Abstract
Food restriction has been reported to have positive effects on cognition. This study examines how another environmental factor, daylength, can alter the impact of food restriction on the brain and behavior. Female California mice (Peromyscus californicus), housed on either long days (16L:8D) or short days (8L:16D) were restricted to 80% of their normal baseline food intake or provided food ad libitum. Testing in a Barnes maze revealed that the effects of food restriction depend on photoperiod, and that these effects differed for acquisition vs. reversal learning. During acquisition testing, food restriction increased latency to find the target hole in short-day mice but not long-day mice. In reversal testing, food restriction decreased latency to find the target hole in long-day mice but not short-day mice. Latency to find the hole was positively and independently correlated with both errors and time spent freezing, suggesting that changes in both spatial learning and anxiety-like behavior contributed to performance. Short days increased hippocampal expression of the synaptic protein, synapsin I, which was reversed by food restriction. Short days also reduced plasma corticosterone levels, but diet had no effect. There was no effect of diet or photoperiod on hippocampal expression of the glial marker, glial acidic fibrillary protein. The present findings suggest that in female California mice the differential effects of food restriction on acquisition and reversal learning are photoperiod-dependent. These results justify further testing of the relationship between food restriction and hippocampal synapsin I in the context of spatial learning.
Introduction
There is growing evidence that food restriction also has important effects on the brain that affect cognition. 12 months of food restriction improved performance of aged mice in a radial maze (Idrobo et al., 1986), while life-long restriction to 60% of baseline food intake improved performance of aged rats in the Morris water maze test (Stewart et al., 1989). Intriguingly, the effects of restricted diets are not limited to aged mice, as FR mice across a wide age range showed more rapid improvement and reduced variability during water maze testing compared to ad libitum mice (Stewart et al., 1989). Another study found that food restriction regimens where male mice were maintained on 80% or 65% of baseline food intake for 6 months improved learning but not memory when tested in a Y-maze (Wu et al., 2003). These studies suggest that a calorie restricted diet may be beneficial for spatial learning. Similar findings were shown in a recent study on elderly women, which showed that restriction to 70% of normal baseline caloric intake improves verbal memory (Witte et al., 2009).
Most food restriction studies reduce caloric intake by providing a certain percentage of the baseline ad libitum diet. It has been argued that in the context of aging, food restriction is hermetic (Masoro, 1998); functioning like a pharmacological agent which may have beneficial effects at low doses but is toxic at higher doses (Boxenbaum et al., 1988). The hypothesis that food restriction is an environmental factor with pharmaceutical characteristics has prompted researchers to investigate the molecular and cellular mechanisms which exert the observed beneficial effects, so that interventions can be developed that exhibit more specific modes of action (Ingram et al., 2004; Anderson et al., 2009). An alternative strategy is to investigate the mechanisms affected by food restriction and how they are affected by other salient environmental stimuli. In many species food availability is seasonally variable, and daylength (photoperiod) is used to anticipate seasonal changes in environmental conditions (Bronson, 1985; Nelson et al., 1995a).
Photoperiod, like food availability, is an environmental factor that can impact spatial learning. Indeed, photoperiodic changes in spatial cognition are well established in mice of the genus Peromyscus. Short days augment spatial learning in female deer mice (Peromyscus maniculatus, Galea et al., 1994), and have been shown to have the opposite effect in male deer mice and white footed mice (Peromyscus leucopus, Galea et al., 1994; Pyter et al., 2005; Pyter et al., 2006; Pyter et al., 2007). Photoperiodic effects on spatial learning and memory are also evident in avian species. Black capped-chickadees (Poecile atricapillus) engage in high levels of seed caching in the autumn, which is dependent on spatial memory. Laboratory studies show increased caching activity when chickadees are transferred from long to short days (Krebs et al., 1995) decreased caching when moved from short to long days (MacDougall-Shackleton et al., 2003). Therefore it is reasonable to hypothesize that photoperiod and food availability would interact in their effects on spatial learning.
The California mouse (Peromyscus californicus) can reproduce year round but demonstrates peak breeding around December and May (Ribble, 1992). It has been reported that this species may integrate food and photoperiod cues to regulate its reproductive timing (Nelson et al., 1995b), and as such, it may serve as a suitable model of seasonal photoperiod*diet interactions. In order to assess whether such interactions apply to spatial learning and learning, we assigned female California mice maintained in either winter like short days (SD), or summer like long days (LD) to either a food restricted (FR) or ad libitum (AL) diet. We then tested spatial learning using a Barnes maze. There is growing awareness that females are understudied in neuroscience research (Zucker & Beery, 2010) and that there are important biological sex differences that affect both brain and behavior (Cahill, 2006). Accordingly, the vast majority of studies on the effects of food restriction on spatial memory employ males, and there is some evidence that food restriction may not benefit female spatial memory to the same extent as males (Wu et al., 2003). The present experimental setup simultaneously explores the effects of food restriction and photoperiod on spatial learning, allowing both factors to be assessed in females.
In addition to examining behavioral parameters, we also sought to determine mechanistically how photoperiod and food restriction affect spatial learning. We assayed plasma corticosterone level because corticosterone can affect spatial memory, enhancing (Pyter et al., 2007; Conboy & Sandi, 2010) or diminishing it (Schwabe et al., 2010). We also examined the proteins synapsin I and glial acidic fibrillary protein (GFAP) in the hippocampus, an important neural locus of spatial memory (Handelmann & Olton, 1981). Synapsin I is localized to the inner membrane of synaptic vesicles and is involved in both regulation of neurotransmitter release and synaptic formation (Greengard et al., 1993; Chin et al., 1995). GFAP is a glial specific cytoskeletal protein that is frequently upregulated in response to spatial memory-impairing hippocampal damage (de la Torre et al., 1992; Eng & Ghirnikar, 1994). We used these approaches to gain insight into how reduced food availability influences spatial learning and whether these effects are photoperiod dependent.
