Abstract
Endocannabinoids are potent regulators of synaptic strength. They are generally thought to modify neurotransmitter release through retrograde activation of presynaptic type 1 cannabinoid receptors (CB1Rs). In the cerebellar cortex, CB1Rs regulate several forms of synaptic plasticity at synapses onto Purkinje cells, including presynaptically expressed short-term plasticity and, somewhat paradoxically, a postsynaptic form of long-term depression (LTD). Here we have generated mice in which CB1Rs were selectively eliminated from cerebellar granule cells, whose axons form parallel fibers. We find that in these mice, endocannabinoid-dependent short-term plasticity is eliminated at parallel fiber, but not inhibitory interneuron, synapses onto Purkinje cells. Further, parallel fiber LTD is not observed in these mice, indicating that presynaptic CB1Rs regulate long-term plasticity at this synapse.
INTRODUCTION
Endocannabinoids (eCBs) mediate multiple forms of synaptic plasticity throughout the brain. Typically, eCBs are released from postsynaptic neurons in response to synaptic activity and act retrogradely on presynaptic terminals to modify neurotransmitter release (Chevaleyre et al. 2006; Kano et al. 2009; Regehr et al. 2009). Most of the synaptic effects of eCBs involve activation of type 1 cannabinoid receptors (CB1Rs) (Matsuda et al. 1990).
In the cerebellar cortex, eCBs mediate several forms of plasticity at synapses onto Purkinje cells (PCs). These include depolarization-induced suppression of inhibition (DSI) and excitation (DSE), in which Purkinje cell depolarization elevates postsynaptic calcium to trigger eCB release, and synaptically evoked suppression of excitation (SSE), in which activation of Gq-coupled receptors and calcium trigger eCB release (Brenowitz and Regehr 2003; Brown et al. 2003; Kreitzer and Regehr 2001a,b; Llano et al. 1991; Maejima et al. 2001). These short-term forms of plasticity are all expressed presynaptically and are eliminated in global CB1R knockout animals, indicating that they are mediated by CB1Rs (Kawamura et al. 2006; Safo and Regehr 2005). Immunohistochemistry has shown that CB1Rs are present in the terminals of all types of synapses made onto PCs (Pettit et al. 1998; Suarez et al. 2008; Tsou et al. 1998). Thus it is likely that DSI, DSE, and SSE all result from suppression of neurotransmitter release following activation of presynaptic CB1Rs.
Recently it was shown that CB1Rs are required for long-term depression (LTD) of parallel fiber (PF) inputs to PCs (Safo and Regehr 2005). This result was surprising because unlike the other forms of CB1R-dependent cerebellar plasticity described in the preceding text, PF-LTD is thought to be induced and expressed postsynaptically (Ito 2001, 2002). Immunohistochemical and in situ hybridization studies, however, have not detected CB1R expression in PCs (Herkenham et al. 1991; Mailleux and Vanderhaeghen 1992; Matsuda et al. 1993; Pettit et al. 1998; Suarez et al. 2008; Tsou et al. 1998). This raises the question: where are the CB1Rs that regulate the induction of LTD? Are they located on presynaptic PFs, PCs (but as yet undetected), or on another cell type?
Here we describe mice in which CB1Rs are eliminated selectively from cerebellar granule cells, whose axons from PF inputs to PCs. These mice are deficient in DSE and SSE at synapses between granule cell PFs and PCs. We also find that long-term depression is not apparent in these mice, suggesting that presynaptic CB1Rs regulate LTD at PF to PC synapses.
