Abstract
Importance of the field
Human immunodeficiency virus (HIV) infection is associated with the development of a wide spectrum of kidney diseases. HIV-associated nephropathy (HIVAN) is the most common cause of chronic kidney disease (CKD) in HIV-infected individuals and predominantly affects patients of African ancestry. HIVAN is a leading cause of end-stage renal disease (ESRD) among African-Americans.
Areas covered in this review
an overview of the spectrum of kidney disease in patients with HIV; current pharmacologic interventions to treat kidney disease in HIV.
What the reader will gain
Knowledge regarding the most common causes of kidney disease in HIV-infected patients and principals related to pharmacotherapy in HIV-infected patients with kidney disease.
Take home message
Kidney disease is an important cause of morbidity and mortality in HIV-infected patients and the most common cause of chronic kidney disease in this population is HIV-associated nephropathy, which is caused by viral infection of the renal epithelium. Several medications that are commonly used in HIV-infected patients can have adverse effects on the kidneys and the doses of many antiretroviral medications need to be adjusted in patients with impaired renal function.
Keywords: HIV associated nephropathy, HIVAN, collapsing glomerulopathy, FSGS, antiretroviral therapy
1. Background
1.1 Historical perspective and epidemiology
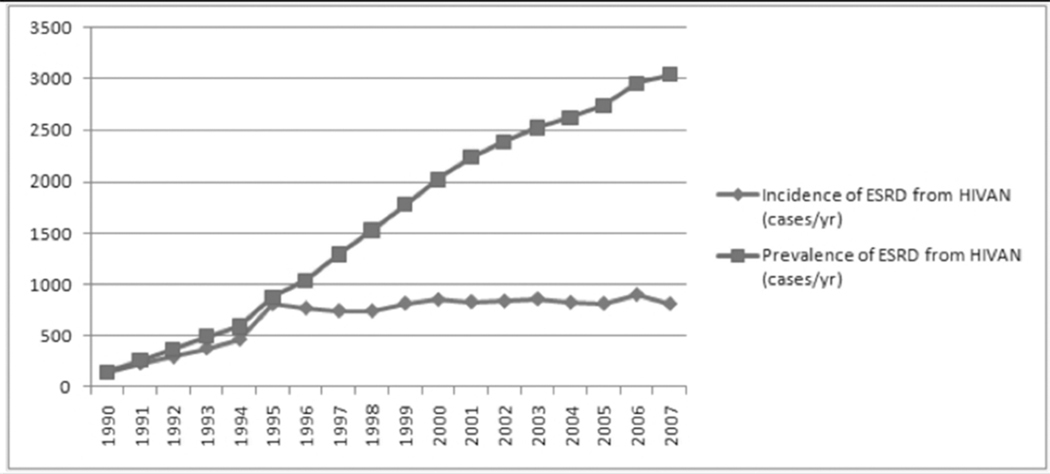
Approximately 33.4 million people were living with Human Immunodeficiency Virus (HIV) infection in 2008. [1] The prevalence of chronic kidney disease (CKD) is 15–30% in some high-risk cohorts [2–5]. Kidney disease in HIV-infected patients is a recognized risk factor not only for progression to end stage renal disease (ESRD) and dialysis but it is also associated with progression to AIDS and death in HIV-infected patients [2,3,5–8]. The most common kidney disease seen in HIV-infected patients is HIV-associated nephropathy (HIVAN), which is characterized histopathologically by a collapsing form of focal segmental glomerulosclerosis (FSGS) with microcystic tubular dilation and interstitial inflammation and fibrosis [9]. After HIVAN was first described in 1984 [10], the incidence of ESRD due to HIVAN rose steadily and peaked in the mid-1990s. Since the introduction of combination anti retroviral therapy (cART) in the mid-1990s, the incidence of ESRD due to HIVAN has remained relatively stable (Figure 1). Access to cART has also resulted in dramatic improvement in survival of the HIV-infected patient population [11,12]. As a result, there is a growing burden of chronic medical diseases in this aging population, including diabetes mellitus, hypertension and hepatitis C – all of which are well-established causes of chronic kidney disease (CKD). Furthermore, several antiretroviral medications are associated with increased risk of developing kidney disease, which further contributes to kidney disease in this population. Thus, despite the overall decrease in morbidity and mortality in HIV-infected patients treated with cART, kidney disease continues to be an important cause of morbidity and mortality in this population.
Figure 1.
Incidence and prevalence of ESRD from HIV-associated nephropathy (1990–2007).
1.2 HIV associated nephropathy (HIVAN)
HIVAN is one of the leading causes of ESRD in African Americans aged 25–64 years. According to the most recent US Renal Data Service (USRDS) annual data report, HIVAN (reported as AIDS nephropathy) accounted for 4,208 incident ESRD cases from 2003 through 2007 [13]. 88% of these cases were in African-American patients, highlighting the strong predilection of HIVAN for the black race [13]. The prevalence of HIVAN among HIV-seropositive black patients is around 12–15% in autopsy studies [14,15].
HIVAN is typically seen in patients with advanced HIV disease and in the absence of ART, presents with rapid decline in renal function and proteinuria which is often, but not necessarily in the nephrotic range [16]. Most patients have moderate to severe renal insufficiency, CD4 counts of less than 200 cell/mm3 and a detectable viral load at the time of diagnosis [17]. Urinalysis is typically characterized by a bland sediment with renal tubular epithelial cells and granular casts [18]. Other notable clinical findings include the absence of hypertension or peripheral edema, which are usually present in patients with other diseases associated with nephrotic range proteinuria and renal failure. Ultrasonongraphic evaluation usually reveals kidneys that are normal or increased in size and highly echogenic.
Renal biopsy is necessary for the diagnosis of HIVAN. In fact, even among African Americans who are at highest risk of developing HIVAN, the majority of patients who undergo diagnostic renal biopsy are diagnosed with renal diseases other than HIVAN. [17,19] Typical histopathologic findings of HIVAN include collapsing focal segmental glomerulosclerosis (FSGS), podocyte proliferation with formation of pseudocrescents, microcystic tubular dilation with atrophy of tubular epithelial cells, and tubulointerstitial inflammation and fibrosis. [9]. Glomerular endothelial tubuloreticular structures may be seen on electron microscopy, indicating high systemic levels of interferon-alpha.
Without treatment with cART, patients usually progress rapidly to ESRD within 8–16 weeks and have a mortality rate that approaches 100% within 6 months of diagnosis. In the cART era, the onset of HIVAN may be more insidious, with stable or slowly progressive renal failure and lower levels of proteinuria. [20] Even after treatment with cART, patients with HIVAN are more likely to progress to ESRD and have a worse survival compared to patients without HIVAN [17]. The one-year mortality rate among incident ESRD patients due to HIVAN was 30.8% from 2003 to 2007 [13].
1.3 Other chronic kidney diseases in HIV-infected patients
Increased longevity of persons living with HIV/AIDS in the cART era has led to aging of this population. As a result, kidney diseases other than HIVAN are increasingly being diagnosed by kidney biopsy in HIV-infected patients. In two large cohort studies of HIV-infected patients who underwent diagnostic kidney biopsies in the United States (the John Hopkins Cohort with 152 patients [17] and a multi-center cohort with 89 patients[21]), though HIVAN was the most common single disease found at biopsy, the prevalence of non-HIVAN lesions was 65.1% and 53%, respectively. And, it was even higher (83.1%) in a prospective cohort of HIV-infected patients who had consented to post-mortem organ donation (the Manhattan HIV Brain Bank Cohort with 89 patients[15]). Results from a prospective cohort from Paris, France[22], which included 60 patients (31 white patients and 29 black patients) who underwent clinically indicated kidney biopsy suggested that FSGS was more common in black patients (23 black patients vs 3 white patients, P<0.001) and HIV associated immune-complex disease (HIVICK) was more frequently seen in white patients (52% versus 21%). However, in a retrospective South-African cohort of 99 black patients, HIVAN accounted for 27% of the cases and HIVICK accounted for 21% cases. HIVICK was also seen in 17.9% and 14.6% patients in the John Hopkins and US multi-center cohorts, which were predominantly African American. Other non-HIVAN lesions seen in HIV-infected patients who underwent kidney biopsies in the above referenced American cohorts include: non-collapsing FSGS (seen in 1.1 to 22.4% of patients), hypertensive arterionephrosclerosis (5% to 16.9%), chronic pyelonephritis (1.1 to 7.9%), diabetic nephropathy (3.4% to 6.7%), mesangial hyperplasia (6.1%), interstitial nephritis (5.6%), ATN (2 to 2.2%), TTP/HUS (2.2%), amyloidosis (0.7 to 2.2%), MCD (0.7–1.1%) and indinavir related acute kidney injury (AKI) (0.7 to 1.1%). Patients non-HIVAN renal diseases are less likely to be African-American and have milder impairment of kidney function, less proteinuria, higher CD4 count, and lower HIV viral load [17,21]. Szczech et al, using multivariate modeling also showed that the time to initiation of renal replacement therapy from the time of renal biopsy was significantly longer in patients with lesions other than HIVAN when compared to patients with HIVAN[21]. Because of this prognostic significance and the broad spectrum of kidney diseases seen in the HIV population (and also therapeutic implications which will be discussed later), it is highly recommended that a kidney biopsy be performed in HIV-infected patients with renal disease, especially when significant proteinuria is present to establish an accurate diagnosis, unless contraindicated.
