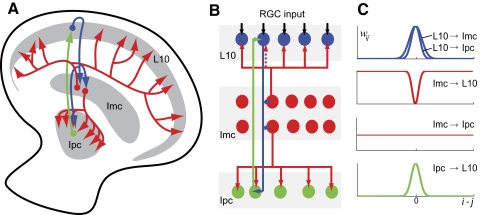
Fig. 2.
Anatomical features of the avian isthmotectal system. A: schematic of the connectivity pattern as seen in a transverse section of the optic tectum and nucleus isthmi. Tectal layer 10 (L10) neurons (blue) receive retinal inputs (data not shown) in upper tectal layers and project topographically to the parvocellular (Ipc, green) and magnocellular (Imc, red) isthmic nuclei. The Ipc feedback projection to the optic tectum is topographic, whereas the Imc feedback projection is antitopographic. In addition, Imc neurons mediate a global projection to the Ipc nucleus. B: schematic of the model circuitry consisting of retinal ganglion cell inputs (RGC, black) and L10 (blue), Ipc (green), and 2 morphological types of Imc (red) neurons. C: in the population model, the topographies of the 5 projections are described by Gaussian spatial distributions of the synaptic weights, wij.
We designed an experimentally constrained model network of the isthmotectal system (Fig. 2, B and C) and undertook a detailed investigation of the structural and physiological parameter space relevant for stimulus competition. Specifically, we show how the superposition of topographic and antitopographic visual information emerges as a key organizational feature of the isthmotectal system, how the antitopographic projection mediates competitive stimulus selection, and how localized top-down inputs modulate this process. The model reproduces in vivo observations of stimulus competition in owl and pigeon and generates experimentally testable and nontrivial predictions. In addition, a model investigation beyond the experimental constraints reveals an alternative mechanism of competitive stimulus selection in a network with homogeneous inhibition and strong topographic excitation.
METHODS
The model network consists of four linear arrays; L10 and Ipc neurons and two types of Imc neurons (Fig. 2). Each array contains 300 neurons. These neurons respond to somatic current injection with regular spiking and show spike-rate adaptation and linear frequency-current curves (Shao et al. 2009). Therefore each neuron in the network is modeled as leaky integrate-and-fire type, including a spike-rate adaptation conductance. In short, the membrane potential Vi of neuron i evolves according to the differential equation, . When the membrane potential Vi reaches the threshold Vθ,i, it is instantaneously reset to Vreset,i, which is interpreted as the occurrence of a spike. The basic cellular parameters are the threshold Vθ,i, the reset potential Vreset,i, the resting membrane potential Ei, the membrane input resistance Ri, and the membrane time constant τi.
Each neuron receives a spike-rate adaptation current Isra,i, a sum of synaptic currents Is,i, and a noise current Inoise,i. The L10 neurons also receive an external excitatory current Ie,i, which represents the stimulus from the retinal ganglion cells. The spike-rate adaptation current is given by Isra,i = gsra,i(t)(Vi − Esra,i) and has the adaptation reversal potential Esra,i. The spike-rate adaptation conductance gsra,i increases by an amount Δgsra,i immediately after a spike, i.e., gsra,i(t+) → gsra,i(t−) + Δgsra,i, and subsequently decays exponentially with adaptation time constant τsra,i, i.e., , until the next spike occurs.
The synaptic current from neuron j to neuron i is proportional to the open probability Pij of the synaptic conductance, where gij is the maximum synaptic conductance, Eij is the synaptic reversal potential, and wij is the weight matrix for the given network. The open probability has the form , where the normalization factor Bij ensures that the peak value of Pij generated by a single spike equals 1. The time constants τ1,ij (fall time) and τ2,ij (τ1,ij > τ2,ij) determine the time course of synaptic current. The synaptic rise time is given by τrise,ij = τ1,ijτ2,ij/(τ1,ij − τ2,ij). The variable tjk represents the time at which neuron j generates the spike k. A summation is performed over all spikes generated by neuron j.
Unless stated otherwise, parameter values of model neurons and synapses are based on previous studies (Shao et al. 2009), where parameter values were tuned within their experimental constraints until the results of model neuron simulations matched results from in vitro intracellular recordings. All conductances are expressed in terms of an average membrane conductance, gm = 2.78 nS. For the basic cellular parameters, the values are as follows: Vθ,L10=−39 mV, Vθ,Ipc=−40 mV, Vθ,Imc=−40 mV, Vreset,L10=−50 mV, Vreset,Ipc=−50 mV, Vreset,Imc=−60 mV, EL10=−55 mV, EIpc=−61 mV, EImc=−64 mV, RL10=480 MΩ, RIpc=135 MΩ, RImc=240 MΩ, τL10=104 ms, τIpc=25 ms, τImc=50 ms. For the spike-rate adaptation the parameter values are: τsra,L10=50 ms, τsra,Ipc=60 ms, τsra,Imc=80 ms, Δgsra,L10=0.375gm, Δgsra,Ipc=2.93gm, Δgsra,Imc=2.25gm, and Esra,L10=Esra,Ipc=Esra,Imc=−70 mV. The synaptic time constants for the excitatory synapses are: τ1,L10→Ipc=τ1,L10→Imc=7.6 ms, τ2,L10→Ipc=τ2,L10→Imc=0.47 ms, τ1,Ipc→L10=10.0 ms, τ2,Ipc→L10=1.0 ms (Shao et al. 2009). The synaptic time constants for the Imc projections are τ1,Imc→L10/Ipc=5.6 ms and τ2,Imc→L10/Ipc=0.3 ms, which are commonly used for GABAergic synapses (Destexhe et al. 1994). Autoradiographic studies indicate that the avian isthmotectal system is rich in GABAA receptors and that GABAB receptors are also present (Veenman et al. 1994). The potential contribution of GABAB receptors to the network dynamics is not considered in this study. The synaptic reversal potentials EL10→Ipc/Imc=0.0 mV, EImc→L10/Ipc=−80.0 mV, and EIpc→L10=−5.0 mV are consistent with literature values for excitatory and inhibitory synapses (Koch 1999) and with in vivo electrophysiological studies, which suggest that GABA acts as an inhibitory neurotransmitter in this system (Felix et al. 1994). The maximum synaptic conductances for the L10 projections are held fixed, gL10→Ipc = 2.1gm and gL10→Imc = 1.5gm. All others are specified in the text.
The anatomical features of the isthmotectal system (Wang et al. 2004, 2006) are incorporated in the weight matrix, wij. The synaptic conductances of the topographic projections (L10 → Ipc, L10 → Imc, Ipc → L10) are assumed to be described by a Gaussian distribution. For instance, the topographic L10 → Ipc projection from L10 neuron j to Ipc neuron i follows a Gaussian distribution, , where ΔL10→Ipc describes the width of the distribution (Fig. 2C). The other two topographic projections, L10 → Imc and Ipc → L10, are generated in the same manner except with different width parameters ΔL10→Imc and ΔIpc→L10, respectively. The chosen widths of the three topographic projections are ΔL10→Ipc = ΔIpc→L10 = 11 and ΔL10→Imc = 16. The antitopographic Imc → L10 projection is generated according to an inverted Gaussian distribution , which dips near i = j. The distribution is specified by two parameters: the width ΔImc→L10 and the depth D of the dip. The synaptic conductance from Imc neuron j to L10 neuron i increases with increasing distance, ⊻i − j⊻, between the two locations i and j. When D = 1, the antitopographic distribution is strict, and there is no feedback from Imc neuron j to L10 neuron i at the same location, i = j. The global Imc → Ipc projection is specified by a uniform distribution, wij = 1.
Each neuron receives a noise current, Inoise,i, which is modeled as uncorrelated white noise, i.e., of SD σi.
The external excitatory current input, Ie,i = I0H[i − (c − s)]H (c + s − i), to L10 neuron i represents the stimulus from the retinal ganglion cell. Here I0 is the input current amplitude, H is a Heaviside step function, c is the location of the input center, and s is the stimulus half-width, i.e., 2s + 1 neurons of the L10 type centered at location c receive current injections. The Heaviside step function, H, expresses that the current to L10 neurons is nonzero between neuron (c − s) and (c + s) and zero elsewhere.
