Abstract
An analysis of the photoacoustic (PA) signal from murine tissue in vivo revealed several benefits of contrast-enhanced PA imaging at a wavelength of 1064 nm. Of all the wavelengths tested in a range from 710 to 1064 nm, the background PA signal from tissue in vivo was lowest and more homogeneous at 1064 nm. For blood-laden tissue, the background PA signal was up to 70% less at 1064 nm. Furthermore, when plasmonic nanoparticles, such as silver nanoplates, were introduced in vivo as contrast agents, the contrast in PA images at 1064 nm increased 38% compared to 750 nm. Therefore, contrast-enhanced PA imaging at 1064 nm is advantageous because of the low and homogeneous signal from native tissue, enabling high contrast in PA imaging when exogenous, molecularly targeted agents are employed.
Photoacoustic (PA) imaging is a noninvasive biomedical imaging technique capable of resolving the optical absorption map of tissue at penetration depths akin with ultrasound (US) imaging [1]. Since the light absorption from native tissue components is minimized at near-IR (NIR) wavelengths, many PA contrast agents, namely plasmonic metal nanoparticles, have been designed to absorb light strongly in this NIR region [2]. Furthermore, nanoparticles can be functionalized with antibodies, proteins, or aptamers on their surface that allow for these contrast agents to molecularly target extracellular and intracellular receptors or pathways. Considering how valuable a molecularly sensitive, noninvasive, and non-ionizing imaging modality could be to researchers and clinicians alike, the topic of exactly which PA imaging wavelengths in the NIR are optimal for enhancing contrast deserves exploration.
One of the least expensive lasers, the Nd:YAG laser, emits light at a primary wavelength of 1064 nm. Considering the Nd:YAG’s reliable and efficient light generation, it could be an appropriate laser for incorporation in clinical PA imaging systems. Therefore, in this study we compare PA imaging at multiple wavelengths in the NIR, including 1064 nm, in vivo in mice. Additionally, most researchers to date have focused on creating PA contrast agents tuned to absorb light at wavelengths in the range of ~680 to 850 nm [3,4], with few studies employing the 1064 nm wavelength to image tissue [5,6]. To explore PA contrast enhancement from nanoparticle agents at multiple wavelengths, silver nanoplates (NPs) were synthesized with different surface plasmon resonance (SPR) peaks, and their ability to enhance PA imaging contrast in vivo was also assessed.
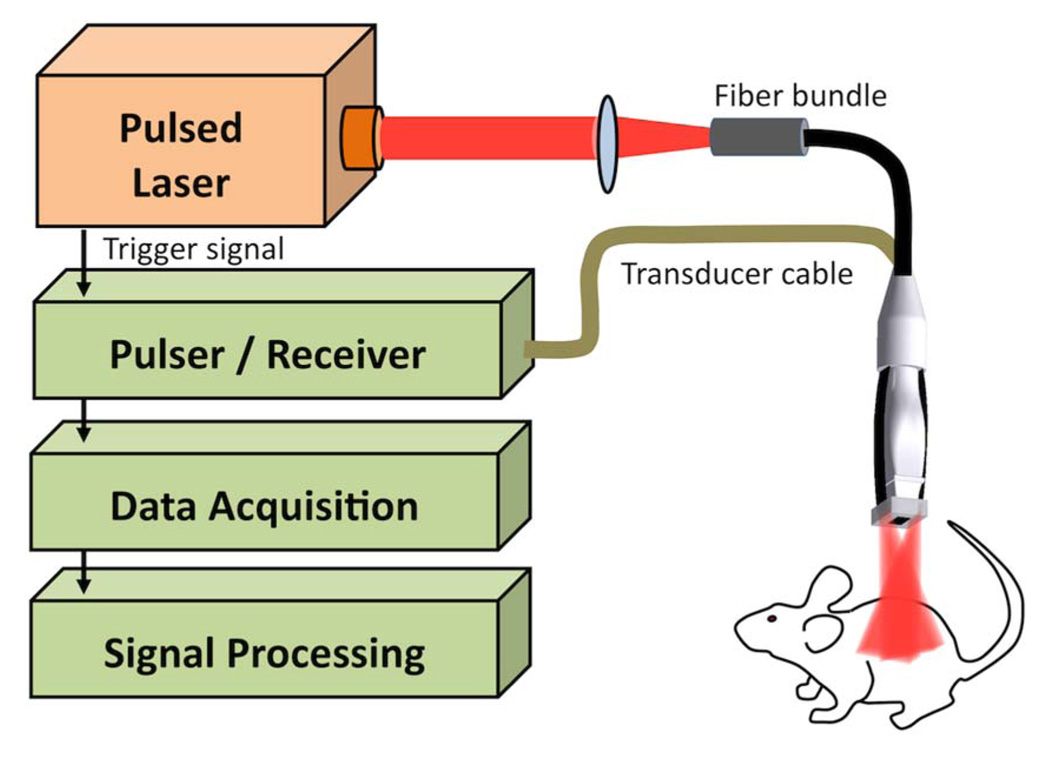
The setup for the combined PA and US (PAUS) imaging system, consisting of a 4–6 ns pulsed optical parametric oscillator laser system and a 7:5 MHz array transducer, is shown in Fig. 1 and explained in detail elsewhere [7]. All experiments were performed in vivo on nude mice (Nu/Nu) that were positioned on a heated electrocardiogram pad. The US system captured rf data for spatially coregistered US and PA rf signals. To form images, rf data was beam formed, and US images (decibel scale) and fluence-compensated PA images (linear scale) were plotted.