1.4 Genetic basis of HIVAN susceptibility
HIVAN almost exclusively affects individuals of African descent [23,24], and patients with HIVAN have an increased familial risk for kidney disease [25]. These observations suggest a hereditary component, but up until recently the genetic basis was elusive. Two studies reported a highly significant peak of excess African ancestry in Chromosome 22 in cohorts with idiopathic FSGS (focal segmental glomerulosclerosis), HIVAN, [26] and non-diabetic ESRD [27] by MALD (mapping by admixture linkage disequilibrium). MYH9 (non-muscle myosin heavy chain 9) was initially proposed as the candidate gene, based on the location of the MALD peak, expression of MYH9 in podocytes, and previously reported syndromes with glomerular involvement attributed to mutations in MYH9. [28]
However, two recent reports using SNP data from the recently released 1000 Genomes Project implicate polymorphisms in the apolipoprotein L-1 (APOL1) gene on chromosome 22 in conferring susceptibility to FSGS and hypertension-attributed ESRD among African-Americans [29,30]. Two APOL1 coding sequence polymorphisms (termed G1 and G2) show the strongest association with FSGS and hypertension-attributed ESRD with an autosomal recessive pattern of inheritance. Importantly, after controlling for APOL1 genotype, there was no residual association of renal disease with MYH9 variants. Given the deleterious effects of renal disease, the high prevalence of APOL1 polymorphisms among Yoruban Africans (38% for G1 and 8% for G2) prompted the investigators to study whether these polymorphisms confer protection against human parasitic diseases. Using in vitro assays, the investigators demonstrated that the G1 and G2 variants of APOL1 are more effective than wild-type APOL1 in lysing certain subspecies of Trypanosoma brucei, the protozoa that causes sleeping sickness [29]. It is therefore possible that the role of APOL1 in protection against parasitic disease in African may have led to a survival advantage for persons with the G1 and G2 genotypes and may explain the disproportionately high incidence of renal disease among African Americans. The mechanism by which APOL1 polymorphisms predispose patients to development of renal disease remains to be determined. While these findings are yet to be validated in a cohort of HIV-infected patients , it is likely that APOL1 polymorphisms confer increased risk of HIVAN.
2. Treatment of HIV associated nephropathy
2.1 Antiretroviral therapy
cART has become the cornerstone of therapy for HIVAN, and is recommended by the IDSA as an indication for treatment of HIVAN, regardless of CD4+ cell count. [31,32] Early reports showed possible utility of treatment with zidovudine (AZT) monotherapy. [33] In the context of cART, one case report [34] described a patient with HIVAN and dialysis-dependent renal failure, who recovered renal function after 13 weeks on cART, and had significant improvements in proteinuria and renal histology. Similarly, Winston et al. [35] reported a patient with acute retroviral syndrome and severe nephrotic syndrome with AKI requiring hemodialysis. Initiation of ART led to resolution of renal failure, a decline in proteinuria, and normalization of tubular architecture and podocyte morphology. In a retrospective cohort study of 19 HIV-infected patients with renal disease [36], the use of protease inhibitors was found to have a benefit on the progression of the nephropathy. In a retrospective cohort study by Atta et al. [37], 26 patients treated with ART were compared to 10 untreated patients. Renal survival was significantly better by multivariate analysis in the ART group compared to no treatment (adjusted HR=0.3, P<0.05). Finally, in a cohort of nearly 4,000 HIV-1 infected patients [38] followed longitudinally at the Johns Hopkins Hospital, the risk for HIVAN in the subgroup with AIDS was 6.8 and 26.4 episodes per 1,000 patient-years among those that received and did not receive ART, respectively. In a multivariate analysis, ART reduced the risk for HIVAN by 60%. No patient developed HIVAN if ART was initiated prior to the diagnosis of AIDS. In a mathematical model studying the dynamics of HIV and ESRD [39], it was estimated that in the period after 1995 (the introduction of ART), the rate of progression to HIV+ ESRD decreased by 38%. However, despite this beneficial effect, the prevalence of HIV-related ESRD was predicted to rise as a result of the increase in the population of African Americans with AIDS.
Given our current understanding that HIVAN pathogenesis is driven by viral replication in renal epithelia and subsequent HIV-1 gene expression, it seems logical that cART should be an effective strategy for the prevention and treatment of HIVAN. It must be recognized, however, that empirical evidence to support the use of cART in this setting is moderate at best. There has been no randomized controlled trial to date assessing the efficacy of cART on renal outcomes in patients with HIV. [40]
2.2 Steroids
There are several reports of improvement in renal function and proteinuria with corticosteroids in patients with HIVAN. Twenty patients with HIVAN [41] were followed prospectively, and received prednisone at a dose of 60 mg/day for 2–11 weeks with a subsequent taper of variable duration. Seventeen patients responded with a significant improvement in renal function, and decrease in serum creatinine from a mean of 8.1 mg/dL to 3 mg/dL (P<0.001). Five patients relapsed, but re-treatment was effective. Twelve of 13 patients with paired 24-hour urine collections had a significant decline in proteinuria (9.1 g/day to 3.2 g/day, P<0.005). However, there was no placebo group in this study and eleven patients died during follow up.
In a retrospective cohort study, Eustace et al. [42] compared 13 patients treated with corticosteroids for one month, followed by several-month taper, with 9 untreated patients. Corticosteroid therapy had a significant protective effect against progressive azotemia (RR=0.2, P<0.05) that was retained after multivariate adjustment. There was a reduction in the levels of proteinuria by a mean of 5.5 g/day with no significant difference in the rates of serious infections. In a retrospective study of 102 patients with biopsy-proven HIVAN, the use of steroids [43] was among the factors associated with better renal outcomes.
2.3 ACE inhibitors and All receptor blockers
Data from animal and human studies suggests that medications that blockage of the renin-angiotensin system (RAS) is an effective approach to slow the progression of several forms of CKD. Large randomized controlled clinical trials have demonstrated that angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) can slow the progression of diabetic nephropathy and non-diabetic CKD [44]. The protective effects of these agents may be mediated via amelioration of intraglomerular hypertension, hyperfiltration, and proteinuria and their use in patients with proteinuria and CKD is supported by Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC7) [45] and National Kidney Foundation Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines on the use of antihypertensive agents in patients with CKD [46], which recommend aggressive control of blood pressure and proteinuria in patients with CKD.
There have been small clinical studies evaluating the role of RAS blocking agents in the treatment of HIVAN. In a case-control study of 18 patients with biopsy-proven HIVAN during the pre-ART era [47] investigators reported that captopril therapy was associated with significantly longer renal survival (156 days) compared with untreated patients (37 days, P<0.002). In a study of 20 patients with HIVAN, Burns, et al. [48] examined the benefits of ACE inhibitors (10 mg of fosinopril daily) on renal function. Of 11 patients with subnephrotic proteinuria, 7 were treated with fosinopril, and 4 were not. Despite similar baseline creatinine and protein excretion, after 24 weeks of treatment, creatinine was 1.5 and 4.9 (P=0.006) and proteinuria was 1.25 g/24h and 8.5 g/24h (P=0.006) in the treated and untreated groups, respectively. Of 9 patients with nephrotic-range proteinuria, 5 were treated and 4 served as controls. Again, despite comparable baseline creatinine and protein excretion, after 12 weeks of therapy, creatinine was 9.2 mg/dL and 2.0 mg/dL (P=0.02), and proteinuria was 10.5 g/24h and 2.8 g/24h (P=0.008) in the treated and untreated groups, respectively.
The same group prospectively followed 44 patients with biopsy-proven HIVAN [49] for 5 years. Fosinopril was offered to all patients and ACE inhibitor-adherent patients (n=28) were compared to patients who refused treatment (controls, n=16). Despite comparable baseline CD4+ counts, creatinine, and levels of proteinuria, all 16 controls progressed to ESRD, while only one of 28 treated patients (3.6%) developed ESRD during follow up (P<0.0001). Median renal survival of untreated and treated patients was 146 and 479 days, respectively (P<0.0001). Interpretation of the results from the above studies is difficult due to selection bias (instead of random allocation, patients that declined therapy were used as controls). In addition, detailed data about ART treatment during the study is lacking. Moreover as patients were enrolled prior to the advent of conventional cART, it is unlikely that they received appropriate antiviral therapy that would have maximally suppressed viral replication for a large portion of the follow-up period [50,50].
Data about the potential benefits of ACE inhibitors or ARBs on the progression of HIVAN in the context of combination ART is lacking. Guidelines for the management of CKD in HIV-infected patients recommend the use of ACE inhibitors or ARBs in the presence of co-existant hypertension and proteinuria, but make no recommendations regarding their use in non-hypertensive patients [31].
2.4 Cyclosporine
In 1991, Igulli et al. reported [51] that treatment of fifteen children with HIV and nephrotic syndrome and variable histologic findings with cyclosporine induced remission of nephrotic syndrome whereas prednisone did not appear to have a beneficial effect. However, no subsequent studies have examined the role of cyclosporine in the treatment for HIVAN or other forms of CKD in HIV-infected patients in the cART era.
