Abstract
Until middle of the last decade, few people had heard of the term microRNA (miRNA), a 21-23bp long conserved RNA. MicroRNAs represent a new paradigm that regulates most physiological processes and has intense potential for medical advancement. Resveratrol, a red wine-derived polyphenolic compound, has been shown to have significant effects in various disease models such as cardioprotection in ischemic heart, diabetes, chemoprevention of cancers, etc. The targets of resveratrol include various pathways and molecules such as sirtuins, FOXOs, and autophagy. The successful application of resveratrol lies in understanding its mechanisms of action through direct and indirect interactions with pathways, including miRNAs. For example, a unique miRNA footprint is present in the heart treated with resveratrol. Targets of those miRNAs have potential implication on the physiological and patho-physiological conditions in health and disease.
Keywords: miRNA expression profile, heart, translational repression or activation, stability of microRNA
Introduction
The rapid pace of outstanding findings in the RNA interference (RNAi) research leads to an expanding array of tools to understand the basic processes of life and disease. After the completion of Human Genome Project, a relatively small number (~5%) of protein-coding genes are found relative to genome size [1]. The rest are non-coding genomes which constitute a variety of small RNAs: miRNA (microRNA, size: 20-22nt), trans-acting endogenous siRNA (small interfering RNA) and piRNA (piwi interacting RNA, size:16–29-nt with repeat sequence). Among them, miRNA is the key major group, which includes over thousands from different species and these are identified by bioinformatics, genetics and molecular biology approach of cloning and characterization [2-4]. Genes for miRNAs are an essential component of the genetic program of all species, many of them also being evolutionarily conserved [5] .
Some RNA has been observed as being part of a complex regulatory system in bacteria for a long time and it regulates target molecule or pathway in many ways [6]. The first report of RNA silencing is found in the plant system [7]. The fundamental study on miRNA is in C. elegans where a gene loci, lin-4, is a regulator of developmental gene expression. Later, the conserved 21-nt RNA let-7, a miRNA, from that locus suppress the expression of target transcript [8].
Resveratrol, a constituent of red wine and many plant roots used for asian medicine, is known for its role of chemoprevention in cancers [Reviewed in 9, 10]. Resveratrol also protects in many tissues injury models like neuronal damage [11, 12]. Overwhelming evidence in literature suggests its key role in cardio-protection [Reviewed in 13, 14].The molecular mechanism of cardio-protection is still under investigation and the role miRNA has not been looked so far.
MicroRNA: Mechanism of action
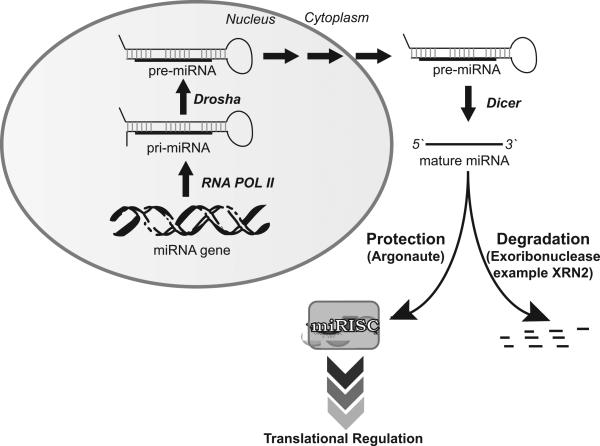
MicroRNAs (miRNA) are the mature form of processed pre-miRNA. Pre-miRNAs are processed by Drosha from bigger poly-adenylated transcripts, known as p-miRNA, in the nucleus and are exported to the cytoplasm by Exportin 5 [15]. Further maturation of pre-miRNA to miRNA occurs in both the nucleus and cytoplasm through Dicer and other protein complexes (Figure1). miRNAs target their mRNA by complimentary base pairing sequences located mainly at 3'UTR (un-translated region). miRNAs also target 5'UTR or coding regions of mRNA [16, 17]. In addition to sequence specific targeting of mRNA, miRNA functions as a ribonucleoprotein complex (miRNPs), also known as miRISCs (miRNA-induced silencing complex). Key components of miRISCs include AGO (Argonaute) and GW182 (glycine-tryptophan repeat-containing protein family), which have conserved domains like PAZ, PIWI and MID. These proteins are found greatly in processing bodies (commonly known as P or GW bodies) for degradation of mRNA[18].
