Abstract
Objective
To report clinical and immunological investigations of contactin-associated protein-like 2 (Caspr2), an autoantigen of encephalitis and peripheral nerve hyperexcitability (PNH) previously attributed to voltage-gated potassium channels (VGKC).
Methods
Clinical analysis of patients with encephalitis, PNH, or both. Immunoprecipitation and mass spectrometry were used to identify the antigen and to develop an assay with Caspr2-expressing cells. Immunoabsorption with Caspr2 and comparative immunostaining of brain and peripheral nerve of wild-type and Caspr2-null mice were used to assess antibody specificity.
Results
Using Caspr2-expressing cells, antibodies were identified in 8 patients but not in 140 patients with several types of autoimmune or viral encephalitis, PNH, or mutations of the Caspr2-encoding gene. Patients’ antibodies reacted with brain and peripheral nerve in a pattern that co-localized with Caspr2. This reactivity was abrogated after immunoabsorption with Caspr2 and was absent in tissues from Caspr2-null mice. Of the 8 patients with Caspr2 antibodies, 7 had encephalopathy or seizures, 5 neuropathy or PNH, and 1 isolated PNH. Three patients had also myasthenia gravis, bulbar weakness, or symptoms that initially suggested motor neuron disease. None of the patients had active cancer; 7 responded to immunotherapy and were healthy or only mildly disabled at last follow-up (median 8 months, range 6–84).
Interpretation
Caspr2 is an autoantigen of encephalitis and PNH previously attributed to VGKC antibodies. The occurrence of other autoantibodies may result in a complex syndrome that at presentation could be mistaken for a motor neuron disorder. Recognition of this disorder is important because it responds to immunotherapy.
Keywords: autoimmune, encephalitis, seizures, neuromyotonia, peripheral nerve hyperexcitability, VGKC, Caspr2
Introduction
We recently reported that leucine-rich glioma inactivated 1 (Lgi1) is the main autoantigen of limbic encephalitis previously attributed to VGKC.1 In that study we also demonstrated that neither Lgi1 nor VGKC are the target antigens in patients with disorders other than limbic encephalitis who also have antibodies previously attributed to VGKC. A direct immunoprecipitation technique showed that one of these antigens is contactin-associated protein-like 2 (Caspr2), but the clinical and immunological associations of these antibodies were not further examined. Here we describe the index patient whose serum was used to isolate Caspr2, and examine the clinical and immunological associations in 7 additional patients with this autoimmunity. We also demonstrate that antibodies to Caspr2 specifically account for the reactivity of these patients’ CSF or sera against brain and peripheral nerve.
Patients and Methods
The term “antibodies attributed to VGKC” is used in this study to define antibodies identified by a radioimmunoassay based on the immunoprecipitation of brain protein complexes containing VGKC labeled with 125I-α-dendrotoxin (125I-α-dendrotoxin RIA) or by immunohistochemical methods.2–4 Because specific assays show that in patients with limbic encephalitis and antibodies attributed to VGKC the main target antigen is Lgi1, and because Caspr2 was precipitated with serum of a patient with encephalitis and peripheral neuropathy,1 a goal of the current study was to determine whether patients with encephalitis or peripheral nerve dysfunction suspected to be related to VGKC antibodies had in fact Caspr2 antibodies. The term peripheral nerve hyperexcitability (PNH) was used to include acquired neuromyotonia (Isaacs’ syndrome) or partial manifestations of this disorder such as cramps and fasciculations without clear evidence of neuromyotonia. The groups of patients examined to identify the seven additional cases included 49 patients with limbic encephalitis previously attributed to VGKC antibodies, 18 with acquired peripheral nerve hyperexcitability (PNH), 15 with encephalitis and atypical antibodies against the neuropil of hippocampus, and 6 with Morvan’s syndrome. As control samples we used serum or CSF from 19 patients with other autoimmune encephalitis associated with NMDA (7), AMPA (6), or GABAB (6) receptor antibodies, 13 patients with Rasmussen’s encephalitis, and 6 patients with viral encephalitis. Additionally, since some disorders with genetic mutations (e.g., hereditary ataxias) associate with tendency to autoimmunity,5 and patients with mutations of CNTNAP2 (the gene that codes for Caspr2) develop symptoms resembling those of patients with Caspr2 antibodies including, seizures, hyperactivity, encephalopathy6 and sometimes neuromyotonia (discussed later), we examined 21 patients with polymorphisms or mutations of CNTNAP2 for antibodies to Caspr2. Studies were approved by the University of Pennsylvania Institutional Review Board.
