Abstract
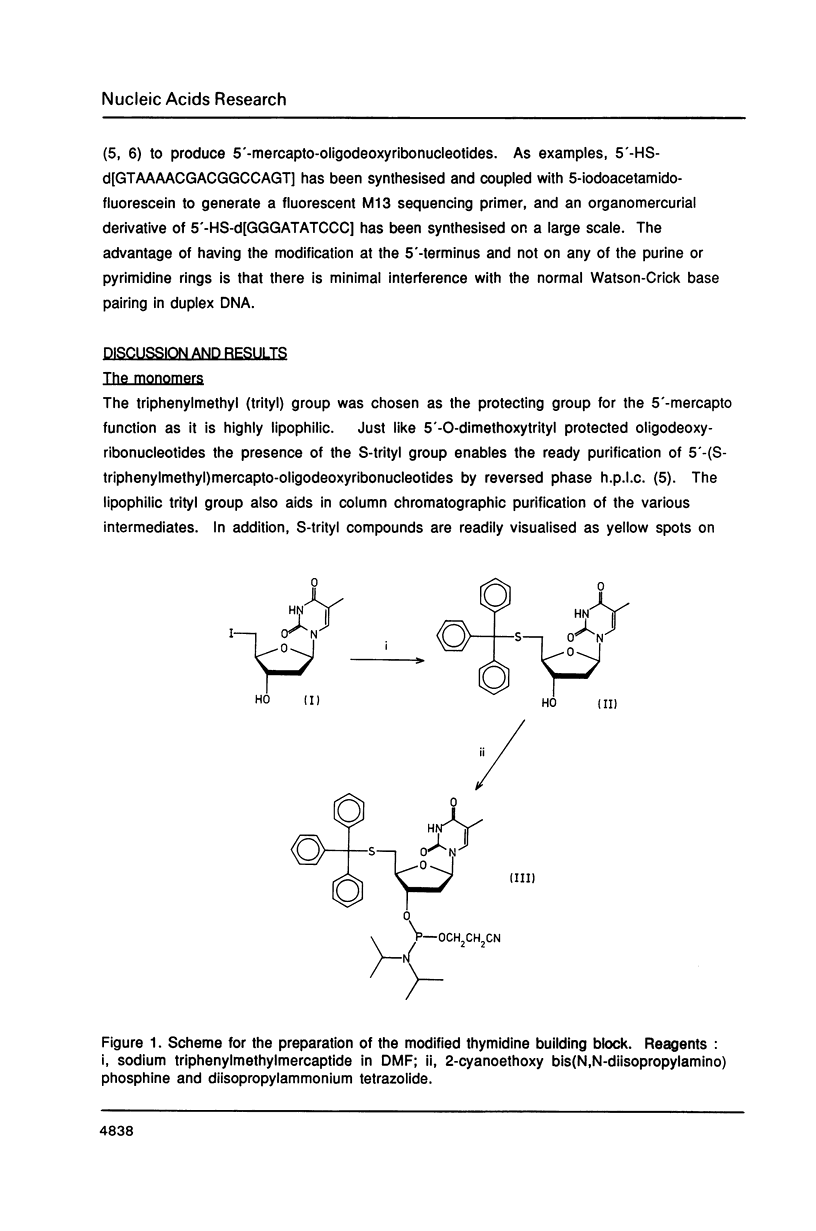
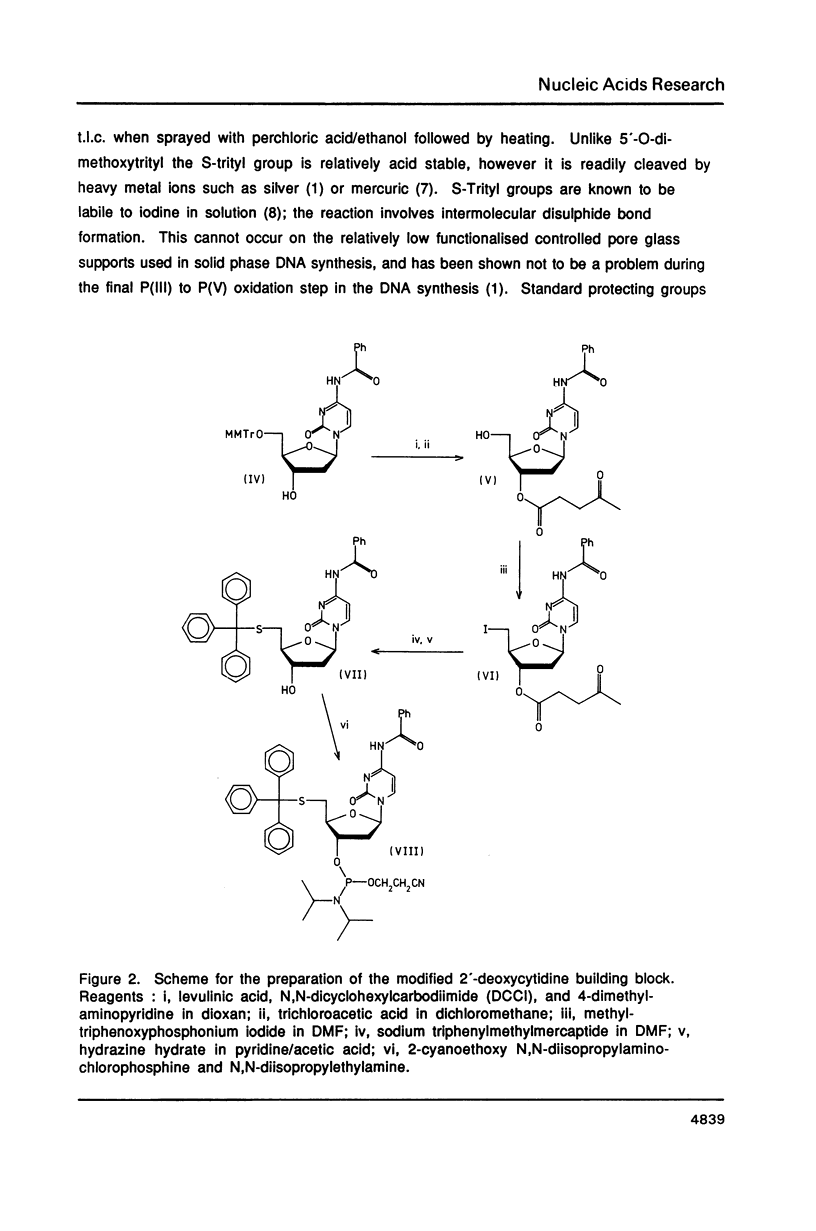
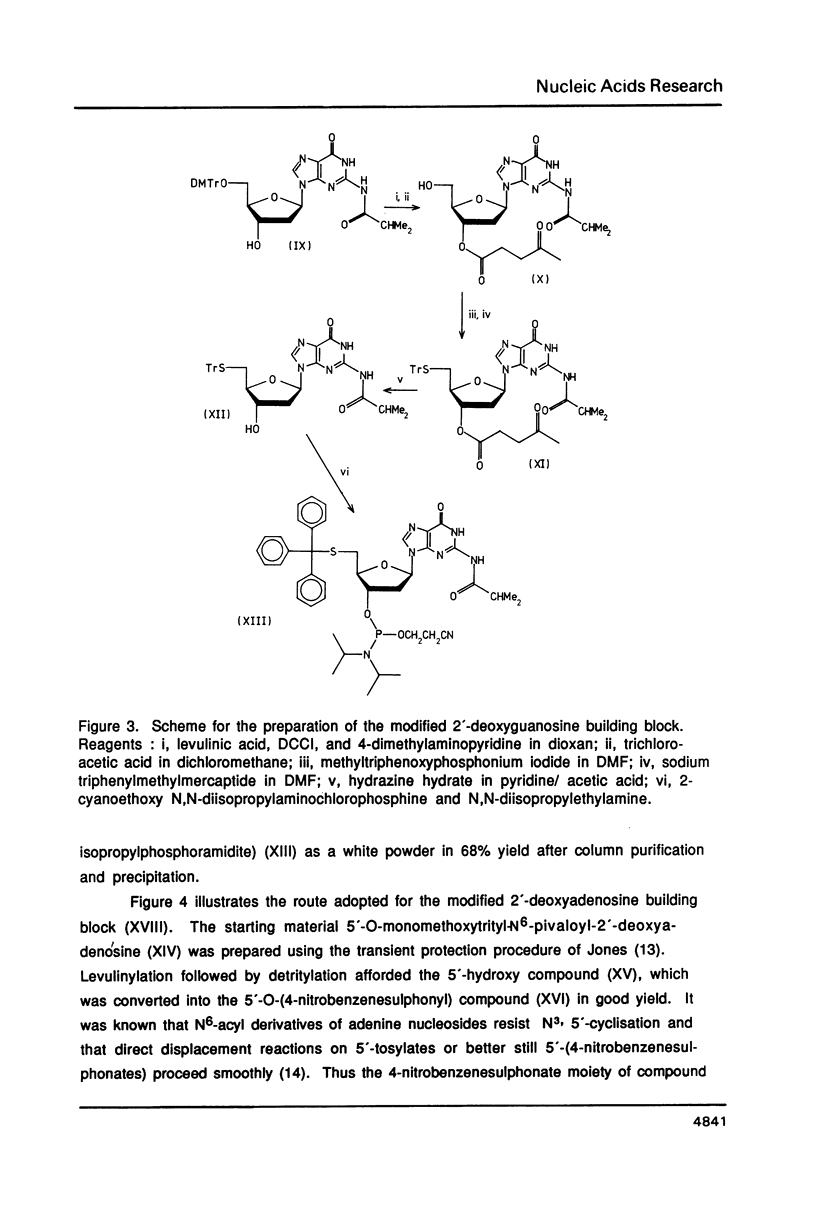
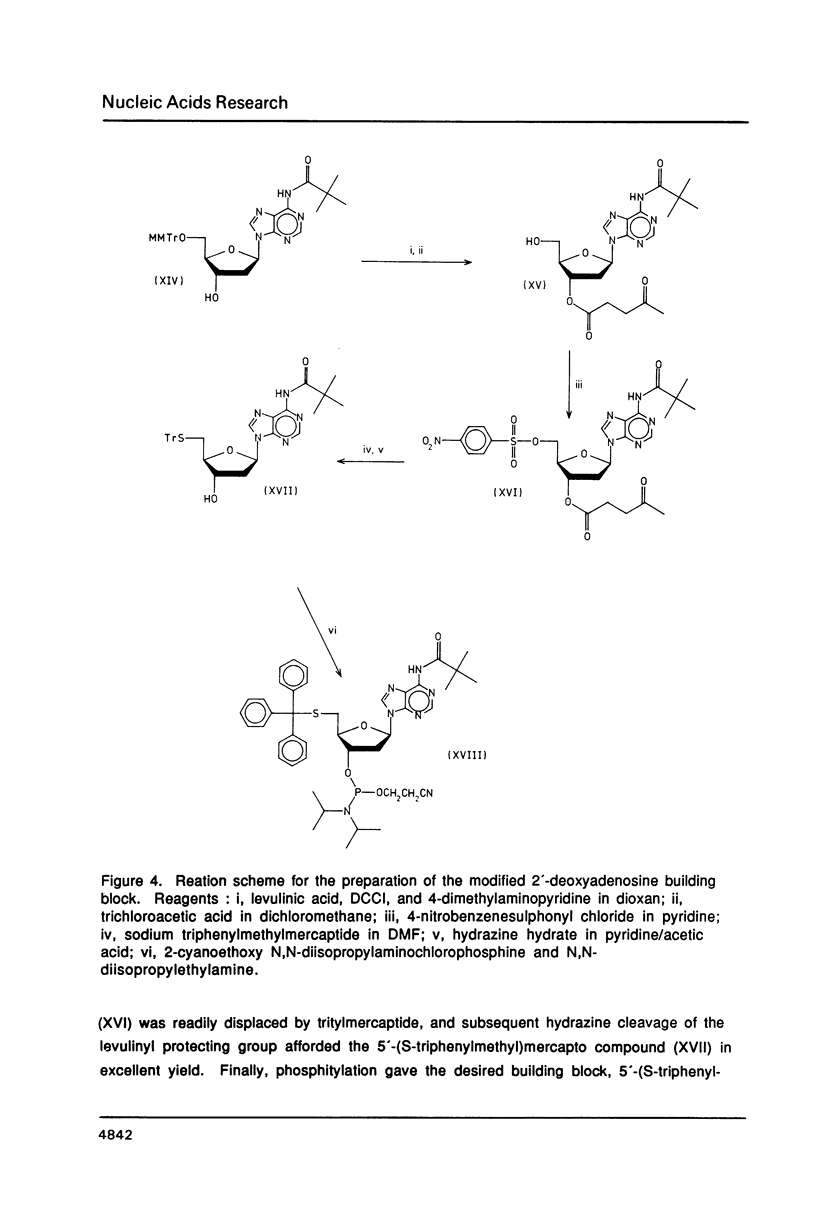
The syntheses of the four novel, base protected 5'-(S-triphenylmethyl)mercapto-2',5'-dideoxyribonucleoside-3 '-O-(2-cyanoethyl N,N-diisopropylphosphoramidites) are described. These compounds have been used to prepare 5'-(S-triphenylmethyl) mercapto-oligodeoxyribonucleotides, which are readily purified by reversed phase h.p.l.c., owing to the highly lipophilic trityl group. After cleavage of the S-trityl group by silver or mercuric ions, the free thiol moiety can be coupled to a wide variety of reagents, generating very useful probes. Fluorescent labelled 5'-mercapto-oligodeoxyribonucleotides are being used for automated DNA sequencing without radioactivity, and heavy metal labelled 5'-mercapto-oligonucleotides will be used in X-ray crystallography.