Abstract
Deletion of N-methyl-d-aspartate receptors (NMDARs) early in development results in an increase in the number of synaptic AMPA receptors (AMPARs), suggesting a role for NMDARs in negatively regulating AMPAR trafficking at developing synapses. Substantial evidence has shown that AMPAR subunits function differentially in AMPAR trafficking. However, the role of AMPAR subunits in the enhancement of AMPARs following NMDAR ablation remains unknown. We have now performed single-cell genetic deletions in double-floxed mice in which the deletion of GluN1 is combined with the deletion of GluA1 or GluA2. We find that the AMPAR enhancement following NMDAR deletion requires the GluA2 subunit, but not the GluA1 subunit, indicating a key role for GluA2 in the regulation of AMPAR trafficking in developing synapses.
INTRODUCTION
Most excitatory synapses in the brain express two types of ionotropic glutamate receptors: AMPARs and N-methyl-d-aspartate receptors (NMDARs). AMPARs mediate the majority of excitatory synaptic transmission and therefore the abundance of AMPARs at a synapse largely determines the strength of the synapse. It is now well accepted that at developed synapses, the presence and number of AMPARs at synapses are tightly regulated by NMDAR activity through mechanisms including long-term potentiation and depression (LTP and LTD) (Barry and Ziff 2002; Bredt and Nicoll 2003; Malinow and Malenka 2002; Newpher and Ehlers 2008; Shepherd and Huganir 2007).
The role of NMDARs in AMPAR trafficking at developing excitatory synapses is less clear. NMDARs appear very early during synapse formation, whereas AMPAR arrival is delayed (Durand and Konnerth 1996; Durand et al. 1996; Hsia et al. 1998; Isaac et al. 1997; Liao et al. 1999; Petralia et al. 1999; Wu et al. 1996). This timing suggests that NMDARs may also play an important role in the regulation of AMPAR trafficking at developing synapses. Indeed recent studies have shown that deletion of NMDARs early in development increases the size of AMPAR excitatory postsynaptic currents (EPSCs) (Adesnik et al. 2008; Hall and Ghosh 2008; Hall et al. 2007; Ultanir et al. 2007), suggesting that NMDARs negatively regulate AMPAR trafficking, perhaps by an LTD-like mechanism (Adesnik et al. 2008; Hall and Ghosh 2008; Hall et al. 2007). However, little is known about the control of AMPAR recruitment following NMDAR ablation. Here we begin to address this issue by combining NMDAR deletion with the deletion of AMPAR subunits. More specifically we have generated double-floxed mice in which the GluN1 floxed gene is combined either with the GluA1 or GluA2 floxed gene to study the role of AMPAR subunits in AMPAR trafficking following NMDAR deletion. As NMDARs are important in the maturation of excitatory synapses, understanding the AMPAR subunit-specific role in synaptic potentiation induced by inhibiting NMDAR function will provide insights into mechanisms underlying synapse development. We find that the enhancement in synaptic AMPARs following NMDAR ablation remains intact in the absence of GluA1 despite the fact that extrasynaptic AMPARs are essentially absent. In contrast, the enhancement is absent when GluA2 is deleted despite a normal level of extrasynaptic AMPARs. These data suggest an important role for GluA2 in NMDAR-dependent AMPAR trafficking in developing synapses.
METHODS
Mouse genetics
Animal housing was performed according to the university guidelines at the University of California at San Francisco (UCSF). All procedures were conducted with the approval of, and in accordance with, the animal use regulations and the IACUC of UCSF. Gene-targeted GRIA1fl/fl, GRIA2fl/fl, and GRIN1fl/fl mice were generated as described previously (Lu et al. 2009). Double GRIAXfl/flGRIN1fl/fl mice were generated by crossing individual GRIAXfl/fl mice with GRIN1fl/fl mice.
In vivo postnatal viral injection
Mice were injected at 0–2 days after birth (P0–P2) with high-titer rAAV-GFP-Cre viral stock (∼1–5 × 1013 vg/ml). Newborns were anesthetized on ice for 1.5–2 min and then mounted in a custom ceramic mold before being injected with 4.2–13.2 nl of viral solution at nine sites targeting the hippocampus at each cerebral hemisphere by Nanoject (Drummond Scientific) and a beveled glass injection pipette. Pups recovered quickly after injection, were returned to home cage and used for recording 13–30 day afterward.