Immunocytochemistry on Caspr2-expressing cells
To generate a diagnostic cell-based assay, human embryonic kidney 293 cells (HEK293) were transiently transfected using lipofectamine 2000 (Invitrogen) and a plasmid containing human Caspr2.1 Patients’ CSF (diluted 1:5) or sera (1:200) and a commercial rabbit antibody to Caspr2 (1:10,000, Abcam ab93228) were applied to cells, followed by FITC-conjugated anti-human-IgG (1:1000, Molecular Probes) and TRITC-conjugated anti-rabbit secondary antibodies (1:1000, Jackson Laboratories). Images of transfected cells were captured with an epifluorescence microscope using Zeiss Axiovision software (Zeiss, Thornwood, NY, USA).
Immunohistochemistry on brain and peripheral nerve
Adult rat brains were prepared for immunohistochemical screening.4 Female Wistar rats were sacrificed without perfusion, the brains removed and immersed in 4% paraformaldehyde at 4°C for 1 hour, cryoprotected in 40% sucrose for 24 hours, snap frozen in chilled isopentane, and sectioned. Caspr2-null mice and their wild-type littermates were generated and genotyped as reported,7 and the brains were prepared and sectioned as above. For studies labeling peripheral nerve with patients’ antibodies, unfixed teased rat or mouse sciatic nerve fibers were prepared as previously described for paraformaldehyde fixed nerves.8
Immunohistochemistry was done using a standard avidin-biotin peroxidase method, using serum (1:200) or CSF (1:5) on 7 μm sagital brain sections followed by the appropriate secondary antibodies.4
Teased nerve fibers were permeabilized with acetone (−20°C for 10 minutes), washed with phosphate buffered saline (PBS, 5 minutes x3), blocked with 5% goat serum (1 hour, room temperature), then incubated with serum (1:200 to 1:4000) or CSF (1:5) and a rabbit antibody to Caspr2 (Abcam, ab93228; diluted 1:300) overnight at 4°C, followed by FITC-conjugated anti-human-IgG (1:200, Molecular Probes) and TRITC-conjugated anti-rabbit secondary antibody (1:300, Jackson Laboratories) for 1 hour at room temperature. An anti-human-IgM antibody (1:200 Molecular Probes) was used for some experiments to confirm that patients’ antibodies are of the IgG type. In some experiments, paranodes were labeled with a monoclonal antibody to CASPR (1:50) described previously,9 followed by a Cy5-conjugated goat anti-mouse secondary antibody (Jackson Laboratories, 1:200). Images of peripheral nerve were captured with an epifluorescence microscope using Openlab 3.1.7 software (Perkin Elmer, Waltham, MA, USA). Immunoabsorption was carried out with serial incubations of patient’s CSF in 6 wells containing fixed, permeabilized HEK293 cells expressing Caspr2 or a control plasmid without an insert. After sequential passes of 1 hour each, the CSF was applied to brain or peripheral nerve as described above.
Results
Index patient (case #1)
A 68 year-old man was evaluated for seizures and memory difficulties. Over the previous 2 years he had had 3 seizures, consisting of staring and unresponsiveness followed by generalized tonic-clonic convulsions. Approximately 1 year before evaluation, he developed progressive memory difficulties. He often repeated himself, and had difficulty learning new information and recalling names of close acquaintances. He occasionally became lost while driving in familiar surroundings. Starting 3 years earlier, he had painful hypersensitivity to warm temperatures affecting his hands and feet that persisted for 2 years and spontaneously resolved. Twelve months after the onset of seizures, he had low-grade, non-invasive bladder cancer that was treated with local scraping and cauterization; this has not recurred. He smoked (40–50 packs/year) and drank approximately 8 alcoholic drinks per week.
His MMSE was 29/30 (missed recall of one item at 5 minutes). He was able to name 12/15 objects on the Boston Naming test. Neuropsychological testing revealed a mild memory deficit, with difficulties in visuospatial and nonverbal memory. Cranial nerve function, strength and muscle tone were normal. Sensation of vibration was decreased on the toes, but sensation of cold, proprioception, and pinprick was intact. Reflexes were 2/5 and symmetrical. His gait was slightly wide-based, and he had difficulty performing tandem gait.