Fig. 1.
(Color online) Photoacoustic and ultrasound imaging setup.
The PAUS imaging system was first used to determine the macroscopic background PA signal from native tissue. In short, PAUS data was collected from four positions (two-dimensional cross sections) in two separate mice (i.e., total of eight imaging planes) over a section of the animal’s back between the lungs and the thighs. For each of the eight imaging planes, PA data was collected at seven different wavelengths (710, 730, 750, 765, 800, 900, and 1064 nm). To calculate the background PA signal, the laser energy compensated PA transients per imaging plane were summed (value of the PA response in each pixel) and divided by its corresponding tissue area. The mean PA signal at each wavelength is shown in Fig. 2(a), where each black square represents the average of eight imaging planes. These data points are overlaid on absorption coefficient data for various native tissue components [8]. The absorption due to blood and skin decreases as wavelength increases, and this trend is likely responsible for the low background PA signal at 1064 nm. In fact, the background PA signal from tissue at 1064 nm is 30% lower than the signal at 900 nm. Thus, for all the wavelengths tested, the background PA signal from native tissue at 1064 nm was lowest.
Fig. 2.
(a) Background PA signal from murine tissue in vivo overlaid on absorption coefficient data for tissue components, and (b) the std divided by the mean for the PA signal from tissue in vivo.
In addition to the background being lower at 1064 nm, there is a trend in the NIR for the absorption of tissue to become more homogeneous. If this trend toward homogeneity is valid, then the standard deviation (std) normalized by the mean of the PA transients within the image should decrease with increasing wavelength. Figure 2(b) shows the results of these std/mean calculations. The trend of increasing std/mean values with the small increase in the deoxygenated hemoglobin curve at 760 nm follows our expectation, as does the decreasing trend in the values at NIR wavelengths, with the lowest being at 1064 nm. Furthermore, we summed the amplitudes of the PA signal along each vertical line (A-line) in all the PA images and compared these various samples of signals using an F test for equality of variances. This right-tailed, two-sample F test revealed that, with a p < 0:04, the PA signals at 710, 730, 750, and 765 nm all had a higher variance than those at 1064 nm, while the signals at 800 and 900 nm were not statistically significantly different from that at 1064 nm. Therefore, the variance in PA signal decreases with increasing wavelength, and wavelengths >800 nm provide amore homogeneous PA signal in vivo.
Since the PA background signal at 1064 nm is low, then contrast enhancement of the PA signal using plasmonic nanoparticles at 1064 nm should be higher than at other wavelengths. To quantify the PA contrast enhancement, two batches of silver NPs with SPR extinction peaks at approximately 750 and 1064 nm, respectively, were injected subcutaneously in separate mice. The NPs were synthesized [9], and the as-prepared NPs were conjugated to poly(ethylene glycol)-thiol (mPEG-SH of 5 Da from Laysan Bio) for stabilization, filtered, and resuspended in 3 ml of water. For subcutaneous injection, the 3 ml solution was heated to 60 °C and 300 mg of gelatin was dissolved in the warm solution while stirring. Subcutaneous injection of 100 μl of the NP solution resulted in an instantly gelled pocket of silver NPs just beneath the skin surface.