Materials and Methods
Animals
Fifty-eight female California mice (Peromyscus californicus) of at least 90 days of age were single-housed in polypropylene cages and randomly assigned to reside under photoschedules mimicking either short (8L:16D, SD) or long (16L:8D, LD) days (lights-off at 1400 hr Pacific standard time [PST] in long and short days). Mice were further randomly subdivided into dietary groups that were restricted to 80% of their individual baseline daily food intake (FR) or that had access to food ad libitum (AL; Harland Teklab 2016 rodent diet, Indianapolis, IN, USA). Baseline daily food intake for each FR restricted mouse was determined by averaging the weights of food consumed each day over a one week period. Mice were maintained for 8-weeks under LD-AL (n = 19), SD-AL (n =15), LD-FR (n = 11) or SD-FR (n =13) conditions, and a subset of mice [LD-AL (n = 10), SD-AL (n = 7), LD-FR (n = 6) and SD-FR (n = 8)] were tested in a Barnes maze starting at 6.5 weeks into the experiment. Approximately 24 hrs following the final Barnes maze trial, mice were anesthetized with isoflurane gas (Minirad Inc., Bethlehem, PA, USA) and euthanized by rapid decapitation. Brains were quickly removed and fixed in 5% acrolein (Sigma, St. Louis MO, USA) in 0.1 M phosphate buffered saline (PBS) overnight at 4°C. The next day, brains were placed in 25% sucrose (Fisher, Pittsburgh, PA, USA) in PBS and then frozen the next day on dry ice. Brains were subsequently stored at −40 °C until being processed for immunohistochemical analysis. Two mice were euthanized as previously described and the brains were rapidly removed and placed in a brain matrix so that 2 mm thick hippocampal sections could be obtained (Trainor et al., 2010). Sections were placed on a freezing plate and 1 mm hippocampal punch samples were taken, flash frozen on dry ice and stored at −40 °C until being homogenized for western blot. At the time of sacrifice stage of estrous cycle was determined by vaginal lavage. Some food restricted mice appeared to have stopped cycling and had closed up reproductive tracts that were inaccessible to lavage. These mice were presumed to be in diestrus because vaginal closure following introitus is indicative of suppressed cycling (Whitsett & Miller, 1982; Wube et al., 2008). All procedures were approved by the UC Davis Institutional Animal Care and Use Committee followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Barnes Maze
Spatial learning was assessed using a Barnes maze, which is generally considered to be a less stressful test of spatial learning and memory compared to the Morris Water Maze (Barnes, 1979; Harrison et al., 2009) The Barnes maze was composed of a white circular board (85 cm in diameter) containing 16 equally spaced holes (5 cm in diameter) that were situated 1.3 cm from the maze’s edge. Since the experiment relies on subjects being motivated to escape from an open, well lit platform, testing was conducted during the light phase of the light cycle between 07:00 and 12:00 hours PST. For each trial, all treatment groups were run in the maze during a single testing period and the order in which mice were tested was randomized daily. The test involved positioning a cage with fresh bedding under one of the holes on the maze (the target hole) and then placing a mouse in the center of the maze. The Barnes maze has been previously used to assess spatial learning with California mice (Bredy et al., 2004). Each mouse was randomly assigned a target hole and was given 5 min to enter. If the mouse did not enter the target hole the experimenter guided the mouse into the target hole and the mouse was allowed to remain in the cage for one min. Day 1 was the habituation phase where mice were run on the maze for the usual 5 min in order to introduce them to the assigned target hole. The same target hole for any given mouse was used on days 2 thru 5, which constituted the acquisition phase of testing. Days 6 thru 9 comprised the reversal phase, wherein the previous target hole was moved 180 °across the maze. The latency to enter the correct hole, the number of times a mouse made an error by poking its head in an incorrect hole, and the total amount of time that the mouse was froze on the maze (starting on day 2) were recorded using Stopwatch+ (Center for Behavioral Neuroscience, Atlanta, GA, USA). Time spent freezing was recorded as an estimate of anxiety-like behavior (Crawley, 2007). On day 10, the mice were tested in a 30 sec probe trial in which the target cage was not placed under any of the holes. The number of head pokes into incorrect holes, and the reversal target hole were recorded.
Immunohistochemistry
Brain tissue was sliced on a microtome at 40 μm. Afterwards, thawed sections were stored at −20 °C in cryoprotectant (50% v/v phosphate buffer, 30% w/v sucrose, 1% w/v polyvinylpyrrolidone, 30% v/v ethylene glycol) until staining. Chromogenic immunostaining for synapsin I (n = 5 per group) and GFAP (n = 6 for LD-AL and SD-FR; n = 5 for SD-AL and LD-FD) was performed on every fourth section beginning with the rostral hippocampus and proceeding caudally approximately 640 μm for synapsin I-stained slices and 800 μm for GFAP-stained slices. All treatment groups were run simultaneously for a given antibody, negating any potential for batch differences. Tissue was washed 3 times in PBS for 5 min/wash and then incubated for 10 min in 0.1 M sodium borohydride in PBS as an antigen retrieval step. Tissue was subsequently blocked in 10% normal goat serum in PBS containing 0.3% hydrogen peroxide to quench endogenous peroxidases. Sections were incubated overnight at 4°C in primary antibody solution consisting of either rabbit anti-synapsin I (ab8, Abcam, Cambridge, MA, USA, concentration 1:500) or rabbit anti-GFAP (1:750, ab7779, Abcam, concentration 1:750) diluted in PBS with 0.5% triton X (Tx) and 2% normal goat serum. The following morning, tissue underwent 3 PBS washes and was incubated for 2 hr at room temperature in a secondary antibody solution consisting of PBS-Tx, 2% normal goat serum, and either biotin-conjugated goat anti-rabbit antibody (BA-1000, Vector Laboratories, Burlingame, CA, USA, diluted 1:500), or horse radish peroxidase-conjugated goat anti-rabbit antibody (PI-1000, Vector Laboratories, diluted 1:350) for synapsin I and GFAP sections, respectively. Synapsin I sections were washed 3 times in PBS and then incubated for 30 min in avidin–biotin complex (ABC Elite Kit, Vector Laboratories). All sections were washed 3 times in PBS before undergoing development in nickel enhanced diaminobenzidine (Vector Laboratories) for 2 min. Following development, tissue was rinsed for 5 min in deionized water. Stained sections were mounted onto Superfrost plus slides (Fisher, Pittsburgh, PA, USA), dehydrated in 100% ethanol, cleared for 3 min in Histoclear (National Diagnostics, Atlanta, GA, USA), and coverslipped with Permount (Fisher). Some sections were processed with primary antibody omitted to serve as a negative control.
Image Analysis
Immunostained sections were photographed through a Zeiss Axioimager (Carl Zeiss Meditec, Inc., Dublin, CA) equipped with an Axiocam MRC camera (Carl Zeiss Meditec). During all quantifying, the observer was blind to treatment. Three-to-four synapsin I-stained sections were analyzed per brain. Photomicrographs were taken at 20X magnification all on the same day and under identical lighting conditions. Mean optical density (O.D.) of immunoreactive synapsin I puncta was quantified using Image J (NIH, Bethesda, MD, USA) calibrated to a calibrated grey scale transmission step tablet (#T2115C, Stauffer, Mishawaka, IN, USA). Images were converted to black and white and 8 circles, each with an area of 0.002 mm2, were placed side-by-side in the stratum lucidum on one side of the hippocampus. Background was accounted for by measuring mean optical density in the corpus callosum of each section using a rectangle that covered an area of 0.05 mm2 and then subtracting this density from the average of the mean O.D. for the 8 circles.
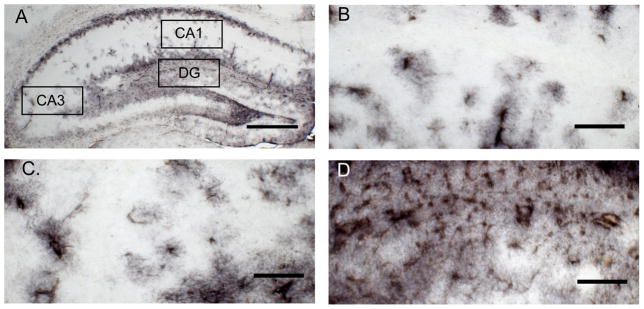
Four to five GFAP-immunostained sections were analyzed per brain. Using Image J, a square box covering an area of 0.14 mm2 was placed in a consistent portion of the CA1, and CA3 while a square box with an area of 0.08 mm2 was positioned consistently in the dentate gyrus (DG). Percent staining of immunoreactive astrocytes within a box were within the box was quantified using the threshold feature of Image J. Threshold was set manually by determining the level at which most fibers were accounted for without including areas that did not contain fibers.