3. Treatment of other kidney diseases in HIV-infected patients
Though few studies have focused on therapy for HIV-associated renal diseases other than HIVAN, it is likely that treatment with cART may be renoprotective in patients with other forms of kidney disease. Studies have demonstrated that initiation of cART is associated with improved renal function in treatment naïve African patients [52,53], though the underlying cause of kidney disease in these studies is unknown. Recent studies have also demonstrated that renal function in HIV-infected patients is correlated with degree of viral suppression [54,55]. It is not known whether the effect of ART is via suppression of viral replication in the kidney or due to changes in the systemic inflammatory milieu which may have adverse effects upon renal function.
In the Strategies for Management of Anti-Retroviral Therapy (SMART) study, participants assigned to the episodic ART treatment strategy had a trend toward fewer fatal and nonfatal renal events than those assigned to continuous ART (9 vs 2, p=0.054) prior to protocol modification. However, participants in the episodic therapy arm had fewer renal events than continuously suppressed participants after protocol modification (1 vs 6, p=0.01) [56]. In a later analysis of SMART participants, discontinuation of ART was associated with a small increase in plasma cystatin C, an alternative marker of kidney function, which persisted during one year of follow up [56]. Taken together, these studies suggest that the salutary effect of ART upon renal function may not be specific to patients with HIVAN. However, little is know regarding which types of renal disease other than HIVAN are likely to improve with ART.
The optimal treatment of proliferative glomerulonephritis, IgA nephropathy and other renal diseases (excluding HIVAN) in the setting of HIV infection is uncertain. [57] In one case report [58], a Caucasian man with HIV infection and biopsy proven IgA nephropathy with nephrotic range proteinuria and normal GFR, captopril therapy induced a significant reduction in proteinuria. In a retrospective cohort study [21], comparing clinical characteristics of 42 patients with HIVAN and 47 patients with other renal diseases, ART was not found to be efficacious in the cohort with non-HIVAN renal disease.
4. Renal toxicity of antiretroviral medications
The widespread use of cART in developed countries has led to a marked reduction in mortality and incidence of AIDS defining illnesses in persons with HIV/AIDS. Though cART has also decreased the incidence of some HIV-associated illnesses such as HIVAN, it can also have significant effects upon the kidney. Table 1 summarizes the reported renal adverse effects with individual anti retroviral medications. In this section, we will focus on tenofovir, indinavir and atazanavir as these drugs are the ART medications most commonly associated with nephrotoxicity.
Table 1.
Adverse Renal Effects of Anti-Retroviral Medications
| Medication (BRAND NAME) |
Nephrotoxicity | References |
|---|---|---|
| NRTIs | ||
| Abacavir (ZIAGEN) | Fanconi, nephrogenic DI, AIN | [81,82] |
| Didanosine (VIDEX EC) | Fanconi, nephrogenic DI | [83,84] |
| Emtricitabine (EMTRIVA) | None | |
| Lamivudine (EPIVIR) | Fanconi | [85] |
| Stavudine (ZERIT) | AKI, Fanconi | [85,86] |
| Tenofovir (VIREAD) | AKI, fanconi, hypophosphatemic osteomalacia, nephrogenic DI |
[66– 69,87,88] |
| Zidovudine (RETROVIR) | None | |
| NNRTIs | ||
| Delavirdine (RESCRIPTOR) | None | |
| Efavirenz (SUSTIVA) | AKI, MCD, urolithiasis, | [89–92] |
| Etravirine (INTELENCE) | None | |
| Nevirapine (VIRAMUNE) | AKI in the setting of DRESS | [93] |
| Protease Inhibitors | ||
| Atazanavir (REYATAZ) | Urolithiasis, AIN | [78,79] |
| Darunavir(PREZISTA) | None | |
| Fosamprenavir (LEXIVA) | Urolithiasis | [94] |
| Indinavir (CRIXIVAN) | Urolithiasis, AIN, , sterile pyuria, AIN, AKI, nephrogenic DI, papillary necrosis, renal atrophy |
[73,75– 77,95–97] |
| Ritonavir (NORVIR) | Urolithiasis, AKI, Pancreatorenal syndrome | [98] |
| Lopinavir/Ritonavir (KALETRA) |
Urolithiasis | [99] |
| Nelfinavir (VIRACEPT) | Urolithiasis | [100] |
| Saquinavir (INVIRASE) | Urolithiasis | [101] |
| Tipranavir (APTIVUS) | None | |
| Entry Inhibitors | ||
| Enfuvirtide (FUZEON) | MPGN | [102] |
| Maraviroc (CELSENTRI) | None | [103] |
| Vicriviroc | None | [104] |
| Integrase inhibitors | ||
| Raltegravir (ISENTRESS) | None | [105] |
| Elvitegravir | None | [106] |
Abbreviations: AIN, acute interstitial nephritis; AKI, acute kidney injury; DRESS, drug rash with eosinophilia and systemic symptoms; DI, diabetes insipidus; MPGN, membranoproliferative glomerulonephritis.
Adapted with permission from Jao J, Wyatt CM. Antiretroviral medications: adverse effects on the kidney. Adv Chronic Kidney Dis;17:72–82.
Nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs) are primarily eliminated unchanged in the urine (except for abacavir, which is metabolized by the liver) and hence require dosage adjustment in patients with compromised renal function. On the other hand, non-nucleoside reverse transcriptase inhibitors (NNRTIs) and protease inhibitors (PIs) undergo hepatic metabolism and do not need dose adjustment with renal impairment. Dose modifications have been recommended for some NNRTIs and PIs with hepatic dysfunction [59]. Table 2 summarizes the dosing recommendations for different anti-retroviral medications in patients with renal insufficiency [59]. It is important to note that many of the dosage recommendations are based on limited data and it is imperative that patients with organ dysfunction should be monitored closely for drug efficacy and toxicity.
Table 2.
ART Dosing In Patients With Renal Insufficiency
| Medication | Daily dosing | Renal dosing for adults |
|---|---|---|
| NRTIs | ||
| Abacavir (ZIAGEN) |
300mg PO BID or 600mg PO QD (if used in combination with other ART) |
No dosage adjustment needed |
| Didanosine (VIDEX EC) |
Body weight <60kg: 250mg PO QD or 125mg PO BID |
CrCl 30–59: 125mg PO QD CrCl 10–29: 125mg PO QD CrCl <10: not recommended HD or PD: not recommended |
| Body weight ≥60kg: 400mg PO QD or 200mg PO BID |
CrCl 30–59: 200mg PO QD CrCl 10–29: 125mg PO QD CrCl <10: 125mg PO QD HD or PD: 125mg PO QD; no supplement. |
|
| Emtricitabine (EMTRIVA) |
200mg PO QD (capsule) | CrCl 30–49: 200mg PO Q48H CrCl 15–29: 200mg PO Q72H CrCl <15: 200mg PO Q96H HD: 200mg PO Q96H; dose after hemodialysis. |
| 240mg PO QD (solution) | CrCl 30–49: 120mg PO Q24H CrCl 15–29: 80mg PO Q24H CrCl <15: 60mg PO Q24H HD: 60mg PO Q24H; dose after hemodialysis. |
|
| Lamivudine (EPIVIR) |
150mg PO BID or 300mg PO QD. If body weight <50kg: 4mg/kg BID (max 150mg BID) |
CrCl 30–49: 150mg PO QD CrCl 15–29: 150mg PO x 1, then 100mg PO QD CrCl 5–14: 150mg PO x 1, then 50mg PO QD CrCl <5: 50mg PO x 1, then 25mg PO QD HD or PD: 50mg PO x 1, then 25mg PO QD; no supplement. |
| Stavudine (ZERIT) |
Body weight <60kg: 30mg PO Q12H |
CrCl 26–50: 15mg PO Q12H CrCl: 10–25: 15mg PO Q24H HD: 15mg PO Q24H; dose after hemodialysis. |
| Body weight ≥60kg: 40mg PO Q12H |
CrCl 26–50: 20mg PO Q12H CrCl 10–25: 20mg PO Q24H HD: 20mg PO Q24H; dose after hemodialysis. |
|
| Tenofovir (VIREAD) |
300mg PO QD | CrCl 30–49: 300mg PO Q48H CrCl 10–29: 300mg PO Q72–96H CrCl <10: 300mg PO Q 7 days HD: 300mg PO Q 7 days; dose after hemodialysis. |
| Zidovudine (RETROVIR) |
Oral: 300mg PO BID | CrCl <15, HD or PD: 100mg PO TID or 300mg PO QD CVVH: 100mg PO Q8H |
| Combination medications | ||
| Abacavir 600mg + Lamivudine 300mg (EPZICOM) |
1 tab PO QD | CrCl < 50: Not recommended |
| Zidovudine 300mg + Lamivudine 150mg (COMBIVIR) |
One tab PO BID | CrCl <50: not recommended |
| Abacavir 300mg + Lamivudine 150mg + Zidovudine 300mg (TRIZIVIR) |
One tab PO BID | CrCl <50: not recommended |
| Lopinavir 200mg + Ritonavir 50mg (KALETRA) |
HAART naïve pts: 4 tabs PO QD or 2 tabs PO BID§ |
No dosage adjustment needed |
| HAART experienced pts: 2 tabs PO BID |
||
| Tenofovir 300mg + Emtricitabine 200mg (TRUVADA) |
One tab PO QD | CrCl 30–49: 1 tab PO Q48H CrCl <30: not recommended HD: not recommended |
| Tenofovir 300mg + Emtricitabine 200mg + Efavirenz 600mg (ATRIPLA) |
One tab PO QD | CrCl <50: not recommended |
NOTE: Dose adjustments are not necessary because of renal insufficiency for non nucleoside reverse transcriptase inhibitors (delavirdine, efavirenz, etravirine, and nevirapine), protease inhibitors (atazanivir, darunavir, fosamprenavir, indinavir, nelfinavir, ritonavir, saqinavir, and tipranavir), entry inhibitors (enfuvirtide, maraviroc), or the integrase inhibitor (raltegravir).