Electrophysiology
Transverse 300 μm hippocampal slices were cut from mice on a vibratome (Ted Pella) in high sucrose cutting solution containing (in mM) 50 NaCl, 2.5 KCl, 0.5 CaCl2, 7 MgCl2, 1.0 NaH2PO4, 25 NaHCO3, 10 glucose, and 150 sucrose. Freshly cut slices were placed in an incubating chamber containing artificial cerebrospinal fluid (ACSF) containing (in mM) 119 NaCl, 2.5 KCl, 26 NaHCO3, 1 Na2PO4, 11 glucose, 4 CaCl2, and 4 MgCl2 and recovered at 35°C for ∼1 h. Slices were maintained in ACSF at room temperature for 0.5–1 h prior to recording and then transferred to a submersion chamber on an upright Olympus microscope, perfused in normal ACSF containing picrotoxin (0.1 mM) and bicuculline (0.01 mM) and saturated with 95% O2-5% CO2, and the perfusion solution was maintained at room temperature (22–25°C). CA1 pyramidal cells were visualized by infrared differential interference contrast microscopy. The intracellular solution contained (in mM) 135 CsMeSO4, 8 NaCl, 10 HEPES, 0.3 Na3GTP, 4 MgATP, 0.3 EGTA, 5 QX-314, and 0.1 spermine. Cells were recorded with 3–5 MΩ borosilicate glass pipettes, following stimulation of Schaffer collaterals with monopolar glass electrodes filled with ACSF placed in stratum radiatum in the CA1 region. Series resistance was monitored and not compensated, and cells in which series resistance varied by 25% during a recording session were discarded. Synaptic responses were collected with a Multiclamp 700A amplifier (Axon Instruments, Foster City, CA), filtered at 2 kHz, digitized at 10 Hz.
GFP positive neurons were identified by epifluorescence microscopy. All paired recordings involved simultaneous whole cell recordings from one GFP positive neuron and a neighboring GFP negative neuron. The stimulus was adjusted to evoke a measurable, monosynaptic EPSC in both cells. AMPAR EPSCs were measured at a holding potential of −70 mV, and NMDAR EPSCs were measured at +40 mV and at 150 ms after the stimulus, at which point the AMPAR EPSC has completely decayed. AMPAR-mediated currents from outside-out somatic patches were recorded at −70 mV by local application of 1 mM glutamate and 100 μM cyclothiazide, in present of 100 μM APV, 0.5 μM TTX and 100 μM picrotoxin, for 2 s. Paired-pulse ratios were measured by giving two pulses at a 50 ms interval and taking the ratio of the two peaks of the EPSCs from an average of 20–50 sweeps. Rectification indices were calculated as the ratio of the slopes of the two lines connecting average EPSC values at −70 and 0 mV and 0 and +40 mV, respectively, in presence of 100 μM APV to block NMDAR-mediated EPSCs. All paired recording data were analyzed statistically with a two-tailed paired Student t-test. For all other analyses, an unpaired two-tailed t-test was used. All errors bars represent SE.
RESULTS
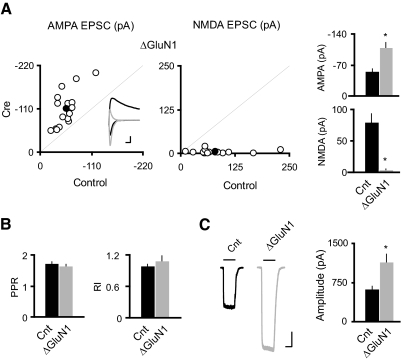
To delete the GluN1 subunit in vivo, we performed transcranial stereotactic injections of a recombinant adeno-associated virus expressing a Cre-GFP fusion protein (rAAV-Cre-GFP) into the hippocampus of P0–2 GluN1 floxed mice (GRIN1fl/fl). After 2–3 wk from the P0 injection, dual whole cell voltage-clamp recordings were made to measure AMPAR- or NMDAR- mediated EPSCs in Cre-expressing CA1 pyramidal cells and nearby control cells evoked by a single stimulating electrode. As reported previously for experiments in which Cre was expressed either embryonically (Adesnik et al. 2008; Ultanir et al. 2007) or in slice culture (Adesnik et al. 2008), Cre-expressing cells had no detectable NMDAR EPSCs, and the AMPAR EPSCs were substantially increased (Fig. 1A). There was no change in the paired-pulse ratio (PPR), a measure of the probability of transmitter release (Fig. 1B) or in rectification, a measure of GluA2 content of AMPARs (B), suggesting that AMPARs trafficked to synapses contain the GluA2 subunit. Interestingly, the size of glutamate-evoked AMPAR-mediated currents in outside-out somatic patches, a measure of the density of extrasynaptic AMPARs, is approximately doubled (Fig. 1C). Collectively, these data show that loss of NMDARs leads to an enhancement of AMPARs at both synaptic and extrasynaptic membranes.
