Abstract
Autosomal dominant hyperimmunoglobulin E syndrome (HIES, Job syndrome) is a rare primary immunodeficiency characterized by elevated immunoglobulin E (IgE), eosinophilia, recurrent skin and pulmonary infections, dermatitis, and connective tissue and skeletal abnormalities. A 26-year-old male with known HIES presented with abdominal pain and diarrhea. Imaging showed sigmoid diverticulitis without abscess or perforation. Conservative management with antibiotics failed, and he developed a peridiverticular abscess, which was percutaneously drained with plans for elective resection. He returned four days later with progression of his diverticulitis, requiring partial colectomy with primary anastomosis. To our knowledge, this is the first case of diverticulitis in HIES. Diverticulitis is rare in younger individuals, raising the possibility that the connective tissue abnormalities of HIES patients may predispose them to colonic diverticula. Although the majority of complications are sinopulmonary and skin infections, diverticulitis should be considered in the differential of intra-abdominal processes in HIES.
Keywords: Hyper IgE Syndrome, diverticulitis
Introduction
Autosomal dominant hyperimmunoglobulin E syndrome (HIES, also known as Job syndrome)is a primary immunodeficiency characterized by recurrent skin and pulmonary infections, as well as numerous skeletal and connective tissue abnormalities. Despite frankly purulent infections, patients often lack “classic” signs of inflammation such as warmth, erythema, and tenderness. Staphylococcus aureus is the most common underlying pathogen, resulting in pyogenic pneumonias, cutaneous abscesses, and folliculitis of the axillae and groin.1 Pyogenic pneumonias often lead to bronchiectasis and pneumatoceles, which can subsequently become secondarily infected with Pseudomonas aeruginosa and molds such as Aspergillus.1 Other infectious sequelae include chronic mucocutaneous candidiasis, Pneumocystis jiroveci pneumonia, and disseminated cryptococcosis and histoplasmosis. HIES has numerous other manifestations, including skeletal abnormalities such as frequent fractures, scoliosis, and hyper extensible joints; distinctive facial features characterized by asymmetry, broad nose, and prominent forehead; dental abnormalities, primarily failure to exfoliate primary teeth; coronary artery tortuosity, dilation, and aneurysm; and increased rate of non-Hodgkin lymphoma (reviewed by Paulson et al).2
Case Report
The patient was a 26-year-old male with known HIES, diagnosed clinically when he was 15 years of age. In 2007, mutations in signal transducer and activator of transcription 3 (STAT3)were identified in patients with autosomal dominant HIES.3, 4 HIES was confirmed recently in this patient with a 1144C-T (R382W) mutation in STAT3, the most common STAT3 mutation in HIES.2 The patient had multiple characteristic findings of HIES, including characteristic facial features, neonatal rash, pneumonia with empyema (at 14 months old), recurrent S aureus skin infections, recurrent bilateral otitis media and sinusitis (requiring multiple surgeries), retained deciduous teeth, and tortuous right coronary artery by cardiac magnetic resonance imaging (MRI). His treatment regimen included monthly infusions of intravenous immunoglobulin and prophylactic antibiotics.
Ten months prior to presentation, the patient developed diarrhea attributed to suppressive antibiotics, which did not resolve after discontinuation of antibiotics. He presented with abdominal pain, worsening diarrhea, and fever. Computed tomography (CT) scan of the abdomen and pelvis demonstrated microperforated sigmoid diverticulitis with no free air (Fig. 1). Laboratory studies demonstrated white blood cell count (WBC) 12,000/uL, immunoglobulin G (IgG)1,625 mg/dL (on intravenous immunoglobulin, within normal limits), immunoglobulin M (IgM)318 mg/dL (within normal limits), immunoglobulin E (IgE) 4,205 mg/dL (elevated), and immunoglobulin A (IgA)was undetectable. All other labs, including an extensive workup for infectious diarrhea, were negative.
Figure 1.
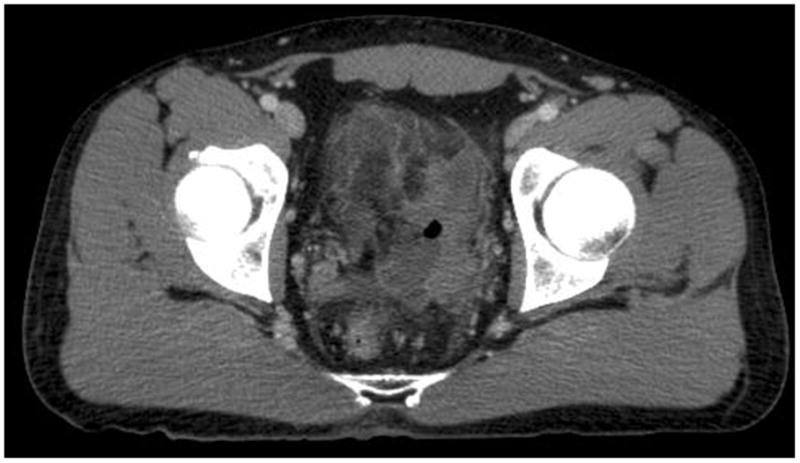
Representative slice of computed tomography (CT) scan of the abdomen and pelvis. Initially, it showed microperforated localized sigmoid diverticulitis with no evidence of abscess formation or free air.
