Abstract
Aim
Angelman syndrome is a neurogenetic disorder characterized by severe intellectual disability, absent speech, seizures, and outbursts of laughter. The aim of this study was to utilize diffusion tensor imaging (DTI) to examine alterations in white matter pathways in Angelman syndrome, with an emphasis on correlations with clinical severity.
Methods
DTI was used to examine the arcuate fasciculus (AF), uncinate fasciculus (UF), inferior longitudinal fasciculus (ILF), inferior fronto-occipital fasciculus (IFOF), and the corpus callosum (CC). We enrolled 14 children aged 8 to 17 years (mean age 10y 8mo; SD 2y 7mo) with Angelman syndrome (seven male; seven female) and 13 typically developing children, aged 8 to 17 years, for comparison (five male; eight female; mean age 12y; SD 2y 9mo). Individuals with Angelman syndrome were assessed using standardized measures of development, language, and behaviour.
Results
The children with Angelman syndrome exhibited lower fractional anisotropy and increased radial diffusivity values than the comparison group for the AF, UF, ILF, and CC (p<0.006 corrected for multiple comparisons). They also had lower fractional anisotropy values for the IFOF and higher radial diffusivity values for the left IFOF (p<0.006). Additionally, children with Angelman syndrome had significantly higher apparent diffusion coefficient values in the AF, CC, ILF, and the left IFOF (p<0.006). Significant correlations were noted between DTI parameters and some of the clinical assessment outcomes (e.g. language, socialization, cognition) for three of the temporal pathways (AF, UF, ILF; p<0.05).
Interpretation
Changes in DTI parameters in individuals with Angelman syndrome suggest decreased/delayed myelination, decreased axonal density or diameter, or aberrant axonal organization. Our findings suggest a generalized white matter alteration throughout the brain in those with Angelman syndrome; however, only the alterations in temporal white matter pathways were associated with language and cognitive and social functioning.
Angelman syndrome is a neurodevelopmental disorder with a known genetic basis that is characterized by severe intellectual disability, lack of speech, ataxia, seizures, hypermotoric behaviours, and frequent outbursts of laughter.1 It has been suggested that the speech/language deficit in Angelman syndrome cannot be accounted for solely by mental retardation* and that oral-motor dyspraxia and deficits in social interactions are quite characteristic of the syndrome.2
Expression of the UBE3A gene from the maternal chromosome is essential if order Angelman sydrome is to be prevented.3 There are four known molecular mechanisms of Angelman syndrome: (1) maternal de novo deletions of 15q11–q13 (70% of cases); (2) paternal uniparental disomy (3–5% of cases); (3) imprinting defects (3–5%); and (4) mutations in the UBE3A gene on the maternally derived chromosome 15 (5–10% of cases).4 A recent study in a mouse model of Angelman syndrome indicates that the maternal Ube3a (YFP) allele is preferentially expressed in neurons and in most brain regions and that dendritic spine development, including spine morphology, number, and length, is affected in cerebellar Purkinje cells and in pyramidal neurons in the hippocampus and cortex.5
The purpose of this study is to examine the relationships between clinical/behavioural profiles and white matter integrity in individuals with Angelman syndrome. One magnetic resonance imaging study demonstrated anomalies in the Sylvian fissure in some individuals with Angelman syndrome, and it was hypothesized that misrouting of long projection axons may be related to these anomalies and the language disorder in Angelman syndrome.6 Another more recent paper demonstrated that infants with Angelman syndrome exhibited myelination delay and white matter deficits.7 The connection between the Angelman syndrome clinical phenotype and alterations in white matter pathways has not yet been explored.
Diffusion tensor imaging (DTI) is a relatively new imaging technique that may be useful in extending knowledge about the consequences of genetic disorders, such as Angelman syndrome, to white matter in vivo by assessing cerebral white matter microstructure. Extracellular water diffusion is based on the characteristics of myelin sheaths and cell membranes of white matter tracts that influence the direction and speed of water molecule movement, whereby molecules tend to move faster in parallel rather than perpendicular to fibres. This characteristic, referred to as anisotropic diffusion, is commonly measured by fractional anisotropy and apparent diffusion coefficient (ADC). These indices reflect differences in fibre diameter, fibre packing, fibre directionality, and fibre myelination, which may enhance our understanding of white matter microstructure. Radial diffusivity is another metric that is useful in determining underlying white matter change as it denotes speed of diffusion perpendicular to the axon and increases with the absence of myelin or increase in extracellular space.
