Abstract
Autophagy is emerging as a central regulator of cellular health and disease and, in the central nervous system (CNS), this homeostatic process appears to influence synaptic growth and plasticity. Herein, we review the evidence that dysregulation of autophagy may contribute to several neurodegenerative diseases of the CNS. Up-regulation of autophagy may prevent, delay or ameliorate at least some of these disorders, and – based on recent findings from our laboratory – we speculate that this goal may be achieved using a safe, simple, and inexpensive approach.
Keywords: autophagy, neuroinflammation, fasting, Multiple Sclerosis, HIV
INTRODUCTION
Autophagy is a critical cellular process that involves the sequestration, degradation and digestion of intracellular components by lysosomes. This process is vital both for metabolic homeostasis, allowing healthy cells to efficiently remove and recycle cellular constituents, and for maintenance of cellular integrity, by preventing the accumulation of misfolded proteins and of damaged organelles [e.g. mitochondria which, when damaged, may release harmful reactive oxygen species (ROS) into the cytosol]. In recent years it has become clear that autophagy is a vital arbiter of death/survival decisions in cells, and constitutes a critical defense against many infections and degenerative states (Kundu and Thompson, 2008; Levine and Klionsky, 2004; Mizushima et al., 2008; Levine and Kroemer, 2009; Orvedahl and Levine, 2009). Studies have shown that autophagy in neurons is a protective mechanism that slows the advance of neurodegenerative disorders, and that its inhibition is associated with neurodegeneration (Martinez-Vicente and Cuervo, 2007). Perhaps related to this, autophagy also appears to play an important role in synaptic growth and plasticity, and may impact learning and memory (Shen and Ganetzky, 2009). Consequently, substantial attention is being paid to the molecular mechanisms by which autophagy limits neurodegenerative diseases, to its role in early stages of disease pathogenesis, and to the development of methods to up-regulate neuronal autophagy for therapeutic benefit (Rubinsztein et al., 2005). However, when discussing neurodegeneration one should not consider only the neurons; neuroinflammation – typified by glial cell activation and lymphocytic infiltration – is a common accompaniment to (and, in some cases, a precipitant of) neurodegenerative disease, and the effects of these non-neuronal cells can be profound (Hauser and Oksenberg, 2006).
Herein, we review the significance of autophagy in neurodegenerative disease, highlighting its possible importance both in infectious neurodegenerative disorders (e.g., HIV-1 associated neurocognitive disorder, HAND) and in immune-mediated neurodegeneration [e.g., multiple sclerosis (MS)]. Furthermore, we discuss how autophagy may modulate neurodegenerative diseases in two ways. First, directly; the level of autophagy within neurons can be altered – in some cases, in response to soluble factors released from glial cells or infiltrating lymphocytes – and this can affect neuronal viability. Second, indirectly; changes in autophagy within CNS-infiltrating lymphocytes may alter the immunopathologic and neurotoxic potential of those cells.
Infections can alter neuronal autophagy, thereby exacerbating neurodegenerative disease
HAND is an acquired cognitive and motor disease that includes three categories of disorders graded by ascending dysfunction: asymptomatic neurocognitive impairment, mild neurocognitive disorder and the most severe manifestation, HIV-associated dementia (HAD). At the beginning of the AIDS epidemic, before effective diagnosis and treatments were available, HAD was commonly observed, primarily in patients with long-term HIV disease and low CD4+ T cell counts. Neuroinflammation, generally termed HIV encephalitis, often was found in these patients and was characterized by activated microglia, infiltrating peripheral macrophages, HIV-infected multinucleated giant cells and pronounced astrocytosis. There is compelling evidence that the above HIV-related neurodegeneration does not result from virus infection of neurons; rather, other cells within the CNS are infected, and neural death is caused by molecules that are released from those infected cells. HIV and SIV can activate and/or infect macrophages/microglia in the brain (Alirezaei et al., 2008a; Gonzalez-Scarano and Martin-Garcia, 2005; Kaul et al., 2005), and also can infect microvascular endothelial cells, which represent an additional source of inflammatory factors (Chaudhuri et al., 2008; Moses et al., 1996). A variety of molecules released from those cells can have neurotoxic effects, including cytokines, chemokines, inducers of oxidative stress, and certain viral proteins (Alirezaei et al., 2007; Gonzalez-Scarano and Martin-Garcia, 2005; Bruce-Keller et al., 2003; Zhang et al., 2003). For example, HIV-1 gp120 has a toxic effect on neuronal primary cells (Alirezaei et al., 2008b). Furthermore, glial cell dysfunction can indirectly harm neurons. Excessive extracellular accumulation of the neurotransmitter glutamate has a profoundly excitotoxic effect that is mediated by increased influx of Ca2+. In the healthy CNS, glutamate is rapidly removed from the synaptic cleft. This task falls largely to astrocytes, which express glutamate transporters (GLT-1 and GLAST) that quickly internalize this amino acid, which then is catabolized within the glial cell. CNS inflammation can cause a functional or numerical deficit in these transporter molecules and, even in the presence of astrocytosis, this transporter-mediated uptake of glutamate often is impaired, leading to glutamate excitotoxicity (Lobsiger and Cleveland, 2007). Thus, inflammatory cells, and pro-inflammatory molecules released by glial cells, play a role in HIV-related neurodegeneration.