Western Blot
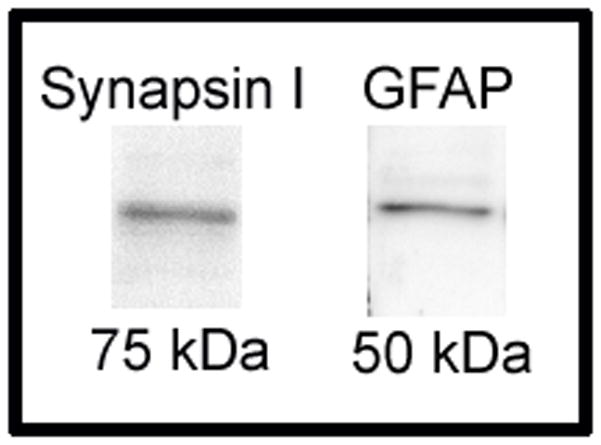
Western blot was used to confirm specificity of antibodies. Hippocampal punch samples were homogenized in lysis buffer [20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 0.4 M NaCl, 5 mM MgCl2, 0.5 mM ethylenediaminetetraacetic acid, 0.1 mM phenylsmethanesulphonyl-fluoride and 20% v/v glycerol]. Sample in lysis buffer was then diluted 1:2 in Laemmli buffer (Sigma) and denatured at 100°C for 3 min, after which proteins were separated by electrophoresis on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel. Proteins were subsequently transferred to a polyvinylidene difluoride membrane (pore size 0.45 μm; Invitrogen, Carlsbad, CA, USA). The membrane was the blocked for 1 hr in 5% skim milk (Oxoid LTD, Basingstoke, Hampshire, England) in tris-buffered saline (TBS; 55 mM tris, 150 mM NaCl, pH 7.4) with 0.1 % Tx. After blocking, the membrane was underwent two 5 min washes in TBS-Tx before being incubated on an orbital shaker overnight at 4°C in rabbit-anti synapsin I antibody diluted 1:1000 in TBS-Tx with 2% normal goat serum. The following day the membrane was washed 3 times for 5 min in TBS-Tx and then incubated for 2 hr at room temperature in horseradish peroxidase-conjugated goat anti-rabbit antibody (PI-1000, Vector Laboratories) diluted 1:2000 in TBS-Tx with 2% normal goat serum. The membrane was then washed for 5 min 3 times in TBS-Tx and developed for 1 min using Immun-Star WesternC Kit (Bio-Rad Laboratories, Hercules, CA, USA). The membrane was viewed and photographed using a ChemiDoc XRS+ molecular Imager with Image Lab software (Bio-Rad Laboratories). The following day the membrane was incubated in stripping buffer [2 % w/v SDS, 0.7% 2-mercaptoethanol, 62.5 mM tris (pH 6.7)] for 30 min at 50°C. The membrane was washed twice for 10 min in TBS-Tx and then reblocked in 5% milk in TBS-Tx at room temperature for 1 hr. After washing twice for 5 min in TBS-Tx, the membrane was incubated overnight at 4°C in rabbit-anti GFAP antibody diluted 1:1000 in TBS-Tx with 2% normal goat serum. The next morning the membrane was washed, incubated in secondary antibody and developed as described for synapsin I. The molecular weight of bands was determined by comparing there position on the membrane with a protein ladder (Precision Plus Protein WesternC Standard; Bio-Rad Laboratories). When the membrane was incubated in anti-synapsin I antibody, a band of approximately 75 kDa (Fig. 1) was detected, an appropriate size for the synapsin 1a isoform (Nicol et al., 1997). A different membrane was stripped after immunoblotting and incubated in anti-GFAP antibody, which revealed a band of approximately 50 kDa (DeArmond et al., 1986).
Figure 1.
Corticosterone Radioimmunoassay
Trunk blood was collected at the time of sacrifice and was stored at −4 °C until being centrifuged at centrifuged at 13.2 rpm for 12 min at −4 °C. Plasma was removed by pipet and stored at −40 °C until use. Duplicated samples were assayed in order to establish intra-assay variation, the mean of which was 2.3%. A single 125I radioimmunoassay kit (ICN Biomedicals, Costa Mesa, CA, USA) was used to evaluate corticosterone levels from plasma samples for each treatment [LD-AL (n = 19), SD-AL (n = 15), LD-FR (n = 11), SD-FR (n = 13)]. This kit has a cross reactivity of under 0.5% and has a lower detection limit of 5 ng/ml. Plasma was suspended at a concentration of 1:2000 in steroid diluent (MP Biomedicals, Solon, OH, USA) to compensate for the fact the kit was developed to for Mus and Rattus, which tend to have significantly lower corticosterone levels than those of Peromyscus (Glasper and Devries, 2005).
Estradiol Enzyme Immunoassay
Plasma estradiol concentrations were determined using an enzyme immunoassay (EIA) kit (Cayman Chemical, Ann Arbor, MI). This assay has a sensitivity of 6.6 pg/mL and the intra-assay coefficient of variation was 12.0 %. Data was log transformed for statistical analysis. Statistical analysis was performed on samples from mice in diestrus only because we obtained insufficient numbers of mice in proestrus and estrus.
Statistics
Latencies, errors, and freezing behavior from the Barnes maze were analyzed with repeated measure ANOVA testing for effects of diet and photoperiod as well as the interaction. Because different neurobiological processes may be involved in reversal learning, we analyzed data for the acquisition and reversal phases separately (Shuai et al., 2010). We also ran independent t-tests for each day of the Barnes maze (Fig. 2). Synapsin I, GFAP and corticosterone levels were analyzed using 2-way ANOVA testing for effects of diet, photoperiod, and photoperiod*diet interaction. Nonparametric Spearman correlations were used to determine associations between various performance measures in the maze and between hippocampal proteins and corticosterone. We used partial correlations to determine whether variance in freezing behavior and errors overlapped with respect to predicting latency. For the probe trial, we used Fisher’s exact test to test whether treatments influenced the likelihood of mice making at least one head poke in the correct hole from the reversal phase.
Figure 2.