The competition score is defined as the ratio (r2 − r1)/(r2 + r1), where r2 and r1 are the average spike rates of 13 Ipc neurons around the two stimulation centers. The average Ipc spike rates are taken over a time window of 100 ms, starting 50 ms after the onset of the novel stimulus. Competition score values can range from −1 (no activity shift) to +1 (complete activity shift). When the two locations show similar activities, the competition score is near zero. Similar activities arise when the two locations do not interact or when the two locations suppress each other.
The top-down input is modeled following anatomical and physiological considerations (Fig. 9). The arcopallial gaze field (AGF) projects strongly and in parallel to the deep layers of the OT and to nuclei of the brain stem, including the n. isthmi (Knudsen et al. 1995). In the AGF, sensory space is organized in a clustered representation in which neighboring neurons encode a similar location, but neighboring groups of neurons encode different, unpredictable locations (Cohen and Knudsen 1995). AGF microstimulation within a cluster tuned to a certain location in sensory space 1) increases the responsiveness of OT neurons tuned to the same location and 2) decreases the responsiveness of OT neurons tuned to all other locations (Winkowski and Knudsen 2006, 2007, 2008). At present, there is no compelling explanation for why clustered organizations exist (Cohen and Knudsen 1999). The two AGF inputs in our model (Fig. 9A, thick arrows) represent two different locations in sensory space.
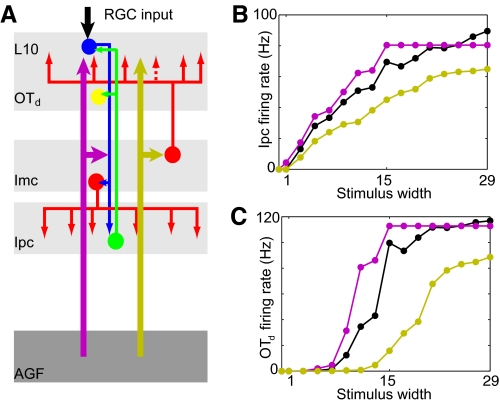
Fig. 9.
Competitive interaction within the isthmotectal system between a sensory input and a top-down input. A: schematic of the isthmotectal model circuitry consisting of retinal ganglion cell inputs (RGC, black) and L10 (blue), Ipc (green), Imc (red) neurons, and 2 external inputs from the AGF onto L10 neurons and the Imc neurons with projections to L10. The 2 AGF inputs (thick arrows) represent 2 different locations in sensory space. A representative deep tectal model neuron is assumed to receive inputs from a group of Ipc neurons. B: average firing rate of the 13 central Ipc neurons (time window, 250–800 ms) as a function of the stimulus width for 3 different situations of top-down modulation: no AGF input (black), aligned AGF input (purple), nonaligned AGF input (yellow). The external currents are Itarget = 0.2 nA, IAGF→L10 = 0.1 nA, and IAGF→Imc = 0.8 nA. The AGF input consists of 2 excitatory current inputs in 13 L10 neurons and 13 Imc neurons. C: average firing rate of the deep tectal model neuron (n = 8, a = 120 Hz, b = 57 Hz) as a function of the stimulus width for the 3 different situations of top-down modulation (colors as in B).
To compare the simulated responses with recordings from deep tectal layers (Winkowski and Knudsen 2008), we introduce an additional read-out neuron, which is modeled as a Hill's function (Fig. 9C). Here r is the input firing rate from presynaptic neurons, a is the saturation firing rate, b is the input firing rate when the output reaches one half of its saturation value, and n describes the steepness of the response curve.
RESULTS
Structure of the isthmotectal model network
To study the dynamics of stimulus competition in the isthmotectal system, we represent the available anatomical (Wang et al. 2004, 2006) and physiological (Shao et al. 2009) information about the avian isthmotectal circuit (Fig. 2A) in a model network (see methods) consisting of four interacting populations of spiking model neurons (Fig. 2B) of the leaky integrate-and-fire type, including a spike-rate adaptation conductance. Tectal L10 neurons receive simulated retinal representations of visual stimuli, which are the sole inputs into this topographically organized model network. The width of an axonal projection is expected to be an important structural feature of any system with competitive interaction. For the feedforward pathway, we describe the lateral spread of individual axons in the L10 → Ipc and L10 → Imc excitatory projections by Gaussian distributions of their synaptic weights (Fig. 2C). The global Imc → Ipc inhibitory projection is described by a uniform distribution of synaptic weights. For the feedback pathway, we represent the Ipc → L10 excitatory projection by a Gaussian distribution of its synaptic weights. Imc neurons, in contrast, project diffusely on L10 neurons, but little to the locus from which they receive input (Wang et al. 2004) This antitopographic GABAergic Imc → L10 feedback projection is described by an inverted Gaussian distribution, where the strength of synaptic weights dips at its center, corresponding to the location from which the Imc neuron receives its input. Thus an Imc model neuron provides weak inhibition on the L10 neurons corresponding to the same location and stronger inhibition on distal L10 neurons. In summary, the experimentally constrained isthmotectal model system consists of a specific combination of excitatory topographic and inhibitory antitopographic and global projections (Fig. 2).
Competitive bottom-up selection of novel stimuli
The network response to two sequentially presented stimuli with temporal overlap shows the competitive nature of the isthmotectal network (Fig. 3A). The isolated target stimulus elicits regular and correlated spiking in L10 neurons, which in turn generates rhythmic bursting in a group of Ipc and Imc neurons corresponding to the target location. Such rhythmic Ipc bursting has been recorded in pigeon Ipc neurons in response to visual stimulation (Marin et al. 2005, 2007).
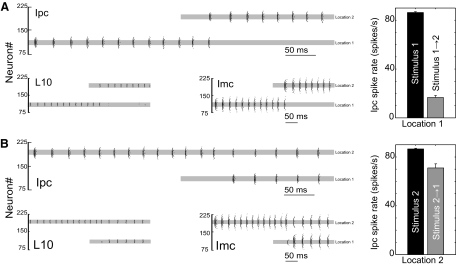
Fig. 3.
Competitive neural interaction between 2 retinal inputs in a population model of the avian isthmotectal system. A: raster plots of spiking activity in L10, Ipc, and Imc model neurons in response to the presentation of a target stimulus followed by the presentation of a novel stimulus of slightly larger amplitude. The target stimulus consists of an input current of 0.4 nA starting at t = 0 ms into 15 L10 neurons centered on neuron #110 (location 1). The novel stimulus consists of an input current of 0.42 nA starting at t = 250 ms into 15 L10 neurons centered on neuron #191 (location 2). The location, width, and timing of the stimuli into L10 neurons are indicated by the gray bar in the L10 raster plots. No current is injected into Ipc and Imc neurons, but, for comparison, the gray bar is also reproduced in the Ipc and Imc raster plots. The synaptic strengths are gIpc→L10 = 0.01gm, gImc→Ipc = 0.12gm, and gImc→L10 = 0.24gm. The depth DImc→L10 and the width ΔImc→L10 of the antitopographic Imc → L10 projection are 0.6 and 8, respectively. The input current has a noise of σ = 0.05 nA. Inset: average spike rate of 13 Ipc neurons (5 trials) centered at neuron #110 (location 1) during isolated target (0.4 nA) presentation (time window 0 to 250 ms) and during concurrent target (0.4 nA) and novel (0.42 nA) stimulus presentation (time window, 250–500 ms). B: raster plots of spiking activity in response to the presentation of a target stimulus (0.42 nA) followed by the presentation of a novel stimulus (0.4 nA) of slightly smaller amplitude. Other parameters are the same as in A. Inset: average spike rate of 13 Ipc neurons (5 trials) centered at neuron #191 (location 2) during isolated target (0.42 nA) presentation and during concurrent target (0.42 nA) and novel (0.4 nA) stimulus presentation.
The additional and delayed presentation of a novel stimulus of equal or larger amplitude (Figs. 3A and 4) at a distant location has two effects on the population activity. The retinal input to L10 neurons at the novel location overcomes the inhibitory input from the Imc neurons at the distal target location. Approximately 50 ms after onset of the novel stimulus, the L10 neurons at the novel location start to spike, which in turn triggers spikes in the corresponding Ipc and Imc neurons. The delay has three causes: the L10 neuron membrane time constant (τL10 = 104 ms), the hyperpolarized level of the L10 membrane potential at the novel location at the onset of the novel stimulus, and the small size of the depolarizing current, which is the difference of the excitatory current (novel stimulus) and the inhibitory current (from the Imc target location). The Imc activity at the novel location provides inhibitory current in L10 neurons at the target location. This new inhibitory current together with the existing adaptation current overcomes the excitatory input current in the L10 target neurons, which ends spiking in these and the corresponding Ipc and Imc neurons at the target location. A complete shift in activity from the target to the novel location has occurred, although stimulation at the target site continues for the entire duration of novel stimulus presentation.