Fig. 1.
Single-cell genetic deletion of N-methyl-d-aspartate receptors (NMDARs) induced potentiation of both synaptic and extrasynaptic AMPA receptors (AMPARs) in CA1 pyramidal neurons. A: scatter plots and bar graphs at right show amplitudes of excitatory postsynaptic currents (EPSCs) for single pairs (open circles) and means ± SE (filled circles) from CA1 pyramidal neurons from acute slices (P17–P24) from GRIN1fl/fl mice injected with AAV-CRE/GFP at P0–P1. Inset: sample traces: black, control; gray, Cre. Scale bar, 40 pA, 0.02 s. EPSC amplitudes in the bar graphs show a significant enhancement in AMPAR EPSCs with the deletion of GluN1 (AMPA: Cnt, −55.75 ± 5.67 pA; ΔGluN1, −109.51 ± 11.49 pA; n = 16, *P < 0.0001; NMDA: Cnt, 80.15 ± 13.33 pA; ΔGluN1, 3.86 ± 1.24 pA, n = 16; *P < 0.0001). B: bar graphs show average paired-pulse ratio (PPR; Cnt: 1.73 ± 0.04, n = 84; ΔGluN1: 1.65 ± 0.05, n = 14; P = 0.39), and average rectification index (RI; Cnt: 0.99 ± 0.03, n = 30; ΔGluN1: 1.09 ± 0.10, n = 14, P = 0.21). C: sample traces of glutamate-evoked currents from outside-out patches in control (black) and Cre cells (gray). Bar graph shows that deletion of GluN1 led to ∼80% enhancement of the AMPAR-mediated outside-out patch currents (Cnt: −630.32 ± 44.68 pA; n = 24; ΔGluN1: −1146.10 ± 144.89 pA; n = 10; *P < 0.001). Scale bar, 200 pA, 1 s.
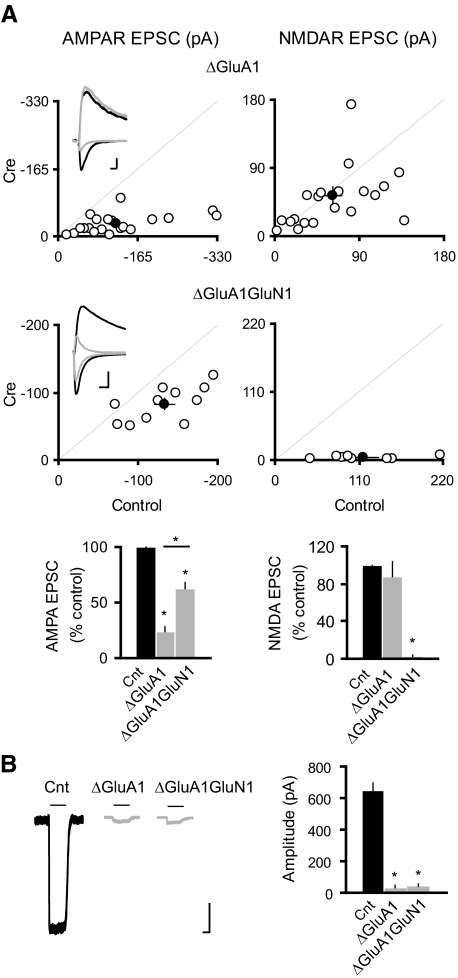
The AMPAR GluA1 subunit is important in the maintenance of both extrasynaptic and synaptic AMPARs (Andrasfalvy et al. 2003; Jensen et al. 2003; Lu et al. 2009; Zamanillo et al. 1999) and is also critical for synaptic delivery of AMPARs during LTP (Boehm et al. 2006; Jensen et al. 2003; Lee et al. 2003; Lin et al. 2009; Shi et al. 2001; Zamanillo et al. 1999). To test whether GluA1 is involved in the increase of surface and synaptic AMPARs following NMDAR deletion, we crossed the GRIN1fl/fl mice to GRIA1fl/fl mice to generate the GRIA1fl/flGRIN1fl/fl double conditional mice. Cre expression in CA1 pyramidal neurons from the GRIA1fl/flGRIN1fl/fl double conditional mice will lead to deletion of both GluN1 and GluA1, thus providing the opportunity to study the role of GluA1 in the enhanced synaptic delivery of AMPARs following NMDAR deletion. First, in agreement with our previous report (Lu et al. 2009), we found that deletion of GluA1 by itself resulted in a selective, severe reduction in AMPAR EPSCs (Fig. 2A) and a profound loss of extrasynaptic AMPARs (B). In GRIA1fl/flGRIN1fl/fl mice, the deletion of both GluN1 and GluA1 results in approximately a doubling of the AMPAR EPSC above that seen with the deletion of GluA1 alone (Fig. 2A). This suggests that the mechanism underlying upregulation of AMPAR trafficking in neurons