Abstract
Plant small RNAs are emerging as significant components of epigenetic processes and of gene networks involved in development and homeostasis. In this paper, to identify small RNAs in wheat, 2,076 small RNAs were identified in a small RNA library from leaf, root, and spike. These small RNAs mapped to non-coding regions the CDS region of protein-coding genes and 5′ UTR and 3′ UTR regions. The expression of small RNAs in seedling leaves, roots, and spikes were analyzed by northern blot, which indicates that some small RNAs were responsive to abiotic stress treatments including heat, cold, salt and dehydration.
Keywords: Small RNA, nat-siRNA, Expressed sequence tag, Wheat, Abiotic stress
Introduction
Small RNAs with the length of 21 to 24 nucleotides function to silence gene expression by multiple mechanisms and are present in diverse eukaryotic organisms (Lu et al. 2005; Sunkar et al. 2005; Jamalkandi and Masoudi-Nejad 2009). More than two million small RNAs from seedlings and inflorescence of Arabidopsis have been sequenced, and among these sequences, more than half represent lower abundance siRNA that match repetitive sequences, intergenic regions, and genes (Lu et al. 2005). The Arabidopsis small RNA project has analyzed the small RNAs from different tissues and genotypes of Arabidopsis and provides a public database of cloned small RNA sequences to assist in analysis of small RNA population (Gustafson et al. 2005). Here, we report that some of the wheat small RNAs exhibit developmental stage-dependent and stress-responsive expression patterns and five putative wheat nat-siRNAs are also identified. We predict 4,249 trans targets for the 1,106 small RNAs, and these predicted target genes include not only transcription factors implicated in development but also other genes involved in broad range of physiological processes.
Materials and methods
A small RNA library ranging in size of 18 to 26 nucleotides using pooled RNA isolated from leaves, roots, and spikes was generated (Yao et al. 2007). Sequencing (454) generated a total of 25,453 unique sequences ranging in length of 18 and 26 nucleotides and were characterized using PLANTGDB with BLASTN. A total of 5,216 sequences were perfectly matched to the wheat ESTs and were used to search against Rfam and NT database to remove the degradation products of other non-coding RNAs such as rRNA and tRNA. Among the 5,216 sequences, the largest class (59.4%) of cloned RNAs represents fragments of abundant non-coding tRNAs and rRNAs. The remaining 2,117 sequences perfectly mapped to wheat ESTs were candidate small RNAs in wheat, among which 41 was classified as microRNAs provided in our previous report (Yao et al. 2007). The majority (62%) of the small RNAs were 21 to 24 nt in size, typical for Dicer-derived products
Results and discussion
The small RNA transcriptomes of bread wheat and its emerging model Brachypodium distachyon have been obtained by using deep sequencing technology (Wei et al. 2009). These studies established that 21-nt small RNAs were the most abundant. In this paper, the identified wheat small RNAs exhibit a biased nucleotide distribution at their 5′ end. A preference for a U is found at the 5′ end of 19–21 nt small RNA classes, which is similar to the siRNAs from other organism (Aravin et al. 2003; Bartel 2004; Girard et al. 2006; Zhao et al. 2007). It is interesting to note that the 22–24 nt small RNAs start with a C. In order to determine whether this nucleotide preference is a common feature in the 21 and 24 nt size classes of plant small RNAs, we analyze the bias of the 5′ end of small RNAs in Arabidopsis, rice, and maize. It is found that most of the Arabidopsis and rice endogenous small RNAs of the 24 nt class begin with a 5′ A, maize begin with C (Fig. 1).
Fig. 1.

Identity of first nucleotide of wheat endogenous siRNA and clone length (nt)
The 2,076 small RNAs (not including the miRNA) were mapped to 12,949 loci on wheat chromosomes, with an average of 6.23 loci per small RNA. A total of 9,887 loci mapped to non-coding genes, and 2,626 were located in the CDS region of protein-coding genes. In addition 360 corresponded to 5′ UTR regions and 76 to 3′ UTR regions. In rice, maize, Arabidopsis, and Chlamydomonas, most small RNAs also match to the non-coding regions.