Fig. 4.
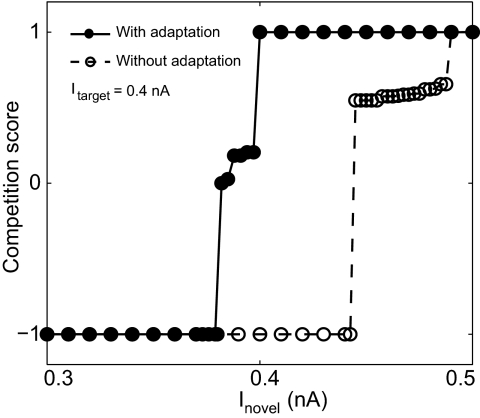
Change of competitive neural interaction between target and novel stimulus with varying novel stimulus amplitude. The competition score is shown as a function of the novel stimulus amplitude. All parameters are the same as in Fig. 3. The transition happens within a narrow range of novel stimulus strength around the value of the target stimulus strength. Without spike-rate adaptation, the transition range shifts to larger values of novel stimulus strength.
Apart from the functionally significant shift in activity to novel stimuli of equal or larger amplitude (Fig. 3A), the population model (Fig. 2) reproduces four important in vivo observations previously recorded in the avian isthmotectal system (Marin et al. 2005, 2007): 1) Ipc neurons respond with rhythmic bursting to visual stimulation, 2) the novel stimulus can be far for the shift in activity to occur, 3) the delay in shift measured from the onset of the novel stimulus is variable between 35 and 100 ms, and 4) Imc neurons fire synchronously and at regular intervals. The retrodiction of these four in vivo observations indicates that the population model captures key anatomical and physiological elements of the avian isthmotectal pathway.
When the novel stimulus is of smaller amplitude than the target stimulus, no shift in activity occurs (Figs. 3B and 4). A small difference in input current causes no significant differences in the responses at the two locations when each stimulus is presented in isolation (Fig. 3, A and B). However, for sequential stimulation with temporal overlap, the response to a weaker novel stimulus (Fig. 3B) is qualitatively different from the response to a stronger novel stimulus (Fig. 3A) described above. The weaker novel stimulus overcomes the inhibitory current from the target Imc neurons and causes L10 spiking. Nevertheless, the corresponding Imc spike rate at the novel location does not generate sufficient inhibitory current in the L10 target neuron. The excitatory current in the target L10 neuron remains larger than the sum of the inhibitory and the adaptation current. As a result, L10 and thus Ipc neurons at target and novel location spike; however, it is at a reduced rate.
This model result reproduces in vivo recordings from pigeon Ipc neurons, which showed that a novel visual stimulus in a superior receptive field strongly suppresses target responses in an inferior receptive field but showed only little suppression vice versa (Marin et al. 2007). We thus show that even though in the population model all locations have equal status, the observed response asymmetry can be implemented by an asymmetry in the retinal representation of the visual stimuli. When the amplitude of the novel stimulus is varied systematically while the target stimulus amplitude remains fixed, the transition from no shift to complete shift occurs within a narrow range of novel stimulus strength below the value of the target stimulus strength (Fig. 4)—a model prediction that is accessible for in vivo tests.
The model further predicts that, in this range of novel stimulus strength, the neural activities synchronize at the two locations (Fig. 3B). The synchrony is independent of the onset timing of the novel stimulus and is robust to noise in the system. Specifically, whether the activities are synchronous (for large inhibition) or antisynchronous (for small inhibition) depends on the strength of the recurrent antitopographic inhibition. The dynamics of synchrony between coupled neurons is complex (Lewis and Rinzel 2003). In the isthmotectal system, synchrony emerges from the reciprocal inhibition (Imc → L10) between groups of neurons at the target and novel location.
Competitive bottom-up selection of stimuli in static visual scenes
To gain insight into the system response to static visual scenes, we studied the model responses to two simultaneously presented stimuli: a target and a distant competitor stimulus. When the competitor is weaker than the target stimulus, the Imc activity at the target location generates a strong inhibitory current in the L10 neurons at the competitor location. The competitor-induced excitatory current in the L10 neurons is too small to trigger spikes. In contrast, when the competitor is stronger than the target, L10 neurons at the competitor location spike. In this case, L10 neurons at the target locations are inhibited, which prevents them from spiking. The model generates three nontrivial predictions for simultaneous stimulus presentation (Fig. 5A). First, when a target and a distant competitor stimulus of different strength are presented simultaneously, the isthmotectal model network selects in a winner-take-all (WTA) manner the strongest stimulus in this static visual scene. Second, for fixed target strength and varying competitor strength, the transition occurs around the value of the target strength. Third, the transition as a function of competitor strength can be gradual or switch-like, because the slope of the transition depends on the strength of the recurrent antitopographic inhibition. In the limit of zero recurrent antitopographic inhibition, no competition occurs. When this inhibition is weak the transition is gradual, when the recurrent antitopographic inhibition is strong the transition is switch-like. Interestingly, transitions around the target strength with a distribution of slopes have been observed in neurons of the isthmotectal system of barn owls in response to visual stimulation with a target and a competitor (Asadollahi et al. 2010; Mysore et al. 2010). This in turn suggests a distribution of the recurrent antitopographic inhibition strength.
Fig. 5.
Competitive neural interaction between a target and a simultaneously presented distant competitor. A: the target stimulus strength is kept fixed at 0.4 nA, the competitor strength is varied, and the results are expressed in terms of a winner-take-all (WTA) score, which is defined as the ratio (r2 − r1)/(r2 + r2). Here r2 and r1 are the average spike rates of 13 Ipc neurons around the 2 stimulation centers. The average Ipc spike rates are taken over a time window of 450 ms starting 50 ms after the onset of the 2 stimuli. Other parameters are the same as in Fig. 3. B: Ipc average firing rate at the target location (averaged over 3 Ipc neurons at the target center) as a function of competitor width for 2 different target widths. The target and competitor stimuli are of the same strength (0.4 nA). Network parameters are the same as in Fig. 3. The simulated results are fitted to a sigmoidal function (solid line).
When the sizes of the target and competitor stimulus are varied, the isthmotectal model network does not select the largest stimulus in a typical WTA manner; rather, the Ipc response at the target location continues to represent information about the target stimulus width (Fig. 5B). We consider two target widths and vary the width of the competitor stimulus. With increasing competitor width, the Ipc target response decreases until it reaches a steady state. Importantly, the steady-state value increases with target width. Two circuit mechanisms cause this deviation from a WTA behavior. First, with increasing competitor width the inhibitory synaptic current in L10 neurons saturates as the membrane potential hyperpolarizes toward the synaptic reversal potential. Second, the number of activated L10 neurons increases with increasing target width. Because of the width of the L10 → Ipc projection (Fig. 2C), this in turn increases the excitatory input to an Ipc neuron at the target location. Thus the Ipc target response increases with target width (Fig. 5B). This model prediction is consistent with recorded responses in owl Ipc in response to looming dots of varying final sizes (Asadollahi et al. 2010).
Recurrent antitopographic inhibition
An intriguing structural feature of the isthmotectal pathway is the combination of global feedforward (Imc → Ipc) and recurrent antitopographic (Imc → L10) inhibition mediated by Imc neurons (Wang et al. 2004), which determines complex responses of this dynamical system (Caudill et al. 2009). Pharmacological inactivation of the Imc showed the essential role of Imc neurons in mediating the shift of Ipc activity to novel stimuli (Marin et al. 2007). This experiment, however, could not determine the individual roles of the global (Imc → Ipc) and antitopographic (Imc → L10) inhibition in the competitive stimulus selection. To address this question, we scanned the model parameter space for varying synaptic strengths, gImc→Ipc and gImc→L10, of the two Imc projections and showed the simulated response to sequential stimulation (Fig. 3A) of equal strength in terms of a competition score (see methods). This score is based on the Ipc activity within a time window after the onset of the novel stimulus. The Ipc response was chosen as a measure of the simulation results, because the Ipc activity gates the processing in the tecto-rotundal visual pathway (Marin et al. 2007).