Figure 1. MicroRNA biogenesis and stability.
After synthesis by RNA Polymerase II (RNA POL II), primary transcript of miRNA (pri miRNA) are recognized by Drosha and Pasha which excise the hairpin generating precursor miRNA (pre miRNA). These are transported to cytoplasm by Exportin 5 and further processed by Dicer to mature ~23nt miRNA. Mature miRNA associated with Argonaute and other factors leads to the targeted translational regulation. Release from Argonaute or absence of protection machinery leaves miRNA prone to degradation by exoribonuclease such as XRN-2 or SDN.
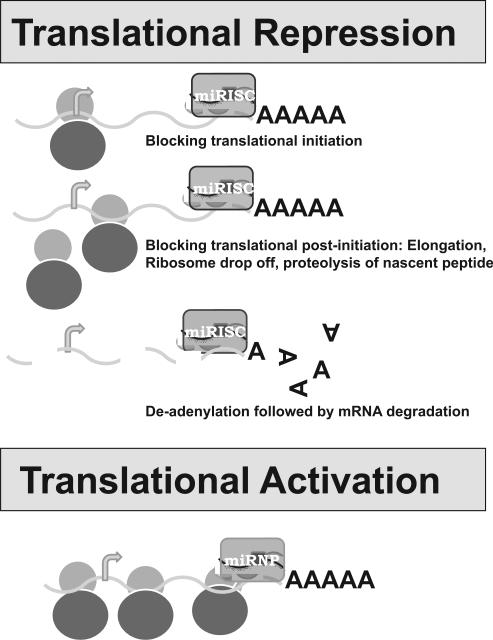
Although mature miRNAs are generally thought to be stable due to their small size, they are prone to degradation by both 5` to 3` and 3` to 5` exoribonucleases present in cells such as XRN2 [19, 20]. miRNA stability is also determined by its sequence complexity [21]. The stability of miRNAs depends on binding to miRISC proteins like Argonaute (Figure1). miRNAs are well known for their role as inhibitors of protein synthesis. The inhibition mechanism of protein synthesis has been hypothesized differently as (1) deadenylation of mRNA followed by degradation (2) blocking of translational initiation (3) blocking at post-initiation stage of translation either by elongation block, ribosome drop-off or proteolysis of nascent polypeptide. Details of the regulation have been reviewed previously [22, 23] and summarized in Figure 2. Some specific initial studies are described briefly in the following paragraphs.
Figure 2. Translational regulation by miRNA.
miRNA modulate translation either by repression or activation although the mechanism is different. miRNA repressed translation has three mechanisms as described in different steps of translational process.
miRNA-mediated deadenylation and degradation of mRNA are initially reported in C. elegans let-7 miRNA targeting lin-41 mRNA and in Zebrafish miR-430 [24, 25]. Various mechanisms of deadenylation and degrdation of mRNA have been proposed after their initial discovery as they appeared in different organisms. mRNA degradation is often mediated by removal of the poly(a) tail by 3'-5' ribonuclease(RNase) and different types that were present in cells such as (1) PARN (2) CCR4p (3) POP (Pop2p) and (4) PAN (Pan2p) [26-29]. mRNA stability is often under the control of cis-acting elements within the 3' untranslated regions(UTR), which recruit enzymes/factors followed by recruitment of deadenylation enzymes.
Blocking of translational initiation is first observed in HeLa cells using reporter mRNAs whose 3' UTRs were targeted by endogenous (let-7) miRNA [30]. Similar observations are reported by inhibiting eukaryotic initiation factors, 4E/cap and poly(A) tail function using artificial miRNA (CXCR4) [31]. Much evidence supports the idea that miRNAs destabilize target mRNAs through deadenylation and subsequent decapping followed by 5'to3'exonucleolytic digestion. Lin-4, the original miRNA in C. elegans, is initially shown to cause inhibition of translation of lin-14.which is important for postembryonic development[8]. Either no reduction in mRNA levels or a shift in polysomes are observed which lead to the conclusion that miRNAs inhibit mRNA translation at the elongation step [8].