ABBREVIATIONS: ART, Anti-retroviral therapy; CrCl, creatinine clearance; HD, hemodialysis;PD, peritoneal dialysis; QD, once daily; BID twice daily; TID three times daily.
QD dosing contraindicated with indinavir, maraviroc, saquinavir, phenytoin, carbamazepine or phenobarbitol.
REFERENCE: Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. December 1, 2009; 1–161. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed Oct 21, 2010.
Tenofovir
Tenofovir is the first and the only nucleotide reverse transcriptase inhibitor approved for treatment of HIV infection. It is primarily excreted in the urine by glomerular filtration and tubular secretion. Uptake of tenofovir into the proximal tubular epithelial cells is mediated by the human organic anion transporters (hOAT) on the basolateral membrane- predominantly by hOAT1 and to a lesser degree by hOAT3[60]. It is then secreted into the tubular lumen by the multi-drug resistance associated-protein[60]. The exact mechanism of NRTI nephrotoxicity is not certain. It has been hypothesized that tenofovir accumulates in the tubular cells and causes apoptosis by direct tubular injury[61] or results in mitochondrial DNA depletion by inhibition of mitochondrial DNA replication[62,63].
Nelson et al[64], examined the incidence of serious adverse effects (SAE) of tenofovir in 10,343 patients who were enrolled in a tenofovir expanded access program. Only 6% of the patients had a SAE after a cumulative patient exposure to tenofovir of 3,700 person-years. The most commonly reported SAE were renal events which were observed in 0.5% of patients. Renal failure was seen in 0.3% of patients. Proximal tubule dysfunction and nephrogenic diabetes insipidus were seen in less than 0.1% of the patients. Bone abnormalities and lactic acidosis were seen in 0.1% of the patients. Median time to onset of renal adverse events was 282 days (IQR: 443) and median time to resolution was 86 days (95% CI: 67–145).
In the EuroSIDA study[65], which is a prospective observational cohort study of more than 16,505 patients followed in 103 hospitals in 32 European countries plus Israel and Argentina, tenofovir use was associated with increased risk of developing CKD (16% relative increase per year of exposure) even after adjustment for baseline eGFR , AIDS and other significant confounders . Among patients who stopped taking tenofovir during follow-up, the risk of CKD remained elevated in the first 12 months (IRR 4.05, 95% CI 2.52–6.53) but did not persist after 12 months (IRR 1.12, 95% CI 0.63 – 1.99). Co
In the John Hopkins HIV Cohort[66], tenofovir use was associated with a 4% relative decline in creatinine clearance (CrCl) compared with other NRTIs. Tenofovir-exposed patients also demonstrated a faster decline in CrCl than tenofovir-unexposed patients in the HIV Outpatient Cohort (−3.2 vs 1.2 ml/min) [67], in the Swiss HIV Cohort (4.1 to 5.5 mL/min) [68] and in an Australian HIV cohort (−5.6 v 1.26 ml/min) [69]. Concomittant use of tenofovir with ritonavir-boosted protease inhibitor regimens is associated with greater decline in estimated GFR than patients treated with tenofovir/ NNRTI regimens [70,71].
Tenofovir toxicity can also result in varying degrees of proximal tubular dysfunction, including Fanconi’s syndrome which is characterized by global dysfunction of the proximal tubule resulting in proteinuria, glycosuria, hypophosphatemia, hypouricemia, hypokalemia, phosphaturia and aminoaciduria. This solute loss leads to acidosis and bone disease. Two cases of tenofovir-induced hypophosphatemia induced osteomalacia have been reported[72]. Both patients presented with bone pain which resolved after drug discontinuation and mineral supplementation. Three cases of tenofovir induced nephrogenic diabetes insipidus have also been reported in literature. In all three cases polyuria resolved after tenofovir was discontinued[63].
Because of these findings the Infectious Disease Society of American (IDSA) recommends biannual screening for proteinuria and glycosuria with urine analysis and measurement of serum creatinine and phosphate in patients receiving tenofovir with a eGFR of < 90 ml/min/1.73m2. [31] Biannual screening is also recommended in tenofovir-treated patients who are exposed to ritonavir boosted protease inhibitor regimens, other medications that undergo renal secretion (e.g., acyclovir, ganciclovir, or cidofovir), and in patients with co morbidities such as diabetes and hypertension. If a tenofovir-treated patient develops AKI, tenofovir should be stopped and evaluation and close follow-up by a nephrologist is recommended. Most cases either have a partial or complete renal recovery after stopping the drug, usually within a few months to a year.
Indinavir
Indinavir is a potent protease inhibitor and is generally well tolerated but can have significant renal side effects. Despite being readily metabolized by the liver, approximately 20% of the drug is excreted unchanged in the urine . [73] Indinavir is poorly soluble in the urine at physiological pH[74]. In a NIH study of 240 patients treated with indinavir, 20% had crystalluria, 8% developed urological symptoms (dysuria, back or flank pain) and 3% developed nephrolithiasis[75]. Risk factors for urological symptoms during indinavir treatment include higher doses (1000mg or more twice daily), low body weight, low lean body mass, warm environmental temperature, concomitant cotrimoxazole use and undetectable HIV RNA levels when starting indinavir therapy[76]. Gagnon et al studied 54 indinavir-treated patients and found that 39% of patients had sterile pyuria, of whom 24% also had a concomitant rise in creatinine[73]. In 2002, Kopp et al found an association between sterile pyuria due to indinavir use and interstitial nephritis[77]. Of the 23 indinavir-treated patients with pyuria in this study, 14 patients had interstitial nephritis and others had urothelial or non-specific inflammation of the urinary tract. Six patients also had elevated serum creatinine levels. Urine abnormalities and serum creatinine normalized in all 20 patients who stopped indinavir. In the EuroSIDA study, indinavir-treated patients had a 12% increased risk of developing CKD per year when compared to those who were not receiving indinavir in multivariate models[65].
The IDSA recommends that indinavir-treated patients should drink a minimum of 1.5 liter of water per day to increase urine flow rates to prevent stone formation[31]. IDSA also recommends periodic monitoring of renal function and screening for pyuria during the first six months of indinavir therapy and biannually thereafter. Routine screening for crystalluria is not recommended unless there is suspicion of nephrolithiasis. In patients who develop indinavir nephrolithiasis, therapy can be restarted once rehydration is achieved. Indinavir should however, be permanently discontinued in patients who develop indinavir induced pyuria, renal insufficiency, hypertension or rhabdomyolysis.
Atazanavir
Atazanavir is a newer protease inhibitor which has recently been reported to be associated with increased risk of nephrolithiasis. According to the product information provided by the manufacturer seven percent of atazanavir is excreted unchanged in the urine after a single 400mg dose. Like indinavir, atazanavir is also poorly soluble at physiological and hence is more prone for precipitation. In 2007, a review of the US Food and Drug Administration’s Adverse Event Reporting System identified 30 cases of nephrolithiasis in atazanavir-treated patients[78]. 14 of these 30 patients had their stones analyzed by infrared spectrophotometry, which determined that in 12 patients, stones were composed of atazanavir. The median time to onset of nephrolithiasis in atazanavir-treated patients was 1.7 years in the 17 cases that had detailed information. 17% of the patients who developed nephrolithiasis had underlying liver disease due to hepatitis B or C. 17% patients developed AKI at the time of nephrolithiasis and all patients recovered renal function after the stone was removed and atazanavir was discontinued. In addition to nephrolithiasis, one case of acute interstitial nephritis (AIN) has also been reported with in patients treated with atazanavir[79]. In the EuroSIDA study, atazanavir-treated patients had a 21% increased risk of developing CKD per year when compared to those who were not receiving atazanavir in multivariate models. This increased risk reverted back to baseline after the drug is stopped and the risk of CKD among patients who stopped atazanavir was similar to those who were not exposed to atazanavir[65]. Though mechanism of the development of reversible CKD in patients treated with atazanavir is not known, since atazanavir can induce crystalluria, which can be associated with acute renal failure [80], it is possible that these patients have subclinical renal crystal deposition.
In light of these data, it is likely that atazanavir can cause renal and urologic complications in patients (at a much lower rate) that are reminiscent of indinavir. Though there are no guidelines suggesting specific measures to prevent atazanavir-induced renal/urologic disease, it is possible that atazanavir-treated patients may benefit from conservative measures to avoid dehydration and preserve adequate urine flow to minimize the risk of crystal and stone formation. It may also be reasonable to periodically monitor of renal function during atazanavir therapy, particularly in patients with liver dysfunction. Discontinuation of atazanavir should be considered in patients who develop nephrolithiasis or kidney failure unless other causes are evident. In patients who develop nephrolithiasis due to atazanvir therapy, it is likely that the medication can be restarted once adequate hydration is achieved, however, there are no published data to determine the likelihood of recurrence.