The present study focused on three temporal white matter pathways, namely the arcuate fasciculus (AF), the uncinate fasciculus (UF), and the inferior longitudinal fasciculus (ILF), which are known to be implicated in language and social functioning and, therefore, postulated to contribute to the Angelman syndrome phenotype. We also included the inferior fronto-occipital fasciculus (IFOF), which connects the frontal and occipital lobes via the temporal stem and is thought to be involved in semantic processing, and the largest white matter fasciculus in the brain, the corpus callosum (CC), as a comparison pathway hypothesized to be less affected in Angelman syndrome and less contributory to the disorder’s phenotype.
The AF is associated with the ability to repeat spoken language, understand words/sentences, and verbalize thought, and so is connected to both receptive and expressive language.8 The ILF connects temporal–occipital regions and has been implicated in translating information about objects, faces, and written words.8 The UF is associated with deficits of cognition, verbal/visual memory, and planning/executive functioning.9,10 These pathways have been insufficiently studied in populations of children with developmental disabilities, although past research has demonstrated that the AF is absent in some children with developmental delays.11 As there is some intergroup variability in functional levels across molecular subtypes in individuals with Angelman syndrome, we focused on studying deletion-positive children because the majority of individuals with Angelman syndrome have deletions.
METHOD
The deletion in the 15q11–q13 region in individuals with Angelman syndrome was confirmed by the results of genetic testing (methylation and fluorescence in situ hybridization testing). The deletion size was confirmed with microarray-based comparative genomic hybridization in all participants.
Participants
Fourteen deletion-positive individuals with Angelman syndrome between the ages of 8 and 17 years (mean age 10y 8mo; SD 2y 7mo; median age 10y) and 13 typically developing individuals of comparable age (mean age 12y; SD 2y 9mo; median age 12y) and sex distribution were enrolled in the study. Of the 14 individuals with Angelman syndrome, seven were male and seven were female. Of the individuals in the comparison group, five were male and eight were female. All of the comparison participants were right-handed – some participants with Angelman syndrome did not have clear hand dominance; those who did were also right-handed. The participants with Angelman syndrome were recruited through the assistance of the Angelman Syndrome Foundation and through local waiting lists of individuals who were interested in participating in studies related to Angelman syndrome. The comparison group was recruited through advertisements and word of mouth (i.e. via relatives of individuals with Angelman syndrome). The Institutional Review Board of Baylor College of Medicine approved the study, and parental consent was obtained for all participants.
Psychological measures
The comparison participants were screened (using the Wechsler Abbreviated Scales of Intelligence) to ensure that they were of normal intelligence (mean IQ 108; SD 8.28). Individuals with Angelman syndrome were given a comprehensive psychological battery that included the Bayley Scales of Infant Development – Third Edition12; the Vineland Adaptive Behaviour Scales – II13; and the Aberrant Behaviour Checklist (ABC) – Community Version,14 a behaviour rating scale that assesses a range of maladaptive behaviours in children, adolescents, and adults with mild to profound intellectual disability and which has been used in children with Angelman syndrome. All measures for individuals with Angelman syndrome were selected based on developmental (not chronological) age. This is because children with developmental disabilities should be assessed using instruments that are appropriate for their developmental age rather than their chronological age. When children score near the floor of a test, there is lack of variability in test scores and the standard scores overestimate true functional abilities. All children with Angelman syndrome completed all direct assessment measures. However, one caregiver did not complete the ABC.
Image acquisition and data processing
Imaging was performed on a Philips 3T Achieva scanner (Cleveland, OH, USA) at Texas Children’s Hospital.
Conventional magnetic resonance imaging
Several conventional sequences were used to examine any areas of abnormality in all participants. Sequences included (1) a coronal T2-weighted fluid-attenuated inversion recovery sequence (11 000ms repetition time [TR]; 120ms echo time [TE]; 2800ms time interval; 5.0mm slices; no sensitivity encoding [SENSE] factor; 230mm field of view [FOV]; acquired voxel size 0.9×1.25×5.0mm; reconstructed voxel size 0.45×0.45×5.0mm); (2) an axial fast field echo (847ms TR; 16ms TE; 4.0mm slices; 0.0mm gap; SENSE factor of 2; 230mm FOV; acquired voxel size 0.9×1.13×4.0mm; reconstructed voxel size 0.45×0.45×4.0mm); (3) a three-dimensional turbo field echo T1-weighted sequence (6.9ms TR; 3.1ms TE; 1.0mm slice thickness; 256mm FOV; acquired and reconstructed voxel size 1.0×1.0×1.0mm; SENSE factor of 2); and (4) a three-dimensional turbo spin echo T2-weighted sequence (5000ms TR; 80ms TE; 1.5mm slice thickness; 256mm FOV; acquired voxel size 1.0×1.0×1.0mm; reconstructed voxel size 1.0×1.0×1.5mm; SENSE factor of 2). Conventional imaging was unremarkable in all of the comparison participants. In individuals with Angelman syndrome, the only finding was a decrease in cerebellar volume in one participant and a mildly foreshortened CC in five participants.