Brain MRI demonstrated bilateral mesial temporal lobe abnormalities, particularly in the amygdala, with extension posteriorly through the hippocampus, as well as mild cortical atrophy. An EEG demonstrated left frontal polar and bitemporal epileptiform discharges but no electrographic seizures. A lumbar puncture revealed an opening pressure of 156 mm H20, 2 WBC/μl, 0 RBC/μl, protein 44 mg/dl, and glucose 65 mg/dl. CSF cytology and flow cytometry were negative, and there were no oligoclonal bands. 125I-α-dendrotoxin RIA for VGKC antibodies in serum was positive (0.19 nmol/L, upper limit of normal 0.02 nmol/L). CSF PCR was negative for West Nile virus, Tropheryma whipplei, and arboviruses. Serum vitamin B12 level was normal and the following antibodies were negative: ANA, SSB, ds-DNA, RNP, Scl 70, and Jo-1. Elevated SSA/Ro antibodies (3 units, upper limit of normal 0.9 units) were noted in serum; complement C3/C4 was within normal limits. Tumor markers CEA, CA19-9, CA125, alpha-fetoprotein, beta-hCG, and paraneoplastic antibodies (Hu, Ma2, CRMP5, amphiphysin, Yo, Ri) were all negative. CT and FDG-PET did not reveal a neoplasm. Nerve conduction velocities showed no evidence of polyneuropathy, and needle EMG showed no evidence of PNH.
One month following this evaluation, he presented with a cluster of 3 generalized tonic-clonic seizures, including one lasting 20 minutes, followed by non-convulsive status epilepticus. His seizures were controlled with lorazepam, levetiracetam, valproic acid, and phenytoin. Repeat CSF and brain MRI studies were unchanged. A 5-day course of methylprednisolone (1 g daily) was initiated, with marked improvement in his cognitive function. He was slowly tapered from prednisone 60 mg to 40 mg, which led to increased confusion. Prednisone 60 mg was reinitiated, with some improvement in mental function. In an effort to decrease his steroid dependence, he was initiated on weekly rituximab for a 4-week cycle. At the last visit (3 months after rituximab), he was seizure-free for over 6 months and was able to fluently converse, balance a check book, and independently manage his daily affairs. His chief concern was continuing difficulty recalling events from several years ago.
Identification of Caspr2 as a target autoantigen
The serum and the CSF from the index case reacted with a neuronal surface antigen expressed on live, non-permeabilized rat hippocampal neurons (data not shown) that was precipitated and characterized by mass spectrometry as Caspr2.1 Subsequently, HEK293 cells transfected to express human Caspr2 were used as a diagnostic assay that confirmed the presence of Caspr2 antibodies in the index patient (Figure 1), and served to identify 7 additional cases among the indicated cohort of patients (Table 1). These 7 patients’ sera did not react with nontransfected cells, cells transfected with the voltage-gated potassium channel subunits Kv1.1 and Kv1.4, or cells transfected with LGI1 (data not shown).
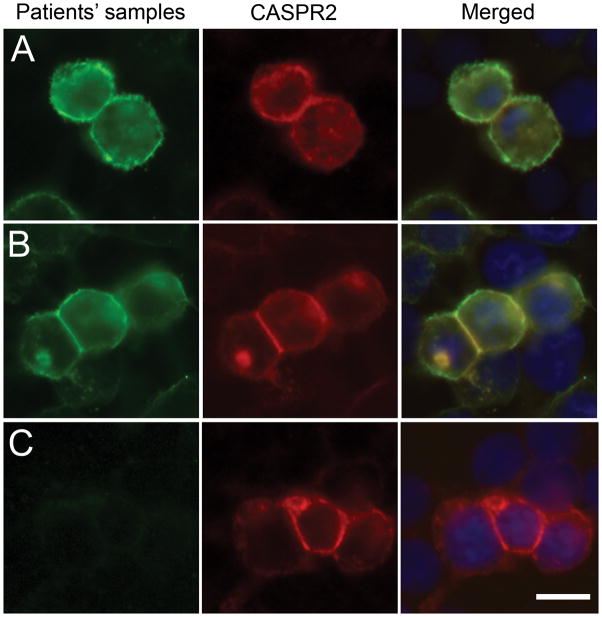
Figure 1. Patients’ antibodies react with cells expressing Caspr2.
HEK cells were transiently transfected to express human Caspr2, and labeled with the index patient’s CSF (A; green) or serum from a different patient (B; green) and a rabbit antibody to Caspr2 (A, B red), and counterstained with DAPI. Merged images (A, B yellow) demonstrate the overlap of patients’ antibody staining with Caspr2 expression. CSF (C) from a control patient did not react with cells expressing Caspr2. Scale bar: 20 μm
Table 1.