Figure 3 shows the result of imaging the NPs with an SPR peak at ~750 nm (NP750) and the NPs with an SPR peak at ~1064 nm (NP1064). Specifically, the extinction spectra for both NP sets are shown in Fig. 3(a) and a transmission electron micrograph (TEM) of a typical NP750 batch is shown in Fig. 3(b). The NP750 were 102 ± 21 nm and the NP1064 were 210 ± 38 nm in their largest dimension, as quantified from TEM images. The thickness of the plates (~12 nm) was more difficult to quantify since the plates tended to lie flat on TEM grids. A display of the PAUS images after injecting 3 × 1010 NP750 per milliliter and 7 × 109 NP1064 per milliliter at the same optical density (OD = 1.5) into two mice encompass the rest of Fig. 3 (molar extinction for the NPs was 3:0 × 1010 cm−1 M−1 and 1.3 × 1011 cm−1 M−1, respectively). In particular, Fig. 3(c) is an US image showing a hypoechoic region in the top left where NP750 was injected (white arrow). As expected, the PA image [Fig. 3(d)] captured at the wavelength corresponding to its SPR at 750 nm exhibited a higher signal than the PA image captured at 1064 nm [Fig. 3(e)]. Likewise, Fig. 3(g) shows an US image of the hypoechoic NP1064 injection region in a different mouse, and the PA image captured at 1064 nm showed the highest PA signal [Fig. 3(i)]. The PA images for the NPs that corresponded to irradiating them at their SPR extinction peaks were overlaid on US images in Figs. 3(f) and 3(j) to show the spatial coregistry of the hypoechoic region containing nanoplates and its corresponding PA signal.
Fig. 3.
(a) Extinction spectra for the NPs and (b) a TEM image of NP750 (scale bar, 100 nm). US images after direct injection of (c) NP750 and (g) NP1064 (arrows indicate injection sites). PA images obtained using (d) and (h) 750 nm and (e) and (i) 1064 nm wavelengths. The injected NPs are even better visualized in combined PAUS images [(f) and (j)]. All images are 10 mm × 10:5 mm.
Qualitatively, the PA signal for both the NP750 and the NP1064 appear similar [Figs. 3(d) and 3(i)], but quantitative assessment of the PA images showed a 38% increase in contrast for the NP1064. Contrast was calculated by summing the intensity of the PA signal from the region with NPs, subtracting the background signal (summed intensity of the PA signal from tissue below the NPs), and dividing by the background signal (all sums were normalized by their respective areas). In fact, this 38% difference in contrast (contrast was 6.28 for NP750 and 8.70 for the NP1064) was directly related to changes in the background PA signal, since the NPs were injected at the same optical density and, as a result, the absolute PA signals from the NPs were similar. Recall that the decrease in the background PA signal from native tissue between the 750 and the 1064 nm PA images in Fig. 2 was 37%. Therefore, the PA contrast enhancement due to plasmonic NPs with resonance at 1064 nm was almost 40% higher than plasmonic NPs whose resonation and irradiation for imaging was 750 nm.
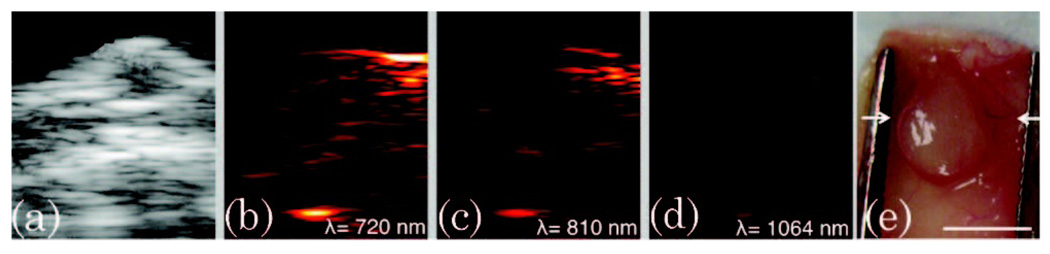
For tissue that is inherently blood laden, such as certain tumors, the background PA signal at 1064 nm was even further reduced. Figure 4 shows images captured from a mouse with a subcutaneous xenograft tumor grown from MPanc96 cells (a human pancreatic cancer line). Figure 4(a) is an US image of the tumor, showing a slight hypoechoic tumor center in the upper right, under the skin. Figures 4(b)–4(d) present 720, 810, and 1064 nm PA images of the same cross section. A decrease in PA signal is evident as the irradiating wavelength is increased. This decrease in PA signal is particularly clear in the upper right of the image, where blood was the dominant absorber. Note the correlation between the high signal from blood in the right upper area of the PA images, and the corresponding tumor photograph showing a major blood vessel feeding the tumor in the imaging plane indicated by white arrows [Fig. 4(e)]. Moreover, the background signals (summed PA intensity over the tissue and tumor area) was 57% lower when comparing the PA images between 810 and 1064 nm and 68% lower when comparing the PA images between 720 and 1064 nm. Therefore, performing PA imaging using plasmonic NPs whose resonance is tuned to 1064 nm should yield 50%–70% higher contrast than performing PA imaging with NPs absorbing in the 700–800 nm wavelength range.