DTI acquisition
Transverse multislice spin echo, single-shot, and echo planar imaging sequences were used (6318.0ms TR; 51ms TE; 2.0mm slices; 0mm gap). A 224mm FOV was used with a measured voxel size of 2.0×2.0×2.0mm, a reconstructed voxel size of 1.75×1.75×2.0mm, and a SENSE factor of 2. Diffusion was measured along 30 directions (number of b-values=2; low b-value=0; high b-value=1000s/mm2). To improve the signal–noise ratio, two acquisitions were taken and averaged. Each acquisition took approximately 4 minutes 50 seconds, and 70 slices were acquired.
Data analysis
Based upon previously published DTI quantitative tractographic methods using Philips Research Independent Research Environment (PRIDE) software v4.0 (Philips; Cleveland, OH),15,16 we calculated the fractional anisotropy, ADC, and radial diffusivity in the right and left AF, right and left UF, right and left ILF, right and left IFOF, and the total CC, using multiple regions of interest applied in previously published protocols.15–18 Although measurements were taken of the genu, splenium, and the total CC, only the total CC is included here owing to the limited statistical power as a result of the small sample size. To examine interrater agreement, each case was measured twice; intraclass correlation coefficients13 exceeded 0.98 for all DTI indices. Interrater agreement was also assessed by measurement of each structure by two different raters in three cases in both groups; intraclass correlation coefficients again exceeded 0.98.
One-way analysis of variance (ANOVA) was performed to assess group differences (between participants with Angelman syndrome and the comparison group) in age; p<0.05 was used as a threshold for significance. For DTI measures, group differences were assessed using analysis of covariance (ANCOVA) with age as a covariate because of the effect of age on fractional anisotropy, ADC, and radial diffusivity. The Bonferroni correction to alpha was used to correct for multiple comparisons as it was the most conservative approach (p=0.006). We also calculated Cohen’s d effect size in order to evaluate the importance of these findings (see Table I for ranges for medium and large effect sizes). For individuals with Angelman syndrome, the DTI measures were correlated with scores from psychological measures. To control for age in these analyses, partial correlation coefficients were determined and a threshold of p<0.05 was used to determine significance. The Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA) was used to analyse these data.
Table I.
Group comparisons of anisotropy and diffusivity
| Angelman syndrome | Comparison group | ANCOVA (age as covariate) | |||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | p-value (uncorrected) | Cohen’s d | |
| Fractional anisotropy | |||||||
| Left AF | 0.37 (n=10) | 0.066 | 0.43 (n=12) | 0.025 | 9.045 | 0.007a | 1.20 |
| Right AF | 0.32 (n=13) | 0.053 | 0.41 | 0.053 | 16.94 | <0.001a | 1.70 |
| Left UF | 0.36 | 0.023 | 0.39 | 0.024 | 12.43 | 0.002a | 1.27 |
| Right UF | 0.34 | 0.031 | 0.38 | 0.024 | 11.49 | 0.002a | 1.43 |
| Left ILF | 0.43 | 0.023 | 0.40 | 0.029 | 7.491 | 0.011 | 1.14 |
| Right ILF | 0.38 | 0.019 | 0.41 | 0.022 | 14.071 | 0.001a | 1.48 |
| Right IFOF | 0.37 | 0.019 | 0.43 | 0.036 | 7.46 | 0.012 | 1.14 |
| Left IFOF | 0.40 | 0.023 | 0.45 | 0.018 | 30.00 | <0.001a | 2.30 |
| CC | 0.45 | 0.016 | 0.48 | 0.015 | 19.97 | <0.001a | 1.88 |
| Radial diffusivity | |||||||
| Left AF | 0.61 (n=10) | 0.031 | 0.55 (n=12) | 0.016 | 30.94 | <0.001a | 2.42 |
| Right AF | 0.66 (n=13) | 0.032 | 0.58 | 0.039 | 29.41 | <0.001a | 2.23 |
| Left UF | 0.63 | 0.026 | 0.60 | 0.031 | 7.475 | 0.012 | 1.05 |