METHODS
Animals
All animal procedures were approved by the Harvard Medical Area Standing Committee on Animals. Gabra6Cre (Funfschilling and Reichardt 2002), CB1f/f (Marsicano et al. 2003), and CB1KO (Zimmer et al. 1999) animals were maintained on a C57BL/6-J background after back crossing into this background for at least six generations. Gabra6cre;CB1f/f males were mated with CB1f/f females to generate Gabra6cre;CB1f/f experimental animals and CB1f/f littermate controls. Mice were genotyped by PCR on tail genomic DNA with sense primer 5′-GAT CTC CGG TAT TGA AAC TCC AGC-3′ and antisense primer 5′-GCT AAA CAT GCT TCA TCG TCG G-3′ to detect Gabra6Cre expression and sense primer 5′-GCT GTC TCT GGT CCT CTT AAA-3′ and antisense primer 5′-GGT GTC ACC TCT GAA AAC AGA-3′ to detect homozygosity for CB1f/f. All control animals were age-matched littermates.
Immunohistochemistry
Mice were perfused with ice-cold paraformaldehyde, and brains were transferred to PBS. Free-floating serial sections (50 μm thick) were collected and incubated in a rabbit polyclonal primary antibody raised against the last 15 amino acids of the C-terminus of the CB1R (Bodor et al. 2005; Nyiri et al. 2005) and Alexa Fluor 488 goat anti-rabbit secondary antibody (Invitrogen). Sections were imaged on a Zeiss 510 M confocal microscope.
Electrophysiology
Parasagittal slices, 200–250 μm thick, were cut from the cerebellar vermis of 12- to 19-day-old mice (Carey and Regehr 2009; Safo and Regehr 2005). The extracellular artificial cerebrospinal fluid (ACSF) contained (in mM) 125 NaCl, 26 NaHCO3, 25 glucose, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, and 2 CaCl2 and was bubbled with 95% O2-5% CO2. Experiments were conducted at 34 ± 1°C (mean ± SD) except where noted.
Whole cell recordings were made from PCs with 1–3 MΩ glass electrodes as previously described (Carey and Regehr 2009; Safo and Regehr 2005). For voltage clamp recordings, the internal solution consisted of (in mM) 145 CsMeSO4, 15 HEPES, 0.2 EGTA, 1 MgCl2, 5 TEA-Cl, 2 Mg-ATP, 10 phosphocreatine (tris), 2 QX-314, and 0.4 Na-GTP (pH 7.3). For inhibitory postsynaptic current (IPSC) measurements, we made the following changes: 19 mM CsMeSO4, 116 mM CsCl, 15 mM TEA-Cl. For the experiments in Fig. 4, recordings were made at 24°C with a K-gluconate internal solution (van Beugen et al. 2006). For current clamp recordings, the internal solution consisted of (in mM) 120 KMeSO3, 5 NaCl, 2 MgCl2, 0.05 CaCl2, 0.1 EGTA, 10 HEPES, 2 Na2ATP, 0.4 NaGTP, and 14 tris-creatine phosphate (pH 7.3).
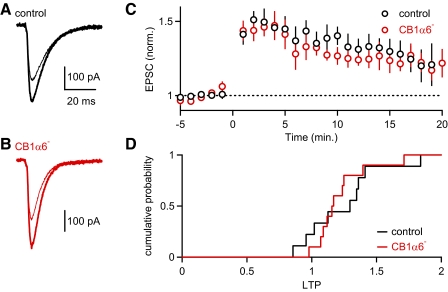
Fig. 4.
Presynaptic PF-LTP is unchanged in CB1α6– mice. PF-EPSCs were recorded from Purkinje cells in response to test pulses delivered at 0.05 Hz before and after a burst of PF stimuli (15 s at 8 Hz) (Salin et al. 1996; van Beugen et al. 2006). Average EPSCs recorded in the 5 min prior to (thin traces) and 15–20 min after the conditioning train (thick traces), are shown for representative experiments for control animals (A) and CB1α6– animals (B). C: the average EPSC amplitude (±SE) is plotted as a function of time for control (n = 9) and CB1α6– (n = 10) animals. D: the cumulative distribution of the amplitudes of long term plasticity (the average EPSP amplitude 15–20 min after the conditioning train/the average EPSP amplitude for the 5 min prior conditioning train) are plotted for the experiments summarized in C. The conditioning train resulted in LTP of PF-EPSCs for both control and CB1α6– animals, and there was no statistically significant difference in the extent of potentiation (25 and 22%, respectively, P = 0.81, t-test).