Demographic and clinical features
| Patient | Age | Sex | Symptoms/signs | Other clinical and immunological features |
|---|---|---|---|---|
| 1 | 68 | Male | 3 years of thermal allodynia, 2 years of intermittent complex partial seizures, and 1 year of progressive memory difficulties | VGKC antibodies† CSF: 2 WBC/μl, protein 44 mg/dl, glucose 65 mg/dl. Oligoclonal bands negative |
| 2 | 46 | Female | 2 years of progressive fatigue, personality changes, increased sweating, dysphagia with substantial weight loss, dysarthria, and intermittent ptosis and diplopia. Progressed to prolonged respiratory failure, visual hallucinations, and delusions of persecution | Diagnosed with myasthenia gravis MUSK antibodies 89 nmol/L (normal <0.5) AchR antibodies 247 nmol/L (normal <0.5) VGKC antibodies† |
| 3 | 66 | Male | 1 year of progressive lower extremity cramps, worse with exercise and sensory- predominant polyneuropathy | VGKC antibodies not detected† |
| 4 | 52 | Male | 5 years of progressive encephalopathy, myokymia and weakness. Progressed to critical illness with seizures, severe bulbar weakness, and encephalitis | VGKC antibodies† GAD65 antibodies, AChR antibodies. SIADH, peripheral neuropathy (type 1 diabetes) |
| 5 | 77 | Male | 1 year of 70 pounds weight loss, diffuse sweating, twitching of hands and feet. Progressed to severe jerking at night, even during sleep, difficulty walking, falls, difficulty concentrating, confusion, and psychomotor slowing |
VGKC antibodies† Serum sodium 129 mmol. CSF: no cells, protein 34 mg/dL, glucose 58 mg/dL |
| 6 | 60 | Male | Agitation, amnestic periods, seizures. No peripheral nervous system symptoms | VGKC antibodies not tested CSF: 4 WBC/μL, protein 35 mg/dL, glucose 75 mg/dL. Elevated PSA negative prostate biopsy, negative PET scan and CT of abdomen and pelvis |
| 7 | 61 | Male | Myokymia, fasciculations, cramps, involuntary lingual movements. Developed seizures and encephalitis after 1 year. Progressive dysphagia, dysarthria, myoclonus and ataxia after 6 years | VGKC antibodies† CSF: 15 WBC/μL, normal protein and glucose |
| 8 | 59 | Male | Generalized seizures and severe amnestic syndrome | VGKC antibodies† |
VGKC antibodies detected by the 125I-α-dendrotoxin RIA; normal values differed in different laboratories. CSF: cerebrospinal fluid.
Patients’ antibodies react with peripheral nerve and co-localize with the juxtaparanodal expression of Caspr2
Since Caspr2 is concentrated at the juxtaparanodal region of myelinated axons7 and the sera of some patients with antibodies previously attributed to VGKC labeled myelinated axons,10 we examined whether patients’ antibodies co-localized with Caspr2 at the juxtaparanodal region of mouse sciatic nerve fibers. The patients’ serum and CSF, but not those from controls, robustly stained the juxtaparanodal regions of myelinated fibers in a pattern that overlapped with that of Caspr2 (Supplementary Figure 1). Patients’ antibodies bound to Caspr2 at the juxtaparanodal region were labeled by a secondary anti-human-IgG antibody but not a secondary anti-human-IgM antibody, indicating that they are of the IgG type (data not shown).
Caspr2 is the target autoantigen in brain and nerve
We next used two different approaches to demonstrate that the reactivity of patients’ antibodies with brain and nerve is directed against Caspr2. First, we immunoabsorbed a patient’s CSF with Caspr2, and found that this specifically abrogated the reactivity of patient’s antibodies with brain and peripheral nerve (Figure 2). As a further test we immunostained brains and teased nerve fibers from Caspr2-null mice and their wild-type littermates. Patients with co-existing antibodies to other antigens (AChR, MuSK, GAD) were excluded from this analysis (#2 and #4). These studies showed that sera from the remaining 5 patients immunostained the neuropil of brain in a pattern expected for Caspr2 (Figure 3). Similar experiments with peripheral nerve showed that patients’ sera also labeled the juxtaparanodes of myelinated peripheral nerve fibers of wild-type mice in a pattern expected for Caspr2 reactivity (Figure 4). In contrast, these patterns of reactivity were not seen in Caspr2-null mice, indicating that the immunostaining was due to specific Caspr2 antibodies (Figures 3 and 4). Peripheral nerve fibers of wild-type and Caspr2-null mice were co-stained with a monoclonal antibody to the paranodal protein CASPR, which is related to Caspr2.9 CASPR staining did not co-localize with patients’ antibody staining and was unchanged in Caspr2-null mice, further confirming the specificity of patients’ antibodies for Caspr2.