Potential trans mRNA targets for the wheat endogenous small RNAs were identified from PLANTGDB database using a criterion of antisense hits with less than four mismatches and no mismatches between positions 10 and 11 from the 5′ end of the small RNA. This process identified 4,249 trans targets for the 1,106 small RNAs and would be predicted to play roles in a broad range of physiological processes.
A total of seven predicted siRNAs that have more than 90% nucleotide acid similarity to rice NAT pairs and as shown in Fig. 2A, both sense and antisense transcripts of the five wheat small RNAs precursors could be amplified from leaf tissue, suggesting that these NAT pairs may serve as one of the major sources of endogenous siRNA. We further detect the expression of mature siRNA by using RNA Northern hybridization or small RNA RT-PCR (Fig. 2B). The results indicate that the five small RNAs are expressed at very low abundance and only one small RNA (005047_0654_1904.1) could be detected on the small RNA blots (Fig. 3). The expressions of other three small RNAs (022002_0602_3314.1, 230674_0416_1173.1, and 029868_3652_2218.1) are determined by RT-PCR (Fig. 2B), which reveal that they are expressed in wheat roots and leaves. The expression of mature small RNA (060074_3246_2220.1) could not be confirmed in this article, suggesting a very low expression level in the tissues tested. The tissue/organ-dependent expression patterns of wheat small RNAs were analyzed by northern blot (Fig. 3). The results showed that all the four small RNAs tested appear to be preferentially expressed in spikes and uniformly expressed in leaves and roots.
Fig. 2.
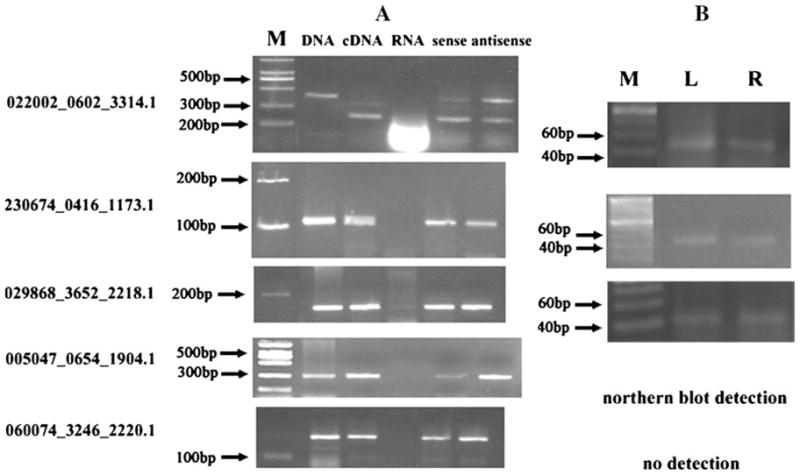
A RT-PCR analysis of wheat nat-siRNA precursor expression in wheat leaf tissue. Reverse transcription performed with primers specific to the precursor allow the detection of both sense and antisense species. The absence of any PCR product amplified from RNA template before reverse transcription testifies to the authenticity of the sense and antisense siRNA precursor. In addition, control amplification reactions are run with each set of primers by using genomic DNA as a template. B PCR-based detection of nat-siRNA. L represents leaves and R represents roots
Fig. 3.