5. Renal dose-adjustment of non-ART medications commonly used in HIV/AIDS
In addition to anti-retroviral therapy, HIV-infected patients are also frequently exposed to antibiotics (including anti-tuberculosis medications), anti-viral agents and anti-fungal agents because these patients are at increased risk of developing infections. Several of these antimicrobial agents are also associated with nephrotoxicity (see Table 3). Clinicians should exercise caution when using these medications in patients with CKD as many of these medications will require dose adjustment to prevent toxicity or may have to be avoided all together.
Table 3.
Adverse renal effects of commonly used antimicrobial medications in patients with HIV/AIDS
| Medication | Renal adverse effects | Reference |
|---|---|---|
| Anti-bacterials | ||
| Beta-lactam antibiotics | AIN | [107] |
| Floroquinoilones | AIN | [107] |
| Rifampin | ATN RPGN AIN Light chain proteinuria |
[108] |
| Streptomycin | Elevated serum creatinine | [109] |
| Trimethoprim + Sulfamethoxazole |
Hyperkalemia RTA AIN Elevated serum creatinine |
[107] |
| Anti-fungals | ||
| Amphotericin (Conventional) |
RTA Elevated serum creatinine |
[110] |
| Anti-virals | ||
| Acyclovir | Crystalluria Obstructive nephropathy ATN |
[111,112] |
| Adefovir | Elevated creatinine | [113] |
| Cidofovir | Proteinuria Elevated creatinine |
[114] |
| Foscarnet | Crystalluria Nephrogenic DI RTA Elevated serum creatinine |
[115,116] |
| Ganciclovir | Elevated serum creatinine | [117] |
ABBREVIATIONS: AIN, acute interstitial nephritis; ATN, acute tubular nephritis; DI, diabetes insipidus; RPGN, rapidly progressive glomerulonephritis; RTA, renal tubular acidosis.
6. Conclusion
HIV-associated nephropathy is the most common kidney disorder in HIV-infected individuals, predominantly affecting patients of African ancestry. A host of other kidney diseases may present in a similar fashion, and kidney biopsy is necessary to establish a definitive diagnosis. Based upon retrospective and observational studies, current guidelines dictate that patients with HIVAN should be treated with cART. Moreover, it is unlikely that such studies will be performed in the future, given the wide acceptance of this practice, and case series and retrospective studies suggesting benefit. The role of cART in kidney diseases other than HIVAN is unclear. Antiretroviral agents, despite their likely renal protective role, can also have substantial nephrotoxic effects, and their dosing must be adjusted with changing renal function.
7. Expert opinion
7.1 Key findings and weaknesses?
Kidney disease is an important cause of morbidity and mortality in HIV-infected patients. HIVAN is the most common cause of chronic kidney disease in HIV-infected patients and is caused by viral infection of the renal epithelium. Observational and retrospective studies suggest that cART significantly attenuates the course of HIVAN though randomized studies are not available. Clinicians who treat these patients should be aware of nephrotoxic effects of several commonly used medications and doses of many ART agents need to be adjusted in patients with impaired kidney function.
7.2 Research potential? Ultimate goal in this field?
HIVAN is an important cause of morbidity and mortality in HIV-infected patients. The recently published association of FSGS and hypertension-attributed ESRD in African Americans with polymorphisms in the APOL1 gene must be verified in a population of patients with HIVAN and, basic studies are needed to explain how polymorphisms in APOL1 predispose to glomerulosclerosis. Further studies are also needed to better understand how HIV infection leads to progressive kidney disease in genetically susceptible individuals. It is likely that advances in the treatment in the prevention and treatment of HIVAN will improve the care of patients with other forms of renal disease, particularly those with focal glomerulosclerosis. More data are also needed to determine whether particular ART agents are protective or deleterious in patients with kidney disease or at risk of kidney disease.
7.3 What is needed to achieve this goal and what is the biggest challenge?
Investigators need to assemble large cohorts of HIV-infected patients with kidney disease and/or at risk of developing kidney disease so that prospective studies can be implemented to determine the optimal treatment strategy for these patients.
7.4 What is going to happen in the next few years?
It is likely that the genetic factors that predispose persons of African descent to HIVAN and other forms of kidney disease will become much better understood and additional risk factors for the development of kidney disease in HIV-infected patients are likely to be elucidated.
7.5 Any particularly interesting areas of research?
Studies that elucidate the mechanisms for increased susceptibility of HIV-infected blacks to kidney disease are likely to yield future treatments for HIV infected and non-infected patients at risk of developing kidney disease. It will also be critical to determine whether modifying the approach to treatment in patients with kidney disease or at risk of developing kidney disease will improve overall clinical outcomes in this population.
Article Highlights
-
-
HIV-infected patients are at increased risk for several forms of chronic kidney disease
-
-
Chronic kidney disease is associated with increased risk of mortality in HIV-infected patients
-
-
Anti-retroviral therapy is the most effective treatment for HIV-associated nephropathy and may have beneficial effects on renal function in patients with some other forms of chronic kidney disease
-
-
Several antiretroviral agents are associated with increased risk of kidney and/or urologic disease, including tenofovir, atazanavir, and indinavir
-
-
Clinicians must exercise caution when prescribing nucleoside and nucleotide reverse transcriptase inhibitors in patients with kidney disease as many of these agents require dose adjustment to reduce the risk of drug toxicity
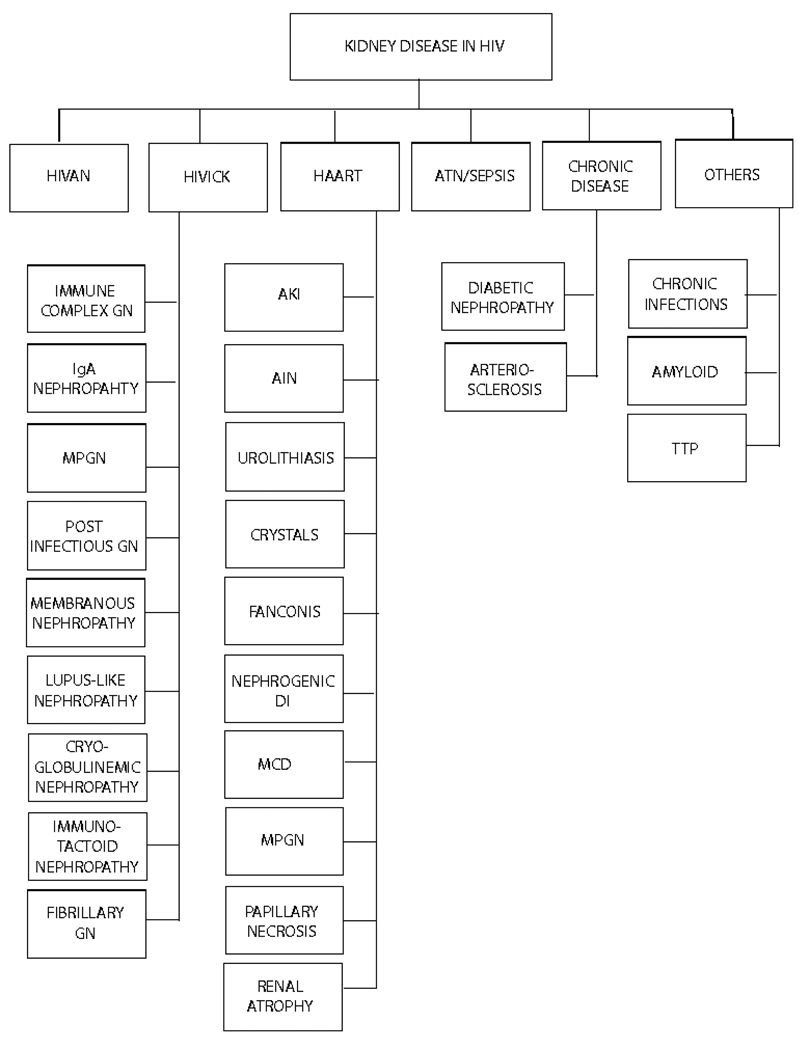
Figure 2.
Spectrum of Kidney Disease in HIV.
Abbreviations: HIVAN, HIV Associated Nephropathy; HIVICK, HIV immune complex kidney disease; HAART, highly active ant retroviral therapy; ATN, acute tubular necrosis; GN, glomerulonephritis; MPGN, membrano-proliferative GN, AKI, acute kidney injury; AIN, acute interstitial nephritis; DI, diabetes insipidus, MCD, minimal change disease; TTP, thrombotic thrombocytopenic purpura.
Footnotes
Declaration of interest
M Ross has received funding from the National Institute of Health
Contributor Information
Zygimantas C. Alsauskas, Assistant Professor of Medicine, Division of Nephrology, University of Louisville, Phone: 502-852-5760, Fax: 502-852-7643, zalsauskas@yahoo.com.
Raj Kiran Medapalli, Fellow in Nephrology, Division of Nephrology, Mt. Sinai School of Medicine, Phone: 212-241-8004, Fax: 212-987-0389, raj.medapalli@mssm.edu.