| Right UF | 0.65 | 0.032 | 0.62 | 0.022 | 13.39 | 0.001a | 1.08 |
| Left ILF | 0.62 | 0.038 | 0.59 | 0.046 | 2.95 | 0.099 | 0.71 |
| Right ILF | 0.65 | 0.037 | 0.61 | 0.029 | 13.59 | 0.001a | 1.21 |
| Right IFOF | 0.62 | 0.021 | 0.61 | 0.043 | 0.36 | 0.553 | 0.31 |
| Left IFOF | 0.61 | 0.023 | 0.56 | 0.019 | 22.29 | <0.001a | 1.95 |
| CC | 0.63 | 0.031 | 0.58 | 0.017 | 19.79 | <0.001a | 2.00 |
| ADC | |||||||
| Left AF | 0.76 (n=10) | 0.017 | 0.73 (n=12) | 0.020 | 11.83 | 0.003a | 1.58 |
| Right AF | 0.81 (n=13) | 0.023 | 0.76 | 0.024 | 22.85 | 0.001a | 2.14 |
| Left UF | 0.79 | 0.028 | 0.78 | 0.028 | 2.437 | 0.132 | 0.36 |
| Right UF | 0.81 | 0.027 | 0.79 | 0.017 | 6.012 | 0.022 | 0.89 |
| Left ILF | 0.81 | 0.038 | 0.79 | 0.048 | 1.19 | 0.286 | 0.46 |
| Right ILF | 0.84 | 0.038 | 0.80 | 0.023 | 9.679 | 0.005a | 1.27 |
| Right IFOF | 0.81 | 0.022 | 0.82 | 0.035 | 0.427 | 0.520 | 0.22 |
| Left IFOF | 0.79 | 0.020 | 0.77 | 0.020 | 6.76 | 0.017 | 1.03 |
| CC | 0.88 | 0.032 | 0.84 | 0.027 | 9.28 | 0.006a | 1.34 |
Effect sizes are reported for Cohen’s d, where d 0.80 indicates a large effect size and d=0.50–0.79 indicates a moderate effect size.
Statistically significant at the threshold uncorrected p<0.008, determined by the Bonferroni correction for multiple comparisons. Least square means are provided adjusted for age as the covariate. ADC, apparent diffusion coefficient; AF, arcuate fasciculus; CC, corpus callosum; ILF, inferior longitudinal fasciculus; IFOF, inferior fronto-occipital fasciculus; UF, uncinate fasciculus.
RESULTS
Analysis of fractional anisotropy, ADC, and radial diffusivity
The mean values of fractional anisotropy, ADC, and radial diffusivity for the AF, UF, ILF, IFOF, and CC are shown in Table I. Because age was used as a covariate, the estimated marginal means are shown. Uncorrected p-values are depicted; however, significance is noted only for those p-values that were significant when using the Bonferroni correction for multiple comparisons for each DTI parameter (p≤0.006). The left AF could not be traced in four individuals with Angelman syndrome and in one of the comparison participants. This was unrelated to the quality of data acquisition or the difficulty in the identification of designated landmarks that were used for the placement of regions of interest; rather, tractography simply did not delineate the tract, even after placement of the regions of interest on multiple alternate slices and expansion of the area of the region of interest that was employed. We note that this occurred more frequently in younger children than in older children. These individuals were excluded from statistical analyses involving the AF. Additionally, the right AF could not be traced in one of the participants with Angelman syndrome (the left AF also could not be traced in this same individual). Figure 1 depicts the differences in tractography in the AF between an individual with Angelman syndrome and an age- and sex-matched comparison individual.
Figure 1.
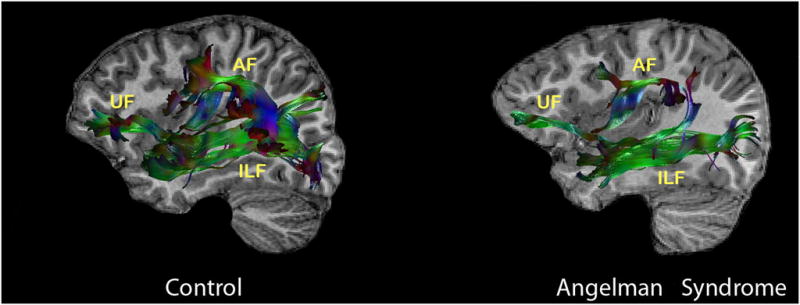
Diffusion tensor imaging tractography of the left uncinate fasciculus (UF), left arcuate fasciculus (AF), and left inferior longitudinal fasciculus (ALF) overlaid on the individual’s sagittal T1-weighted image in an age- and sex-matched comparison child versus a child with Angelman syndrome demonstrating abnormality in pattern or paucity of ‘streamlines’ generated by tractography that reflect the presumed fasciculi. Green colour indicates fibres coursing in an anterior to posterior direction, red colour indicates fibres coursing in a right to left direction, and blue colour represents fibres coursing in a superior to inferior direction.