Picrotoxin (20 μM) was added to the ACSF to block inhibitory currents. For the experiments in Fig. 2C, picrotoxin was omitted and 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX; 10 μM) was added to block excitatory currents. For the experiments in Fig. 2D, NBQX (250–350 nM) was bath applied to reduce the amplitude and voltage-clamp errors of CF-EPSCs. For experiments using high-frequency conditioning trains, CGP55845A (2 μM) was added to the ACSF to block GABAB receptors.
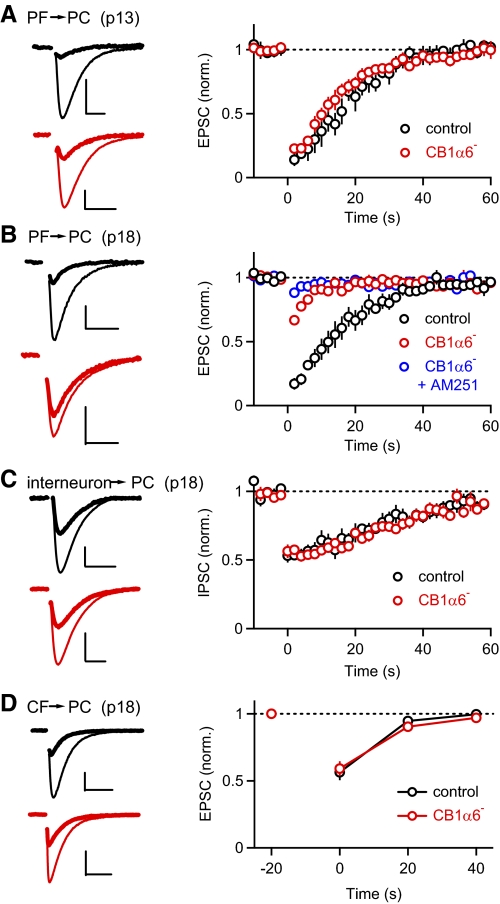
Fig. 2.
CB1R function is eliminated from parallel fibers in a neuron and age-specific manner. Depolarization-induced suppression of excitatory and inhibitory synapses (DSE/DSI) was examined in control and CB1α6 – mice. Purkinje cells were depolarized to 0 mV for 2 s and the effect on PF-excitatory postsynaptic currents (EPSCs, A and B), interneuron IPSCs (C), and climbing fiber EPSCs (D) were assessed. Experiments were conducted in p18 animals (B–D) and in p13 animals before full Cre expression (A). A–D, left: traces from representative experiments are shown for control (black) and CB1α6– (red) mice with EPSCs prior to (thin traces) and following depolarization (thick traces) superimposed. A–D, right: summaries of the average amplitudes of synaptic responses (±SE) are shown for control and CB1α6– mice (n = 4–10 neurons per condition). For the electrophysiological traces, vertical scale bars correspond to 200 pA in A–C and 300 pA in D, and horizontal scale bars correspond to 5 ms in A and B, 10 ms in C, and 20 ms in D.
RESULTS
We used the cre-lox system (Kuhn and Torres 2002) to generate mice lacking CB1Rs selectively in cerebellar granule cells, the source of excitatory PF synapses onto PCs. A previous study (Funfschilling and Reichardt 2002) described mice expressing Cre recombinase under the control of the alpha6 subunit of the GABAA receptor (Gabra6Cre). In the cerebellum of these animals, Cre expression is limited to granule cells and does not begin until the second postnatal week. We crossed Gabra6Cre mice with mice carrying floxed alleles of the Cnr 1 gene that encodes the CB1R (CB1f/f) (Marsicano et al. 2003). All electrophysiological experiments were carried out in Gabra6Cre;CB1f/f animals (henceforth CB1α6−) and their CB1f/f age-matched littermates (controls).