Recently, miRNAs are also shown to activate protein synthesis [17, 32, 33] . AU-rich elements (AREs) and microRNA target sites are conserved sequences in 3′UTRs of mRNA. During the cell cycle arrest, the AREs in tumor necrosis factor–a, mRNA is transformed into a translation activation signal, recruiting many factors associated with micro-ribonucleoproteins (miRNPs) (Figure 2). Vasudevan et al have shown that human microRNA miR369-3 directs the association of these proteins with the AREs to activate translation.[34].
MicroRNA in cardiovascular research
The development of various cardiovascular disease models is a complex process involving different cell types including fibroblasts, cardimyocytes, endothelial cells, smooth muscle cells, and many others. The patterns of miRNA expression are different in those cell types and thus can be explained based on different models.
Cardiac fibrosis, where cardiac fibroblasts take the lead role in the development of many diseases like cardiomyopathy, hypertension, myocardial infraction, chronic DOX induced cardiomyopathy, etc and regulate the cardiac extracellular matrix components [35-37]. Initial studies demonstrates the dysfunction of miRNA metabolism using a conditional deletion mutant (of dicer) which lead to hypertrophy and ventricular fibrosis [38].In acute myocardial infarction model, dysregulation of a specific miRNA (miR-29) family is observed and targeting miR-29 leads to reduced collagen expression in fibroblasts [39].In addition to miR-29 dysregulation, increased expression of miR-21, miR-214 and miR-224 and reduced expression of miR-29b and miR-149 are found in myocardial infarction based on microarray analyses followed by Real-time PCR [39]. Similar studies with microarray and northern blot analyses lead to the discovery of miR-21 over-expression in failing heart and miR-21 observed to regulate ERK-MAP kinase pathways [40]. Later, miR-21 also regulate MMP2 in fibroblast in myocardial infraction model [41]. One of the key players in fibrosis, CTGF was regulated at post-translational level by miR-133 and miR-30 [42].
In Ischemic heart disease, miR-1 has been shown to be upregulated in human studies. Overexpression studies in rat correlate miR-1 expression with arrhythmogenesis, cardiac conduction disturbance and membrane potential abnormality [43]. Another miRNA (miR-133), encoded by the same loci of miR-1, induces myoblast proliferation in vitro and proliferate skeletal as well as cardiac muscle after overexpression in Xenopus embryos [44]. Thus miRNA can be used in understanding the development of the pathophysiological condition of heart disease as well as therapeutics.
Recently, comparative profile studies by microarray analyses between healthy patients and patients with coronary artery disease (CAD) lead to the discovery of many circulating miRNA in blood[45, 46]. Some miRNAs such as miR-126, miR-17, miR-92a, mir155 are reduced in CAD patients whereas miR-133 and miR-208 are increased in blood [45]. These studies can be used in the future as development miRNA biomarkers in cardiac disease models.
Resveratrol and French Paradox
In their seminal article in 1992 about the French paradox, Renaud and de Lorgeril presented evidence that dietary fat and blood cholesterol may not be the determining factors for mortality and morbidity due to heart disease[47]. The mortality due to coronary heart disease is only 78 per 100,1000 population in Toulouse, France and 105 in Lille, France, compared to 182 in Stanford, USA, 348 in Belfast, UK, and 380 in Glasgow, UK. Despite the same saturated fat intake of 15% of the total calories and similar serum cholesterol in other parts of Europe (Belfast 232, Glasgow 244 compared to Toulouse 230 and Lille 252) or even lower in USA (Stanford 209), the French had the lowest mortality due to myocardial infarction [47]. The authors noted that the countries having lowest mortality due to heart disease had one thing in common, the population of these countries had regular wine consumption, this suggests that wine provided cardioprotection.They described this phenomenon as French Paradox. Comparing the population of several countries, the authors concluded that the wine consumption inhibits platelet aggregation, which in turn lowers mortality due to ischemic heart disease [48].
It was not until 1991 when the incidence of French Paradox was televised by NBC's 60 minutes. French Paradox was attributed to red wine, which is routinely consumed by the French with their meals. Subsequent studies determined that wine, especially red wine is rich in certain flavonoids and antioxidants, which can neutralize the damaging free radicals that are continuously being generated by human body [49]. Within a short period of time, one of the polyphenolic compounds, resveratrol, present in red wine showed amazing results and the cardioprotective property of red wine was attributed to resveratrol [49].