lacking NMDARs is preserved in neurons lacking both NMDARs and the AMPAR GluA1 subunit. In addition, there is no change of PPR and rectification in neurons lacking of either GluA1 or both GluA1 and GluN1 (PPR: Cnt, 1.73 ± 0.04, n = 84; ΔGluA1, 1.69 ± 0.09, n = 15; ΔGluA1GluN1, 1.64 ± 0.12, n = 9; P > 0.05 for both conditions as compared with control; Rectification index: Cnt, 0.99 ± 0.03, n = 30; ΔGluA1, 1.02 ± 0.08, n = 14; ΔGluA1GluN1: 0.95 ± 0.03, n = 4; P > 0.05 for both conditions as compared with control). Furthermore, there was no significant recovery of the extrasynaptic AMPAR responses (Fig. 2B). The lack of detectable change of extrasynaptic AMPAR currents in neurons devoid of both GluA1 and GluN1 may be due to the fact that outside-out patch currents are typically extremely small in neurons lacking of GluA1 (<30 pA) as compared with wild-type neurons (>600 pA; Fig. 2) (see also Lu et al. 2009). As outside-out patch currents can be influenced by channel density in the excised membranes, positioning of perfusion system and the size of the opening of the patch pipette, small currents as observed in neurons lacking of GluA1 or lacking of both GluA1 and GluN1 make rigorous comparisons difficult. Taken together, we conclude that the GluA1 subunit is not required for the potentiation of synaptic AMPARs induced by NMDAR deletion.
Fig. 2.
GluA1 subunit is not required for the NMDAR ablation-induced potentiation of synaptic AMPARs. A: scatter plots show amplitudes of EPSCs for single pairs (open circles) and means ± SE (filled circles) from CA1 pyramidal neurons from acute slices (P19–P25) from GRIA1fl/fl (top) and GRIA1fl/flGRIN1fl/fl (bottom) mice injected with AAV-CRE/GFP at P0–P1. Inset: sample traces: black, control; gray, Cre. Scale bar, 50 pA, 0.02 s. EPSC amplitudes in the bar graphs show a significant reduction in AMPAR EPSCs for the deletion of GluA1 or both GluA1 and GluN1 (ΔGluA1: −24.18 ± 4.30; n = 21, *P < 0.0001 as compared with control; ΔGluA1GluN1: −62.20 ± 5.90; n = 11; *P < 0.005 as compared with control). AMPAR EPSCs were significantly higher in ΔGluA1GluN1 than that in ΔGluA1 (*P < 0.0005). There was no change in the NMDAR EPSCs in ΔGluA1: 87.29 ± 16.90, n = 21; n = 21; P = 0.87; but NMDAR EPSCs were essentially eliminated in ΔGluA1GluN1: 3.10 ± 0.70, n = 8; *P < 0.0001). B: sample traces of glutamate-evoked currents from outside-out patches in control (black) and Cre cells (gray). Bar graph shows that deletion of GluA1 or both GluA1 and GluN1 eliminated the AMPAR-mediated outside-out patch currents (Cnt: −630.32 ± 44.68 pA; n = 24; ΔGluA1: −35.31 ± 13.05 pA, n = 16; ΔGluA1GluN1: −42.09 ± 16.66, n = 11; *P < 0.001 for both conditions). Scale bar, 100 pA, 1 s.