Results
Barnes Maze
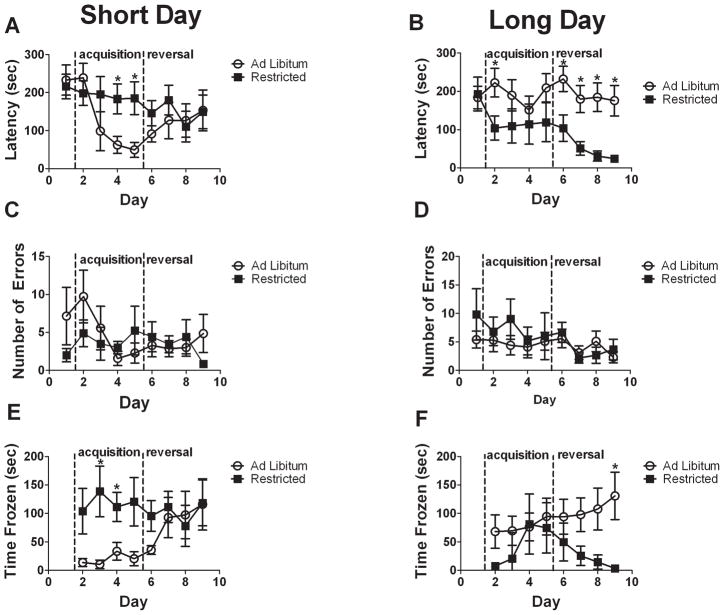
During the acquisition phase of the Barnes maze there was a significant photoperiod*diet interaction (Fig. 2A,B repeated measures ANOVA, F1,27 = 5.96, p = 0.021). There were no significant main effects of photoperiod or diet (all p’s > 0.96). In short days food restriction increased latency to find the target hole on days 4 (Fig. 2A, p = 0.023) and 5 (Fig. 2A, p= 0.017) during the acquisition phase. In contrast, LD-FR had a shorter latency to find the target hole on day 2 than LD-AL mice (Fig. 2B, p = 0.046). Repeated measure ANOVA indicated that only SD-AL mice showed a significant reduction in latency to enter the target hole over the course of the acquisition phase (Fig. 2A, Table 1). There was a non-significant trend toward reduced latency in SD-AL mice as compared with LD-AL mice (repeated measures ANOVA, F1,15 = 3.86, p = 0.068). Neither errors nor freezing time changed for any group over the course of testing, although a trend toward a reduced number of errors approached significance for SD-AL mice (Table 1, F3,18 = 3.0, p = 0.057).
Table 1.
| Latency | |||||
|---|---|---|---|---|---|
| Acquisition | F | p | Reversal | F | p |
| SD-AL | F3, 18 = 9.0 | 0.001* | SD-AL | F3, 18 = 0.4 | 0.77 |
| SD-FR | F3, 21 = 0.1 | 0.97 | SD-FR | F3, 21 = 0.7 | 0.58 |
| LD-AL | F3, 27 = 1.3 | 0.30 | LD-AL | F3, 27 = 1.0 | 0.41 |
| LD-FR | F3, 15 = 0.1 | 0.97 | LD-FR | F3, 15 = 4.4 | 0.02* |
| Errors | |||||
| Acquisition | F | p | Reversal | F | p |
| SD-AL | F3, 18 = 3.0 | 0.06 | SD-AL | F3, 18 = 0.4 | 0.75 |
| SD-FR | F3, 21 = 0.3 | 0.83 | SD-FR | F3, 21 = 1.0 | 0.41 |
| LD-AL | F3, 27 = 0.2 | 0.90 | LD-AL | F3, 27 = 0.2 | 0.10 |
| LD-FR | F3, 15 = 0.4 | 0.76 | LD-FR | F3, 15 = 1.7 | 0.21 |
| Freezing | |||||
| Acquisition | F | p | Reversal | F | p |
| SD-AL | F3,18 = 1.1 | 0.37 | SD-AL | F3, 18 = 1.2 | 0.33 |
| SD-FR | F3,21 = 0.4 | 0.75 | SD-FR | F3, 21 = 0.4 | 0.76 |
| LD-AL | F3,27 = 0.6 | 0.62 | LD-AL | F3, 27 = 0.7 | 0.59 |
| LD-FR | F3,15 = 2.1 | 0.15 | LD-FR | F3, 15 =1.8 | 0.20 |
A significant photoperiod*diet interaction was also observed during the reversal phase of the experiment (Fig. 2A,B, F1,27 = 9.65, p = 0.004) which differed from that seen in the acquisition phase. Food restriction reduced latency in LD mice on each day of reversal testing (Fig. 2B, all p’s < 0.025). In contrast, food restriction had no effect on latency on any day of reversal testing for SD mice (all p’s > 0.2). Latency to find the hole progressively decreased only in LD-FR (Table 1, F4,15 = 4.44, p = 0.02). LD-FR mice also demonstrated shorter latencies than SD-FR mice (F1,12 = 9.19, p = 0.011). There was no change in number of errors or freezing with progressive trials (Table 1).
Repeated measures ANOVA indicated that number of errors made in the Barnes maze was neither affected by diet or photoperiod, nor by interactions between these treatments, in either phase of testing (Fig. 2C,D, all p’s > 0.4). There were, however, consistent significant positive correlations between latency and errors which were observed on days 3 (r = 0.41, p = 0.022), 4 (r = 0.41, p = 0.021), 5 (Fig. 3A, r = 0.55, p = 0.001), 7 (Fig. 3B, r = 0.49, p = 0.005) and 8 (r = 0.37, p = 0.042). There was a non-significant trend for a correlation on day 6 (r = 0.35, p = 0.051).
Figure 3.
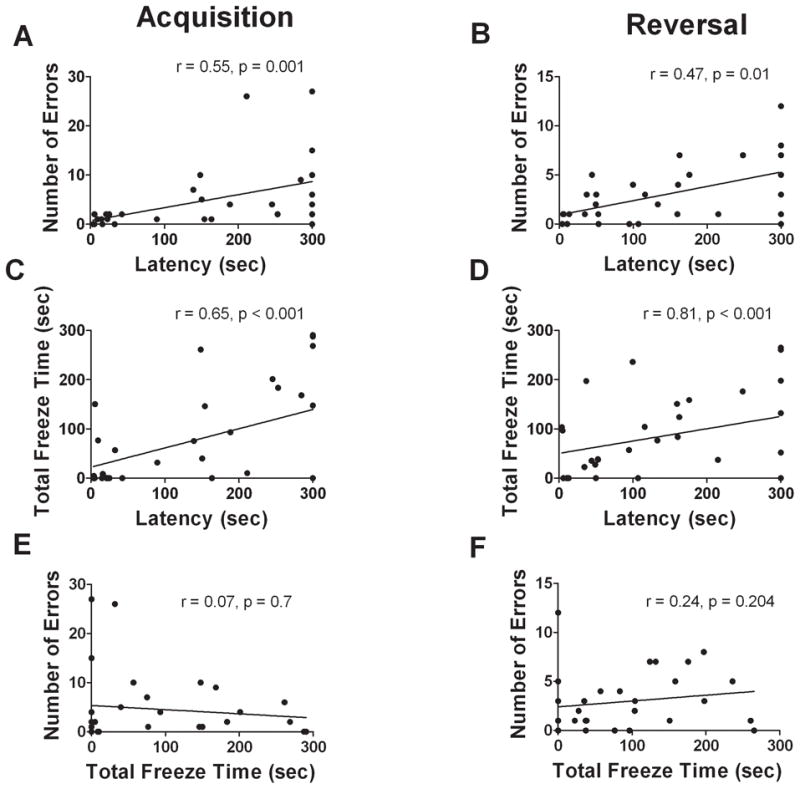
Analyses of total freezing time revealed a significant photoperiod*diet interaction during the acquisition phase (Fig. 2E,F, F1,27 = 6.02, p = 0.021) with SD-AL mice freezing significantly less than SD-FR mice (F1,13 = 7.48, p = 0.017). During the reversal phase there were no treatment effects or interactions on freezing time (all p’s > 0.05). Freezing showed a significant positive association with latency on each day of acquisition and reversal testing (days 5 and 7 shown in Fig. 3C,D, all p’s ≤ 0.005). There were, however, no significant correlations between freezing time and errors (Fig. 3E,F, all p’s > 0.1), suggesting that even though some animals froze more, they still explored the maze and examined incorrect holes. Partial correlations confirmed that the frequent positive correlations between latency and errors were present when controlling for freezing on each day (S1, all p’s ≤ 0.004) except day 2 (S1, p = 0.270) and that the positive correlations between freezing and latency were maintained when controlling for errors (S1, all p’s ≤ 0.002).