Resveratrol is a trihydroxystilbene present in grape vines and skins. They protect the grapes from fungal infection [50]. The grape vines produce increasing amounts of resveratrol when they are subjected to stresses in their environment, such as dehydration, nutrient deprivation, and attacks by pathogenic organisms. These defensive molecules are called phytoalexins, a Greek words meaning plant and protector and resveratrol fulfils this definition. Resveratrol is also present in peanuts and certain berries. The main source of resveratrol is the dried roots of Polygonum cuspidatum that is used in traditional Japanese and Chinese medicine Kojo-kon from the time immomemorial to treat fungal diseases of heart, liver and blood vessels [51]. Resveratrol soon became the central issue of French Paradox, similar to wine. Resveratrol was found to possess heart health, anti-obesity and anti-aging properties [52-54]. However, whether resveratrol alone fulfils the definition of French Paradox is still under considerable debate.
Striking similarities exist between the cardioprotective properties of wine and resveratrol. Both can reduce blood pressure [52], increase HDL cholesterol and decrease LDL cholesterol [55, 56], possess anti-platelet and anti-thrombin activities (aspirin-like effects)[57, 58], exhibit insulin like effects and lower blood sugar [59, 60], reduce obesity [61, 62], and activate longevity genes [63, 64]. While overwhelming evidence exists supporting the effects of resveratrol on heart health [33-63, 64], the concentration of resveratrol necessary to achieve the positive effects is under considerable debate. The amount of wine necessary for maintaining a healthy heart is about two glasses of wine a day [65], which may contain about 3-6 mg of resveratrol depending on the source of the wine. American red wine only contains about 2-3 mg of resveratrol while wines from Spain, Italy and Germany may contain double the amount of resveratrol [66-68]. Several scientists published articles stating that one needs hundreds of bottles of red wine to obtain the amount of resveratrol necessary to maintain a healthy heart [69]. In fact, higher amounts of resveratrol could even be cytotoxic as resveratrol appears to behave like a hormetin [70, 71]. Similar to alcohol and wine, resveratrol also exhibits an inverted U-shaped or a J-shaped curve, indicating that like high doses of alcohol and wine, resveratrol might also exert harmful effects on the heart [70, 71]. Further research is necessary to resolve the problem of the correct amount of resveratrol necessary to achieve heart health.
One of the most important findings in recent years is that resveratrol induces autophagy [72]. This supports many previous reports that resveratrol combats heart disease by preconditioning, i.e., adapting the heart to stress [73]. Resveratrol at lower doses (0.1 to 1 μM in H9c2 cardiac myoblast cells and 2.5 mg/kg/day in rats) induces autophagy in the ischemic mycardium as evidenced by the formation of autophagosomes and its creditable markers, LC3-II and beclin-1 [74]. Autophagy is attenuated with the higher doses of resveratrol, which induce apoptotic cell death. The induction of autophagy with low doses of resveratrol is correlated with enhanced cell survival and decreased apoptosis [72]. The activation of mammalian target of rapamycin (mTOR) is differentially regulated with low doses of resveratrol i.e., the phosphorylation of mTOR at serine 2448 is inhibited whereas the phosphorylation of mTOR at serine 2481 is enhanced, which is attenuated with a higher dose of resveratrol [72]. Interestingly, even though resveratrol attenuates the activation of mTOR complex 1, low dose resveratrol significantly induces the expression of Rictor, a component of mTOR complex 2 leading to the activation of its downstream survival kinase Akt (Ser 473) [72]. Resveratrol-induced Rictor eventually binds to mTOR and Rictot siRNA and attenuates resveratrol-induced autophagy. Thus suggesting that at lower dose, resveratrol-mediated cell survival, at least in part, is mediated through the induction of autophagy[72].