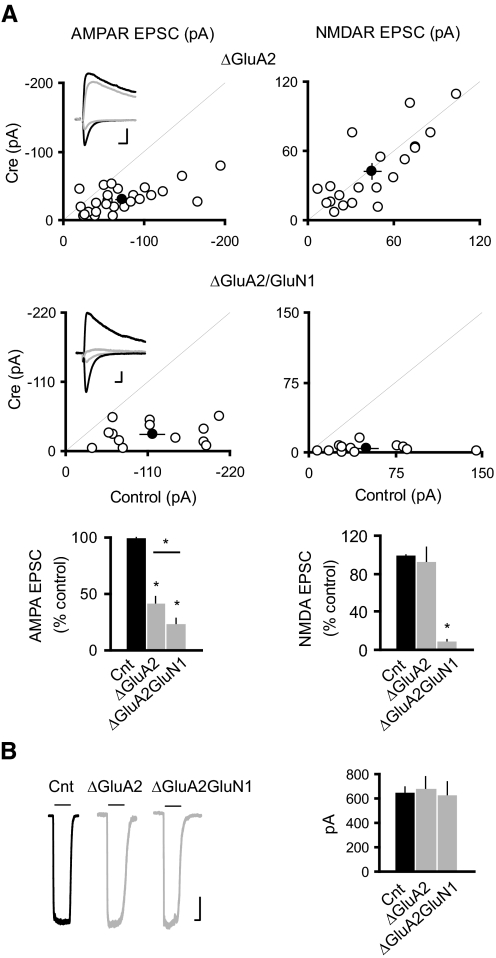
Synaptic AMPARs at excitatory synapses onto CA1 pyramidal neurons contain the GluA2 subunit (Lu et al. 2009). In addition, the GluA2 subunit has been shown to play important roles in various aspects of AMPAR trafficking, including receptor maturation from endoplasmic reticulum, endocytosis and exocytosis, and synaptic plasticity (Barry and Ziff 2002; Bredt and Nicoll 2003; Greger and Esteban 2007; Malinow and Malenka 2002; Newpher and Ehlers 2008; Shepherd and Huganir 2007). We thus tested whether GluA2 is required for the potentiation of AMPAR EPSCs following the loss of NMDARs. In the GRIA2fl/fl mice, deleting GluA2 caused about a 50% reduction in the AMPAR EPSC (Fig. 3A) with no change in the glutamate-evoked AMPAR-mediated outside-out patch currents (B). Interestingly, in GRIA2fl/flGRIN1fl/fl mice the loss of NMDARs failed to enhance AMPAR EPSCs. In fact, there was a significant further decrease (Fig. 3A). Neither genetic manipulation changes the PPR (PPR: Cnt, 1.73 ± 0.04, n = 84; ΔGluA2, 1.72 ± 0.06, n = 29; ΔGluA2GluN1, 1.74 ± 0.16, n = 6; P > 0.05 for both conditions as compared with control). In addition, as expected, deletion of GluA2 caused strong inward rectification (rectification index: Cnt, 0.99 ± 0.03, n = 30; ΔGluA2, 0.15 ± 0.02, n = 19; ΔGluA2GluN1, 0.14 ± 0.05, n = 8; *P < 0.05 for both conditions as compared with control). Finally, no difference was found in glutamate evoked AMPAR-mediated currents in outside-out patches (Fig. 3B). Therefore in contrast to GluA1, the GluA2 subunit is critical for synaptic potentiation of AMPARs following NMDAR deletion.