In the probe trial there was a significant interaction (F1,27 = 9.8, p = 0.004) on the total number of errors, with LD-FR mice making more errors than LD-AL mice, while diet had no effect in short days. There were no main effects on errors (Table 2, all p’s > 0.2). There were also no main effects or interactions of diet or photoperiod on freezing time (Table 2, all p’s > 0.06). During the probe trial, LD-FR mice were more likely to visit the correct hole than SD-FR mice (Table 3, Fisher’s exact test p = 0.026) while photoperiod had no effect in AL mice (Fisher’s exact test p > 0.99).
Table 2.
| Short Day Ad Lib | Short Day Restricted | Long Day Ad Lib | Long Day Restricted | |
|---|---|---|---|---|
| # of Errors | 3.1±1.8 | 0.8±0.9 | 0.6±0.5 | 6±2.3* |
| Freezing (sec) | 10±5 | 18±4 | 13±3 | 6±5 |
Table 3.
| Entered ≥ 1 time | Long Day | Short Day | |
|---|---|---|---|
| Yes | 7 | 5 | |
| Ad Lib | No | 3 | 2 |
| Yes | 1 | 7 | |
| Restricted | No | 5 | 1 |
Corticosterone Radioimmunoassay
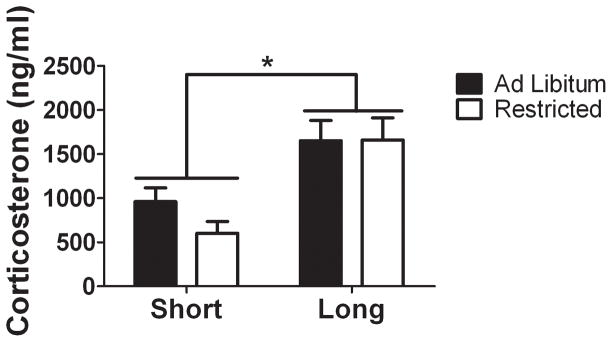
There was a main effect of photoperiod (Fig. 4, F1,57 = 13.92, p < 0.001) with short days reducing plasma corticosterone as compared with long days. There was neither an effect of diet nor any interaction (all p’s > 0.1).
Figure 4.
Estradiol immunoassay
There was no effect of photoperiod or diet on plasma estradiol concentrations in diestrus mice, nor was there an interaction (Table 4, all p’s > 0.15).
Table 4.
| Estrous Stage | Short Day Ad Lib | Short Day Restricted | Long Day Ad Lib | Long Day Restricted | ||||
|---|---|---|---|---|---|---|---|---|
| E2 (pg/ml) | n | E2 (pg/ml) | n | E2 (pg/ml) | n | E2 (pg/ml) | n | |
| Diestrus | 18.8±3.3 | 7 | 21.4±4.5 | 8 | 21.3±3.0 | 7 | 27.1±3.8 | 6 |
| Proestrus | 16.8 | 1 | N/A | 0 | 13.2 | 1 | N/A | 0 |
| Estrus | N/A | 0 | N/A | 0 | 18.8±5.3 | 4 | 10.5 | 1 |
Synapsin I immunoreactivity
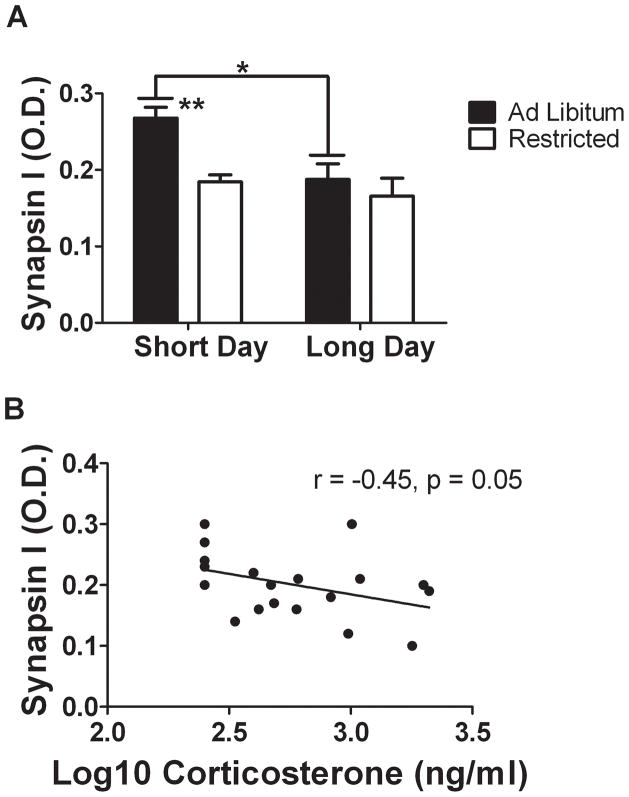
Synapsin I expression was conspicuous throughout the stratum lucidum (Fig. 5) of the hippocampus as previously described (Morioka et al., 1997; Iwata et al., 2006). Within this hippocampal structure, synapsin I mean O.D. was significantly altered by both photoperiod (F1,16 = 7.75, p = 0.013) and diet (F1,16 = 8.8, p = 0.009). In AL animals, synapsin I protein was significantly upregulated by short days (Fig. 6A, p = 0.012), however food restriction abolished this SD induced increase in synapsin I immunoreactivity (Fig 6A, p = 0.001). In LD mice, food restriction had no effect on synapsin I expression within the S.L. (p > 0.05). Synapsin I mean O.D. correlated negatively with corticosterone levels (Fig. 6B, r = −0.45, p = 0.048).
Figure 5.
Figure 6.
GFAP Immunoreactivity
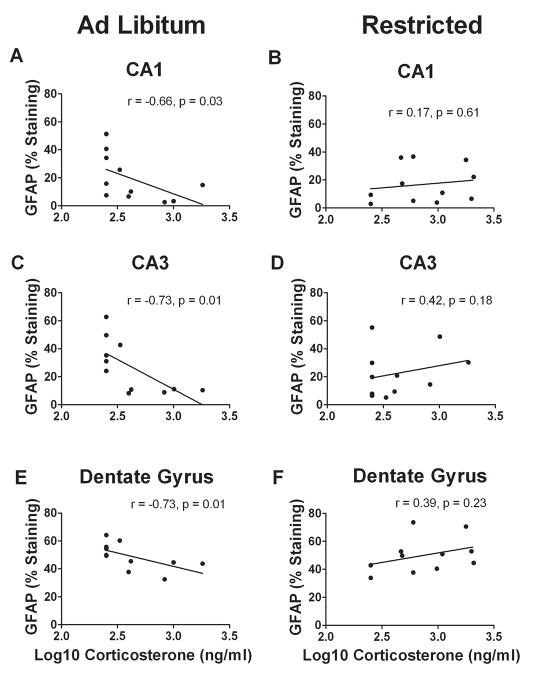
Hippocampal GFAP expression was assessed both by counting GFAP-immunoreactive cells and analyzing percent staining (Fig. 7). Within the CA1, CA3 and DG, no main effects or interactions were indentified for either cell number or percent staining (Table 5, all p’s > 0.1). Nevertheless, an interesting interaction emerged when GFAP-immunoreactivity was correlated with corticosterone levels as there were significant negative correlations between GFAP expression and corticosterone concentration for all hippocampal regions in AL mice [CA1 (Fig 8A, r = −0.658, p = 0.028); CA3 (Fig 8C, r = −0.73, p = 0.012); DG (Fig 8E, r = −0.73, p = 0.010)], while such correlations were nonexistent in FR mice [Fig 8B, CA1 (r = 0.17, p =0.610); CA3 (Fig 8D, r = 0.43, p = 0.183); DG (Fig 8F, r = 0.39, p = 0.232)].