Michael J. Ross, Associate Professor of Medicine, Division of Nephrology, Mt. Sinai School of Medicine, Phone: 212-241-0131, Fax: 212-987-0389.
References
- 1.UNAIDS. AIDS epidemic update : November 2009. 2009 [Google Scholar]
- 2.Szczech LA, Gange SJ, van der Horst C, et al. Predictors of proteinuria and renal failure among women with HIV infection. Kidney Int. 2002;61:195–202. doi: 10.1046/j.1523-1755.2002.00094.x. [DOI] [PubMed] [Google Scholar]
- 3.Gupta SK, Mamlin BW, Johnson CS, et al. Prevalence of proteinuria and the development of chronic kidney disease in HIV-infected patients. Clin Nephrol. 2004;61:1–6. doi: 10.5414/cnp61001. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt CM, Winston JA, Malvestutto CD, et al. Chronic kidney disease in HIV infection: an urban epidemic. AIDS. 2007;21:2101–2103. doi: 10.1097/QAD.0b013e3282ef1bb4. [DOI] [PubMed] [Google Scholar]
- 5.Mulenga LB, Kruse G, Lakhi S, et al. Baseline renal insufficiency and risk of death among HIV-infected adults on antiretroviral therapy in Lusaka, Zambia. AIDS. 2008;22:1821–1827. doi: 10.1097/QAD.0b013e328307a051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gardner LI, Holmberg SD, Williamson JM, et al. Development of proteinuria or elevated serum creatinine and mortality in HIV-infected women. J Acquir Immune Defic Syndr. 2003;32:203–209. doi: 10.1097/00126334-200302010-00013. [DOI] [PubMed] [Google Scholar]
- 7.Szczech LA, Hoover DR, Feldman JG, et al. Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis. 2004;39:1199–1206. doi: 10.1086/424013. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt CM, Hoover DR, Shi Q, et al. Microalbuminuria Is Associated With All-Cause and AIDS Mortality in Women With HIV Infection. J Acquir Immune Defic Syndr. 2010 doi: 10.1097/QAI.0b013e3181cc1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Agati V, Appel GB. Renal pathology of human immunodeficiency virus infection. Semin Nephrol. 1998;18:406–421. [PubMed] [Google Scholar]
- 10.Rao TK, Filippone EJ, Nicastri AD, et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:669–673. doi: 10.1056/NEJM198403153101101. [DOI] [PubMed] [Google Scholar]
- 11.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 12.Ross MJ, Klotman PE. Recent progress in HIV-associated nephropathy. J Am Soc Nephrol. 2002;13:2997–3004. doi: 10.1097/01.asn.0000040750.40907.99. [DOI] [PubMed] [Google Scholar]
- 13.USRDS. USRDS 2009 Annual Data Report. 2009 [Google Scholar]
- 14.Shahinian V, Rajaraman S, Borucki M, et al. Prevalence of HIV-associated nephropathy in autopsies of HIV-infected patients. Am J Kidney Dis. 2000;35:884–888. doi: 10.1016/s0272-6386(00)70259-9. [DOI] [PubMed] [Google Scholar]
- 15.Wyatt CM, Morgello S, Katz-Malamed R, et al. The spectrum of kidney disease in patients with AIDS in the era of antiretroviral therapy. Kidney Int. 2009;75:428–434. doi: 10.1038/ki.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han TM, Naicker S, Ramdial PK, et al. A cross-sectional study of HIV-seropositive patients with varying degrees of proteinuria in South Africa. Kidney Int. 2006;69:2243–2250. doi: 10.1038/sj.ki.5000339. [DOI] [PubMed] [Google Scholar]
- 17.Berliner AR, Fine DM, Lucas GM, et al. Observations on a cohort of HIV-infected patients undergoing native renal biopsy. Am J Nephrol. 2008;28:478–486. doi: 10.1159/000112851. [DOI] [PubMed] [Google Scholar]
- 18.Ross MJ, Klotman PE, Winston JA. HIV-associated nephropathy: case study and review of the literature. AIDS Patient Care STDS. 2000;14:637–645. doi: 10.1089/10872910050206559. [DOI] [PubMed] [Google Scholar]
- 19.Fine DM, Perazella MA, Lucas GM, et al. Kidney biopsy in HIV: beyond HIV-associated nephropathy. Am J Kidney Dis. 2008;51:504–514. doi: 10.1053/j.ajkd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Izzedine H, Deray G. The nephrologist in the HAART era. AIDS. 2007;21:409–421. doi: 10.1097/QAD.0b013e328011ec40. [DOI] [PubMed] [Google Scholar]
- 21.Szczech LA, Gupta SK, Habash R, et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 22.Nochy D, Glotz D, Dosquet P, et al. Renal disease associated with HIV infection: a multicentric study of 60 patients from Paris hospitals. Nephrol Dial Transplant. 1993;8:11–19. doi: 10.1093/oxfordjournals.ndt.a092263. [DOI] [PubMed] [Google Scholar]
- 23.Kopp JB, Winkler C. HIV-associated nephropathy in African Americans. Kidney Int Suppl. 2003;83:S43–S49. doi: 10.1046/j.1523-1755.63.s83.10.x. [DOI] [PubMed] [Google Scholar]
- 24.Leventhal JS, Ross MJ. Pathogenesis of HIV-Associated Nephropathy. Semin Nephrol. 2008;28:523–534. doi: 10.1016/j.semnephrol.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Freedman BI, Soucie JM, Stone SM, et al. Familial clustering of end-stage renal disease in blacks with HIV-associated nephropathy. Am J Kidney Dis. 1999;34:254–258. doi: 10.1016/s0272-6386(99)70352-5. [DOI] [PubMed] [Google Scholar]
- 26.Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008 doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008 doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong F, Li S, Pujol-Moix N, et al. Genotype-phenotype correlation in MYH9-related thrombocytopenia. Br J Haematol. 2005;130:620–627. doi: 10.1111/j.1365-2141.2005.05658.x. [DOI] [PubMed] [Google Scholar]
- 29.Genovese G, Friedman DJ, Ross MD, et al. Association of Trypanolytic ApoL1 Variants with Kidney Disease in African-Americans. Science. 2010 doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010 doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta SK, Eustace JA, Winston JA, et al. Guidelines for the management of chronic kidney disease in HIV-infected patients: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2005;40:1559–1585. doi: 10.1086/430257. [DOI] [PubMed] [Google Scholar]
- 32.Hammer SM, Eron JJJ, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 33.Ifudu O, Rao TK, Tan CC, et al. Zidovudine is beneficial in human immunodeficiency virus associated nephropathy. Am J Nephrol. 1995;15:217–221. doi: 10.1159/000168835. [DOI] [PubMed] [Google Scholar]
- 34.Wali RK, Drachenberg CI, Papadimitriou JC, et al. HIV-1-associated nephropathy and response to highly-active antiretroviral therapy [letter] Lancet. 1998;352:783–784. doi: 10.1016/S0140-6736(98)24037-2. [DOI] [PubMed] [Google Scholar]
- 35.Winston JA, Bruggeman LA, Ross MD, et al. Nephropathy and establishment of a renal reservoir of HIV type 1 during primary infection. N Engl J Med. 2001;344:1979–1984. doi: 10.1056/NEJM200106283442604. [DOI] [PubMed] [Google Scholar]
- 36.Szczech LA, Edwards LJ, Sanders LL, et al. Protease inhibitors are associated with a slowed progression of HIV- related renal diseases. Clin Nephrol. 2002;57:336–341. doi: 10.5414/cnp57336. [DOI] [PubMed] [Google Scholar]
- 37.Atta MG, Gallant JE, Rahman MH, et al. Antiretroviral therapy in the treatment of HIV-associated nephropathy. Nephrol Dial Transplant. 2006;21:2809–2813. doi: 10.1093/ndt/gfl337. [DOI] [PubMed] [Google Scholar]
- 38.Lucas GM, Eustace JA, Sozio S, et al. Highly active antiretroviral therapy and the incidence of HIV-1-associated nephropathy: a 12-year cohort study. Aids. 2004;18:541–546. doi: 10.1097/00002030-200402200-00022. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz EJ, Szczech LA, Ross MJ, et al. Highly active antiretroviral therapy and the epidemic of HIV+ end-stage renal disease. J Am Soc Nephrol. 2005;16:2412–2420. doi: 10.1681/ASN.2005040340. [DOI] [PubMed] [Google Scholar]
- 40.Yahaya I, Uthman AO, Uthman MM. Interventions for HIV-associated nephropathy. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD007183.pub2. CD007183. [DOI] [PubMed] [Google Scholar]
- 41.Smith MC, Austen JL, Carey JT, et al. Prednisone improves renal function and proteinuria in human immunodeficiency virus-associated nephropathy. Am J Med. 1996;101:41–48. doi: 10.1016/s0002-9343(96)00065-4. [DOI] [PubMed] [Google Scholar]