Figure 2. Immunoabsorption with Caspr2 abrogates the reactivity of the index patient’s antibodies with rodent brain and peripheral nerve.
Top row: Rat brain sections were immunolabeled with the CSF of the index patient after immunoabsorption with sham transfected HEK cells (A) or HEK cells transfected to express Caspr2 (B); scale bar: 300 μm. Bottom rows: Teased mouse sciatic nerve fibers were immunolabeled with the CSF (green) of the index patient after immunoabsorption with sham transfected HEK cells (C) or HEK cells transfected to express Caspr2 (D); a rabbit antibody against Caspr2 (red) labels the endogenous Caspr2. Note that the reactivity of the patient’s CSF for both brain and teased fibers is abrogated when the CSF is preabsorbed with Caspr2. Scale bar: 10 μm.
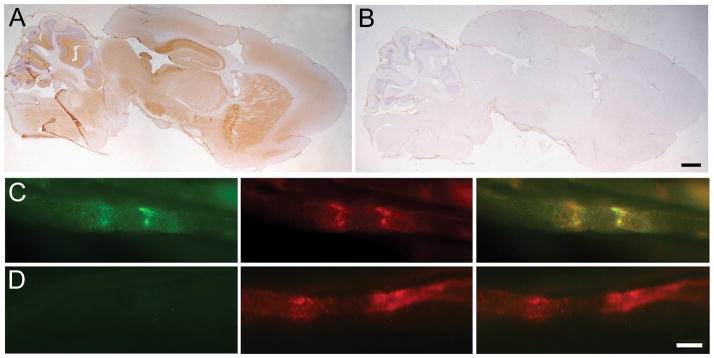
Figure 3. Patients’ antibodies react with wild-type but not Caspr2-null mouse brains.
The CSF (A) and serum (B) from 2 different patients with Caspr2 antibodies immunostain the hippocampus of a wild-type mouse in a pattern identical to that obtained with a rabbit polyclonal antibody against Caspr2 (C). This staining is not seen in sections from a Caspr2-null mouse (A–C, columns on the right). The pattern of staining with a CSF from a patient with limbic encephalitis associated with LGI1 antibodies is subtly different from Caspr2 and it is not affected using wild-type or Caspr2-null mice (D). The CSF of a control individual without Caspr2 or LGI1 antibodies does not label either sample (E). Scale bar: 100 μm
Figure 4. Patients’ antibodies label teased fibers from wild-type but not Caspr2-null nerves.

The sera (green) of 2 patients with Caspr2 antibodies (A and B) immunolabel the juxtaparanodes of teased sciatic nerve fibers from wild-type mice in a pattern similar to that of a rabbit antibody against Caspr2 (red), but different than the paranodal staining seen with a mouse monoclonal against CASPR (blue). In contrast, neither these patients’ sera nor the rabbit antibody against Caspr2 label teased sciatic nerve fibers from Caspr2-null mice (columns on the right). Sera from 2 controls (C and D) do not label teased nerve fibers. Scale bar is 5 μm.
Characteristics of patients with Caspr2 antibodies
The clinical characteristics of the patients with Caspr2 antibodies are described in Tables 1 and 2. Five patients had symptoms involving both the CNS (encephalitis, seizures) and PNS (hyperexcitability, neuropathy, thermal allodynia). Two additional patients had a purely CNS syndrome. Only one patient (#3) had acquired neuromyotonia without CNS symptoms. Two patients (#2 and #4) had bulbar weakness consistent with myasthenia gravis, and other autoantibodies against the skeletal muscle AchR and/or MuSK. One of these patients (#2) was described in a case report prior to the identification of Caspr2 antibodies.11 Case #7 had severe progressive bulbar weakness but was not evaluated for myasthenia. A diagnosis of motor neuron disease was initially considered in these 3 patients (#2, #4, and #7).
Table 2.