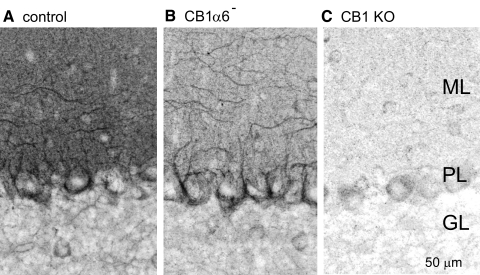
We visualized CB1R expression in the cerebella of control, CB1α6−, and CB1R global knockout animals (CB1KO, Fig. 1). In control animals, as in wild-type animals, CB1R immunofluorescence was dense in the molecular layer and in the large inhibitory presynaptic boutons known as basket cell Pinceau formations that surround the cell body and initial segment of PCs (Suarez et al. 2008). In CB1α6− mice, molecular layer staining was reduced, consistent with the elimination of CB1Rs from PFs (Fig. 1B). However, Pinceau formations and individual processes running perpendicular to PF beams were still stained, suggesting normal expression of CB1Rs in molecular layer interneurons (basket cells and stellate cells). Comparison with CB1KO animals (Fig. 1C) confirms the selective elimination of CB1Rs from PFs in CB1α6− mice.
Fig. 1.
Immunostaining reveals selective elimination of CB1Rs from cerebellar granule cells in CB1α6− animals. Sagittal sections (50 μm thick, ≥4 per animal) from 2-mo-old mice were stained with a rabbit polyclonal antibody raised against the last 15 amino acids of the CB1R C-terminus (Bodor et al. 2005; Nyiri et al. 2005) and imaged at ×40. Images (z-projections of 11 images taken at 1-μm intervals) of the cerebellar cortex are shown for a representative experiment of control (A), CB1α6− (B), and global CB1 knockout (C) animals (n = 5 each). The molecular layer (ML), the Purkinje cell layer (PL), and the granular layer (GL) are indicated in C.
To further assess the completeness and selectivity of the genetic manipulation, we examined DSE and DSI in control and CB1α6− mice. Depolarizing PCs to 0 mV for 2 s led to robust suppression of PF synaptic responses in control animals (Fig. 2A, black, 0.19 ± 0.03, n = 4) (Kreitzer and Regehr 2001b). This DSE reflects a decrease in presynaptic transmitter release mediated by CB1Rs. PCs from CB1α6− mice recorded at postnatal day 13 (P13) also exhibited normal DSE (Fig. 2A, red, 0.23 ± 0.02, n = 5; P = 0.47). By P18, PF-DSE was prominent in control animals (Fig. 2A, black, 0.21 ± 0.04, n = 5) but was almost completely eliminated in CB1α6− animals (Fig. 2B, red; 0.78 ± 0.03, n = 7; P = 5.6E-14, compared with “P18 control”). The small, short-lived suppression that remained in CB1α6− mice was reduced in the presence of the CB1R antagonist AM251 (Fig. 2B, blue, 0.93 ± 0.03, n = 5; P = 0.003, compared with “P18 CB1α6−”), indicating that it was partly CB1R dependent. This residual suppression in the face of a strong DSE stimulus is likely due to the continued presence of small amounts of CB1R at P18. The finding that CB1R function in granule cells is intact at P13, but disrupted in P18 animals, confirms the late onset of Cre expression in CB1α6− mice (Funfschilling and Reichardt 2002) and minimizes potential developmental complications (Berghuis et al. 2007).