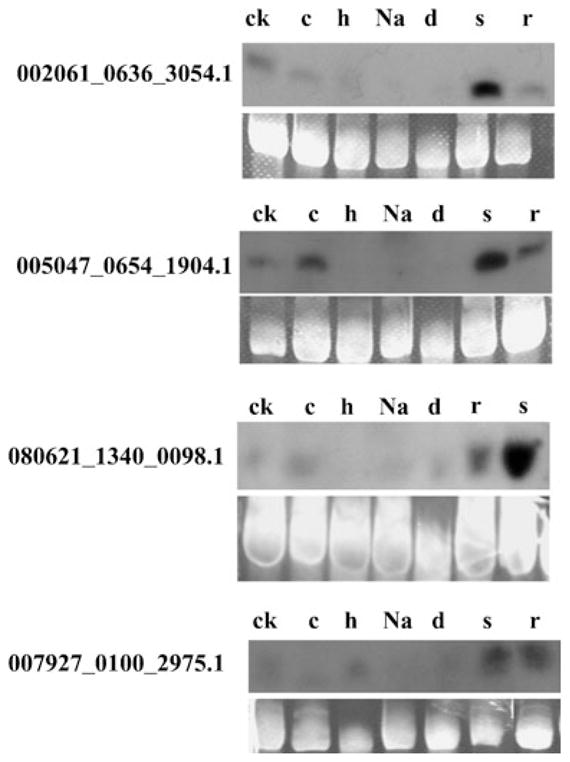
Expression analysis of wheat siRNAs by northern blot. The 30 μg low molecular weight RNA isolated from different tissues and tissues in different stress are probed with labeled oligonucleotides. The tRNA and 5S RNA bands are visualized by ethidium bromide staining of polyacrylamide gels and serve as loading controls. Wheat seedling roots (r), leaves (ck), and spikes (s) are collected and used. The 3-week-old seedlings grown in normal nutrient solution (ck) are treated with cold (4°C) for 2 h (c), heat (40°C) for 2 h (h), NaCl (200 mM) for 2 h (Na), dehydration stress for 2 h (d), with the leaves then collected for RNA extraction
In order to test whether the expression of wheat small RNA is regulated by abiotic stresses, RNA gel blot is performed by using wheat Line 3338 seedling treated with cold (4°C for 2 h), heat (40°C for 2 h), NaCl (200 mM), and dehydration stress (Fig. 3). The northern blot analysis revealed that the expressions of four siRNAs change greatly under various stress treatments. SiRNA 002061_0636_3054.1 is strongly down-regulated by heat, NaCl, and dehydration stress; 005047_0654_1904.1 is greatly up-regulated by cold stress and down-regulated by heat, NaCl, and dehydration stress; 080621_1340_0098.1 is slightly up-regulated by cold and down-regulated by heat but not by NaCl and dehydration stress; and 007927_0100_2975.1 is down-regulated by cold, NaCl, and dehydration stress but not by heat stress. The genes targeted by these abiotic stress-responsive small RNA could play roles in regulating stress responses in wheat. However, the mechanisms of wheat small RNAs in regulating gene expression and the biological function of target genes need further investigation.
Acknowledgments
The authors thank Dr. Thomas Girke (Institute for Integrative Genome Biology, University of California, Riverside) for bioinformatics analysis of 454 raw sequences data. We gratefully thank Haiyan Wu from China Agricultural University for assistance in bioinformatic analysis. This research is supported by National Basic Research Program of China (2007CB109000 to Q.S., Z.N., and Y.Y), 863 Project of China (2007A10Z100 to Y.Y and H.P), and the Programme of Introducing Talents of Discipline to Universities (B06003).
Contributor Information
Yingyin Yao, Key Laboratory of Crop Heterosis and Utilization (MOE) and State Key Laboratory for Agrobiotechnology, Key Laboratory of Crop Genomics and Genetic Improvement (MOA), Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, Beijing 100094, China, National Plant Gene Research Centre (Beijing), Beijing 100094, China.
Zhongfu Ni, Key Laboratory of Crop Heterosis and Utilization (MOE) and State Key Laboratory for Agrobiotechnology, Key Laboratory of Crop Genomics and Genetic Improvement (MOA), Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, Beijing 100094, China, National Plant Gene Research Centre (Beijing), Beijing 100094, China.
Huiru Peng, Key Laboratory of Crop Heterosis and Utilization (MOE) and State Key Laboratory for Agrobiotechnology, Key Laboratory of Crop Genomics and Genetic Improvement (MOA), Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, Beijing 100094, China, National Plant Gene Research Centre (Beijing), Beijing 100094, China.