Imaging, clinical electrophysiology, treatment, and outcome
| Patient | Brain MRI | EEG | EMG/NCS | Treatments | Outcome (duration of follow-up) |
| 1 | Bilateral increased T2 signal involving mesial temporal lobes, and mild cortical atrophy | Left frontal polar and bitemporal epileptiform discharges but no electrographic seizures | Normal motor and sensory responses (aside from median neuropathy at the wrist). No evidence of peripheral nerve hyperexcitability | Corticosteroids, rituximab | Improving cognition; mild residual memory deficits; seizures controlled with antiepileptics (6 months) |
| 2 | Normal | - | Normal motor and sensory nerve conduction studies. Needle EMG showed spontaneous motor unit discharges and fibrillation potentials, but no neuromyotonia. Repetitive stimulation was normal | Prednisone, cyclosporine, thymectomy, rituximab | All symptoms resolved. Mental status normal (5 years) |
| 3 | - | - | Decreased sensory and motor amplitudes, normal conduction velocities, fasciculations, 5–150 Hz spontaneous motor unit discharges, increased motor unit duration, amplitude, and polyphasic motor units. | carbamazepine, amitriptyline, pregabalin (all for peripheral nerve hyperexcitability). | Mild disability from continuing sensory- motor polyneuropathy and cramps (4 years). |
| 4 | Normal | Mild diffuse background slowing | Methylprednisolone , plasma exchange, IVIG, low-dose cyclophosphamide, rituximab | Markedly improved cognition, seizures controlled, bulbar weakness improved (6 months) | |
| 5 | Age-appropriate atrophy | Normal | At presentation: myokymia, fasciculations, chronic and acute denervation. After one month of treatment: marked improvement of myokymia, no signs of denervation | Corticosteroids, plasma exchange | Cognitive symptoms persist (not oriented to time), improving muscle movements (12 months) |
| 6 | T2 increased intensities affecting left medial temporal lobe | Focal slowing and sharp waves over left temporal region | methylprednisolone | Recovered from seizures and memory deficits; mild mood instability (6 months) | |
| 7 | At presentation: normal. At 2 years: diffuse cortical and subcortical atrophy | Right temporal sharp waves | At presentation: normal. At 5 years: normal NCS, diffuse fasciculations, normal motor unit morphology. At 7 years: normal sensory responses, decreased motor amplitudes, normal conduction velocities, wide-spread fasciculations, fibrillation potentials, and 5–150 Hz motor unit discharges |
None | Lost to follow-up after 7 years of worsening symptoms. |
| 8 | Normal | - | - | IVIG (6 monthly cycles) | Normal (6 months) |
Of the 5 patients with CNS symptoms who had brain MRI, 2 had T2 hyperintensities affecting the medial temporal lobes, 1 developed generalized atrophy, and 2 were normal. EEG studies of these 5 patients showed focal epileptiform activity in 3, diffuse slowing in 1, and no abnormality in 1. Of the 5 patients with PNS symptoms who had EMG/NCS studies, 4 had electrophysiological evidence of PNH, and 1 (who only had transient thermal allodynia) had a normal study.
Six of 7 patients tested for VGKC antibodies using the 125I-α-dendrotoxin RIA had positive results, although none of them recognized HEK293 cells transfected with VGKC subunits (data not shown). None of the patients had a tumor except for past history of low-grade bladder cancer in the index case. Seven patients improved with immunotherapy and were either healthy or only mildly disabled at the last follow-up (median 8 months, range 6–84 months). The patient with poor outcome (# 7) had progressive bulbar weakness, never received immunotherapy, and was lost to follow-up after 7 years.
Other groups of patients
Among the groups of patients studied, Caspr2 antibodies were not identified in 2 of 6 patients with Morvan’s syndrome, 17 of 18 patients with acquired PNH, and 13 of 15 patients with encephalitis associated with atypical antibodies against the neuropil of hippocampus. None of the 49 patients with limbic encephalitis and antibodies attributed to VGKC had Caspr2 antibodies; however they all had Lgi1 antibodies. Two patients with PNH and one patient with Morvan’s syndrome without Caspr2 or Lgi1 antibodies had a positive 125I-α-dendrotoxin RIA, suggesting they had antibodies against other components of the VGKC-protein complex.
Discussion
This study demonstrates that Caspr2 is a brain and peripheral nerve autoantigen in a subgroup of disorders previously attributed to VGKC antibodies, including encephalitis, peripheral nerve dysfunction, or a combination of both (Morvan’s syndrome). Symptoms may include cognitive impairment, memory loss, hallucinations, delusions, seizures, PNH, and axonal sensorimotor neuropathy. The presence of other autoantibodies may result in a complex syndrome with clinical and electrophysiological features suggesting a motor neuron disorder. Three different sets of experiments establish Caspr2 as an autoantigen of these disorders, 1) specific immunostaining of HEK293 cells expressing Caspr2 with serum or CSF of patients, 2) specific abrogation of patients’ serum or CSF antibody reactivity after immunoabsorption with Caspr2-expressing cells, and 3) comparative brain and nerve immunostaining of wild-type and Caspr2-null mice, demonstrating lack of reactivity of patients’ serum and CSF with Caspr2-null mice.