The fact that resveratrol renders numerous health benefits from chemoprevention to cardioprotection makes it a suitable target for stem cell therapy. Recent studies have indicated that resveratrol can prolong stem cell survival by altering the intracellular redox environment of the heart thus resolving one of the major problems associated with cell therapy,. Resveratrol was introduced in two ways. The first was by feeding the animals with resveratrol for up to three weeks, this changed the intracellular redox environment and was followed by cell therapy [75]. The second was by pre-treating the stem cells with low concentration of resveratrol before the cell therapy [75]. In both protocols, resveratrol prolonged the life of the stem cells in the infracted heart as evidenced by active proliferation and differentiation, this suggests that resveratrol can regenerate the infracted myocardium.
microRNA expression profile in response to resveratrol
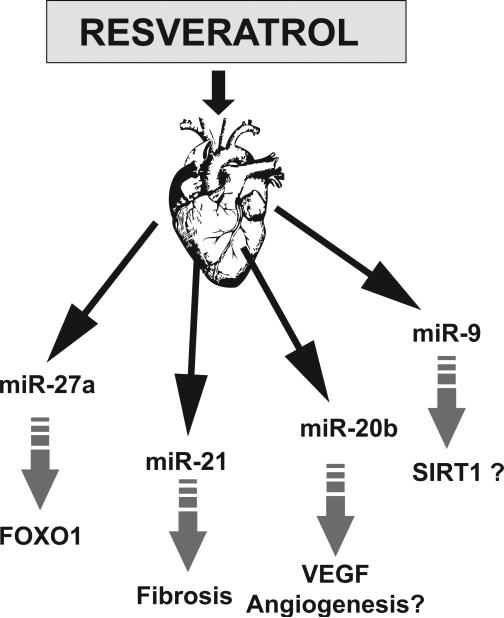
Differential expression of over 50 miRNAs was observed in ischemic reperfused (IR) myocardium, some of them were previously implicated in cardiac remodeling. The target genes for the differentially expressed miRNA include genes of various molecular functions such as metal ion modulation, transcription factors, which may play key role in reducing ischemic reperfusion I/R injury (Mukhopadhyay and Das, unpublished data). IR samples pretreated with resveratrol or its commercial formulation longevinex both reversed the up or down regulation in IR samples in the opposite direction in more than 50% of differentially expressed miRNAs. Resveratrol modulation of miRNAs in IR includes miR-21, miR-20b, mir-27a, miR-9 and many more (Mukhopadhyay and Das, unpublished data). miR-21 was shown to regulate the ERK-MAP kinase signalling pathway in cardiac fibroblasts regulating cardiac structure and function [40]. VEGF was reported to be modulated by mir20b through HIF1α whereas FOXO1 is regulated by mir-27a in cancer cells [76, 77]. SIRT1 were observed to be regulated by miR-9 in stem cells [78]. Thus, the resveratrol treatment leads to a unique signature of miRNA expression. Some of the miRNA were demonstrated previously in various cardiac remodeling like fibrosis. Few differentially expressed miRNA target key transcription factors like FOXO1, SIRT1 based on bioinformatics analyses and observations found in different cell types (Figure 3). Future studies will be based on the mechanistic action and stability of miRNA as described before. Further detailed in vivo and in vitro studies like targeting those miRNA followed by loss/gain of function will able to explore the complex mechanism underlying the cardioprotection of resveratrol. Targeting miRNA responsible for cardiac damage may lead to exploration of new therapeutic potential.
Figure 3. Role of miRNA in cardioprotection.
Preconditioning of heart is mediated by resveratrol as significant miRNAs are up or down when treated with resveratrol for 3 weeks in rats. Few miRNAs were shown as key regulators in cardioprotection by various pathways.