Fenglong Sun, Key Laboratory of Crop Heterosis and Utilization (MOE) and State Key Laboratory for Agrobiotechnology, Key Laboratory of Crop Genomics and Genetic Improvement (MOA), Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, Beijing 100094, China, National Plant Gene Research Centre (Beijing), Beijing 100094, China.
Mingming Xin, Key Laboratory of Crop Heterosis and Utilization (MOE) and State Key Laboratory for Agrobiotechnology, Key Laboratory of Crop Genomics and Genetic Improvement (MOA), Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, Beijing 100094, China, National Plant Gene Research Centre (Beijing), Beijing 100094, China.
Ramanjulu Sunkar, Department of Biochemistry and Molecular Biology, Oklahoma State University, Stillwater, OK 74078, USA.
Jian-Kang Zhu, Department of Botany and Plant Sciences, University of California, Riverside, CA 92521, USA.
Qixin Sun, Email: qxsun@cau.edu.cn, Key Laboratory of Crop Heterosis and Utilization (MOE) and State Key Laboratory for Agrobiotechnology, Key Laboratory of Crop Genomics and Genetic Improvement (MOA), Beijing Key Laboratory of Crop Genetic Improvement, China Agricultural University, Beijing 100094, China, National Plant Gene Research Centre (Beijing), Beijing 100094, China, Department of Plant Genetics and Breeding, China Agricultural University, Yuanmingyuan Xi Road No. 2, Haidian District, Beijing 100193, China.
References
- Aravin AA, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, Snyder B, Gaasterland T, Meyer J, Tuschl T. The small RNA profile during Drosophila melanogaster development. Dev Cell. 2003;5:337–350. doi: 10.1016/s1534-5807(03)00228-4. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Girard A, Sachidanandam R, Hannon GJ, Carmell MA. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature. 2006;442:199–202. doi: 10.1038/nature04917. [DOI] [PubMed] [Google Scholar]
- Gustafson AM, Allen E, Givan S, Smith D, Carrington JC, Kasschau KD. ASRP: the Arabidopsis Small RNA Project Database. Nucleic Acids Res. 2005;33:D637–D640. doi: 10.1093/nar/gki127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamalkandi SA, Masoudi-Nejad A. Reconstruction of Arabidopsis thaliana fully integrated small RNA pathway. Funct Integr Genomics. 2009;9:419–432. doi: 10.1007/s10142-009-0141-z. [DOI] [PubMed] [Google Scholar]
- Lu C, Tej SS, Luo S, Haudenschild CD, Meyers BC, Green PJ. Elucidation of the small RNA component of the transcriptome. Science. 2005;309:1567–1569. doi: 10.1126/science.1114112. [DOI] [PubMed] [Google Scholar]
- Sunkar R, Girke T, Zhu JK. Identification and characterization of endogenous small interfering RNAs from rice. Nucleic Acids Res. 2005;33:4443–4454. doi: 10.1093/nar/gki758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei B, Cai T, Zhang RZ, Li AL, Huo NX, Li S, Gu YQ, Vogel J, Jia JZ, Qi YJ, Mao L. Novel microRNAs uncovered by deep sequencing of small RNA transcriptomes in bread wheat (Triticum aestivum L.) and Brachypodium distachyon (L.) Beauv Funct Integr Genomics. 2009 doi: 10.1007/s10142-009-0128-9. [DOI] [PubMed] [Google Scholar]
- Yao Y, Guo G, Ni Z, Sunkar R, Du J, Zhu JK, Sun Q. Cloning and characterization of microRNAs from wheat (Triticum aestivum L.) Genome Biol. 2007;8:R96. doi: 10.1186/gb-2007-8-6-r96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Li G, Mi S, Li S, Hannon GJ, Wang XJ, Qi Y. A complex system of small RNAs in the unicellular green alga Chlamydomonas reinhardtii. Genes Dev. 2007;21:1190–1203. doi: 10.1101/gad.1543507. [DOI] [PMC free article] [PubMed] [Google Scholar]