For the AF, participants with Angelman syndrome exhibited lower fractional anisotropy values than the comparison group (left: F=9.045; p<0.00; right: F=16.94; p<0.001), had a significantly higher ADC than the comparison group (left: F=11.83; p=0.003; right: F=22.85; p<0.001) with large effect sizes noted (see Table I), and had significantly higher radial diffusivity than the comparison group (left: F=30.94; p<0.001; right: F=29.41; p<0.001; Table I). For the UF, participants with Angelman syndrome had lower fractional anisotropy (left: F=12.43; p=0.002; right: F=11.49; p=0.002) and higher radial diffusivity than the comparison group in the right UF (F=13.39; p<9.001; see Fig. 1). Individuals with Angelman syndrome had lower fractional anisotropy for the right ILF (F=14.07; p=0.001; see Table I and Fig. 1), higher ADC for the right ILF (F=13.59; p<0.001), and higher radial diffusivity for the right ILF (F=13.59; p<0.001; see Table I) than the comparison group. Participants with Angelman syndrome exhibited lower fractional anisotropy than the comparison group only for the left IFOF at the threshold for statistical significance (left: F=30.00; p<0.001; right: F=7.46; p=0.012) and exhibited higher radial diffusivity only for the left IFOF (left: F=22.29; p<0.001; right: F=0.36; p=NS). For the CC, individuals with Angelman syndrome had lower fractional anisotropy (F=19.97; p<0.001), higher ADC (F=9.28; p=0.006), and higher radial diffusivity (F=19.79; p<0.001) than the comparison group.
Correlations with clinical variables
Correlations with AF
The results of partial correlations revealed that decreased fractional anisotropy in the left AF was associated with lower receptive and expressive language (see Table II). Decreased fractional anisotropy in the left AF was also associated with increased impairment in socialization and play skills and a higher degree of lethargy/social withdrawal. No significant clinical correlations were noted for fractional anisotropy in the right AF. Increased radial diffusivity in the left AF was associated with lower cognition, receptive language, expressive language (see Fig. 2), greater impairments in social affect, and higher rates of lethargy/social withdrawal (see Table II).
Table II.
Summary of significant partial correlations between diffusion tensor imaging (DTI) variables and clinical parameters (controlling for age)
| DTI measures | Clinical measures | r-coefficient | p-value | n |
|---|---|---|---|---|
| Left AF fractional anisotropy | Receptive language | 0.66 | 0.05 | 10 |
| Expressive language | 0.63 | 0.05 | 10 | |
| ADOS socialization | −0.60 | 0.05 | 10 | |
| ABC lethargy/social withdrawal | −0.67 | 0.05 | 9 | |
| Left AF radial diffusivity | Cognition | −0.70 | 0.04 | 10 |
| Receptive language | −0.75 | 0.02 | 10 | |
| Expressive language | −0.71 | 0.03 | 10 | |
| ADOS socialization | 0.70 | 0.03 | 10 | |
| ABC lethargy/social withdrawal | 0.82 | 0.01 | 9 | |
| Left UF fractional anisotropy | Cognition | 0.66 | 0.01 | 14 |
| ADOS socialization | −0.56 | 0.04 | 14 | |
| ABC lethargy/social withdrawal | −0.63 | 0.03 | 13 | |
| Left UF radial diffusivity | ABC lethargy/social withdrawal | 0.65 | 0.02 | 13 |
| Right UF radial diffusivity | ABC lethargy/social withdrawal | 0.62 | 0.03 | 13 |
| Right UF ADC | ABC lethargy/social withdrawal | 0.74 | 0.006 | 13 |
| Right ILF fractional anisotropy | ABC irritability/agitation | −0.56 | 0.05 | 13 |
| Right ILF radial diffusivity | ABC irritability/agitation | 0.70 | 0.01 | 13 |
| Right ILF ADC | ABC irritability/agitation | 0.64 | 0.02 | 13 |
| Left ILF ADC | ABC irritability/agitation | 0.65 | 0.02 | 13 |
Measures of cognition, language, and motor skills are based upon the Bayley Scales of Infant and Toddler Development III (BSID-III), where high scores are associated with good performance. High scores on the Autism Diagnostic Observation Schedule (ADOS) are indicative of greater impairment. High scores on the Aberrant Behaviour Checklist (ABC) are also indicative of greater impairment. ADC, apparent diffusion coefficient; AF, arcuate fasciculus; ILF, inferior longitudinal fasciculus; UF, uncinate fasciculus.