Fig. 3.
GluA2 subunit is required for the NMDAR ablation-induced potentiation of synaptic AMPARs. A: scatter plots show amplitudes of EPSCs for single pairs (open circles) and means ± SE (filled circles) from CA1 pyramidal neurons from acute slices (P17–P24) from GRIA2fl/fl (top) and GRIA2fl/flGRIN1fl/fl (bottom) mice injected with AAV-CRE/GFP at P0–P1. Inset: sample traces: black, control; gray, Cre. Scale bar, 50 pA in ΔGluA2 and 20 pA in ΔGluA2GluN1, 0.02 s. EPSC amplitudes in the bar graphs show a significant reduction in AMPAR EPSCs for the deletion of GluA2 or both GluA2 and GluN1 (ΔGluA2: −41.38 ± 5.20; n = 26, *P < 0.0001 as compared with control; ΔGluA2GluN1: −23.46 ± 4.70; n = 13; *P < 0.0001 as compared with control). AMPAR EPSCs were significantly smaller in ΔGluA2GluN1 than that in ΔGluA2 (*P < 0.05). There was no change in the NMDAR EPSCs in ΔGluA2: 92.98 ± 15.00, n = 20; P = 0.85; but NMDAR EPSCs were essentially eliminated in ΔGluA2GluN1: 9.01 ± 2.20, n = 13; *P < 0.0001). B: sample traces of glutamate-evoked currents from outside-out patches in control (black) and Cre cells (gray). Bar graph shows that deletion of GluA2 or both GluA2 and GluN1 did not change the AMPAR-mediated outside-out patch currents (Cnt: −630.32 ± 44.68 pA; n = 24; ΔGluA2: −684.27 ± 92.17 pA, n = 11; ΔGluA2GluN1: −615.29 ± 106.16, n = 7; P > 0.05 for both conditions). Scale bar, 100 pA, 1 s.
DISCUSSION
Excitatory glutamatergic synapses have received considerable attention, not only because of their abundance in the brain but also for their remarkable plasticity. Much of this plasticity involves the trafficking of the AMPARs that mediate the majority of fast excitatory synaptic transmission. At mature synapses, AMPAR trafficking is tightly regulated by NMDAR activity that controls synaptic insertion or removal of AMPARs (Barry and Ziff 2002; Bredt and Nicoll 2003; Malinow and Malenka 2002; Newpher and Ehlers 2008; Shepherd and Huganir 2007). Convincing evidence has shown that NMDARs regulate AMPAR trafficking through different AMPAR subunits and AMPAR accessory subunits (Barry and Ziff 2002; Bredt and Nicoll 2003; Chen et al. 2000; Malinow and Malenka 2002; Newpher and Ehlers 2008; Nicoll et al. 2006; Shepherd and Huganir 2007). However, little is known about the role of NMDARs in AMPAR trafficking in developing neurons, and it is also unclear whether AMPAR trafficking at young synapses requires specific subunit of the AMPAR. Recently, genetic deletion of the GluN1 subunit, the obligatory subunit for the formation of the NMDAR, in developing neurons causes upregulation of synaptic AMPARs in both hippocampus and cortex (Adesnik et al. 2008; Hall and Ghosh 2008; Ultanir et al. 2007), suggesting an important role of NMDARs in the regulation of AMPAR abundance at developing synapses. It has been proposed that the presence of NMDARs and their basal activity suppresses synaptic delivery of AMPARs perhaps via an LTD-like mechanism. Genetic deletion of NMDARs relieves this suppression, thus leading to enhanced AMPAR trafficking to synapses (Adesnik et al. 2008; Hall and Ghosh 2008). We have now shown that the enhancement of AMPAR-mediated synaptic transmission in neurons lacking NMDARs requires the existence of the GluA2, but not the GluA1 subunit, indicating a critical role for GluA2 in AMPAR trafficking in developing synapses. These data also suggest that the basal activity of NMDARs may inhibit AMPAR-mediated synaptic transmission through the downregulation of GluA2-dependent synaptic delivery of AMPARs. In addition, it is worth noting that in GRIA2fl/flGRIN1fl/fl double conditional mice, AMPAR-mediated synaptic transmission is smaller than that in GRIA2fl/fl (Fig. 3A), indicating that synaptic trafficking of GluA2-lacking receptors is less efficient in neurons devoid of NMDARs.