When both inhibitory projections are weak, neurons at the target and novel stimulus location respond largely independently to their local inputs (Fig. 6A). This model result reproduces the in vivo observation that Imc neurons are required for the shift in activity to happen (Marin et al. 2007). For increased strength of the feedforward inhibition, the global Imc → Ipc projection does not contribute to competitive interaction, but rather merely regulates the level of Ipc activity. With increasing strength of the antitopographic Imc → L10 feedback projection, however, stimulus competition sets in. The Imc activity at the novel location generates sufficient inhibitory current in the L10 target neurons, such that the sum of the inhibitory and the adaptation current overcomes the excitatory current. The L10 target neurons cease to spike, i.e., the Ipc competition score is +1. Further increase of the Imc → L10 projection strength mediates large inhibitory currents at both locations and dynamically reduces the L10 spike rate. Depending on the relative strength of excitation and antitopographic inhibition, either L10 neurons at the novel location do not spike (competition score equals −1) or L10 neurons spike at both locations (competition score near 0). In conclusion, given the weak Ipc → L10 excitatory feedback, the Imc → L10 antitopographic inhibition at intermediate strength is essential for the isthmotectal selection of novel stimuli. The experimental test of this model prediction requires the selective block of the Imc → L10 pathway within the avian isthmotectal system.
Fig. 6.
Recurrent antitopographic inhibition mediates competitive stimulus selection between 2 distant sensory inputs of equal strength. Parameter dependence of novelty detection in the isthmotectal model network is expressed by the competition score as a function of A, global inhibition gImc→Ipc/gm and recurrent antitopographic inhibition gImc→L10/gm; B, the strength gImc→L10/gm and depth DImc→L10 of the recurrent antitopographic inhibition; and C, the strength gImc→L10/gm and width ΔImc→L10 of the recurrent antitopographic inhibition. The competition score is color coded representing −1 (blue, no shift in activity), 0 (green, similar spike rates at both locations) to 1 (red, complete shift in activity to the novel location). The novel stimulus starts 250 ms after the onset of the target stimulus. Both stimuli are of the same amplitude (Itarget = Inovel = 0.4 nA). A–C: 3 cross-sections through the multidimensional parameter space. The cross-sections intersect at the point indicated by the white asterisk, which is also the parameter set chosen for Figs. 3 and 9. No noise is included in the simulations. All other parameters are the same as in Fig. 3.
The importance of the antitopographic inhibitory Imc → L10 projection within the isthmotectal system raises the question to what extent the two structural parameters, the depth DImc→L10 and the width ΔImc→L10 of the dip in this feedback projection, influence the competitive interaction (Fig. 6, B and C). When the depth or width of the dip is small, an Imc neuron exerts a strong inhibition on the L10 neuron from which it receives input. Thus the L10 and Imc activity at the target location is too small for the spike-rate adaptation current to reach a significant level. Similarly, Imc activity at the target location mediates only a small inhibitory current in L10 neurons at the novel location. As a result, when the novel stimulus occurs, neurons at both locations fire at a reduced but similar rate (competition score near 0). With increasing depth or width parameter, the inhibition of the L10 neurons at the target location is reduced. This leads to an increased spike rate with a concurrent increase in the spike-rate adaptation current at the target location and an increased inhibitory current at distal locations. When a novel stimulus occurs at a distant location, the corresponding L10 and Imc neurons start spiking. Consequently, the L10 neurons at the target location cease spiking as the sum of the currents from the continuing adaptation and the new antitopographic inhibition cancels the excitatory stimulus current (competition score equals +1). Further increase of the depth (width) of the antitopographic inhibition, gImc→L10, decreases the inhibition of the L10 neurons at the target location, thus increasing the local spike rate. For large strength of the antitopographic Imc → L10 feedback projection, the Imc-mediated inhibitory current at distal locations prevents L10 neurons at these distant locations to spike in response to novel stimuli. As a result, there is no shift in activity in this parameter range (competition score equals −1). Consequently, a large depth or width narrows the range of inhibitory feedback strengths for which a shift in activity occurs.
Motivated by in vitro studies of the isthmotectal system (Shao et al. 2009), this model investigation assumed a weak Ipc excitatory feedback onto L10 neurons. With increasing strength of the Ipc → L10 excitatory feedback, an appropriate level of recurrent inhibition is required to stabilize the system and for novelty shifts to occur (Fig. 7).
Fig. 7.
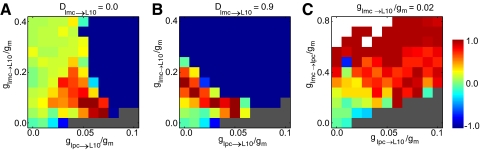
A new role arises for the topographic Ipc → L10 excitatory feedback when the inhibitory Imc projections are more homogeneous. The parameter dependence of novelty detection in these isthmotectal model networks is expressed by the competition score (color code) as a function of A, the strength gImc→L10/gm of a global Imc → L10 inhibition (DImc→L10 = 0.0) and the topographic excitatory feedback gIpc→L10/gm; B, the strength gImc→L10/gm of an antitopographic Imc → L10 inhibition (DImc→L10 = 0.9) and the topographic excitatory feedback gIpc→L10/gm; and C, the strength gImc→Ipc/gm of a global Imc → Ipc inhibition and the topographic excitatory feedback gIpc→L10/gm. The color code and stimulus settings are as in Fig. 6. Gray represents diverging activity, defined as an average firing rate of the center Ipc neuron >1,000 Hz. White represents vanishing activity.
A new role arises for the topographic Ipc → L10 excitatory feedback when the inhibitory Imc → L10 projection is homogeneous (DImc→L10 = 0), i.e., no dip in the inhibitory projection. In this case, the inhibition of L10 neurons at the target location reduces spiking, thus limiting the spike-rate adaptation current. The local inhibitory feedback thus prevents novelty shifts to occur for stimuli of equal strength. However, with increasing local Ipc → L10 excitatory feedback, the local inhibition can be overcome. This increases the spike rate and the concurrent spike-rate adaptation current, which promotes the occurrence of activity shifts in response to novel stimuli at distant locations (Fig. 7A). Interestingly, in this case competitive stimulus selection occurs only within a narrow parameter range of inhibition and excitation strength. When the inhibitory Imc → L10 projection is antitopographic DImc→L10 = 0.9), the neural activity at the target location is sufficiently high, even without Ipc → L10 excitatory feedback, to allow for activity shifts in response to novel stimuli (Fig. 7B). In general, with increasing strength of the Ipc → L10 feedback, the Ipc activity plays a larger role in L10 responses and thus the nature of the inhibitory global Imc → Ipc projection changes from feedforward (Imc → Ipc) to feedback (Imc → Ipc → L10 → Imc). To illustrate this point, we consider a model with excitatory topographic Ipc → L10 projection and inhibitory homogenous Imc → Ipc projection, but with a weak Imc → L10 projection. In this case, competitive stimulus selection is possible but requires strong excitation and inhibition (Fig. 7C).
In conclusion, these parameter scans show that stimulus selection in the avian isthmotectal system requires a careful balance of the physiological and anatomical parameters in the antitopographic Imc → L10 feedback projection.
Adaptation
Isthmotectal neuron firing rates adapt to somatic current injection (Shao et al. 2009). To quantify the role of adaptation in competitive stimulus selection in the avian isthmotectal system, we analyzed neural competition for varying amplitude of spike-rate adaptation, Δgsra,L10 and Δgsra,Imc, for L10 and Imc neurons, respectively. Because of their weak synaptic strength on L10 neurons (Shao et al. 2009), the role of Ipc spike-rate adaptation was not considered. For small values of spike-rate adaptation, Δgsra,L10 and Δgsra,Imc, the L10 and Imc spike frequency at the target location is high. As a result, the Imc-mediated inhibitory current in L10 neurons prevents the L10 neurons at the novel location from spiking in response to the novel stimulus. Consequently, no shift in activity occurs, i.e., the Ipc competition score is −1 (Fig. 8). However, for intermediate values of spike-rate adaptation, Δgsra,L10 and Δgsra,Imc, the rate of L10 and Imc spiking at the target location is reduced, thus reducing the inhibitory synaptic current in L10 neurons at the novel location. The novel excitatory input to L10 neurons overcomes the inhibition. Consequently, the L10 and thus the Imc neurons at the novel location start to spike. The resulting Imc-mediated inhibitory current (together with the adaptation current) at the target L10 neurons reduce further the L10 response to the target excitatory input. As a result, Ipc neurons spike at both the target and the novel location, which leads to a competition score near 0. With further increase of the spike-rate adaptation, the sum of the inhibitory synaptic current from the antitopographic projection together with the increased adaptation current in L10 neurons overcomes the target excitatory current. The L10 target neurons cease spiking, i.e., a shift in activity occurs and the Ipc competition score is near +1. In conclusion, spike-rate adaptation facilitates the isthmotectal selection of novel stimuli.