In what way might these neurodegenerative outcomes intersect with neuronal autophagy? Inflammatory mediators such as nitric oxide (NO) and other ROS have pleiotropic effects, one of which is their diversion or disruption of autophagic functions (Berliocchi et al., 2007; Scherz-Shouval and Elazar, 2007; Kiffin et al., 2006). The influx of Ca2+, acting via ROS, activates caspases and calpains, and calpain activation reduces neuronal autophagy (Demarchi et al., 2006; Yousefi et al., 2006). It is well-established that deficits in autophagy in differentiated cells such as neurons can have a profoundly negative impact by, for example, provoking aggregation or misfolding of proteins, with consequent neurotoxicity (Alirezaei et al., 2008a; Hara et al., 2006; Komatsu et al., 2006).
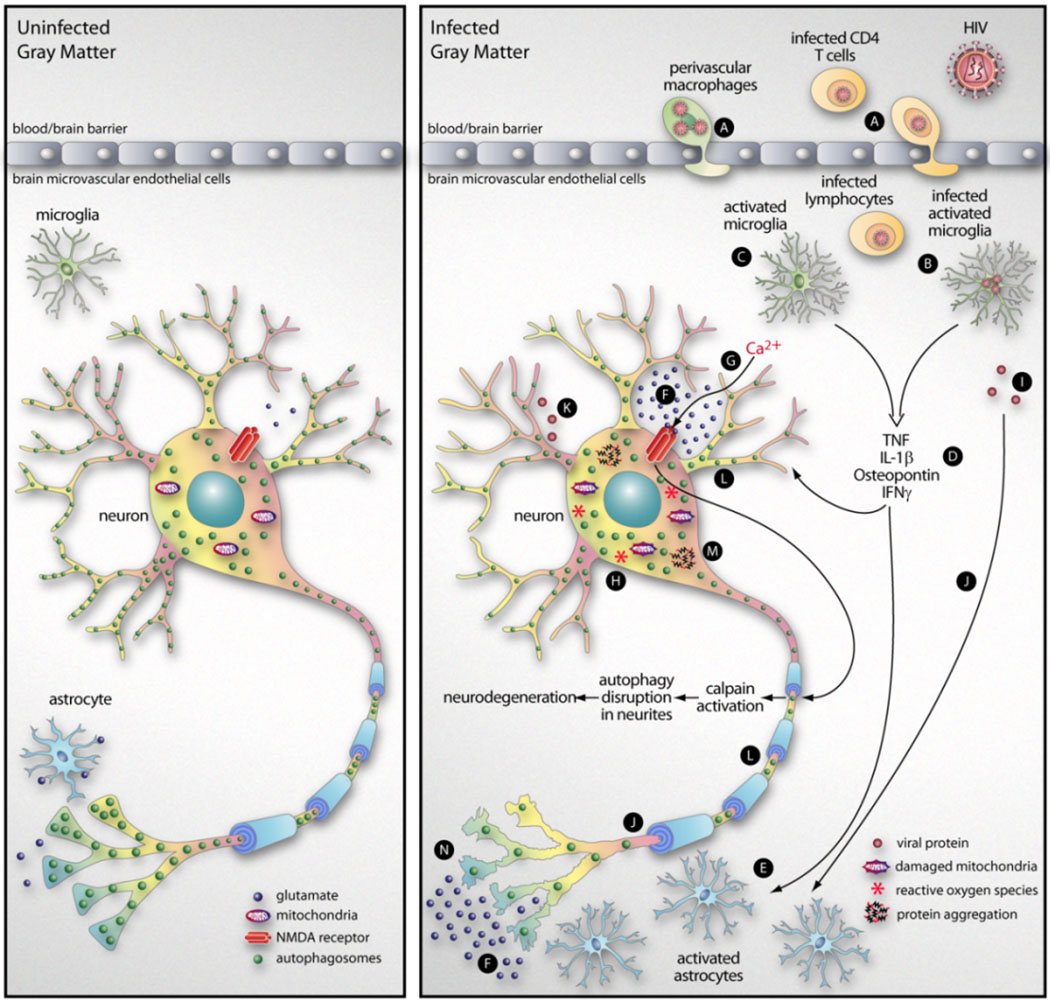
Studies of primary neurons have shown that autophagy is down-modulated by soluble products shed by activated microglia from the brain of an SIV-infected monkey (Alirezaei et al., 2008a); exposure to microglial supernatant led to a reduction in autophagosome numbers within the neurites of cortical neurons, while the number of autophagosomes in the cell bodies was not significantly altered. This effect was reversed when the cells were treated with rapamycin, a potent inducer of autophagy. Together with further biochemical analyses (decrease of Atg12-Atg5 complex and LC3-II levels, increase of sequestosome-1/p62), these experiments indicated that microglial products induced a disruption of autophagy in neuronal cultures (Alirezaei et al., 2008a). The observed disruption in autophagy could be mimicked using excitotoxic and/or pro-inflammatory molecules such as glutamate and TNF, both of which are present in the brain during HIV encephalitis (Alirezaei et al., 2008a) or during neuroinflammation in general. Thus, we propose that, in infectious neurodegenerative diseases, some of the deleterious changes may result from the inhibition of neuronal autophagy, driven by neurotoxic soluble mediators that are released as a response to infection. A cartoon is provided summarizing how these proposed effects may occur during HIV infection (Figure 1).