The mechanisms for the role of GluA2 in the enhancement of AMPAR-mediated synaptic transmission in neurons lacking NMDARs are unclear at this stage. It is possible that GluA2-specific trafficking mechanisms may play a role in this process. For example, the unique interaction between GluA2 and NSF may underlie synaptic trafficking of AMPARs in neurons lacking of NMDARs (Nishimune et al. 1998; Osten et al. 1998; Song et al. 1998). Alternatively, in neurons lacking both GluA2 and GluN1, the remaining GluA2-lacking and calcium-permeable AMPARs can mediate calcium influx, which may trigger LTD-like mechanisms to prevent AMPARs from delivery to synapses. In this scenario, the importance of GluA2 may lie in the fact that the presence of GluA2 prevents calcium influx through AMPARs (Burnashev et al. 1992; Hume et al. 1991). Future experiments, such as molecular replacement of endogenous AMPAR subunits with GluA1 or GluA2 containing mutations of the Q/R editing site in the channel pore, could provide insight into these issues.
In addition to the synaptic trafficking of AMPARs following NMDAR deletion, AMPAR levels on extrasynaptic membranes are also enhanced. This indicates that along with a localized synaptic role, NMDAR activation in developing neurons has a global role in AMPAR trafficking. It has been reported that in the dendritic regions of pyramidal neurons, synaptic activation of NMDARs can induce local NMDA spikes (Larkum et al. 2009; Schiller et al. 2000). Through interactions with dendritic calcium spikes and axosomatic sodium spikes, these local NMDA spikes eventually lead to firing of the neuron (Larkum et al. 2009). NMDA spikes may especially be important in modulating neuronal firing in developing neurons as the majority of excitatory synaptic transmission in developing neurons is mediated by NMDARs (Durand and Konnerth 1996; Durand et al. 1996; Hsia et al. 1998; Isaac et al. 1997; Liao et al. 1999; Petralia et al. 1999; Wu et al. 1996). Therefore it is conceivable that loss of NMDARs may cause chronic inhibition of neuronal firing during development, leading to adaptations of AMPARs at synaptic and extrasynaptic membranes in neurons lacking NMDARs. Intriguingly, it has recently been shown that synaptic scaling during homeostatic plasticity also requires the GluA2 subunit (Gainey et al. 2009; Goold and Nicoll 2010), suggesting that these two forms of neuronal plasticity may share common pathways mediated by the GluA2 subunit.
In summary, our in vivo single-cell genetic study demonstrates that deletion of NMDARs in developing neurons causes enhanced trafficking of AMPARs to both synaptic and extrasynaptic membranes, which depends on the GluA2 subunit. A prominent role for the GluA2 subunit in synaptic trafficking of AMPARs regulated by NMDARs in developing synapses may underlie the maturation of excitatory synapses that are characterized by high GluA2 content in mature neurons.
GRANTS
W. Lu is funded by a postdoctoral fellowship from the American Heart Association, J. A. Gray is funded by a NARSAD Young Investigator Award, and is the NARSAD Hammerschlag Family Investigator, and A. J. Granger is funded by a predoctoral fellowship from National Science Foundation Graduate Research Fellowship Program, and R. A. Nicoll is funded by grants from National Institute of Mental Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We are grateful to the generosity of P. Seeburg and R. Sprengel (Max Planck Institute for Medical Research, Heidelberg, Germany) for the GRIA1fl/fl and GRIA2fl/fl mice, and S. Tonegawa (Howard Hughes Medical Institute, Massachusetts Institute of Technology) for the GRIN1fl/fl mice. We thank K. Bjorgan and M. Cerpas for help in mouse colony maintenance and colleagues from the Nicoll lab for discussions during this project.