- 42.Eustace JA, Nuermberger E, Choi M, et al. Cohort study of the treatment of severe HIV-associated nephropathy with corticosteroids. Kidney Int. 2000;58:1253–1260. doi: 10.1046/j.1523-1755.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 43.Laradi A, Mallet A, Beaufils H, et al. HIV-associated nephropathy: outcome and prognosis factors. Groupe d' Etudes Nephrologiques d'Ile de France. J Am Soc Nephrol. 1998;9:2327–2335. doi: 10.1681/ASN.V9122327. [DOI] [PubMed] [Google Scholar]
- 44.Sarafidis PA, Khosla N, Bakris GL. Antihypertensive therapy in the presence of proteinuria. Am J Kidney Dis. 2007;49:12–26. doi: 10.1053/j.ajkd.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Jones DW, Hall JE. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure and evidence from new hypertension trials. Hypertension. 2004;43:1–3. doi: 10.1161/01.HYP.0000110061.06674.ca. [DOI] [PubMed] [Google Scholar]
- 46.K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–S290. [PubMed] [Google Scholar]
- 47.Kimmel PL, Mishkin GJ, Umana WO. Captopril and renal survival in patients with human immunodeficiency virus nephropathy. Am J Kidney Dis. 1996;28:202–208. doi: 10.1016/s0272-6386(96)90302-9. [DOI] [PubMed] [Google Scholar]
- 48.Burns GC, Paul SK, Toth IR, et al. Effect of angiotensin-converting enzyme inhibition in HIV-associated nephropathy. J Am Soc Nephrol. 1997;8:1140–1146. doi: 10.1681/ASN.V871140. [DOI] [PubMed] [Google Scholar]
- 49.Wei A, Burns GC, Williams BA, et al. Long-term renal survival in HIV-associated nephropathy with angiotensin-converting enzyme inhibition. Kidney Int. 2003;64:1462–1471. doi: 10.1046/j.1523-1755.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- 50.Szczech LA, Winston JA. The impact of antiretroviral therapy on HIVAN. Kidney Int. 2004;65:1114. doi: 10.1111/j.1523-1755.2004.501_7.x. author reply 1115. [DOI] [PubMed] [Google Scholar]
- 51.Ingulli E, Tejani A, Fikrig S, et al. Nephrotic syndrome associated with acquired immunodeficiency syndrome in children. J Pediatr. 1991;119:710–716. doi: 10.1016/s0022-3476(05)80284-7. [DOI] [PubMed] [Google Scholar]
- 52.Reid A, Stohr W, Walker AS, et al. Severe renal dysfunction and risk factors associated with renal impairment in HIV-infected adults in Africa initiating antiretroviral therapy. Clin Infect Dis. 2008;46:1271–1281. doi: 10.1086/533468. [DOI] [PubMed] [Google Scholar]
- 53.Peters PJ, Moore DM, Mermin J, et al. Antiretroviral therapy improves renal function among HIV-infected Ugandans. Kidney Int. 2008 doi: 10.1038/ki.2008.305. [DOI] [PubMed] [Google Scholar]
- 54.Kalayjian RC, Franceschini N, Gupta SK, et al. Suppression of HIV-1 replication by antiretroviral therapy improves renal function in persons with low CD4 cell counts and chronic kidney disease. AIDS. 2008;22:481–487. doi: 10.1097/QAD.0b013e3282f4706d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Longenecker CT, Scherzer R, Bacchetti P, et al. HIV viremia and changes in kidney function. AIDS. 2009 doi: 10.1097/QAD.0b013e32832a3f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mocroft A, Wyatt C, Szczech L, et al. Interruption of antiretroviral therapy is associated with increased plasma cystatin C. AIDS. 2009;23:71–82. doi: 10.1097/QAD.0b013e32831cc129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rachakonda AK, Kimmel PL. CKD in HIV-infected patients other than HIV-associated nephropathy. Adv Chronic Kidney Dis. 2010;17:83–93. doi: 10.1053/j.ackd.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Gorriz JL, Rovira E, Sancho A, et al. IgA nephropathy associated with human immuno deficiency virus infection: antiproteinuric effect of captopril. Nephrol Dial Transplant. 1997;12:2796–2797. doi: 10.1093/ndt/12.12.2796. [DOI] [PubMed] [Google Scholar]
- 59.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2009. Dec 1, pp. 1–161. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed Oct 21, 2010, page 159–160. [Google Scholar]
- 60.Ray AS, Cihlar T, Robinson KL, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50:3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ortiz A, Justo P, Sanz A, et al. Tubular cell apoptosis and cidofovir-induced acute renal failure. Antivir Ther. 2005;10:185–190. [PubMed] [Google Scholar]
- 62.Bendele RA, Richardson FC. Adefovir nephrotoxicity and mitochondrial DNA depletion. Hum Pathol. 2002;33:574. doi: 10.1053/hupa.2002.124012. [DOI] [PubMed] [Google Scholar]
- 63.Gitman MD, Hirschwerk D, Baskin CH, et al. Tenofovir-induced kidney injury. Expert Opin Drug Saf. 2007;6:155–164. doi: 10.1517/14740338.6.2.155. [DOI] [PubMed] [Google Scholar]
- 64.Nelson MR, Katlama C, Montaner JS, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. AIDS. 2007;21:1273–1281. doi: 10.1097/QAD.0b013e3280b07b33. [DOI] [PubMed] [Google Scholar]
- 65.Mocroft A, Kirk O, Reiss P, et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24:1667–1678. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 66.Gallant JE, Parish MA, Keruly JC, et al. Changes in renal function associated with tenofovir disoproxil fumarate treatment, compared with nucleoside reverse-transcriptase inhibitor treatment. Clin Infect Dis. 2005;40:1194–1198. doi: 10.1086/428840. [DOI] [PubMed] [Google Scholar]
- 67.Young B, Buchacz K, Baker RK, et al. Renal Function in Tenofovir-Exposed and Tenofovir-Unexposed Patients Receiving Highly Active Antiretroviral Therapy in the HIV Outpatient Study. J Int Assoc Physicians AIDS Care (Chic Ill) 2007;6:178–187. doi: 10.1177/1545109707300676. [DOI] [PubMed] [Google Scholar]
- 68.Fux CA, Simcock M, Wolbers M, et al. Tenofovir use is associated with a reduction in calculated glomerular filtration rates in the Swiss HIV Cohort Study. Antivir Ther. 2007;12:1165–1173. [PubMed] [Google Scholar]
- 69.Winston A, Amin J, Mallon P, et al. Minor changes in calculated creatinine clearance and anion-gap are associated with tenofovir disoproxil fumarate-containing highly active antiretroviral therapy. HIV Med. 2006;7:105–111. doi: 10.1111/j.1468-1293.2006.00349.x. [DOI] [PubMed] [Google Scholar]
- 70.Goicoechea M, Liu S, Best B, et al. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis. 2008;197:102–108. doi: 10.1086/524061. [DOI] [PubMed] [Google Scholar]
- 71.Gallant JE, Moore RD. Renal function with use of a tenofovir-containing initial antiretroviral regimen. AIDS. 2009;23:1971–1975. doi: 10.1097/QAD.0b013e32832c96e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parsonage MJ, Wilkins EG, Snowden N, et al. The development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapy. HIV Med. 2005;6:341–346. doi: 10.1111/j.1468-1293.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 73.Gagnon RF, Tecimer SN, Watters AK, et al. Prospective study of urinalysis abnormalities in HIV-positive individuals treated with indinavir. Am J Kidney Dis. 2000;36:507–515. doi: 10.1053/ajkd.2000.9791. [DOI] [PubMed] [Google Scholar]
- 74.Jao J, Wyatt CM. Antiretroviral medications: adverse effects on the kidney. Adv Chronic Kidney Dis. Jan;17:72–82. doi: 10.1053/j.ackd.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Kopp JB, Miller KD, Mican JA, et al. Crystalluria and urinary tract abnormalities associated with indinavir. Ann Intern Med. 1997;127:119–125. doi: 10.7326/0003-4819-127-2-199707150-00004. [DOI] [PubMed] [Google Scholar]
- 76.Dieleman JP, Sturkenboom MC, Jambroes M, et al. Risk factors for urological symptoms in a cohort of users of the HIV protease inhibitor indinavir sulfate: the ATHENA cohort. Arch Intern Med. 2002;162:1493–1501. doi: 10.1001/archinte.162.13.1493. [DOI] [PubMed] [Google Scholar]
- 77.Kopp JB, Falloon J, Filie A, et al. Indinavir-associated interstitial nephritis and urothelial inflammation: clinical and cytologic findings. Clin Infect Dis. 2002;34:1122–1128. doi: 10.1086/339486. [DOI] [PubMed] [Google Scholar]
- 78.Chan-Tack KM, Truffa MM, Struble KA, et al. Atazanavir-associated nephrolithiasis: cases from the US Food and Drug Administration's Adverse Event Reporting System. AIDS. 2007;21:1215–1218. doi: 10.1097/QAD.0b013e32813aee35. [DOI] [PubMed] [Google Scholar]
- 79.Brewster UC, Perazella MA. Acute interstitial nephritis associated with atazanavir, a new protease inhibitor. Am J Kidney Dis. 2004;44:e81–e84. [PubMed] [Google Scholar]