Acknowledgements
This study was supported in part by NIH HL 34360, HL 22559 and HL 33889 (DKD) and Intramural Research Program of the NIAAA/NIH.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Venter JC, et al. The sequence of the human genome. Science. 2001;291:1304–1351. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 2.Siomi H, Siomi MC. On the road to reading the RNA-interference code. Nature. 2009;457:396–404. doi: 10.1038/nature07754. [DOI] [PubMed] [Google Scholar]
- 3.Katiyar-Agarwal S, et al. A pathogen-inducible endogenous siRNA in plant immunity. Proc Natl Acad Sci U S A. 2006;103:18002–18007. doi: 10.1073/pnas.0608258103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Q, Paroo Z. Biochemical principles of small RNA pathways. Annu Rev Biochem. 2010;79:295–319. doi: 10.1146/annurev.biochem.052208.151733. [DOI] [PubMed] [Google Scholar]
- 5.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 6.Beisel CL, Storz G. Base pairing small RNAs and their roles in global regulatory networks. FEMS Microbiol Rev. 2010;34:866–882. doi: 10.1111/j.1574-6976.2010.00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 8.Olsen PH, Ambros V. The lin-4 regulatory RNA controls developmental timing in Caenorhabditis elegans by blocking LIN-14 protein synthesis after the initiation of translation. Dev Biol. 1999;216:671–680. doi: 10.1006/dbio.1999.9523. [DOI] [PubMed] [Google Scholar]
- 9.Delmas D, et al. Resveratrol as a chemopreventive agent: a promising molecule for fighting cancer. Curr Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 10.Goswami SK, Das DK. Resveratrol and chemoprevention. Cancer Lett. 2009;284:1–6. doi: 10.1016/j.canlet.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 11.de Almeida LM, et al. Resveratrol protects against oxidative injury induced by H2O2 in acute hippocampal slice preparations from Wistar rats. Arch Biochem Biophys. 2008;480:27–32. doi: 10.1016/j.abb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Quincozes-Santos A, et al. The janus face of resveratrol in astroglial cells. Neurotox Res. 2009;16:30–41. doi: 10.1007/s12640-009-9042-0. [DOI] [PubMed] [Google Scholar]
- 13.Das DK, Maulik N. Resveratrol in cardioprotection: a therapeutic promise of alternative medicine. Mol Interv. 2006;6:36–47. doi: 10.1124/mi.6.1.7. [DOI] [PubMed] [Google Scholar]
- 14.Das S, Das DK. Resveratrol: a therapeutic promise for cardiovascular diseases. Recent Pat Cardiovasc Drug Discov. 2007;2:133–138. doi: 10.2174/157489007780832560. [DOI] [PubMed] [Google Scholar]
- 15.Hammond SM. Dicing and slicing: the core machinery of the RNA interference pathway. FEBS Lett. 2005;579:5822–5829. doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- 16.Rigoutsos I. New tricks for animal microRNAS: targeting of amino acid coding regions at conserved and nonconserved sites. Cancer Res. 2009;69:3245–3248. doi: 10.1158/0008-5472.CAN-09-0352. [DOI] [PubMed] [Google Scholar]
- 17.Orom UA, Nielsen FC, Lund AH. MicroRNA-10a binds the 5'UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell. 2008;30:460–471. doi: 10.1016/j.molcel.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Tolia NH, Joshua-Tor L. Slicer and the argonautes. Nat Chem Biol. 2007;3:36–43. doi: 10.1038/nchembio848. [DOI] [PubMed] [Google Scholar]
- 19.Kai ZS, Pasquinelli AE. MicroRNA assassins: factors that regulate the disappearance of miRNAs. Nat Struct Mol Biol. 2010;17:5–10. doi: 10.1038/nsmb.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 21.Bail S, et al. Differential regulation of microRNA stability. RNA. 2010;16:1032–1039. doi: 10.1261/rna.1851510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated posttranscriptional regulation in animal cells. Curr Opin Cell Biol. 2009;21:452–460. doi: 10.1016/j.ceb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of posttranscriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 24.Bagga S, et al. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Giraldez AJ, et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 26.Korner CG, Wahle E. Poly(A) tail shortening by a mammalian poly(A)-specific 3'-exoribonuclease. Journal of Biological Chemistry. 1997;272:10448–10456. doi: 10.1074/jbc.272.16.10448. [DOI] [PubMed] [Google Scholar]