To determine the specificity of CB1R elimination, we assessed depolarization-induced suppression of synaptic transmission at two other types of synapses onto PCs in P18 animals: those of inhibitory interneurons (Fig. 2C) (Kreitzer and Regehr 2001a; Llano et al. 1991) and climbing fibers (CF, Fig. 2D) (Kreitzer and Regehr 2001b). IPSCs recorded from PCs of control and CB1α6− mice demonstrated identical, robust DSI (0.54 ± 0.03, n = 9 and 0.57 ± 0.03, n = 6, respectively, P = 0.39), suggesting that CB1R expression and signaling in molecular layer interneurons within the cerebellar cortex is not perturbed in CB1α6− mice. Moreover, CF synapses, which are a second type of excitatory input to PCs, also exhibited robust DSE in both control and CB1α6− animals (0.56 ± 0.06, n = 8 and 0.59 ± 0.05, n = 10, respectively, P = 0.74). Taken together, these results indicate that in the cerebellar cortex of CB1α6− mice, CB1R signaling is eliminated selectively from PF synapses without perturbing eCB signaling at other inputs onto PCs.
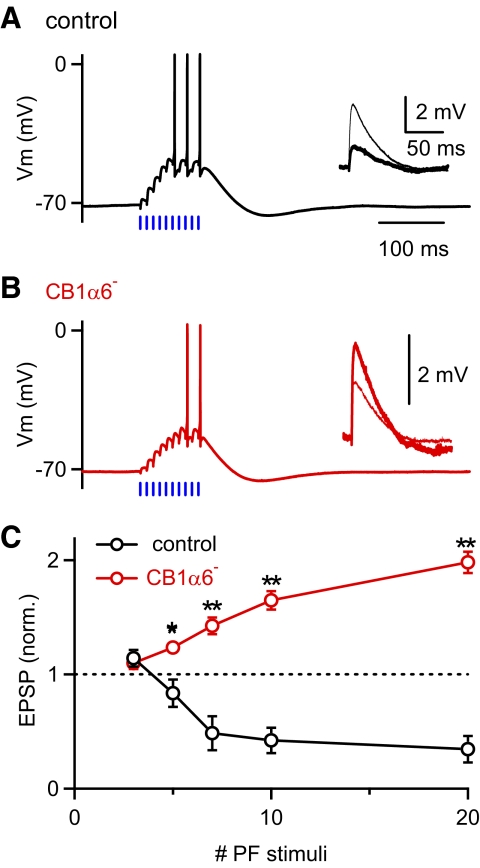
We next conducted a series of current-clamp experiments to assess the effects of CB1R elimination on PC responses to patterns of synaptic activity. Under normal conditions, short-term plasticity at the PF-PC synapse reflects a balance of posttetanic potentiation (PTP) and short-term synaptic suppression of excitation (SSE) (Brown et al. 2003). PTP is a presynaptic enhancement that is independent of eCB signaling, and SSE reflects CB1R-dependent suppression of neurotransmitter release. The magnitude of PTP increases with the number of stimuli in the conditioning train, but more prolonged PF stimulation evokes eCB release in wild-type animals. As a result, in wild-type animals, small PTP is observed for brief PF stimulus trains, and SSE is observed for more prolonged stimulus trains.
We compared SSE in control and CB1α6− mice. In control mice, a train of 10 PF stimuli delivered at 100 Hz elicited robust SSE (Fig. 3A). In CB1α6− mice, an identical PF stimulus train produced PTP (Fig. 3B) as is the case for wild-type animals in the presence of a CB1R antagonist (Brown et al. 2003). We compared the effects of stimulus trains of varying length in control and CB1α6− mice to determine how our genetic manipulation affected the balance of PTP and eCB-mediated SSE. We found that the difference between control and CB1α6− mice became more extreme as the number of PF stimuli increased, suggesting that CB1R function in CB1α6− mice is not rescued by increasingly prolonged stimulation (Fig. 3C).
Fig. 3.
Elimination of presynaptic CB1Rs alters short-term synaptic plasticity at PF-PC synapses. PF-excitatory postsynaptic potentials (EPSPs) were measured before and after the presentation of a conditioning train consisting of 3–20 PF stimuli at 100 Hz. Traces from representative experiments are shown for a control (A) and a CB1α6– mouse (B). Typical responses to conditioning trains with 10 PF stimuli are shown for each experiment, and insets show the average PF-EPSPs measured before (thin) and 2–4 s after (thick) the train. C: the short-term plasticity in Purkinje cells recorded from control (n = 7, black) and CB1α6– mice (n = 4, red) are summarized as a function of the number of stimuli in the conditioning train. Error bars = SE. (*, P < 0.05; **, P < 0.01; t-test).