Fig. 4.
(a) US image of a xenograft tumor grown subcutaneously in a mouse. PA images of the tumor taken at (b) 720, (c) 810, and (d) 1064 nm wavelengths. (e) A photograph of the tumor showing that the bright PA signal was due to a large blood vessels that were feeding the tumor (white arrows indicate the imaging plane, and the scale bar is 5 mm). PAUS images are 13 mm × 10:5 mm.
We have demonstrated several advantages of contrast-augmented PA imaging at 1064 nm, but there are also some challenges. For example, to create metal nanoparticles that are resonant at 1064 nm, the aspect ratio of the longitudinal to the transverse cross section of the particle must be increased. Particles with high aspect ratios are generally known for reduced thermodynamic stability and shape instability under pulsed light irradiation. Unfortunately, shape change simultaneously induces a blue-shift of the longitudinal SPR peak that can be detrimental to PA imaging. Fortunately, silica coating of gold nanorods significantly enhances their stability for PA imaging [10], offering one possible solution to the stability problem. Another challenge is related to the increase in overall size of nanoparticles with increasing aspect ratio. For the silver NPs described here, the largest dimension of the NP must increase by >100 nm in order to shift the SPR peak from 750 to 1064 nm. If these particles were used as intravenously injectable, molecularly targeted contrast agents for applications such as tumor imaging, their increased size might cause (1) premature clearance from the blood stream and/or (2) a decrease in tumor uptake due to restricted diffusion of the particles through the endothelial gap junctions in tumor vasculature. Therefore, thermodynamic stability and nanoparticle size are the major challenges to performing contrast-enhanced PA imaging at 1064 nm. However, improvements in nanoparticle design, including the synthesis of large-aspect-ratio particles of small overall size and the use of capping agents like silica to stabilize them, will mitigate these issues and help to realize the advantages of molecular PAUS imaging at 1064 nm in vivo.
Acknowledgments
The authors thank Dr. Craig Logsdon for providing the MPanc96 cell line, Dr. Andrei Karpiouk for building the PAUS imaging probe, and the Animal Resource Center at UT Austin. Partial support from the National Institutes of Health (NIH) under grants CA141203, HL096981, and EB008101 is acknowledged.
Footnotes
OCIS codes: 170.0110, 170.5120, 170.6930, 110.5125, 160.1050, 160.4236.
References
- 1.Emelianov SY, Aglyamov SR, Shah J, Sethuraman S, Scott WG, Schmitt R, Motamedi M, Karpiouk A, Oraevsky A. Proc. SPIE. 2004;5320:101. [Google Scholar]
- 2.Mallidi S, Larson T, Aaron J, Sokolov K, Emelianov S. Opt. Express. 2007;15:6583. doi: 10.1364/oe.15.006583. [DOI] [PubMed] [Google Scholar]
- 3.Eghtedari M, Oraevsky A, Copland J, Kotov N, Conjusteau A, Motamedi M. Nano Lett. 2007;7:1914. doi: 10.1021/nl070557d. [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Skrabalak S, Li Z, Xia Y, Wang L. Nano Lett. 2007;7:3798. doi: 10.1021/nl072349r. [DOI] [PubMed] [Google Scholar]
- 5.Li P, Wei C, Liao C, Chen C, Pao K, Wang C, Wu Y, Shieh D. Proc. SPIE. 2006;6086:60860M. [Google Scholar]
- 6.Manohar S, Kharine A, van Hespen JC, Steenbergen W, van Leeuwen TG. Phys. Med. Biol. 2005;50:2543. doi: 10.1088/0031-9155/50/11/007. [DOI] [PubMed] [Google Scholar]
- 7.Homan K, Shah J, Gomez S, Gensler H, Karpiouk A, Brannon-Peppas L, Emelianov S. J. Biomed. Opt. 2010;15:021316. doi: 10.1117/1.3365937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prahl SA. [(2001, retrieved 2010)];Optical property spectra compiled by Scott Prahl. http://omlc.ogi.edu/spectra/
- 9.Zou X, Ying E, Chen H, Dong S. Colloids Surf. A. 2007;303:226. [Google Scholar]
- 10.Chen Y-S, Kruizinga PP, Joshi PP, Kim S, Homan KA, Sokolov K, Frey W, Emelianov S. Proc. SPIE. 2010;7564:75641Q. [Google Scholar]