- 27.Brown CE, Sachs AB. Poly(A) tail length control in Saccharomyces cerevisiae occurs by message-specific deadenylation. Molecular and Cellular Biology. 1998;18:6548–6559. doi: 10.1128/mcb.18.11.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tollervey D. Genetic and biochemical analyses of yeast RNase MRP. Mol Biol Rep. 1996;22:75–79. doi: 10.1007/BF00988709. [DOI] [PubMed] [Google Scholar]
- 29.Collart MA. Global control of gene expression in yeast by the Ccr4-Not complex. Gene. 2003;313:1–16. doi: 10.1016/s0378-1119(03)00672-3. [DOI] [PubMed] [Google Scholar]
- 30.Pillai RS, et al. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 31.Humphreys DT, et al. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc Natl Acad Sci U S A. 2005;102:16961–16966. doi: 10.1073/pnas.0506482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchan JR, Parker R. Molecular biology. The two faces of miRNA. Science. 2007;318:1877–1878. doi: 10.1126/science.1152623. [DOI] [PubMed] [Google Scholar]
- 33.Vasudevan S, Steitz JA. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 2007;128:1105–1118. doi: 10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 35.Mukhopadhyay P, et al. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010;85:773–784. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raman SV. The hypertensive heart. An integrated understanding informed by imaging. J Am Coll Cardiol. 2010;55:91–96. doi: 10.1016/j.jacc.2009.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jellis C, et al. Assessment of nonischemic myocardial fibrosis. J Am Coll Cardiol. 2010;56:89–97. doi: 10.1016/j.jacc.2010.02.047. [DOI] [PubMed] [Google Scholar]
- 38.da Costa Martins PA, et al. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation. 2008;118:1567–1576. doi: 10.1161/CIRCULATIONAHA.108.769984. [DOI] [PubMed] [Google Scholar]
- 39.van Rooij E, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thum T, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 41.Roy S, et al. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duisters RF, et al. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–178. doi: 10.1161/CIRCRESAHA.108.182535. 176p following 178. [DOI] [PubMed] [Google Scholar]
- 43.Yang B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 44.Chen JF, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fichtlscherer S, et al. Circulating MicroRNAs in Patients With Coronary Artery Disease. Circ Res. 2010 doi: 10.1161/CIRCRESAHA.109.215566. [DOI] [PubMed] [Google Scholar]
- 46.Kumarswamy R, Anker SD, Thum T. MicroRNAs as circulating biomarkers for heart failure: questions about MiR-423-5p. Circ Res. 2010;106:e8. doi: 10.1161/CIRCRESAHA.110.220616. author reply e9. [DOI] [PubMed] [Google Scholar]
- 47.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 48.Renaud S, de Lorgeril M. The French paradox: dietary factors and cigarette smoking-related health risks. Ann N Y Acad Sci. 1993;686:299–309. doi: 10.1111/j.1749-6632.1993.tb39191.x. [DOI] [PubMed] [Google Scholar]
- 49.Troup GJ, et al. Free radicals in red wine, but not in white? Free Radic Res. 1994;20:63–68. doi: 10.3109/10715769409145626. [DOI] [PubMed] [Google Scholar]
- 50.Farina A, Ferranti C, Marra C. An improved synthesis of resveratrol. Nat Prod Res. 2006;20:247–252. doi: 10.1080/14786410500059532. [DOI] [PubMed] [Google Scholar]
- 51.Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005;49:472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 52.Wallerath T, et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 53.Das DK, et al. Cardioprotection of red wine: role of polyphenolic antioxidants. Drugs Exp Clin Res. 1999;25:115–120. [PubMed] [Google Scholar]
- 54.de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res. 2005;49:405–430. doi: 10.1002/mnfr.200500022. [DOI] [PubMed] [Google Scholar]
- 55.Fan E, et al. Beneficial effects of resveratrol on atherosclerosis. J Med Food. 2008;11:610–614. doi: 10.1089/jmf.2007.0091. [DOI] [PubMed] [Google Scholar]
- 56.Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341:1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 57.Bertelli AA, et al. Antiplatelet activity of synthetic and natural resveratrol in red wine. Int J Tissue React. 1995;17:1–3. [PubMed] [Google Scholar]