Fig. 8.
Spike-rate adaptation facilitates the selection of novel stimuli. Novelty detection is expressed by the competition score as a function of spike-rate adaptation Δgsra,Imc/gm and Δgsra,L10/gm. The competition score is color coded representing −1 (blue, no shift in activity), 0 (green, similar spike rates at both locations) to 1 (red, complete shift in activity to the novel location). The novel stimulus starts 250 ms after the onset of the target stimulus. Both stimuli are of the same amplitude (Itarget = Inovel = 0.4 nA). The white asterisk indicates the parameter set chosen for Figs. 3 and 9. No noise is included in the simulations. All other parameters are the same as in Fig. 3.
This facilitation is documented further, when the amplitude of the novel stimulus is varied systematically while the target stimulus amplitude remains fixed (Fig. 4). Without spike-rate adaptation, the novel stimulus has to be larger than the target stimulus for activity shifts to occur. In contrast, competition between simultaneously presented target and competitor stimuli is not significantly influenced by spike-rate adaptation (Fig. 5A).
Competitive interaction between top-down and sensory inputs
Electrical microstimulation in the avian AGF (putative homolog of the primate frontal eye field region) increases the sensitivity of sensory responses for deep tectal neurons with a receptive field aligned to the AGF stimulation site but decreases the gain of deep tectal neurons representing stimuli at other locations (Winkowski and Knudsen 2008). The AGF projects to both the optic tectum and the isthmic nuclei (Knudsen et al. 1995), suggesting that the mechanisms for the top-down control of tectal gain and sensitivity emerge from the interaction of the AGF and the sensory input within the isthmotectal circuit. The isthmic output, e.g., Ipc spiking, modulates the responses of postsynaptic deep tectal neurons.
To elucidate these mechanisms, we simulated the interaction of a sensory input (from retinal ganglion cells) and a top-down input (from AGF) in the isthmotectal model network for varying stimulus strength (Fig. 9A). Given the spatially broad tuning curves of sensory pathways, we assume that, at the level of the tectum, stimulus strength is usefully represented by the number of activated L10 neurons. We refer to the latter as the stimulus width.
The isthmic responses to sensory stimuli of varying width emerge from the recurrent interaction of excitatory (L10) and inhibitory (Imc) neurons. In response to a sensory stimulus alone, the number of activated L10 neurons increases with increasing stimulus width and thus the average L10 firing rate increases. This in turn activates more Imc neurons and, because of the recurrent antitopographic inhibition, the average L10 firing rate of a group of neurons around the stimulation center saturates with increasing stimulus strength. Because Ipc neuron activity largely follows their L10 input (Fig. 3), the Ipc stimulus response function displays a qualitatively similar sigmoidal form (Fig. 9B).
Guided by the available anatomical information (Knudsen et al. 1995), we represent the AGF control with two excitatory current inputs in a group of L10 and Imc neurons (Fig. 9A). When the sensory stimulus and the AGF control are aligned, the L10 response to the sensory input is increased because of the additional excitation from the AGF. Because of the antitopographic feedback of Imc neurons, the AGF excitation of Imc neurons has little effect on the local L10 responses. In contrast, when the sensory stimulus and the AGF control are nonaligned, the AGF input to distant Imc (and L10) neurons activates an antitopographic inhibition, which reduces the L10 response to the sensory stimulus. Consequently, compared with the control case, the Ipc stimulus response function is slightly shifted to smaller stimulus width for the aligned case and overall reduced for the nonaligned case (Fig. 9B).
With these results at hand, we evaluated the stimulus-width response function of a deep tectal model neuron that is assumed to receive inputs from a group of Ipc neurons. A single-neuron rate model (see methods) with the simulated Ipc activity as inputs qualitatively reproduces (Fig. 9C) the stimulus-width response function of deep tectal neurons in owls for the aligned and nonaligned top-down control scenarios (Winkowski and Knudsen 2008) and thereby indicates two distinct mechanisms for the top-down control of neural sensitivity and gain, respectively. Aligned AGF input increases the sensitivity of the deep tectal neuron via the excitation of L10 neurons at the sensory stimulation site. Nonaligned AGF input decreases the gain of the deep tectal neuron via the excitation of distant Imc neurons, which provide antitopographic inhibition of L10 neurons at the sensory stimulation site. The top-down control of the L10 neurons is then communicated from L10 to Ipc to the deep tectal neurons.
In addition to its modulatory effect on steady-state firing rate, top-down inputs can also modulate temporal aspects of neural responses to sensory inputs (Fries et al. 2001; Mitchell et al. 2007; Reynolds et al. 2000). To evaluate the dynamics of competitive interaction between a sensory input and a nonaligned top-down input in the isthmotectal system, we stimulate a group of L10 target neurons with uncorrelated Poisson current pulses and mimic the top-down input with constant current input to a group of distant L10 and Imc neurons. When the nonaligned AGF input is sufficiently strong, Ipc and Imc responses at the target location become periodic and synchronize to the oscillatory bursts at the distant location, which is activated by the nonaligned top-down AGF input (Fig. 10). The synchrony is mediated by the Imc (nonaligned) → L10 (target) inhibitory projection, which imposes a temporal structure onto the otherwise irregularly firing L10 neurons.
Fig. 10.
Ipc and Imc responses to a sensory input synchronize to distant isthmic activity induced by a top-down AGF input. A target stimulus consisting of uncorrelated Poisson current pulses [I0,i(t) = as/τst exp(−t/τs), f = 100 Hz, as = 0.6 nA, τs = 5.6 ms] delivered to 25 L10 neurons (centered on neuron 110; indicated by the gray bar in the L10 raster plots) triggers irregular spiking in this group of L10 neurons. The AGF input consists of continuous current inputs in 37 L10 neurons (0.5 nA) and 37 Imc neurons (0.8 nA) centered on neuron 191 (green bars in the L10 and Imc raster plots). The cellular and synaptic parameters of the network are the same as in Figs. 3 and 9.
DISCUSSION
Mechanisms of competitive stimulus selection
Attention is a crucial component of sensory processing, yet the circuit mechanisms that mediate attentional stimulus selection have remained elusive. The superior colliculus (mammals) and the optic tectum (other vertebrates) are intricately involved in stimulus selection and share common circuit features, including the nucleus isthmi. Investigating an anatomically and physiologically constrained network model of the avian isthmotectal system, we found that recurrent antitopographic inhibition mediates competitive stimulus selection and that cellular spike-rate adaptation facilitates the selection of novel stimuli. Moreover, forebrain influences are integrated by their modulation of the isthmotectal circuitry, uniting both bottom-up and top-down mechanisms into a common control system.
Confidence in the validity of plausible model assumptions is supported by the fact that the model reproduces a wide range of in vivo observations from pigeons and owls (Asadollahi et al. 2010; Marin et al. 2007; Mysore et al. 2010; Winkowski and Knudsen 2008). The observations include shifts in activity to a novel stimulus, sharp transitions to strong stimuli, global competition, and changes in gain and sensitivity mediated by top-down control.
The extension of our investigation to a model network not constrained by the isthmotectal system showed an alternative mechanism of competition, where competitive stimulus selection is mediated by homogeneous inhibition combined with a strong topographic excitation.
Recurrent antitopographic inhibition
The recurrent antitopographic inhibition extends throughout the entire structure, with a dip only at the center. This antitopographic organization is fundamentally different from the better-known surround inhibition in the center-surround organization. Here, the surround inhibition is largest at the center and vanishes asymptotically with increasing distance. Although this local organization is limited to local computations (contrast enhancement, edge detection, gain control); the far-reaching nature of the antitopographic inhibitory organization is ideally suited to mediate competitive interaction between distant sensory stimuli.