Caspr2 has a critical role in concentrating VGKCs and other proteins in the juxtaparanodal region of myelinated axons in both the PNS and the CNS.9, 12 One patient with homozygous deletion of CNTNAP2 (OMIM 604569), the human gene that encodes Caspr2, had history of seizures and developed over 6 months progressive painful peripheral neuropathy and neuromyotonia to the point that it interfered with the patient’s gait (K Strauss, unpublished data). However, knockout mice with disruption of the murine homolog encoding Caspr27 had normal peripheral nerve conduction, suggesting that in patients with genetic or autoimmune disruption of Caspr2 the occurrence of peripheral neuropathy may be due to the involvement of other proteins that interact with Caspr2. Caspr2 is also expressed in the hippocampus,13 and mutations and polymorphisms of CNTNAP2 have been linked to schizophrenia, psychosis, intractable focal seizures, autism, mental retardation, and cortical dysplasia.6 14–16 In this respect, the CNS symptoms of patients with antibodies to Caspr2 recapitulate some of the clinical features of genetic disruption of the gene. In one patient with mutated CNTNAP2 the resemblance of symptoms with “VGKC-antibody associated encephalitis” led his neurologist to suspect this disorder (S. Markx, unpublished observation). Subsequent testing for VGKC antibodies using 125I-α-dendrotoxin RIA as well as for Caspr2 antibodies resulted to be negative. We examined 20 additional patients with CNTNAP2 mutations or polymorphisms and all were negative for Caspr2 antibodies.
Juxtaparanodal immunostaining of peripheral nerve by sera of patients with PNH has been previously attributed to antibodies against VGKC.10 Our data show that an actual antigenic target is Caspr2. Other investigators recently confirmed that Lgi1 is a major CNS target of VGKC complex-binding autoantibodies, and independently reported the paranodal protein Caspr2 as a second antigenic target.17 In clinical practice the diagnosis of patients with Caspr2 antibodies can be more complicated than that of patients with classical limbic encephalitis and Lgi1 antibodies. This is due to the frequent occurrence of Caspr2 with other autoantibodies resulting in a complex disorder that can manifest with motor weakness, atrophy, fasciculations, and bulbar symptoms, leading to suspect an irreversible motor neuron disorder (see pictures and detailed information of case #2 in11). Yet, this patient and most patients of the current study had dramatic responses to immunotherapy. It has been suggested that Caspr2 antibodies often occur in association with tumors (mostly thymoma) but our study shows otherwise.17 Extensive tumor screening, sometimes with a long follow-up, did not reveal a tumor in most patients, an observation that is emphasized by our referral pattern that likely favors patients with paraneoplastic disorders.
Our findings help to clarify the perplexing diversity of symptoms in patients previously diagnosed with VGKC antibodies. We suspect that other components of the Lgi1 or Caspr2 protein complex may be target antigens in other subgroups of patients pending characterization. For example, Lgi1 is a neuronal secreted protein that interacts with pre- and post-synaptic proteins, organizing a trans-synaptic protein complex with multiple components.18 There are cases of epilepsy or rapidly progressive dementia, different from limbic encephalitis, reported in association with antibodies to VGKC.19 It is unclear whether in these cases the target antigen is Lgi1, Caspr2, or another cell surface protein. Since only 1 of 18 patients with acquired PNH had Caspr2 antibodies and another 2 had positive 125I-α-dendrotoxin RIA, other autoantigens may account for the majority of these cases.
Additional work is required to clarify the pathogenic mechanisms of autoantibodies to Caspr2, and determine how they may cause central and peripheral nerve dysfunction. Based upon the response of most patients with Caspr2 antibodies to immunotherapy and what is known about the direct effects of other cell surface antibodies on the target antigens,20–22 functional disruption of Caspr2, as opposed to neuronal destruction, is a likely mechanism.
Supplementary Material
Sera from 3 patients with Caspr2 antibodies (A–C) label teased mouse sciatic nerve axons (green) in a pattern similar to that of a rabbit polyclonal antibody to Caspr2 (red). Sera from 3 controls (D–F) do not stain mouse sciatic nerve fibers. Merged images demonstrate the co-localization. Scale bar is 10 μm.
Acknowledgments
This work was supported by (RO1CA89054-06A2 and 1RC1NS068204) to JD, (NIH NS43174) to SSS, (NINDS NS50220, and the Israel Academy of Sciences) to EP, and a Dana Foundation Neuro-immunology Award to EL. We thank the physicians who provided clinical information. We also thank the patients and their families.