REFERENCES
- Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA. NMDA receptors inhibit synapse unsilencing during brain development. Proc Natl Acad Sci USA 105: 5597–5602, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrasfalvy BK, Smith MA, Borchardt T, Sprengel R, Magee JC. Impaired regulation of synaptic strength in hippocampal neurons from GluR1-deficient mice. J Physiol 552: 35–45, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol 12: 279–286, 2002 [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron 51: 213–225, 2006 [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron 40: 361–379, 2003 [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8: 189–198, 1992 [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 408: 936–943, 2000 [DOI] [PubMed] [Google Scholar]
- Durand GM, Konnerth A. Long-term potentiation as a mechanism of functional synapse induction in the developing hippocampus. J Physiol 90: 313–315, 1996 [DOI] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature 381: 71–75, 1996 [DOI] [PubMed] [Google Scholar]
- Gainey MA, Hurvitz-Wolff JR, Lambo ME, Turrigiano GG. Synaptic scaling requires the GluR2 subunit of the AMPA receptor. J Neurosci 29: 6479–6489, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goold CP, Nicoll RA. Single-cell optogenetic excitation drives homeostatic synaptic depression. Neuron 68: 512–528, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger IH, Esteban JA. AMPA receptor biogenesis and trafficking. Curr Opin Neurobiol 17: 289–297, 2007 [DOI] [PubMed] [Google Scholar]
- Hall BJ, Ghosh A. Regulation of AMPA receptor recruitment at developing synapses. Trends Neurosci 31: 82–89, 2008 [DOI] [PubMed] [Google Scholar]
- Hall BJ, Ripley B, Ghosh A. NR2B signaling regulates the development of synaptic AMPA receptor current. J Neurosci 27: 13446–13456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Malenka RC, Nicoll RA. Development of excitatory circuitry in the hippocampus. J Neurophysiol 79: 2013–2024, 1998 [DOI] [PubMed] [Google Scholar]
- Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science 253: 1028–1031, 1991 [DOI] [PubMed] [Google Scholar]
- Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron 18: 269–280, 1997 [DOI] [PubMed] [Google Scholar]
- Jensen V, Kaiser KM, Borchardt T, Adelmann G, Rozov A, Burnashev N, Brix C, Frotscher M, Andersen P, Hvalby O, Sakmann B, Seeburg PH, Sprengel R. A juvenile form of postsynaptic hippocampal long-term potentiation in mice deficient for the AMPA receptor subunit GluR-A. J Physiol 553: 843–856, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science 325: 756–760, 2009 [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112: 631–643, 2003 [DOI] [PubMed] [Google Scholar]
- Liao D, Zhang X, O'Brien R, Ehlers MD, Huganir RL. Regulation of morphological postsynaptic silent synapses in developing hippocampal neurons. Nat Neurosci 2: 37–43, 1999 [DOI] [PubMed] [Google Scholar]
- Lin DT, Makino Y, Sharma K, Hayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci 12: 879–887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62: 254–268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103–126, 2002 [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron 58: 472–497, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science 311: 1253–1256, 2006 [DOI] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron 21: 87–97, 1998 [DOI] [PubMed] [Google Scholar]
- Osten P, Srivastava S, Inman GJ, Vilim FS, Khatri L, Lee LM, States BA, Einheber S, Milner TA, Hanson PI, Ziff EB. The AMPA receptor GluR2 C terminus can mediate a reversible, ATP-dependent interaction with NSF and alpha- and beta-SNAPs. Neuron 21: 99–110, 1998 [DOI] [PubMed] [Google Scholar]
- Petralia RS, Esteban JA, Wang YX, Partridge JG, Zhao HM, Wenthold RJ, Malinow R. Selective acquisition of AMPA receptors over postnatal development suggests a molecular basis for silent synapses. Nat Neurosci 2: 31–36, 1999 [DOI] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature 404: 285–289, 2000 [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol 23: 613–643, 2007 [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331–343, 2001 [DOI] [PubMed] [Google Scholar]
- Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir RL. Interaction of the N-ethylmaleimide-sensitive factor with AMPA receptors. Neuron 21: 393–400, 1998 [DOI] [PubMed] [Google Scholar]
- Ultanir SK, Kim JE, Hall BJ, Deerinck T, Ellisman M, Ghosh A. Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proc Natl Acad Sci USA 104: 19553–19558, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science 274: 972–976, 1996 [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science 284: 1805–1811, 1999 [DOI] [PubMed] [Google Scholar]