Figure 7.
Table 5.
| % Stain | Short Day Ad Lib | Short Day Restricted | Long Day Ad Lib | Long Day Restricted |
|---|---|---|---|---|
| CA1 | 22±9 | 18±6 | 17±6 | 15±6 |
| CA3 | 33±9 | 24±7 | 22±8 | 20±9 |
| DG | 54±3 | 51±5 | 45±4 | 49±6 |
Figure 8.
Discussion
We hypothesized that photoperiod and diet would exert interacting effects on spatial learning in female California mice. Barnes maze testing showed that food restriction increased latency to find the target hole during acquisition testing in SD mice but shortened latency for one trial in LD animals. Furthermore, food restriction had no effect on performance during reversal learning in SD mice, but improved performance in LD mice. During the acquisition phase, only SD-AL mice showed overall improvement in finding the target hole with progressive trials, while in the reversal phase, only the LD-FR mice showed improvement. Our data suggest that differences in performance in the Barnes maze are affected by changes in both spatial memory (estimated by errors) and anxiety-like behavior (estimated by freezing). In AL mice, acquisition learning was generally improved under short days as compared with long days, consistent with previous data (Galea et al., 1994). Our results demonstrate that the effects of food restriction on spatial learning are not uniform, but depend on photoperiod.
Reversal vs. Acquisition Learning
Reversal learning has been used as a model of adaptive forgetting, facilitating the replacement of older memories. This process appears to rely on molecular mechanisms and neuroanatomical structures that are distinct from those used to acquire memories (Thompson et al., 1981; Shuai et al., 2010). Previous studies in rats housed under photoschedules of 12L:12D have reported that food restricted diets (Gyger et al., 1992) diets improve reversal but not acquisition leaning. Our data suggest that this effect depends on the environment as we observed that food restriction improved reversal learning when California mice were housed in long days, but not short days. This is consistent with results from the probe trial, which demonstrated that under food restriction, long day mice were more likely to head poke the correct hole than short day mice.
Contributions of Spatial Strategies and Anxiety to Barnes Maze Performance
The latency to find the target hole in the Barnes maze can be affected by spatial learning and other processes such as activity or anxiety-like behavior. Our analyses suggest that both spatial learning and anxiety-like behavior contributed to latency data. Latency was positively correlated with errors, suggesting that individuals with long latencies had difficulty remembering the location of the target hole. In the probe trial, LD-FR mice were more likely than SD-FR mice to head poke the correct hole, further suggesting a role for spatial learning. However, latency was also positively correlated with freezing. This suggests that individuals with long latencies were also showing increases in anxiety-like behavior. Partial correlations demonstrated that latency was correlated with both errors and freezing independently. Similarly, errors and freezing were never correlated with each other. These data indicate that freezing and errors make independent contributions to variability in the latency to find the correct hole. There was a significant photoperiod*diet interaction on freezing time during the acquisition phase that paralleled the interaction seen on latency, but no interaction during the reversal phase. Previous studies show that short days increased anxiety-like behavior in an elevated plus maze in both collared lemmings (Dicrostonyx groenlandicus), (Weil et al., 2007), and Siberian hamsters (Phodopus sungorus) (Prendergast & Nelson, 2005). In contrast the effects of food restriction on anxiety-like behavior have been inconsistent (Jahng et al., 2007; Yamamoto et al., 2009), further supporting the hypothesis that effects of food restriction on behavior may depend on the environment.
Although the number of errors did not vary with treatment, a previous study with male and female California mice also reported stronger changes in latency compared to number of errors (Bredy et al., 2004). Freezing behavior was not reported in that study. Although we did not measure path length, it is possible that changes in search strategy may have also affected performance. Increased glucocorticoids induce a switch from a spatial strategy to a stimulus-response strategy in C57BL/6J mice (Schwabe et al., 2010). In our study it is possible that in some cases a spatial strategy was not the primary strategy used, which will need to be addressed in future studies. Although LD mice had been in the light phase of the light cycle longer than SD mice, it is unlikely that this significantly altered maze performance. Circadian timing of testing does not impact spatial learning in a Morris water maze, or performance in various anxiety based tests (Beeler et al., 2006), nor does it affect locomotion in an open field test or habituation to a novel environment (Valentinuzzi et al., 2000).
Effects of Photoperiod and Food Restriction on the Hippocampus
Previous studies have established a link between synapsin I expression and spatial memory formation (Gomez-Pinilla et al., 2001; John et al., 2009), so we measured synapsin I-immunoreactivity. We focused on the stratum lucidum of the CA3 because this structure is a putative neural locus of spatial memory (Sudhof et al., 1989; Villacres et al., 1998; Holahan et al., 2006) Under ad libitum conditions, short day mice had shorter latencies than long day mice during the acquisition phase and also had increased synapsin I activity in the stratum lucidum. In contrast food restriction blocked the augmentation of synapsin I expression by short days, which could be linked to the longer latencies observed in SD-FR mice. This, taken with the finding that food restriction improved spatial memory in LD mice without affecting synapsin I expression, suggests that synapsin I in the stratum lucidum is more important for acquisition than reversal spatial memory. Studies in Kunming mice found that a moderate restricted diet regime (80% of AL) showed increased brain levels of synapsin I but that more severe food restriction (20–40% AL) decreased synapsin I expression (Deng et al., 2009). Therefore, short days may make California mice more susceptible to food restriction-induced downregulation of synapsin I. Further work is necessary to determine whether synapsin I directly influences spatial memory.
Effects of Photoperiod and Food Restriction on Steroid Hormones
Studies examining the effects of ovarian steroids on spatial learning and memory ability in female rodents have yielded inconsistent results (Heikkinen et al., 2004; Ping et al., 2008; Hammond et al., 2009), (Luine & Rodriguez, 1994; Singh et al., 1994). We did not detect any treatment differences on estradiol, suggesting that its levels do not underlie the behavioral differences observed here. The lack of a photoperiodic effect during diestrus in the present study contrasts with previous work from our lab in which plasma was collected during lights-off (Silva et al., 2010). Furthermore, it is likely that the number of mice in proestrus and estrus was too small for estrous cycle-dependent changes in estradiol levels to emerge. California mice spend the majority of the cycle in diestrus (Gubernick, 1988), but food restriction (Tropp & Markus, 2001) likely impacted the distribution of stages as well by impairing cyclicity. Mice were not staged during maze testing because lavage itself alters behavior (Davis & Marler, 2003; Silva et al., 2010).