To date, the anatomical evidence for recurrent antitopographic inhibition is best documented for the avian isthmotectal system (Wang et al. 2004). The emergent role of the antitopographic inhibitory organization for stimulus selection raises the question whether this structural principle generalizes to other systems. In all species studied, reciprocal connections exist between the optic tectum (superior colliculus) and the nucleus isthmi (parabigeminal nucleus) (reviewed in Gruberg et al. 2006; Isa and Hall 2009; May 2006; Wang 2003). In reptiles and birds, the nucleus isthmi consist of spatially separate cholinergic and GABAergic groups of neurons with topographic and diffuse, respectively, projections to the optic tectum (reptile: Powers and Reiner 1993; Saha et al. 2010; Sereno and Ulinski 1987; bird: Wang et al. 2004, 2006). The GABAergic diffuse isthmotectal projection modulates tectal cells and thereby, as shown here, can mediate the competitive interaction of visual stimuli in the avian isthmotectal system (Asadollahi et al. 2010; Marin et al. 2005, 2007; Mysore et al. 2010; Winkowski and Knudsen 2006). In mammals, the superior colliculus maintains topographic reciprocal connections with the parabigeminal nucleus (Baizer et al. 1991; Graybiel 1978; Jiang et al. 1996; Sherk 1979). The parabigeminal nucleus to SC projection is cholinergic (Hall et al. 1989; Hashikawa 1989; Wang et al. 1988), thus providing an excitatory topographic feedback. In contrast to birds and reptiles, for mammals, no diffuse GABAergic projection from the parabigeminal nucleus to the superior colliculus has been reported. Rather, ACh (acetylcholine) release from parabigeminal nucleus axon terminals in SC activates GABAergic interneurons in the intermediate layers with broad projections (Binns and Salt 2000; Lee et al. 2001), which in turn inhibit projection neurons (Endo et al. 2005). Thus in mammals, the diffuse projection seems to be mediated by GABAergic interneurons within the superior colliculus that are activated by parabigeminal nucleus input.
Shifting spatial attention
The simulated competitive stimulus selection has an immediate functional significance for the avian isthmotectal system and the retino-tecto-rotundal visual pathway. Ipc axons form narrow tectal columns with hundreds of presynaptic terminals (Wang et al. 2006) (Fig. 2A), enabling Ipc neurons to gate tectal signal processing at that location (Marin et al. 2007; Wang 2003).
Attention is the selection of relevant signals from an enormous amount of often topographically organized information. The isthmotectal system mediates a competitive stimulus selection, which results in a shift of Ipc axon terminal activity to the novel stimulus location in the retino-tecto-rotundal visual pathway. To date, the organization of the nucleus rotundus (pulvinar in mammals) has remained puzzling (Mahani et al. 2006; Marin et al. 2003). The simulated shift of Ipc activity may help to clarify this puzzle. This shift results in a selective mapping from the tectal topographic representation into the rotundal representation. This implies that at any instant the rotundal representation contains the properties of only a single location in the visual scene, the selected location.
Interestingly, the isthmic activity that mediates the gating of tectal signals is not itself involved in the visual processing. Rather, isthmic activity selects the area of visual space that will be analyzed by tectal circuitry. This observation supports an early hypothesis of competitive stimulus selection, which postulates that the selection system itself is not responsible for the information processing relevant to the visual task but merely selects which area of visual space should be inspected (Koch and Ullman 1985; Posner et al. 1980). This focal attention hypothesis was further popularized by the searchlight or spotlight metaphor (Crick 1984).
Perhaps surprisingly, psychophysical experiments indicate that focal selection does not necessarily involve contiguous parts of the visual field (Pylyshyn and Storm 1988; Sperling and Weichselgartner 1995). In other words, the spotlight does not seem to sweep continuously across the visual field. In this respect, the isthmotectal model results are consistent with focal selection in humans. Isthmic activity decreases at the target location while increasing at the distant novel location (Fig. 3A). In terms of the spotlight metaphor, two (or more) spotlights are required; one spotlight fades at the target location, whereas another spotlight brightens at the novel location. Neurons corresponding to locations between the target and novel location remain quiet during this process. From the model investigation, we know that in the isthmotectal system, the jump in activity is mediated by the long-range antitopographic inhibitory Imc feedback. This insight is likely to provide a useful clue in the search for the underlying mechanisms of human focal selection.
The jump in isthmic activity, corresponding to a shift in spatial covert attention (no eye movements), is reminiscent of saccades and the concurrent shift in spatial overt attention (with eye movements). This may not be coincidental, because experimental evidence suggests a close relationship between covert and overt attention (Kustov and Robinson 1996; Sheliga et al. 1995; Shepherd et al. 1986). This relationship is particularly strong in the superior colliculus, which directs saccadic eye movements (Lovejoy and Krauzlis 2010; Müller et al. 2005). The excitatory isthmic feedback with axonal terminals in both upper collicular layers of sensory processing and lower collicular layers of motor control is thus ideally suited to communicate stimulus selection to the superior colliculus and to trigger concurrent jumps in spatial attention and saccadic eye movement to the same location.
Role of adaptation in stimulus competition
We show that adaptation within the isthmotectal circuitry, namely L10 and Imc spike-rate adaptation, can facilitate the competitive selection of novel stimuli (Figs. 4 and 8). We included cellular spike-rate adaptation in the model, because this implementation of adaptation is experimentally constrained in the avian isthmotectal circuit (Shao et al. 2009). However, our model investigation cannot exclude that other implementations of adaptation, such as synaptic plasticity or delayed inhibition, contribute to isthmotectal stimulus competition as well.
Spike-rate adaptation depends on the activation history of the neuron. Thus a single neuron alone, receiving inputs from two locations, cannot achieve novelty sensitivity. Rather, the enhanced sensitivity to a stimulus at a novel location is mediated by stimulus-specific adaptation. This adaptation depends on the stimulus history rather than on the activation history and has been described in visual and auditory pathways (Dragoi et al. 2002; Hosoya et al. 2005; Reches and Gutfreund 2008; Sharpee et al. 2006; Ulanovsky et al. 2003), although it has been challenging to pinpoint the biophysical implementation of adaptation in these complex circuits. In general, the computations to achieve stimulus-specific responses require a network to compare between current and past stimulus conditions (Abbott et al. 1997). In such a network, different stimuli activate separate paths to the stimulus-specific neuron, and the adaptation is localized to the activated path (Eytan et al. 2003). This characteristic feature of stimulus-specific adaptation is present in the isthmotectal network, where stimuli at different locations activate adaptation in separate paths.
WTA networks
When a target and a distant competitor stimulus of different strength are presented simultaneously, the isthmotectal system can serve as a WTA network (Fig. 5A). We showed that the observed distribution of slopes in the transition as a function of competitor strength (Asadollahi et al. 2010) can be reproduced by a distribution of the recurrent antitopographic inhibition strength (Fig. 5A). Interestingly, when the target and competitor stimulus width are varied, the Ipc response at the target location continues to represent information about the target stimulus width (Asadollahi et al. 2010). Our model investigations show how this deviation from a WTA behavior can be mediated by a combination of cellular and circuit mechanisms (Fig. 5B).
The theoretical analysis of neurally implemented maximum detectors has a long history (Koch and Ullman 1985). The architecture of model WTA networks falls into two broad categories: 1) lateral inhibition without self-inhibition (Mao and Massaquoi 2007; Sum et al. 1999; Yuille and Grzywacz 1989) and 2) global inhibition with self-excitation (Brandt and Wessel 2007; Hahnloser et al. 1999). The anatomically constrained model (Fig. 2) of the isthmotectal system includes elements of both network categories. The recurrent antitopographic inhibition (Imc → L10) belongs to category 1, whereas the global inhibition (Imc → Ipc) with self-excitation (Ipc → L10) belongs to category 2. Depending on the relative strength of the inhibition and excitation, the stimulus maximum selection can be based on a combination of the two basic network categories. WTA selection and the selection of novel stimuli are related in that both require competitive interaction. However, the selection of novel stimuli poses higher requirements on the network. In a WTA network, strong inhibition usually ensures the selection of the winner (Hahnloser et al. 1999) (Fig. 5A). In contrast, for inhibition above a certain level, the network fails to respond to novel stimuli. Thus a network has to maintain its inhibition at an appropriate level to execute the task of novelty detection (Fig. 6).