Figure 2.
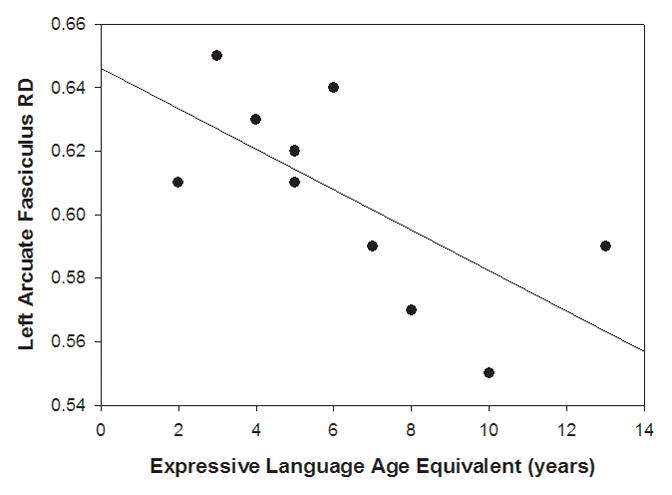
Scatterplot of individual children with Angelman syndrome showing the relation of mean radial diffusivity values of the left arcuate fasciculus to expressive language age equivalent on the Bayley Scales of Infant Development III (BSID-III).
Correlations with UF
Reduced fractional anisotropy in the left UF was associated with lower cognition (see Table II), higher lethargy/social withdrawal, and greater impairment in socialization skills. A higher ADC in the right UF and higher radial diffusivity in the left and right UF were associated with increased lethargy/social withdrawal.
Correlations with inferior longitudinal fasciculus
Lower fractional anisotropy, a higher ADC, and radial diffusivity in the right ILF were associated with increased irritability/agitation (see Table II).
Correlations with inferior fronto-occipital fasciculus and CC
There were no significant correlations at the p<0.05 level for any DTI parameters in IFOF or CC and clinical variables.
DISCUSSION
In this study, we observed alterations in white matter pathways throughout the brain in individuals with Angelman syndrome. Effect sizes were large for group differences on several DTI parameters. The combination of mechanisms that account for these changes in children with Angelman syndrome remains unknown, but generally decreased or delayed myelination, decreased axonal density or diameter, and abnormal axonal organization can cause decreased fractional anisotropy and increased ADC.19 The results from this study indicate generalized aberrant or decreased neural connectivity in Angelman syndrome. In spite of alteration in multiple pathways throughout the brain in Angelman syndrome, significant correlations with clinical severity were noted for three of the temporal pathways. Moreover, among lateralized fibre bundles only the AF showed differences for all DTI parameters (fractional anisotropy, radial diffusivity, ADC) in Angelman syndrome. Altogether our findings indicate a relatively greater anatomical and functional involvement in pathways related to the neurobehavioural phenotype of Angelman syndrome.
The significant imaging–clinical correlations in this study corresponded to the related functions of the specific pathway (i.e. language, cognition, social functioning). Reduced fractional anisotropy and increased radial diffusivity in the left AF in children with Angelman syndrome correlated with greater impairment in language and cognition. A recent study found that a large percentage of language sites were closely related to the AF, and the results also demonstrated the importance of the AF for language fluency.20 The AF has also been implicated in intelligence and reasoning tasks and is correlated with verbal intelligence, recall, and phonological processing. Thus, abnormalities in the left AF may account for the significant language and cognitive delays that are evident in Angelman syndrome. These same patterns were not noted for the right AF, perhaps indicating that performance on these tasks (i.e. expressive/receptive language, cognition) is lateralized to the left AF in most individuals with Angelman syndrome, as has been demonstrated in studies with other populations.17 Similar to the findings in our present study, past studies have demonstrated that the AF is absent in some children with developmental delays.11 The AF matures slowly compared with other white matter fibre tracts, and in a recent study was found to mature after other language-related regions.21 Notably, the four children with Angelman syndrome in whom the AF could not be traced were among our youngest participants.