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal S., Christodoulou C., Gait M. J. Efficient methods for attaching non-radioactive labels to the 5' ends of synthetic oligodeoxyribonucleotides. Nucleic Acids Res. 1986 Aug 11;14(15):6227–6245. doi: 10.1093/nar/14.15.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge W., Sproat B. S., Stegemann J., Schwager C. A non-radioactive automated method for DNA sequence determination. J Biochem Biophys Methods. 1986 Dec;13(6):315–323. doi: 10.1016/0165-022x(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Barone A. D., Tang J. Y., Caruthers M. H. In situ activation of bis-dialkylaminophosphines--a new method for synthesizing deoxyoligonucleotides on polymer supports. Nucleic Acids Res. 1984 May 25;12(10):4051–4061. doi: 10.1093/nar/12.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly B. A., Rider P. Chemical synthesis of oligonucleotides containing a free sulphydryl group and subsequent attachment of thiol specific probes. Nucleic Acids Res. 1985 Jun 25;13(12):4485–4502. doi: 10.1093/nar/13.12.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn W. Synthese 5'-substituierter Adenosinderivate. Chem Ber. 1965 Jun;98(6):1705–1708. doi: 10.1002/cber.19650980604. [DOI] [PubMed] [Google Scholar]
- Kamber B. Die Synthese von Insulinfragmenten mit intakter interchenarer Disulfidbrücke A20-B19. Helv Chim Acta. 1971;54(1):398–422. doi: 10.1002/hlca.19710540143. [DOI] [PubMed] [Google Scholar]
- Kuhn R., Jahn W. Vom Adenosin abgeleitete Thioäther und S-Oxide. Chem Ber. 1965 Jun;98(6):1699–1704. doi: 10.1002/cber.19650980603. [DOI] [PubMed] [Google Scholar]
- Michniewicz J. J., Bahl C. P., Itakura K., Katagiri N., Narang S. A. Fractionation of synthetic deoxyribopolynucleotides on silica gel thin-layer plates. J Chromatogr. 1973 Oct 10;85(1):159–161. doi: 10.1016/s0021-9673(01)91880-1. [DOI] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproat B. S., Brown D. M. A new linkage for solid phase synthesis of oligodeoxyribonucleotides. Nucleic Acids Res. 1985 Apr 25;13(8):2979–2987. doi: 10.1093/nar/13.8.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyden J. P., Moffatt J. G. Halo sugar nucleosides. I. Iodination of the primary hydroxyl groups of nucleosides with methyltriphenoxyphosphonium iodide. J Org Chem. 1970 Jul;35(7):2319–2326. doi: 10.1021/jo00832a047. [DOI] [PubMed] [Google Scholar]