Top-down modulation of the stimulus-response function
Our model provides new insight into the microcircuit mechanisms of top-down stimulus-response modulation (Fig. 9). Directing attention to a target stimulus enhances visual responses of cortical neurons (V4) to the attended stimulus while suppressing the responses to other stimuli (reviewed in Reynolds and Heeger 2009). The effects of focal attention on V4 visual responses can be mimicked by low-level electrical microstimulation of the macaque frontal eye field region (Moore and Armstrong 2003). This microstimulation paradigm was extended to the owl forebrain AGF (putative homolog of the primate frontal eye field region), which projects to both the nucleus isthmi and the deep tectal layers (Knudsen et al. 1995). Stimulating an AGF site aligned with the receptive field of a recorded deep tectal neuron caused a leftward shift of the neurons stimulus-response function. In contrast, stimulating an AGF site outside the receptive field of a recorded deep tectal neuron reduced the responses across all stimulus levels (Winkowski and Knudsen 2008).
The observed differential effect of aligned and nonaligned AGF stimulation has led to the suggestion that AGF inputs involve two distinct mechanisms modulating tectal responses (Winkowski and Knudsen 2008). Our model investigation indicates two such mechanisms. The aligned AGF stimulation causes a leftward shift of the neurons stimulus response function via the direct AGF excitation of tectal L10 neurons. The nonaligned AGF stimulation, however, reduces the responses across all stimulus levels dynamically via the competitive interaction of the target stimulus and the distal top-down input onto L10 and Imc neurons. The long-range interaction is mediated by the antitopographic inhibitory Imc → L10 projection. The isthmotectal circuit thus integrates the top-down influence into the bottom-up competitive interaction network.
The top-down modulation results in stimulus-response functions may be mimicked, at least for simple stimuli, by the normalization model of attention. However, the phenomenological normalization model (Reynolds and Heeger 2009) and the circuit-based competitive interaction model (Figs. 2, B and C, and 9A) are fundamentally different. The former is based on feedforward mechanisms and steady-state firing rates alone, whereas the latter includes feedback and temporal aspects of the responses. Specifically, in response to simple stimuli, the circuit-based model predicts that nonaligned top-down input imposes periodic bursting on target responses that synchronize with the activity at the top-down input location (Fig. 10). We hypothesize that, in natural viewing, the differences in predictions of stimulus-response modulation between the two classes of models will become more apparent.
In conclusion, based on the detailed anatomical information of the avian isthmotectal system, this study shows a set of neural mechanisms for competitive stimulus selection. Given the parallels between attentional phenomenology of barn owls and rhesus monkey (Reynolds 2008; Winkowski and Knudsen 2008), it will be interesting to see to what extent this detailed circuit insight will assist useful working hypotheses for the investigation of attentional selection in monkeys and humans.
GRANTS
This research was supported by National Eye Institute Grant R01 EY-018818 to R. Wessel.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank M. Ariel, A. Carlsson, B. Raman, L. Snyder, J. Zacks, and members of the Neurophysics Laboratory for critical reading of the manuscript.
REFERENCES
- Abbott LF, Varela JA, Sen K, Neslon SB. Synaptic depression and cortigal gain control. Science 275: 220–224, 1997 [DOI] [PubMed] [Google Scholar]
- Asadollahi A, Mysore SP, Knudsen EI. Stimulus-driven competition in a cholinergic midbrain nucleus. Nat Neurosci 13: 889–895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baizer JS, Whitney JF, Bender DB. Bilateral projections from the parabigeminal nucleus to the superior colliculus in monkey. Exp Brain Res 86: 467–470, 1991 [DOI] [PubMed] [Google Scholar]
- Binns KE, Salt TE. The functional influence of nicotinic cholinergic receptors on the visual responses of neurons in the superficial superior colliculus. Vis Neurosci 17: 283–289, 2000 [DOI] [PubMed] [Google Scholar]
- Bisley JW. The neural basis of visual attention. J Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt SF, Wessel R. Winner-take-all selection in a neural system with delayed feedback. Biol Cybern 97: 221–228, 2007 [DOI] [PubMed] [Google Scholar]
- Caudill MS, Brandt SF, Nussinov Z, Wessel R. An intricate phase diagram of a prevalent visual circuit reveals universal dynamics, phase transitions and resonances. Phys Rev E 80: 051923, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen YE, Knudsen EI. Binaural tuning of auditory units in the forebrain archistriatal gaze fields of the barn owl: local organization but no space map. J Neurosci 15: 5152–5168, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen YE, Knudsen EI. Maps versus clusters: different representations of auditory space in the midbrain and forebrain. Trends Neurosci 22: 128–35, 1999 [DOI] [PubMed] [Google Scholar]
- Crick F. Function of the thalamic reticular complex: the searchlight hypothesis. Proc Natl Acad Sci USA 81: 4586–4590, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci 18: 193–222, 1995 [DOI] [PubMed] [Google Scholar]
- Destexhe A, Mainen ZF, Sejnowski TJ. Synthesis of models for excitable membranes, synaptic transmission and neuromodulation using a common kinetic formalism. J Comput Neurosci 1: 195–230, 1994 [DOI] [PubMed] [Google Scholar]
- Dragoi V, Sharma J, Miller EK, Sur M. Dynamics of neuronal sensitivity in visual cortex and local feature discrimination. Nat Neurosci 5: 883–891, 2002 [DOI] [PubMed] [Google Scholar]
- Endo T, Yanagawa Y, Obata K, Isa T. Nicotinic acetylcholine receptor subtypes involved in facilitation of GABAergic inhibition in mouse superficial superior colliculus. J Neurophysiol 94: 3893–3902, 2005 [DOI] [PubMed] [Google Scholar]
- Eytan D, Brenner N, Marom S. Selective adaptation in networks of cortical neurons. J Neurosci 23: 9340–9356, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix D, Wu GY, Wang SR. GABA as an inhibitory transmitter in the pigeon isthmotectal pathway, Neurosci Lett 169: 212–214, 1994 [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R. Selective visual attention modulation of oscillatory neuronal synchronization. Science 291: 1560–1563, 2001 [DOI] [PubMed] [Google Scholar]
- Graybiel AM. A satellite system of the superior colliculus: the parabigeminal nucleus and its projection to the superficial collicular layers. Brain Res 145: 365–374, 1978 [DOI] [PubMed] [Google Scholar]
- Gruberg ER, Dudkin EA, Wang Y, Marin G, Salas C, Sentis E, Letelier JC, Mpodozis J, Malpeli J, Cui H, Ma R, Northmore D, Udin S. Influencing and interpreting visual input: the role of a visual feedback system. J Neurosci 26: 10368–10371, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser R, Douglas RJ, Mahowald M, Hepp K. Feedback interactions between neuronal pointers and maps for attentional processing. Nat Neurosci 2: 746–752, 1999 [DOI] [PubMed] [Google Scholar]
- Hall WC, Fitzpatrick D, Klatt LL, Raczkowski D. Cholinergic innervation of the superior colliculus in the cat. J Comp Neurol 287: 495–514, 1989 [DOI] [PubMed] [Google Scholar]
- Hall WC, Moschovakis A. (eds). The Superior Colliculus: New Approaches for Studying Sensorimotor Ontegration. London: CRC Press, 2004 [Google Scholar]
- Hashikawa T. Regional and laminar distribution of choline acetyltransferase immunoreactivity in the cat superior colliculus. Neurosci Res 6: 426–437, 1989 [DOI] [PubMed] [Google Scholar]
- Hosoya T, Baccus SA, Meister M. Dynamic predictive coding by the retina. Nature 436: 71–77, 2005 [DOI] [PubMed] [Google Scholar]
- Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci 7: 56–64, 2004 [DOI] [PubMed] [Google Scholar]
- Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol 102: 2581–2593, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modeling of visual attention. Nat Rev Neurosci 2: 194–229, 2001 [DOI] [PubMed] [Google Scholar]
- Jiang ZD, King AJ, Moore DR. Topographic organization of projection from the parabigeminal nucleus to the superior colliculus in the ferret revealed with fluorescent latex microspheres. Brain Res 743: 217–232, 1996 [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider G. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci 23: 315–341, 2000 [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci 30: 57–78, 2007 [DOI] [PubMed] [Google Scholar]