The patient was treated with parenteral ciprofloxacin and metronidazole. Repeat CT scan for worsening pain and fever demonstrated a 4.5 × 2.3 cm inflamed diverticulum with thickening of the sigmoid wall, but no abscess. He remained on vancomycin, piperacillin/tazobactam, and fluconazole, with resolution of his abdominal pain and diarrhea. A screening CT demonstrated abscess formation (Fig. 2). CT-guided percutaneous drain placement with drainage of purulent fluid demonstrated light growth of pansensitive Eschereschia coli and Enterococcus faecalis. Two days later, CT scan showed abscess resolution; the drain was removed and the patient discharged with parenteral antibiotics and plans for later elective resection.
Figure 2.
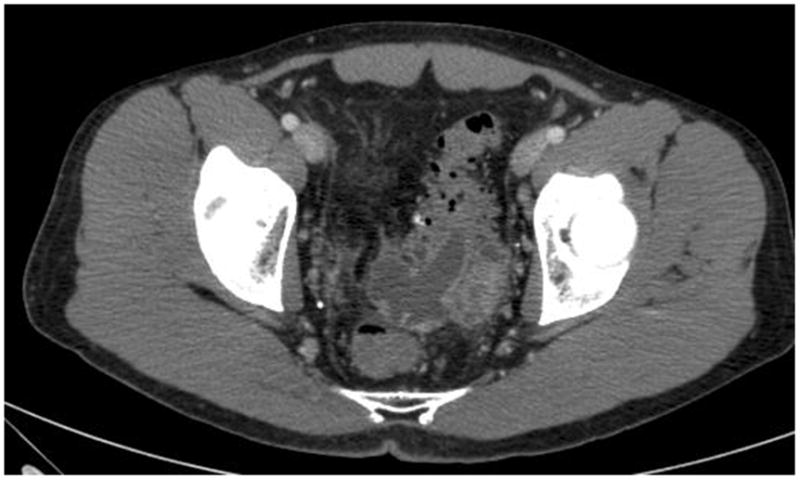
Representative slice of computed tomography (CT) scan of the abdomen and pelvis. After the patient was asymptomatic for seven days, diverticulitis was complicated by abscess formation.
Four days later, the patient presented with abdominal pain and fever. He underwent sigmoid colectomy with primary anastomosis and evacuation of pelvic abscess, which was adherent to the anterior peritoneal reflection, as well as the mid rectum. Pathology demonstrated a mixed inflammatory response consisting of acute, chronic, and focally granulomatous inflammation. The areas showing granulomatous inflammation demonstrated polarizable plant material within the abscess. Special stains were negative for a significant fungal, bacterial, or mycobacterial infection; cultures grew no predominant organism. He was treated with an extended course of intravenous antibiotics and oral antifungals. Despite the emergent nature of his surgical intervention, the patient had successful healing of his primary anastomosis and made a complete recovery.
Discussion
Hyperimmunoglobulin E syndrome is a primary immunodeficiency caused by mutations in STAT3, and is characterized by recurrent infections and connective tissue and skeletal abnormalities. This patient represents, to our knowledge, the first reported case of diverticulitis associated with HIES. Intra-abdominal processes are rare in HIES. There are two reports of spontaneous colonic perforation in HIES—cecal perforation in an 18-year-old male with pneumonia, and transverse colon perforation in an 8-year-old female admitted with neck abscess.5,6 Both patients had a prolonged hospitalization prior to perforation, and neither were associated with diverticulitis.
There are isolated case reports of localized intra-abdominal fungal infections in HIES, including colonic and esophageal cryptococcosis and two cases of ileocecal histoplasmosis.7–9 Interestingly, the cases of ileocecal histoplasmosis mimicking Crohn disease in patients with HIES were remarkably similar. As in our case, both demonstrated granulomatous inflammation. In all three cases, an atypical course of infection led to difficulty in establishing a clear diagnosis, suggesting that intra-abdominal infections could mimic inflammatory bowel disease in patients with HIES.
Less than 20% of patients who present with diverticulitis are younger than age 45, based on a large retrospective review of hospitalizations for diverticulitis.10 This raises the question: why did our patient developed diverticular disease at age 26? Gastrointestinal diverticula may be seen more frequently in young patients with genetic disorders affecting connective tissue, including patients with Marfan, Ehlers-Danlos, or Williams-Beuren syndromes. Giant multiple diverticula involving the stomach, duodenum, small intestine, and colon occurred in one patient with Marfan syndrome, suggesting that connective tissue laxity may result in a more severe diverticulitis phenotype.11 Connective tissue abnormalities are part of the spectrum of HIES, including coronary tortuosity and aneurysm development, scoliosis, and hyperextensibility of joints.