The anatomical and electrophysiological data presented in the preceding text (Figs. 1–3) suggest that CB1Rs are effectively eliminated from PF synapses in CB1α6− mice, while CB1R function is left intact in other cell types within the cerebellum.
The selective removal of CB1Rs from PF synapses allowed us to test the role of these receptors in long-term plasticity of the PF to PC synapse. We first examined a presynaptic form of plasticity that does not require CB1R activation. Presynaptic PF-LTP is typically induced at room temperature by stimulating PF synapses at 8 Hz for 15 s (Salin et al. 1996). We found that the extent of LTP in control animals (1.25 ± 0.1, n = 9) and in CB1α6− mice (1.22 ± 0.07, n = 10) was not significantly different (Fig. 4, P = 0.81) as expected for this eCB-independent form of plasticity.
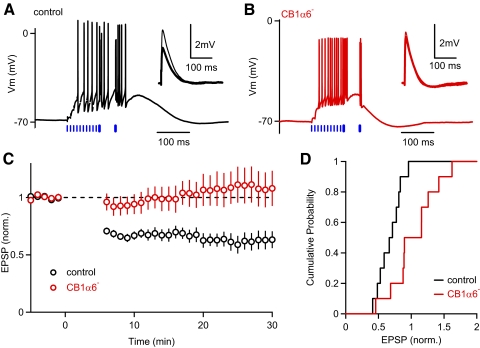
Finally, we asked whether PF CB1Rs are involved in PF-LTD. Repeated presentation of bursts of PF stimuli followed by CF activation have been shown to induce a postsynaptic form of LTD at PF synapses that requires activation of CB1Rs (Safo and Regehr 2005, 2008). We tested whether PF CB1Rs specifically are required for PF-LTD by comparing the plasticity induced by this conditioning stimulus in control and CB1α6− mice (Fig. 5). A stimulus consisting of 10 PF stimuli at 100 Hz followed by 2 CF stimuli at 20 Hz, presented every 10 s for 5 min, induced LTD of PF-EPSPS in PCs from control animals (Fig. 5A). As shown for a representative experiment, however, the same conditioning train failed to induce LTD in PCs from CB1α6− mice (Fig. 5B). On average, 15–20 min after the conditioning stimulation the PF-EPSC amplitude was 0.68 ± 0.05 (n = 10) in control animals, and 1.04 ± 0.11 (n = 10) in CB1α6− mice, and there was a significant difference in control animals and CB1α6− mice (P = 0.008, Fig. 5, C and D). These results suggest that CB1Rs located at PF-PC synapses provide an important means of regulating LTD at PF synapses.
Fig. 5.
PF-LTD is eliminated in CB1α6– mice. PF-long-term depression (LTD) was induced with a conditioning train consisting of 10 PF stimuli at 100 Hz (thin blue bars in A and B) followed by 2 CF stimuli at 20 Hz (thick blue bars in A and B), repeated every 10 s for 5 min. Traces from representative experiments are shown for a control (A) and a CB1α6– mouse (B). Typical responses to conditioning stimuli are shown for each experiment. Insets: average PF-EPSPs measured for the 5 min before (thin) and 15–20 min after (thick) the induction protocol. C: summary of the average normalized EPSP amplitude (±SE) is plotted as a function of time recorded for control (n = 10, black) and CB1α6– (n = 10, red) mice. D: the cumulative distributions of the normalized EPSP amplitudes 15–20 min after the conditioning train are plotted for the experiments summarized in C.