Corticosterone is known to suppress GFAP transcription and expression in the hippocampus (Laping et al., 1994). The present findings in AL mice support this view as there were significant negative correlations between corticosterone levels and GFAP-immunoreactivity in all examined hippocampal regions. Food restriction eliminated these correlations, appearing to alter the relationship between corticosterone and GFAP.
Conclusions
Our data from female California mice indicate that the specific effects of food restriction on performance in the Barnes maze are sensitive to photoperiod. It appears that the beneficial effects of food restriction on spatial learning are biased toward reversal learning and are evident only in LD mice. In contrast, food restriction impairs acquisition learning during short photoperiods. The negative effects of food restriction in short days are associated with reduced expression of synapsin I levels within the hippocampus. Future studies should further test the involvement of enhanced hippocampal synapsin I expression in spatial acquisition memory of SD mice. It is also likely that treatment induced changes in anxiety-like behavior affected latency to find the target hole. Taken as a whole, this study suggests that the effects of food restriction on learning are context dependent and influenced by the environment. Food restriction can have different effects on acquisition versus reversal learning, and these effects may depend on salient environmental cues such as photoperiod.
Supplementary Material
Acknowledgments
The authors would like to thank Jennifer Cai, Jennifer New and Andrea Silva for technical help, Cindy Clayton for assisting with animal care, Doug Bean for assistance constructing the Barnes Maze and Andy Yonelinas for manuscript advice. Supported by Sigma Xi Grant-in-Aid to MQS and NIH R01 MH085069-01 to BCT.
Works Cited
- Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37:47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Beeler JA, Prendergast B, Zhuang XX. Low amplitude entrainment of mice and the impact of circadian phase on behavior tests. Physiology & Behavior. 2006;87:870–880. doi: 10.1016/j.physbeh.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Boxenbaum H, Neafsey PJ, Fournier DJ. Hormesis, Gompertz functions, and risk assessment. Drug Metab Rev. 1988;19:195–229. doi: 10.3109/03602538809049623. [DOI] [PubMed] [Google Scholar]
- Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus) Horm Behav. 2004;46:30–38. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Mammalian reproduction: an ecological perspective. Biol Reprod. 1985;32:1–26. doi: 10.1095/biolreprod32.1.1. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Chin LS, Li L, Ferreira A, Kosik KS, Greengard P. Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci U S A. 1995;92:9230–9234. doi: 10.1073/pnas.92.20.9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy L, Sandi C. Stress at learning facilitates memory formation by regulating AMPA receptor trafficking through a glucocorticoid action. Neuropsychopharmacol. 2010;35:674–685. doi: 10.1038/npp.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Wha’t Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. Wiley; New Jersey: 2007. [Google Scholar]
- Davis ES, Marler CA. The progesterone challenge: steroid hormone changes following a simulated territorial intrusion in female Peromyscus californicus. Horm Behav. 2003;44:185–198. doi: 10.1016/s0018-506x(03)00128-4. [DOI] [PubMed] [Google Scholar]
- de la Torre JC, Fortin T, Park GA, Butler KS, Kozlowski P, Pappas BA, de Socarraz H, Saunders JK, Richard MT. Chronic cerebrovascular insufficiency induces dementia-like deficits in aged rats. Brain research. 1992;582:186–195. doi: 10.1016/0006-8993(92)90132-s. [DOI] [PubMed] [Google Scholar]
- DeArmond SJ, Lee YL, Kretzschmar HA, Eng LF. Turnover of glial filaments in mouse spinal cord. J Neurochem. 1986;47:1749–1753. doi: 10.1111/j.1471-4159.1986.tb13084.x. [DOI] [PubMed] [Google Scholar]
- Deng L, Wu ZN, Han PZ. Effects of different levels of food restriction on passive-avoidance memory and the expression of synapsin I in young mice. Int J Neurosci. 2009;119:291–304. doi: 10.1080/00207450802328250. [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS. GFAP and astrogliosis. Brain pathology (Zurich, Switzerland) 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- Galea LA, Kavaliers M, Ossenkopp KP, Innes D, Hargreaves EL. Sexually dimorphic spatial learning varies seasonally in two populations of deer mice. Brain Res. 1994;635:18–26. doi: 10.1016/0006-8993(94)91419-2. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, So V, Kesslak JP. Spatial learning induces neurotrophin receptor and synapsin I in the hippocampus. Brain Res. 2001;904:13–19. doi: 10.1016/s0006-8993(01)02394-0. [DOI] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Gubernick DJ. Reproduction in the California Mouse, Peromyscus-Californicus. Journal of Mammalogy. 1988;69:857–860. [Google Scholar]
- Gyger M, Kolly D, Guigoz Y. Aging, modulation of food intake and spatial memory: a longitudinal study. Arch Gerontol Geriatr. 1992;15(Suppl 1):185–195. doi: 10.1016/s0167-4943(05)80018-4. [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelmann GE, Olton DS. Spatial memory following damage to hippocampal CA3 pyramidal cells with kainic acid: impairment and recovery with preoperative training. Brain Res. 1981;217:41–58. doi: 10.1016/0006-8993(81)90183-9. [DOI] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behav Brain Res. 2009;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T, Puolivali J, Tanila H. Effects of long-term ovariectomy and estrogen treatment on maze learning in aged mice. Exp Gerontol. 2004;39:1277–1283. doi: 10.1016/j.exger.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Rekart JL, Sandoval J, Routtenberg A. Spatial learning induces presynaptic structural remodeling in the hippocampal mossy fiber system of two rat strains. Hippocampus. 2006;16:560–570. doi: 10.1002/hipo.20185. [DOI] [PubMed] [Google Scholar]
- Idrobo F, Nandy K, Mostofsky DI, Blatt L, Nandy L. Dietary Restriction Effects on Radial Maze Learning and Lipofuscin Pigment Deposition in the Hippocampus and Frontal Cortex. Age (Media) 1986;9:117. doi: 10.1016/0167-4943(87)90014-8. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Anson RM, de Cabo R, Mamczarz J, Zhu M, Mattison J, Lane MA, Roth GS. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–423. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- Iwata M, Shirayama Y, Ishida H, Kawahara R. Hippocampal synapsin I, growth-associated protein-43, and microtubule-associated protein-2 immunoreactivity in learned helplessness rats and antidepressant-treated rats. Neuroscience. 2006;141:1301–1313. doi: 10.1016/j.neuroscience.2006.04.060. [DOI] [PubMed] [Google Scholar]
- Jahng JW, Kim JG, Kim HJ, Kim BT, Kang DW, Lee JH. Chronic food restriction in young rats results in depression- and anxiety-like behaviors with decreased expression of serotonin reuptake transporter. Brain Res. 2007;1150:100–107. doi: 10.1016/j.brainres.2007.02.080. [DOI] [PubMed] [Google Scholar]
- John JP, Sunyer B, Hoger H, Pollak A, Lubec G. Hippocampal synapsin isoform levels are linked to spatial memory enhancement by SGS742. Hippocampus. 2009;19:731–738. doi: 10.1002/hipo.20553. [DOI] [PubMed] [Google Scholar]