Our patient was treated according to current recommendations for diverticulitis (reviewed by Jacobs).12 Unfortunately, our patient required emergent sigmoid colectomy. There is little prospective data regarding management of diverticulitis in younger patients, and management remains unclear, particularly in the context of HIES. Given the high risk of recurrence and complications associated with recurrent diverticulitis, aggressive management is recommended when it occurs in young patients, including consideration of elective colectomy after the first episode of diverticulitis.13
Conclusion
To our knowledge, we present the first case of diverticulitis in hyperimmunoglobulin E syndrome. Given the growing list of apparent connective tissue dysfunction in association with HIES, this case raises the possibility that early-onset colonic diverticula and associated diverticulitis may be another manifestation of the disease.
Key Points.
Hyperimmunoglobulin E syndrome (HIES) is a primary immunodeficiency caused by mutation of the signal transducer and activator of transcription 3 (STAT3) that manifests clinically with recurrent skin and lung infections.
Despite recurrent infections in HIES, intra-abdominal infections are rare.
Young and immunocompromised patients have a high rate of recurrent diverticulitis.
Aggressive management is recommended in young and immunocompromised patients with diverticulitis, including consideration of elective colectomy after the first episode of diverticulitis.
Footnotes
Disclosures: A.F. Freeman is employed by the National Institutes of Health. D.G. Stover, P.W. Wright, D.S. Hummell, and R.M. Ness do not have any financial relationship to the subject matter associated with this manuscript.
References
- 1.Grimbacher B, Holland SM, Gallin JI, et al. Hyper-IgE syndrome with recurrent infections--an autosomal dominant multisystem disorder. N Engl J Med. 1999;340(9):692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 2.Paulson ML, Freeman AF, Holland SM. Hyper IgE syndrome: an update on clinical aspects and the role of signal transducer and activator of transcription 3. Curr Opin Allergy Clin Immunol. 2008;8(6):527–33. doi: 10.1097/ACI.0b013e3283184210. [DOI] [PubMed] [Google Scholar]
- 3.Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448(7157):1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 4.Holland SM, DeLeo FR, Elloumi HZ, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357(16):1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 5.Chen CM, Lai HS, Lin CL, Hsieh KS. Colon perforation in a patient with hyperimmunoglobulin E (Job’s) syndrome. J Pediatr Surg. 1995;30(10):1479–80. doi: 10.1016/0022-3468(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 6.Hwang EH, Oh JT, Han SJ, Kim H. Colon perforation in hyperimmunoglobulin E syndrome. J Pediatr Surg. 1998;33(9):1420–2. doi: 10.1016/s0022-3468(98)90025-2. [DOI] [PubMed] [Google Scholar]
- 7.Alberti-Flor JJ, Granda A. Ileocecal histoplasmosis mimicking Crohn’s disease in a patient with Job’s syndrome. Digestion. 1986;33(3):176–80. doi: 10.1159/000199290. [DOI] [PubMed] [Google Scholar]
- 8.Hutto JO, Bryan CS, Greene FL, et al. Cryptococcosis of the colon resembling Crohn’s disease in a patient with the hyperimmunoglobulinemia E-recurrent infection (Job’s) syndrome. Gastroenterology. 1988;94(3):808–12. doi: 10.1016/0016-5085(88)90257-0. [DOI] [PubMed] [Google Scholar]
- 9.Steiner SJ, Kleiman MB, Corkins MR, et al. Ileocecal histoplasmosis simulating Crohn disease in a patient with hyperimmunoglobulin E syndrome. Pediatr Infect Dis J. 2009;28(8):744–6. doi: 10.1097/INF.0b013e31819b65e0. [DOI] [PubMed] [Google Scholar]
- 10.Etzioni DA, Mack TM, Beart RW, Jr, Kaiser AM. Diverticulitis in the United States: 1998–2005: changing patterns of disease and treatment. Ann Surg. 2009;249(2):210–7. doi: 10.1097/SLA.0b013e3181952888. [DOI] [PubMed] [Google Scholar]
- 11.Shapira O, Mavor E, Simon D, et al. Multiple Giant Gastrointestinal Diverticula Complicated by Perforated Jejunoileal Diverticulitis in Marfan Syndrome. Digestive Surgery. 1992;9(1):58–60. [Google Scholar]
- 12.Jacobs DO. Clinical practice. Diverticulitis. N Engl J Med. 2007;357(20):2057–66. doi: 10.1056/NEJMcp073228. [DOI] [PubMed] [Google Scholar]
- 13.Stollman N, Raskin JB. Diverticular disease of the colon. Lancet. 2004;363(9409):631–9. doi: 10.1016/S0140-6736(04)15597-9. [DOI] [PubMed] [Google Scholar]