In our participants with Angelman syndrome, reduced fractional anisotropy, higher radial diffusivity, and a higher ADC in the UF corresponded to increased social withdrawal. Similar patterns are noted in social anxiety disorder, bipolar disorder, and schizotypal personality disorder.9,22 Researchers hypothesize that altered fractional anisotropy in the UF, which connects the amygdala to the orbitofrontal cortex, may reflect a common endophenotype of affect dysregulation and/or impaired social interactions.22 Some individuals with Angelman syndrome do have greater impairment in social interactions, and these findings illustrate some of the intergroup variability in Angelman syndrome in this regard (i.e. individuals with Angelman syndrome who are more socially interactive have a lower ADC and radial diffusivity in the UF and higher fractional anisotropy in the UF). Taken together, these findings seem to be indicative of aberrant or decreased fronto-amygdala structural connectivity in individuals with Angelman syndrome. Additionally, reduced fractional anisotropy in the left UF in individuals with Angelman syndrome corresponded to lower levels of cognition. This mirrors findings in other studies, where the left UF has been correlated with measures of intelligence and executive function.9,10
Our participants with Angelman syndrome also had reduced fractional anisotropy and an increased ADC and radial diffusivity in the ILF that corresponded to increased levels of irritability/agitation. The functions of the ILF have not been fully characterized; however, this tract appears to be responsible for transmitting emotional signals to visual areas that pertain to the salience of visual stimuli so that visual processing of emotionally significant stimuli is enhanced. Thus, structural abnormalities of the ILF may be associated with impairments in recognizing and responding to facial expressions.23 A recent study demonstrated that Ube3a (the gene responsible for Angelman syndrome)-deficient mice exhibited aberrant development of visual cortical circuits and reduced functional connectivity of excitatory neurons in the visual cortex.24 The relevance of this work in animal models to our findings of alterations in the ILF in humans requires further investigation.
Although we demonstrated clear differences between individuals with Angelman syndrome and the comparison group for DTI parameters in the IFOF, particularly the left side, and the CC, these alterations were not significantly correlated with any of our clinical variables. Thus, these white matter abnormalities seem to support a relatively generalized developmental disruption in Angelman syndrome, although their functional significance is unclear.
Our study was conducted in deletion-positive individuals with Angelman syndrome between the ages of 8 and 17 years; DTI studies should also be conducted using a younger cohort of participants with Angelman syndrome in order to determine the developmental trajectory of the diffuse white matter changes found in this investigation. The present study also included a small sample size and therefore had limited statistical power; future studies should aim to include a larger sample size. It will also be important in future studies to determine the specificity of these findings with regard to Angelman syndrome by comparison with other phenotypically similar populations (e.g. Prader–Willi syndrome, Rett syndrome).
Taken together, our findings seem to suggest a generalized white matter alteration in Angelman syndrome, with greater functional/clinical consequences when affecting temporal pathways. It is possible that a lack of UBE3A leads to greater alterations in temporal white matter pathways that contribute to language, cognition, and social functioning in individuals with Angelman syndrome. Although reduced or absent UBE3A expression may lead to a disruption in the development of dendritic targets of developing axons in multiple brain regions through the disruption of ubiquitin processes implicated in axonal guidance and synapse development,5 the preferential impact on temporal pathways is not surprising as deficits in general synaptic regulators such as MeCP2 result in selective cortical involvement in Rett syndrome.25 Studies like this one could contribute to a better understanding of the neurobiology of UBE3A and the pathophysiology of Angelman syndrome.
What this paper adds.
This is the first study to use diffusion tensor imaging techniques to examine differences in the white matter microstructure underlying the clinical phenotype in Angelman syndrome.
Individuals with Angelman syndrome exhibit changes in white matter fibre tracts that are connected to language and cognition, social affect/emotion regulation, and processing of visual stimuli.
The significant differences in the left arcuate fasciculus between individuals with Angelman syndrome and the comparison group may explain why children with Angelman syndrome experience significant delays in expressive language and cognition.
Although there seems to be a generalized white matter alteration throughout the brain in Angelman syndrome, correlations with clinical severity (cognition, language, social functioning) are most apparent in temporal pathways.
Acknowledgments
This work was supported by a grant from the Angelman Syndrome Foundation (to SUP). This study was also supported by NIH U54 grants RR019478 (NCRR) and HD061222 (NICHD; to Alan Percy PI) as well as 5P30HD015052-30 (to Elisabeth Dykens PI). We acknowledge the hard work of our study coordinator, Beverly Feldman, as well as the individuals and families who so graciously participated in this study. We also wish to thank Stacey K Martin for her assistance in preparing the manuscript.
LIST OF ABBREVIATIONS
- AF
Arcuate fasciculus
- ADC
Apparent diffusion coefficient
- CC
Corpus callosum
- DTI
Diffusion tensor imaging
- FOV
Field of view
- IFOF
Inferior fronto-occipital fasciculus
- ILF
Inferior longitudinal fasciculus
- SENSE
Sensitivity encoding (factor)
- UF
Uncinate fasciculus
Footnotes
North Americanusage for learning disability.