- 80.Izzedine H, M'rad MB, Bardier A, et al. Atazanavir crystal nephropathy. AIDS. 2007;21:2357–2358. doi: 10.1097/QAD.0b013e3282f17503. [DOI] [PubMed] [Google Scholar]
- 81.Ahmad M. Abacavir-induced reversible Fanconi syndrome with nephrogenic diabetes insipidus in a patient with acquired immunodeficiency syndrome. J Postgrad Med. 2006;52:296–297. [PubMed] [Google Scholar]
- 82.Krishnan M, Nair R, Haas M, et al. Acute renal failure in an HIV-positive 50-year-old man. Am J Kidney Dis. 2000;36:1075–1078. doi: 10.1053/ajkd.2000.19114. [DOI] [PubMed] [Google Scholar]
- 83.Crowther MA, Callaghan W, Hodsman AB, et al. Dideoxyinosine-associated nephrotoxicity. AIDS. 1993;7:131–132. [PubMed] [Google Scholar]
- 84.Izzedine H, Launay-Vacher V, Deray G. Fanconi syndrome associated with didanosine therapy. AIDS. 2005;19:844–845. doi: 10.1097/01.aids.0000168985.05209.b8. [DOI] [PubMed] [Google Scholar]
- 85.Nelson M, Azwa A, Sokwala A, et al. Fanconi syndrome and lactic acidosis associated with stavudine and lamivudine therapy. AIDS. 2008;22:1374–1376. doi: 10.1097/QAD.0b013e328303be50. [DOI] [PubMed] [Google Scholar]
- 86.Morris AA, Baudouin SV, Snow MH. Renal tubular acidosis and hypophosphataemia after treatment with nucleoside reverse transcriptase inhibitors. AIDS. 2001;15:140–141. doi: 10.1097/00002030-200101050-00027. [DOI] [PubMed] [Google Scholar]
- 87.Van Rompay KK, Brignolo LL, Meyer DJ, et al. Biological effects of short-term or prolonged administration of 9-[2-(phosphonomethoxy)propyl]adenine (tenofovir) to newborn and infant rhesus macaques. Antimicrob Agents Chemother. 2004;48:1469–1487. doi: 10.1128/AAC.48.5.1469-1487.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Judd A, Boyd KL, Stohr W, et al. Effect of tenofovir disoproxil fumarate on risk of renal abnormality in HIV-1-infected children on antiretroviral therapy: a nested case-control study. AIDS. 2010;24:525–534. doi: 10.1097/QAD.0b013e3283333680. [DOI] [PubMed] [Google Scholar]
- 89.Angel-Moreno-Maroto A, Suarez-Castellano L, Hernandez-Cabrera M, et al. Severe efavirenz-induced hypersensitivity syndrome (not-DRESS) with acute renal failure. J Infect. 2006;52:e39–e40. doi: 10.1016/j.jinf.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 90.Barbour TD, Furlong TJ, Finlayson RJ. Efavirenz-associated podocyte damage. AIDS. 2007;21:257–258. doi: 10.1097/QAD.0b013e32801120d1. [DOI] [PubMed] [Google Scholar]
- 91.Wirth GJ, Teuscher J, Graf JD, et al. Efavirenz-induced urolithiasis. Urol Res. 2006;34:288–289. doi: 10.1007/s00240-006-0052-6. [DOI] [PubMed] [Google Scholar]
- 92.Curry E, Thomas M, Yehia M. Renal impairment and hypersensitivity reaction due to efavirenz. Nephrology (Carlton) 2008;13:541 doi: 10.1111/j.1440-1797.2008.00968.x. [DOI] [PubMed] [Google Scholar]
- 93.Knudtson E, Para M, Boswell H, et al. Drug rash with eosinophilia and systemic symptoms syndrome and renal toxicity with a nevirapine-containing regimen in a pregnant patient with human immunodeficiency virus. Obstet Gynecol. 2003;101:1094–1097. doi: 10.1016/s0029-7844(02)02620-0. [DOI] [PubMed] [Google Scholar]
- 94.Feicke A, Rentsch KM, Oertle D, et al. Same patient, new stone composition: amprenavir urinary stone. Antivir Ther. 2008;13:733–734. [PubMed] [Google Scholar]
- 95.Hanabusa H, Tagami H, Hataya H. Renal atrophy associated with long-term treatment with indinavir. N Engl J Med. 1999;340:392–393. doi: 10.1056/NEJM199902043400515. [DOI] [PubMed] [Google Scholar]
- 96.Iba-Ba J, Yombi JC, Danse E, et al. [Bilateral papillary necrosis during indinavir treatment] Presse Med. 2008;37:967–969. doi: 10.1016/j.lpm.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 97.van Rossum AM, Dieleman JP, Fraaij PL, et al. Persistent sterile leukocyturia is associated with impaired renal function in human immunodeficiency virus type 1-infected children treated with indinavir. Pediatrics. 2002;110:e19. doi: 10.1542/peds.110.2.e19. [DOI] [PubMed] [Google Scholar]
- 98.Stricker RB, Man KM, Bouvier DB, et al. Pancreatorenal syndrome associated with combination antiretroviral therapy in HIV infection. Lancet. 1997;349:1745–1746. doi: 10.1016/S0140-6736(05)62957-1. [DOI] [PubMed] [Google Scholar]
- 99.Doco-Lecompte T, Garrec A, Thomas L, et al. Lopinavir-ritonavir (Kaletra) and lithiasis: seven cases. AIDS. 2004;18:705–706. doi: 10.1097/00002030-200403050-00022. [DOI] [PubMed] [Google Scholar]
- 100.Engeler DS, John H, Rentsch KM, et al. Nelfinavir urinary stones. J Urol. 2002;167:1384–1385. [PubMed] [Google Scholar]
- 101.Green ST, McKendrick MW, Schmid ML, et al. Renal calculi developing de novo in a patient taking saquinavir. Int J STD AIDS. 1998;9:555. [PubMed] [Google Scholar]
- 102.Zhang X, Lalezari JP, Badley AD, et al. Assessment of drug-drug interaction potential of enfuvirtide in human immunodeficiency virus type 1-infected patients. Clin Pharmacol Ther. 2004;75:558–568. doi: 10.1016/j.clpt.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 103.Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gulick RM, Su Z, Flexner C, et al. Phase 2 study of the safety and efficacy of vicriviroc, a CCR5 inhibitor, in HIV-1-Infected, treatment-experienced patients: AIDS clinical trials group 5211. J Infect Dis. 2007;196:304–312. doi: 10.1086/518797. [DOI] [PubMed] [Google Scholar]
- 105.Steigbigel RT, Cooper DA, Kumar PN, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359:339–354. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 106.DeJesus E, Berger D, Markowitz M, et al. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr. 2006;43:1–5. doi: 10.1097/01.qai.0000233308.82860.2f. [DOI] [PubMed] [Google Scholar]
- 107.Parkhie SM, Fine DM, Lucas GM, et al. Characteristics of Patients with HIV and Biopsy-Proven Acute Interstitial Nephritis. Clin J Am Soc Nephrol. 2010 doi: 10.2215/CJN.08211109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Vriese AS, Robbrecht DL, Vanholder RC, et al. Rifampicin-associated acute renal failure: pathophysiologic, immunologic, and clinical features. Am J Kidney Dis. 1998;31:108–115. doi: 10.1053/ajkd.1998.v31.pm9428460. [DOI] [PubMed] [Google Scholar]
- 109.de Jager P, van Altena R. Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. Int J Tuberc Lung Dis. 2002;6:622–627. [PubMed] [Google Scholar]
- 110.Wingard JR, Kubilis P, Lee L, et al. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin Infect Dis. 1999;29:1402–1407. doi: 10.1086/313498. [DOI] [PubMed] [Google Scholar]
- 111.Becker BN, Fall P, Hall C, et al. Rapidly progressive acute renal failure due to acyclovir: case report and review of the literature. Am J Kidney Dis. 1993;22:611–615. doi: 10.1016/s0272-6386(12)80939-5. [DOI] [PubMed] [Google Scholar]
- 112.Sawyer MH, Webb DE, Balow JE, et al. Acyclovir-induced renal failure. Clinical course and histology. Am J Med. 1988;84:1067–1071. doi: 10.1016/0002-9343(88)90313-0. [DOI] [PubMed] [Google Scholar]
- 113.Ha NB, Garcia RT, Trinh HN, et al. Renal dysfunction in chronic hepatitis B patients treated with adefovir dipivoxil. Hepatology. 2009;50:727–734. doi: 10.1002/hep.23044. [DOI] [PubMed] [Google Scholar]
- 114.Ljungman P, Deliliers GL, Platzbecker U, et al. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. The Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2001;97:388–392. doi: 10.1182/blood.v97.2.388. [DOI] [PubMed] [Google Scholar]
- 115.Maurice-Estepa L, Daudon M, Katlama C, et al. Identification of crystals in kidneys of AIDS patients treated with foscarnet. Am J Kidney Dis. 1998;32:392–400. doi: 10.1053/ajkd.1998.v32.pm9740154. [DOI] [PubMed] [Google Scholar]
- 116.Navarro JF, Quereda C, Gallego N, et al. Nephrogenic diabetes insipidus and renal tubular acidosis secondary to foscarnet therapy. Am J Kidney Dis. 1996;27:431–434. doi: 10.1016/s0272-6386(96)90369-8. [DOI] [PubMed] [Google Scholar]
- 117.Schmidt GM, Horak DA, Niland JC, et al. A randomized, controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants; The City of Hope-Stanford-Syntex CMV Study Group. N Engl J Med. 1991;324:1005–1011. doi: 10.1056/NEJM199104113241501. [DOI] [PubMed] [Google Scholar]