DISCUSSION
We took advantage of the Cre/loxP system to eliminate CB1Rs selectively from PF synapses in the cerebellum. Immunohistochemical and electrophysiological analyses verified the elimination of CB1Rs selectively from PFs of CB1α6− mice. Two forms of eCB-dependent short-term plasticity, DSE and SSE, were eliminated at PF synapses onto PCs. In contrast, DSI at inhibitory interneuron and DSE at climbing fiber synapses were unaffected by PF CB1R elimination. Thus our results are consistent with the existing idea of presynaptic CB1Rs acting to reduce neurotransmitter release in these forms of short-term plasticity. Finally, we found that a stimulus protocol previously shown to induce a postsynaptically expressed form of LTD had no net effect on synaptic strength in CB1α6− mice.
Previous studies demonstrating that activation of CB1Rs is required for the normal expression of LTD did not identify the location of the CB1Rs involved (Safo and Regehr 2005, 2008). It was possible that the CB1Rs responsible for regulating LTD were located on postsynaptic PCs, presynaptic PFs, or another cell type, such as molecular layer intereneurons. Given the postsynaptic nature of PF-LTD (Ito 2001, 2002), the most straightforward explanation would seemingly have been that CB1Rs in PCs regulated LTD. However, immunohistochemical and in situ hybridization studies have not found CB1Rs in PCs (Herkenham et al. 1991; Mailleux and Vanderhaeghen 1992; Matsuda et al. 1993; Pettit et al. 1998; Suarez et al. 2008; Tsou et al. 1998). This suggested that either CB1Rs were present in PCs, but as yet undetected for technical reasons (e.g., Kawamura et al. 2006), or that the CB1Rs that regulate LTD were located in another cell type. For instance, it has been proposed that molecular layer interneurons produce nitric oxide (Shin and Linden 2005), which is required for PF-LTD. While our findings do not rule out the additional involvement of CB1Rs on molecular layer interneurons or other cell types, they establish that CB1Rs on PFs regulate LTD at PF synapses.
What is the mechanism through which presynaptic CB1Rs regulate LTD? During LTD induction, calcium elevation and activation of mGluR1 in PCs leads to release of eCBs that bind to presynaptic CB1Rs. Then there are two main possibilities, depending on whether CB1Rs regulate PF-LTD directly or indirectly. The first is that activation of CB1Rs is a necessary step in the induction of LTD (Safo and Regehr 2005). According to this hypothesis, activation of PF CB1Rs promotes release of an anterograde messenger from the PFs, such as nitric oxide, which then acts in the PC to activate the kinases responsible for AMPA receptor downregulation. The second possibility is that elimination of CB1Rs does not directly interfere with LTD but rather influences the net effect on synaptic strength through disinhibition of presynaptic PF-LTP (van Beugen et al. 2006). According to this scheme, if the amplitude of LTP were sufficiently large, it could mask LTD. However, we think it is unlikely that our LTD induction protocol would generate sufficient LTP to mask LTD in CB1α6− mice. LTP is most often studied at room temperature using a prolonged low frequency stimulation (8 Hz for 15 s) (Salin et al. 1996; van Beugen et al. 2006). For our high temperature experiments and PF stimuli (bursts of 10 stimuli at 100 Hz), the extent of LTP is ∼10%, seemingly insufficient to mask LTD (Safo and Regehr 2008).
CB1Rs are known to play important roles in many forms of short- and long-term plasticity throughout the brain (Chevaleyre et al. 2006). PF-LTD in the cerebellum appears to be an unusual case in which CB1Rs located presynaptically are required for the expression of a postsynaptic form of plasticity. Genetic approaches like the one used here will be useful for further studies examining CB1Rs in identified cell types and their roles in different forms of synaptic plasticity.
GRANTS
This study was supported by National Institute Health Grants R37NS 032405 and R0DA024090 to W. G. Regehr and a Helen Hay Whitney postdoctoral fellowship and Harvard University Research Enabling Grant to M. R. Carey.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank G. Kozorovitskiy for help with immunostaining and M. Antal, A. Best, J. Crowley, D. Fioravante, L. Glickfeld, C. Hull, T. Pressler, and M. Thanawala for helpful comments on the manuscript.