- Krebs JR, Clayton NS, Hampton RR, Shettleworth SJ. Effects of photoperiod on food-storing and the hippocampus in birds. Neuroreport. 1995;6:1701–1704. doi: 10.1097/00001756-199508000-00026. [DOI] [PubMed] [Google Scholar]
- Laping NJ, Nichols NR, Day JR, Johnson SA, Finch CE. Transcriptional control of glial fibrillary acidic protein and glutamine synthetase in vivo shows opposite responses to corticosterone in the hippocampus. Endocrinology. 1994;135:1928–1933. doi: 10.1210/endo.135.5.7956913. [DOI] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav Neural Biol. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- MacDougall-Shackleton SA, Sherry DF, Clark AP, Pinkus R, Hernandez AM. Photoperiodic regulation of food storing and hippocampus volume in black-capped chickadees, Poecile atricapillus. Animal Behaviour. 2003;65:805–812. [Google Scholar]
- Masoro EJ. Hormesis and the antiaging action of dietary restriction. Exp Gerontol. 1998;33:61–66. doi: 10.1016/s0531-5565(97)00071-5. [DOI] [PubMed] [Google Scholar]
- Morioka M, Nagahiro S, Fukunaga K, Miyamoto E, Ushio Y. Calcineurin in the adult rat hippocampus: different distribution in CA1 and CA3 subfields. Neuroscience. 1997;78:673–684. doi: 10.1016/s0306-4522(96)00626-4. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE, Klein SL, Kriegsfeld LJ. The influence of season, photoperiod, and pineal melatonin on immune function. J Pineal Res. 1995a;19:149–165. doi: 10.1111/j.1600-079X.1995.tb00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson RJ, Gubernick DJ, Blom JM. Influence of photoperiod, green food, and water availability on reproduction in male California mice (Peromyscus californicus) Physiol Behav. 1995b;57:1175–1180. doi: 10.1016/0031-9384(94)00380-n. [DOI] [PubMed] [Google Scholar]
- Nicol S, Rahman D, Baines AJ. Ca-2+-dependent interaction with calmodulin is conserved in the synapsin family: Identification of a high-affinity site. Biochemistry. 1997;36:11487–11495. doi: 10.1021/bi970709r. [DOI] [PubMed] [Google Scholar]
- Ping SE, Trieu J, Wlodek ME, Barrett GL. Effects of estrogen on basal forebrain cholinergic neurons and spatial learning. J Neurosci Res. 2008;86:1588–1598. doi: 10.1002/jnr.21609. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ. Affective responses to changes in day length in Siberian hamsters (Phodopus sungorus) Psychoneuroendocrinology. 2005;30:438–452. doi: 10.1016/j.psyneuen.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Adelson JD, Nelson RJ. Short days increase hypothalamic-pituitary-adrenal axis responsiveness. Endocrinology. 2007;148:3402–3409. doi: 10.1210/en.2006-1432. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Reader BF, Nelson RJ. Short photoperiods impair spatial learning and alter hippocampal dendritic morphology in adult male white-footed mice (Peromyscus leucopus) J Neurosci. 2005;25:4521–4526. doi: 10.1523/JNEUROSCI.0795-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Trainor BC, Nelson RJ. Testosterone and photoperiod interact to affect spatial learning and memory in adult male white-footed mice (Peromyscus leucopus) Eur J Neurosci. 2006;23:3056–3062. doi: 10.1111/j.1460-9568.2006.04821.x. [DOI] [PubMed] [Google Scholar]
- Ribble DO. Lifetime Reproductive Success and Its Correlates in the Monogamous Rodent Peromyscus-Californicus. Journal of Animal Ecology. 1992;61:457–468. [Google Scholar]
- Schwabe L, Schachinger H, de Kloet ER, Oitzl MS. Corticosteroids operate as a switch between memory systems. J Cogn Neurosci. 2010;22:1362–1372. doi: 10.1162/jocn.2009.21278. [DOI] [PubMed] [Google Scholar]
- Shuai Y, Lu B, Hu Y, Wang L, Sun K, Zhong Y. Forgetting is regulated through Rac activity in Drosophila. Cell. 2010;140:579–589. doi: 10.1016/j.cell.2009.12.044. [DOI] [PubMed] [Google Scholar]
- Silva AL, Fry WH, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav Brain Res. 2010;208:528–534. doi: 10.1016/j.bbr.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–312. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Stewart J, Mitchell J, Kalant N. The effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the eight-arm radial and the Morris water mazes. Neurobiol Aging. 1989;10:669–675. doi: 10.1016/0197-4580(89)90003-1. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Czernik AJ, Kao HT, Takei K, Johnston PA, Horiuchi A, Kanazir SD, Wagner MA, Perin MS, De Camilli P, et al. Synapsins: mosaics of shared and individual domains in a family of synaptic vesicle phosphoproteins. Science. 1989;245:1474–1480. doi: 10.1126/science.2506642. [DOI] [PubMed] [Google Scholar]
- Thompson R, Kao L, Yang S. Rapid forgetting of individual spatial reversal problems in rats with parafascicular lesions. Behav Neural Biol. 1981;33:1–16. doi: 10.1016/s0163-1047(81)92189-0. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Crean KK, Fry WHD, Sweeney C. Activation of extracellular signal-regulated kinases in social behavior circuits during resident-intruder aggression tests. Neuroscience. 2010;165:325–336. doi: 10.1016/j.neuroscience.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropp J, Markus EJ. Effects of mild food deprivation on the estrous cycle of rats. Physiol Behav. 2001;73:553–559. doi: 10.1016/s0031-9384(01)00487-5. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Buxton OM, Chang AM, Scarbrough K, Ferrari EA, Takahashi JS, Turek FW. Locomotor response to an open field during C57BL/6J active and inactive phases: differences dependent on conditions of illumination. Physiol Behav. 2000;69:269–275. doi: 10.1016/s0031-9384(00)00219-5. [DOI] [PubMed] [Google Scholar]
- Villacres EC, Wong ST, Chavkin C, Storm DR. Type I adenylyl cyclase mutant mice have impaired mossy fiber long-term potentiation. J Neurosci. 1998;18:3186–3194. doi: 10.1523/JNEUROSCI.18-09-03186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Bowers SL, Nelson RJ. Photoperiod alters affective responses in collared lemmings. Behav Brain Res. 2007;179:305–309. doi: 10.1016/j.bbr.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett JM, Miller LL. Photoperiod and reproduction in female deer mice. Biol Reprod. 1982;26:296–304. doi: 10.1095/biolreprod26.2.296. [DOI] [PubMed] [Google Scholar]
- Witte AV, Fobker M, Gellner R, Knecht S, Floel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106:1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Sun X, Liu Y. Effects of caloric restriction on cognition and behavior in developing mice. Neurosci Lett. 2003;339:166–168. doi: 10.1016/s0304-3940(03)00008-9. [DOI] [PubMed] [Google Scholar]
- Wube T, Fares F, Haim A. A differential response in the reproductive system and energy balance of spiny mice Acomys populations to vasopressin treatment. Comp Biochem Physiol A Mol Integr Physiol. 2008;151:499–504. doi: 10.1016/j.cbpa.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Tanahashi T, Kawai T, Chikahisa S, Katsuura S, Nishida K, Teshima-Kondo S, Sei H, Rokutan K. Changes in behavior and gene expression induced by caloric restriction in C57BL/6 mice. Physiol Genomics. 2009;39:227–235. doi: 10.1152/physiolgenomics.00082.2009. [DOI] [PubMed] [Google Scholar]
- Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465:690. doi: 10.1038/465690a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.