References
- 1.Williams CA, Beaudet AL, Clayton-Smith J, et al. Angelman syndrome 2005: updated consensus for diagnostic criteria. Am J Med Genet A. 2006;140:413–8. doi: 10.1002/ajmg.a.31074. [DOI] [PubMed] [Google Scholar]
- 2.Penner KA, Johnston J, Faircloth BH, Irish P, Williams CA. Communication, cognition, and social interaction in the Angelman syndrome. Am J Med Genet. 1993;46:34–9. doi: 10.1002/ajmg.1320460108. [DOI] [PubMed] [Google Scholar]
- 3.Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15:70–3. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Lev-Lehman E, Bressler J, Tsai TF, Beaudet AL. Genetics of Angelman syndrome. Am J Hum Genet. 1999;65:1–6. doi: 10.1086/302473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17:111–8. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- 6.Leonard CM, Williams CA, Nicholls RD, et al. Angelman and Prader–Willi syndrome: a magnetic resonance imaging study of differences in cerebral structure. Am J Med Genet. 1993;46:26–33. doi: 10.1002/ajmg.1320460107. [DOI] [PubMed] [Google Scholar]
- 7.Harting I, Seitz A, Rating D, et al. Abnormal myelination in Angelman syndrome. Eur J Paediatr Neurol. 2009;13:271–6. doi: 10.1016/j.ejpn.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Catani M, Mesulam M. The arcuate fasciculus and the disconnection theme in language and aphasia: history and current state. Cortex. 2008;44:953–61. doi: 10.1016/j.cortex.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura M, McCarley RW, Kubicki M, et al. Fronto-temporal disconnectivity in schizotypal personality disorder: a diffusion tensor imaging study. Biol Psychiatry. 2005;58:468–78. doi: 10.1016/j.biopsych.2005.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitropoulou V, Harvey PD, Maldari LA, et al. Neuropsychological performance in schizotypal personality disorder: evidence regarding diagnostic specificity. Biol Psychiatry. 2002;52:1175–82. doi: 10.1016/s0006-3223(02)01426-9. [DOI] [PubMed] [Google Scholar]
- 11.Sundaram SK, Sivaswamy L, Makki MI, Behen ME, Chugani HT. Absence of arcuate fasciculus in children with global developmental delay of unknown etiology: a diffusion tensor imaging study. J Pediatr. 2008;152:250–5. doi: 10.1016/j.jpeds.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 12.Bayley N. Bayley Scales of Infant Development. 3. San Antonio, TX: PsychCorp; 2006. [Google Scholar]
- 13.Sparrow SS, Chicchetti DV, Balla DA. Vineland Adaptive Behaviour Scales. Circle Pines, MN: AGS; 2005. [Google Scholar]
- 14.Aman MG, Singh NN. Aberrant Behaviour Checklist. East Aurora, NY: Slosson; 1986. [Google Scholar]
- 15.Wilde EA, McCauley SR, Chu Z, et al. Diffusion tensor imaging of hemispheric asymmetries in the developing brain. J Clin Exp Neuropsychol. 2009;31:205–18. doi: 10.1080/13803390802098118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilde EA, Chu Z, Bigler ED, et al. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma. 2006;23:1412–26. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- 17.Lebel C, Beaulieu C. Lateralization of the arcuate fasciculus from childhood to adulthood and its relation to cognitive abilities in children. Hum Brain Mapp. 2009;30:3563–73. doi: 10.1002/hbm.20779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–44. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–39. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Ellmore TM, Beauchamp MS, O’Neill TJ, Dreyer S, Tandon N. Relationships between essential cortical language sites and subcortical pathways. J Neurosurg. 2009;111:755–66. doi: 10.3171/2009.3.JNS081427. [DOI] [PubMed] [Google Scholar]
- 21.Su P, Kuan CC, Kaga K, Sano M, Mima K. Myelination progression in language-correlated regions in brain of normal children determined by quantitative MRI assessment. Int J Pediatr Otorhinolaryngol. 2008;72:1751–63. doi: 10.1016/j.ijporl.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Phan KL, Orlichenko A, Boyd E, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009;66:691–4. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126(Pt 9):2093–107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 24.Yashiro K, Riday TT, Condon KH, et al. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neurosci. 2009;12:777–83. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter JC, Lanham DC, Pham D, Bibat G, Naidu S, Kaufmann WE. Selective cerebral volume reduction in Rett syndrome: a multiple-approach MR imaging study. AJNR Am J Neuroradiol. 2008;29:436–41. doi: 10.3174/ajnr.A0857. [DOI] [PMC free article] [PubMed] [Google Scholar]