- Knudsen EI, Cohen YE, Masino T. Characterization of a forebrain gaze field in the archistriatum of the barn owl: Microstimulation and anatomical connections. J Neurosci 15: 5139–5151, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C. Biophysics of Computation. New York: Oxford, 1999 [Google Scholar]
- Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Human Neurobiol 4: 219–227, 1985 [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature 384: 74–77, 1996 [DOI] [PubMed] [Google Scholar]
- Lee DK, Itti L, Braun J. Attention activates winner-take-all competition among visual filters. Nat Neurosci 2: 375–381, 1999 [DOI] [PubMed] [Google Scholar]
- Lee PH, Schmidt M, Hall WC. Excitatory and inhibitory circuitry in the superficial gray layer of the superior colliculus. J Neurosci 21: 8145–8153, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T, Rinzel J. Dynamics of spiking neurons connected by both inhibitory and electrical coupling. J Comput Neurosci 14: 283–309, 2003 [DOI] [PubMed] [Google Scholar]
- Li X, Basso MA. Competitive stimulus interactions within single response fields of superior colliculus neurons. J Neurosci 25: 11357–11373, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci 13: 261–266, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahani AS, Khanbabaie R, Luksch H, Wessel R. Sparse spatial sampling for the computation of motion in multiple stages. Biol Cybern 94: 276–287, 2006 [DOI] [PubMed] [Google Scholar]
- Mao ZH, Massaquoi SG. Dynamics of winner-take-all competition in recurrent neural networks with lateral inhibition. IEEE Trans Neural Netw 18: 55–69, 2007 [DOI] [PubMed] [Google Scholar]
- Marin G, Letelier JC, Henny P, Sentis E, Farfan G, Fredes F, Pohl N, Karten H, Mpodozis J. Spatial organization of the pigeon tecto-rotundal pathway: an interdigitating topographic arrangement. J Comp Neurol 458: 361–380, 2003 [DOI] [PubMed] [Google Scholar]
- Marin G, Mpodozis J, Sentis E, Ossandon T, Letelier JC. Oscillatory bursts in the optic tectum of birds represent re-entrant signals from the nucleus isthmi pars parvocellularis. J Neurosci 25: 7081–7089, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin G, Salas C, Sentis E, Rojas X, Letelier JC, Mpodozis J. A cholinergic gating mechanism controlled by competitive interactions in the optic tectum of the pigeon. J Neurosci 27: 8112–8121, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res 151: 321–378, 2006 [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci 7: 757–763, 2004 [DOI] [PubMed] [Google Scholar]
- Mitchell JF, Sundberg KA, Reynolds JH. Differential attention dependent response modulation across cell classes in macaque visual area V4. Neuron 55: 131–141, 2007 [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature 421: 370–373, 2003 [DOI] [PubMed] [Google Scholar]
- Mulckhuyse M, Theeuwes J. Unconscious attentional orienting to exogenous cues: a review of the literature. Acta Psychol 134: 299–309, 2010 [DOI] [PubMed] [Google Scholar]
- Müller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci USA 102: 524–529, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mysore SP, Asadollahi A, Knudsen EI. Global inhibition and stimulus competition in the owl optic tectum. J Neurosci 30: 1727–1738, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshausen BA, Anderson CH, Essen DCV. A neurobiological model of visual attention and invariant pattern recognition based on dynamic routing of information. J Neurosci 13: 4700–4719, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quart J Exp Psychol 32: 3–25, 1980 [DOI] [PubMed] [Google Scholar]
- Powers AS, Reiner A. The distribution of cholinergic neurons in the central nervous system of turtles. Brain Behav Evol 41: 326–345, 1993 [DOI] [PubMed] [Google Scholar]
- Pylyshyn ZW, Storm RW. Tracking multiple independent targets: evidence for a parallel tracking mechanism. Spatial Vis 3: 1–19, 1988 [DOI] [PubMed] [Google Scholar]
- Rabinovich M, Huerta R, Laurent G. Transient dynamics for neural processing. Science 321: 48–50, 2008 [DOI] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci 4: 193–201, 2003 [DOI] [PubMed] [Google Scholar]
- Reches A, Gutfreund Y. Stimulus-specific adaptations in the gaze control system of barn owl. J Neurosci 28: 1523–1533, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci 19: 1736–1753, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH. Three hundred million years of attentional selection. Neuron 60: 528–530, 2008 [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron 61: 168–185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron 26: 703–714, 2000 [DOI] [PubMed] [Google Scholar]
- Saha D, Morton D, Ariel M, Wessel R. Visual response properties of a cholinergic neuron in turtle nucleus isthmi. J Comp Physiol A In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Ulinski PS. Caudal topographic nucleus isthmi and the rostral nontopographic nucleus isthmi in the turtle, Pseudemys scripta. J Comp Neurol 261: 319–346, 1987 [DOI] [PubMed] [Google Scholar]
- Shao J, Lai D, Meyer U, Luksch H, Wessel R. Generating oscillatory bursts from a network of regular spiking neurons without inhibition. J Comput Neurosci 27: 591–606, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpee TO, Sugihara H, Kurgansky AV, Rebrik SP, Stryker MP, Miller KD. Adaptive filtering enhances information transmission in visual cortex. Nature 439: 936–942, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Craighero L, Rizzolatti G. Spatial attention-determined modifications in saccade trajectories. Neuroreport 6: 585–588, 1995 [DOI] [PubMed] [Google Scholar]
- Shepherd M, Findlay JM, Hockey RJ. The relationship between eye movements and spatial attention. Quarterly J Exp Psychol 38A: 475–491, 1986 [DOI] [PubMed] [Google Scholar]
- Sherk H. Connections and visual field mapping in cat's tectoparabigeminal circuit. J Neurophysiol 42: 1656–1668, 1979 [DOI] [PubMed] [Google Scholar]
- Shpiro A, Curtu R, Rinzel J, Rubin N. Dynamical characteristics common to neuronal competition models. J Neurophysiol 97: 462–473, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling G, Weichselgartner J. Episodic theory of the dynamics of spatial attention. Psychol Rev 102: 503–532, 1995 [Google Scholar]
- Stein BE, Meredith MA. The Merging of the Senses. Cambridge, MA: MIT Press, 1993 [Google Scholar]
- Sum JPF, Leung CS, Tam PKS, Young GH, Kan WK, Chan LW. Analysis for a class of winner-take-all model. IEEE Trans Neural Netw 10: 64–71, 1999 [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Las L, Nelken I. Processing of low-probability sounds by cortical neurons. Nat Neurosci 6: 391–398, 2003 [DOI] [PubMed] [Google Scholar]
- Usher M, Niebur E. Modeling the temporal dynamics of IT neurons in visual search: A mechanism for top-down selective attention. J Cogn Neurosci 8: 311–327, 1996 [DOI] [PubMed] [Google Scholar]
- Veenman CL, Albin RL, Richfield EK, Reiner A. Distributions of GABAA, GABAB, and benzodiazepine receptors in the forebrain and midbrain of pigeons. J Comp Neurol 344: 161–189, 1994 [DOI] [PubMed] [Google Scholar]
- Wang SR. The nucleus isthmi and dual modulation of the receptive field of tectal neurons in non-mammals. Brain Res Rev 41: 13–25, 2003 [DOI] [PubMed] [Google Scholar]
- Wang Y, Luksch H, Brecha NC, Karten HJ. Columnar projections from the cholinergic nucleus isthmi to the optic tectum in chicks (gallus gallus): a possible substrate for synchronizing tectal channels. J Comp Neurol 494: 7–35, 2006 [DOI] [PubMed] [Google Scholar]
- Wang Y, Major DE, Karten HJ. Morphology and connections of nucleus isthmi pars magnocellularis in chicks. J Comparative Neurol 469: 275–297, 2004 [DOI] [PubMed] [Google Scholar]
- Wang YQ, Takatsuji K, Yamano M, Tohyama M. Localization of neuroactive substances in the rat parabigeminal nucleus: an immunohistochemical study. J Chem Neuroanat 1: 195–204, 1988 [PubMed] [Google Scholar]
- Winkowski DE, Knudsen EI. Top-down gain control of the auditory space map by gaze control circuitry in the barn owl. Nature 439: 336–339, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski DE, Knudsen EI. Top-down control of multimodal sensitivity in the barn owl optic tectum. J Neurosci 27: 13279–13291, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski DE, Knudsen EI. Distinct mechanisms for top-down control of neural gain and sensitivity in the owl optic tectum. Neuron 60: 698–708, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR, Krupa B, Wilkinson F. Dynamics of perceptual oscillations in form vision. Nat Neurosci 3: 170–176, 2000 [DOI] [PubMed] [Google Scholar]
- Yuille AL, Grzywacz NM. A winner-take-all mechanism based on presynaptic inhibition feedback. Neural Comput 1: 334–347, 1989 [Google Scholar]