Present address for M. R. Carey: Champalimaud Neuroscience Programme, Champalimaud Centre for the Unknown, Av. Brasília, Doca de Pedrouços, 1400-038 Lisboa, Portugal (Email: mcarey@ineurophd.org).
REFERENCES
- Berghuis P, Rajnicek AM, Morozov YM, Ross RA, Mulder J, Urban GM, Monory K, Marsicano G, Matteoli M, Canty A, Irving AJ, Katona I, Yanagawa Y, Rakic P, Lutz B, Mackie K, Harkany T. Hardwiring the brain: endocannabinoids shape neuronal connectivity. Science 316: 1212–1216, 2007 [DOI] [PubMed] [Google Scholar]
- Bodor AL, Katona I, Nyiri G, Mackie K, Ledent C, Hajos N, Freund TF. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci 25: 6845–6856, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Calcium dependence of retrograde inhibition by endocannabinoids at synapses onto Purkinje cells. J Neurosci 23: 6373–6384, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci 6: 1048–1057, 2003 [DOI] [PubMed] [Google Scholar]
- Carey MR, Regehr WG. Noradrenergic control of associative synaptic plasticity by selective modulation of instructive signals. Neuron 62: 112–122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci 29: 37–76, 2006 [DOI] [PubMed] [Google Scholar]
- Funfschilling U, Reichardt LF. Cre-mediated recombination in rhombic lip derivatives. Genesis 33: 160–169, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Groen BG, Lynn AB, De Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors and second messengers in mutant mouse cerebellum. Brain Res 552: 301–310, 1991 [DOI] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev 81: 1143–1195, 2001 [DOI] [PubMed] [Google Scholar]
- Ito M. The molecular organization of cerebellar long-term depression. Nat Rev Neurosci 3: 896–902, 2002 [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89: 309–380, 2009 [DOI] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci 26: 2991–3001, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci 21: RC174, 2001a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron 29: 717–727, 2001b [DOI] [PubMed] [Google Scholar]
- Kuhn R, Torres RM. Cre/loxP recombination system and gene targeting. Methods Mol Biol 180: 175–204, 2002 [DOI] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron 6: 565–574, 1991 [DOI] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron 31: 463–475, 2001 [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience 48: 655–668, 1992 [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302: 84–88, 2003 [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol 327: 535–550, 1993 [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346: 561–564, 1990 [DOI] [PubMed] [Google Scholar]
- Nyiri G, Cserep C, Szabadits E, Mackie K, Freund TF. CB1 cannabinoid receptors are enriched in the perisynaptic annulus and on preterminal segments of hippocampal GABAergic axons. Neuroscience 136: 811–822, 2005 [DOI] [PubMed] [Google Scholar]
- Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res 51: 391–402, 1998 [DOI] [PubMed] [Google Scholar]
- Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron 63: 154–170, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron 48: 647–659, 2005 [DOI] [PubMed] [Google Scholar]
- Safo P, Regehr WG. Timing dependence of the induction of cerebellar LTD. Neuropharmacology 54: 213–218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Malenka RC, Nicoll RA. Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron 16: 797–803, 1996 [DOI] [PubMed] [Google Scholar]
- Shin JH, Linden DJ. An NMDA receptor/nitric oxide cascade is involved in cerebellar LTD but is not localized to the parallel fiber terminal. J Neurophysiol 94: 4281–4289, 2005 [DOI] [PubMed] [Google Scholar]
- Suarez J, Bermudez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, de Fonseca FR. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol 509: 400–421, 2008 [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83: 393–411, 1998 [DOI] [PubMed] [Google Scholar]
- van Beugen BJ, Nagaraja RY, Hansel C. Climbing fiber-evoked endocannabinoid signaling heterosynaptically suppresses presynaptic cerebellar long-term potentiation. J Neurosci 26: 8289–8294, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA 96: 5780–5785, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]