Footnotes
Conflicts of Interest
EL has received grant support from Talecris, a company which sells human immunoglobulin. A patent application for the use of Lgi1 antibody detection in patients’ CSF and sera has been filed by JD. No other authors have conflicts of interest.
References
- 1.Lai M, Huijbers MG, Lancaster E, et al. Investigation of Lgi1 as the antigen of limbic encephalitis previously attributed to potassium channels. Lancet Neurol. 2010;9:776–785. doi: 10.1016/S1474-4422(10)70137-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckley C, Oger J, Clover L, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol. 2001;50:73–78. doi: 10.1002/ana.1097. [DOI] [PubMed] [Google Scholar]
- 3.Tan KM, Lennon VA, Klein CJ, et al. Clinical spectrum of voltage-gated potassium channel autoimmunity. Neurology. 2008;70:1883–1890. doi: 10.1001/archneur.65.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ances BM, Vitaliani R, Taylor RA, et al. Treatment-responsive limbic encephalitis identified by neuropil antibodies: MRI and PET correlates. Brain. 2005;128:1764–1777. doi: 10.1093/brain/awh526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushara KO, Goebel SU, Shill H, et al. Gluten sensitivity in sporadic and hereditary cerebellar ataxia. Ann Neurol. 2001;49:540–543. [PubMed] [Google Scholar]
- 6.Strauss KA, Puffenberger EG, Huentelman MJ, et al. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 7.Poliak S, Salomon D, Elhanany H, et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown AA, Xu T, Arroyo EJ, et al. Molecular organization of the nodal region is not altered in spontaneously diabetic BB-Wistar rats. J Neurosci Res. 2001;65:139–149. doi: 10.1002/jnr.1137. [DOI] [PubMed] [Google Scholar]
- 9.Poliak S, Gollan L, Martinez R, et al. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–1047. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- 10.Kleopa KA, Elman LB, Lang B, et al. Neuromyotonia and limbic encephalitis sera target mature Shaker-type K+ channels: subunit specificity correlates with clinical manifestations. Brain. 2006;129:1570–1584. doi: 10.1093/brain/awl084. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Manera J, Rojas-Garcia R, Gallardo E, et al. Antibodies to AChR, MuSK and VGKC in a patient with myasthenia gravis and Morvan's syndrome. Nat Clin Pract Neurol. 2007;3:405–410. doi: 10.1038/ncpneuro0526. [DOI] [PubMed] [Google Scholar]
- 12.Scherer SS, Arroyo EJ. Recent progress on the molecular organization of myelinated axons. J Peripher Nerv Syst. 2002;7:1–12. doi: 10.1046/j.1529-8027.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- 13.Bel C, Oguievetskaia K, Pitaval C, et al. Axonal targeting of Caspr2 in hippocampal neurons via selective somatodendritic endocytosis. J Cell Sci. 2009;122:3403–3413. doi: 10.1242/jcs.050526. [DOI] [PubMed] [Google Scholar]
- 14.Alarcon M, Abrahams BS, Stone JL, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman JI, Vrijenhoek T, Markx S, et al. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry. 2008;13:261–266. doi: 10.1038/sj.mp.4002049. [DOI] [PubMed] [Google Scholar]
- 16.Zweier C, de Jong EK, Zweier M, et al. CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am J Hum Genet. 2009;85:655–666. doi: 10.1016/j.ajhg.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irani SR, Alexander S, Waters P, et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010 July 27; doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukata Y, Lovero KL, Iwanaga T, et al. Disruption of LGI1-linked synaptic complex causes abnormal synaptic transmission and epilepsy. Proc Natl Acad Sci U S A. 2010;107:3799–3804. doi: 10.1073/pnas.0914537107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geschwind MD, Tan KM, Lennon VA, et al. Voltage-gated potassium channel autoimmunity mimicking creutzfeldt-jakob disease. Arch Neurol. 2008;65:1341–1346. doi: 10.1001/archneur.65.10.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai M, Hughes EG, Peng X, et al. AMPA receptor antibodies in limbic encephalitis alter synaptic receptor location. Ann Neurol. 2009;65:424–434. doi: 10.1002/ana.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2009;9:67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sera from 3 patients with Caspr2 antibodies (A–C) label teased mouse sciatic nerve axons (green) in a pattern similar to that of a rabbit polyclonal antibody to Caspr2 (red). Sera from 3 controls (D–F) do not stain mouse sciatic nerve fibers. Merged images demonstrate the co-localization. Scale bar is 10 μm.