- 58.Stef G, et al. Resveratrol inhibits aggregation of platelets from high-risk cardiac patients with aspirin resistance. J Cardiovasc Pharmacol. 2006;48:1–5. doi: 10.1097/01.fjc.0000238592.67191.ab. [DOI] [PubMed] [Google Scholar]
- 59.Szkudelska K, Szkudelski T. Resveratrol, obesity and diabetes. Eur J Pharmacol. 2010;635:1–8. doi: 10.1016/j.ejphar.2010.02.054. [DOI] [PubMed] [Google Scholar]
- 60.Chen WP, et al. Resveratrol enhances insulin secretion by blocking K(ATP) and K(V) channels of beta cells. Eur J Pharmacol. 2007;568:269–277. doi: 10.1016/j.ejphar.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 61.Bertelli AA, Das DK. Grapes, wines, resveratrol, and heart health. J Cardiovasc Pharmacol. 2009;54:468–476. doi: 10.1097/FJC.0b013e3181bfaff3. [DOI] [PubMed] [Google Scholar]
- 62.Pfluger PT, et al. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das DK, Mukherjee S, Ray D. Resveratrol and red wine, healthy heart and longevity. Heart Fail Rev. 2010 doi: 10.1007/s10741-010-9163-9. [DOI] [PubMed] [Google Scholar]
- 64.Mukherjee S, et al. Expression of the longevity proteins by both red and white wines and their cardioprotective components, resveratrol, tyrosol, and hydroxytyrosol. Free Radic Biol Med. 2009;46:573–578. doi: 10.1016/j.freeradbiomed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 65.Goldberg IJ, et al. AHA Science Advisory: Wine and your heart: a science advisory for healthcare professionals from the Nutrition Committee, Council on Epidemiology and Prevention, and Council on Cardiovascular Nursing of the American Heart Association. Circulation. 2001;103:472–475. doi: 10.1161/01.cir.103.3.472. [DOI] [PubMed] [Google Scholar]
- 66.Stervbo U, Vang O, Bonnesen C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. 2007;101:449–457. [Google Scholar]
- 67.Lamuelaraventos RM, et al. Resveratrol and Piceid Levels in Wine Production and in Finished Wines. Abstr Pap Am Chem S. 1995;210:10–Agfd. [Google Scholar]
- 68.Goldberg DM, et al. A Global Survey of Trans-Resveratrol Concentrations in Commercial Wines. Am J Enol Viticult. 1995;46:159–165. [Google Scholar]
- 69.E W. Wine ingredient resveratrol as anti-aging pill? Not just yet. USA TODAY. 2006 11/29/2006. [Google Scholar]
- 70.Mukherjee S, L. I, Das DK. Hormetic response of resveratrol against cardioprotection. J Exp Clin Cardiology. In Press. [Google Scholar]
- 71.Juhaz B, M. S, Juhasz A, Kertesz A, Varga B, Das DK. Hormetic response of resveratrol aginst cardioprotection. Exp Clin Cardiol. 2010 In Press. [PMC free article] [PubMed] [Google Scholar]
- 72.Gurusamy N, et al. Cardioprotection by resveratrol: a novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc Res. 2010;86:103–112. doi: 10.1093/cvr/cvp384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Das S, et al. Pharmacological preconditioning with resveratrol: role of CREB-dependent Bcl-2 signaling via adenosine A3 receptor activation. Am J Physiol Heart Circ Physiol. 2005;288:H328–335. doi: 10.1152/ajpheart.00453.2004. [DOI] [PubMed] [Google Scholar]
- 74.Lekli I, R. D, Mukherjee S, Gurusamy N, Ahsan MK, Juhasz B, Bak I, Tosaki A, Gherghiceanu M, Popescu L, Das DK. Coordinated autophagy with resveratrol and gamma tocotrienol confers synergetic cardioprotection. J Cellular Molecular Medicine. 2010 doi: 10.1111/j.1582-4934.2009.00921.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gurusamy N, R. D, Lekli I, Kim Y-H, Das DK. Regeneration of infracted myocardium by nutritionally modified cardiac stem cells. J Cell Mol Medicine. 2010 doi: 10.1111/j.1582-4934.2010.01140.x. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cascio S, et al. miR-20b modulates VEGF expression by targeting HIF-1 alpha and STAT3 in MCF-7 breast cancer cells. J Cell Physiol. 2010;224:242–249. doi: 10.1002/jcp.22126. [DOI] [PubMed] [Google Scholar]
- 77.Guttilla IK, White BA. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J Biol Chem. 2009;284:23204–23216. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saunders LR, et al. miRNAs regulate SIRT1 expression during mouse embryonic stem cell differentiation and in adult mouse tissues. Aging (Albany NY) 2010 doi: 10.18632/aging.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]