Figure 1. Summary of proposed mechanism of HIV-mediated neurodegeneration.
HIV-infected cells, in particular infected monocytes/macrophages and CD4+ T cells, enter the CNS by crossing the blood-brain barrier in a process termed diapedesis (A). The introduction of infection into the CNS leads to the activation of microglia, either directly (by infection, B) or indirectly (C). These activated microglia produce a variety of cytokines and chemokines (D) with pleiotropic effects, including the activation of astrocytes (E). This leads to an increase in astrocyte abundance (astrocytosis) but, despite the increase in cell numbers, there is a decrease in a key astrocytic function: the resorption of glutamate (F). The increase in extracellular glutamate, acting via the N-methyl-D-aspartic acid (NMDA) receptor, can produce an excessive influx of Ca2+ into neurons (G) with consequent free-radical formation (H), leading to Ca2+ excitotoxicity (Berliocchi et al., 2007). In addition, viral proteins shed from infected microglia (I) may have neurotoxic effects that can be indirect (acting via astrocytes, J) or direct (K). ROS formation also activates intracellular calpain and, as described in the text, this causes a marked disruption of neuronal autophagy, with a reduction in the abundance of autophagosomes in neurites (L). This, in turn, leads to an increase in intra-neuronal protein aggregates (M) and neurodegeneration (N).
Potential impact of neuronal autophagy in non-infectious neurodegenerative diseases
It is thought that glia-driven neurotoxicity also may be a prominent cause of a variety of non-infectious neurodegenerative disorders, contributing to both the initiation and progression of disease (Lobsiger and Cleveland, 2007), and here, too, autophagy appears to play an important role. Complex interactions between glial cells, extracellular matrix, neurons, endothelia and host immune cells regulate homeostasis and orchestrate neuroinflammation and degeneration. These interactions can contribute to disease initiation, for example, in spinocerebellar ataxia (Custer et al., 2006; Garden et al., 2002; Lobsiger and Cleveland, 2007). Autophagy has recently been implicated in another non-infectious neurodegenerative disorder, amyotrophic lateral sclerosis (ALS), a disease in which ~90% of human cases are sporadic and of unknown etiology (Gal et al., 2009; Hetz et al., 2009). In the MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of Parkinson’s disease, there is marked microgliosis, and these cells produce a variety of pro-inflammatory cytokines, as well as inducible nitric oxide synthase (iNOS) (Croisier and Graeber, 2006) and, as noted above, these ROS can disrupt autophagy. ROS also appear to be involved in Huntington’s disease (HD), in which iNOS levels are elevated in the human brain (Chen et al., 2000). Protein aggregation, commonly observed when autophagy is disrupted, is another characteristic of HD (Davies et al., 1997; Scherzinger et al., 1997) and, in a mouse model of HD, mutant huntingtin protein accumulated in the nuclei of astrocytes; this was accompanied by a decrease in glutamate transporter expression, with a parallel increase in glutamate excitotoxicity (Shin et al., 2005). Minocycline, a semisynthetic tetracycline that inhibits iNOS, confers protection against several neurodegenerative disorders, including ALS and Parkinson’s disease (Zhu et al., 2002; Du et al., 2001), and the effect of this drug on ALS is currently being evaluated in clinical trials (Siciliano et al., 2010). Similarly, minocycline delays mortality in the mouse model of HD, and the delay is accompanied by decreased activation of iNOS in the brain (Chen et al., 2000). We propose that the minocycline-driven reduction in NO and other ROS restores autophagy within the neurons, limiting the aggregation of proteins, and delaying or preventing irrevocable harm. Furthermore, others have reported that, in Alzheimer’s disease, there is an accumulation of neuronal autophagosomes that appears to result from a failure in their fusion with lysosomes (Cataldo et al., 1996; Nixon et al., 2005). Thus, autophagy may be involved in a variety of non-infectious neurodegenerative diseases, and we speculate below that changes in neuronal autophagy also may contribute to multiple sclerosis (MS), a common chronic inflammatory, demyelinating and neurodegenerative disease of CNS.
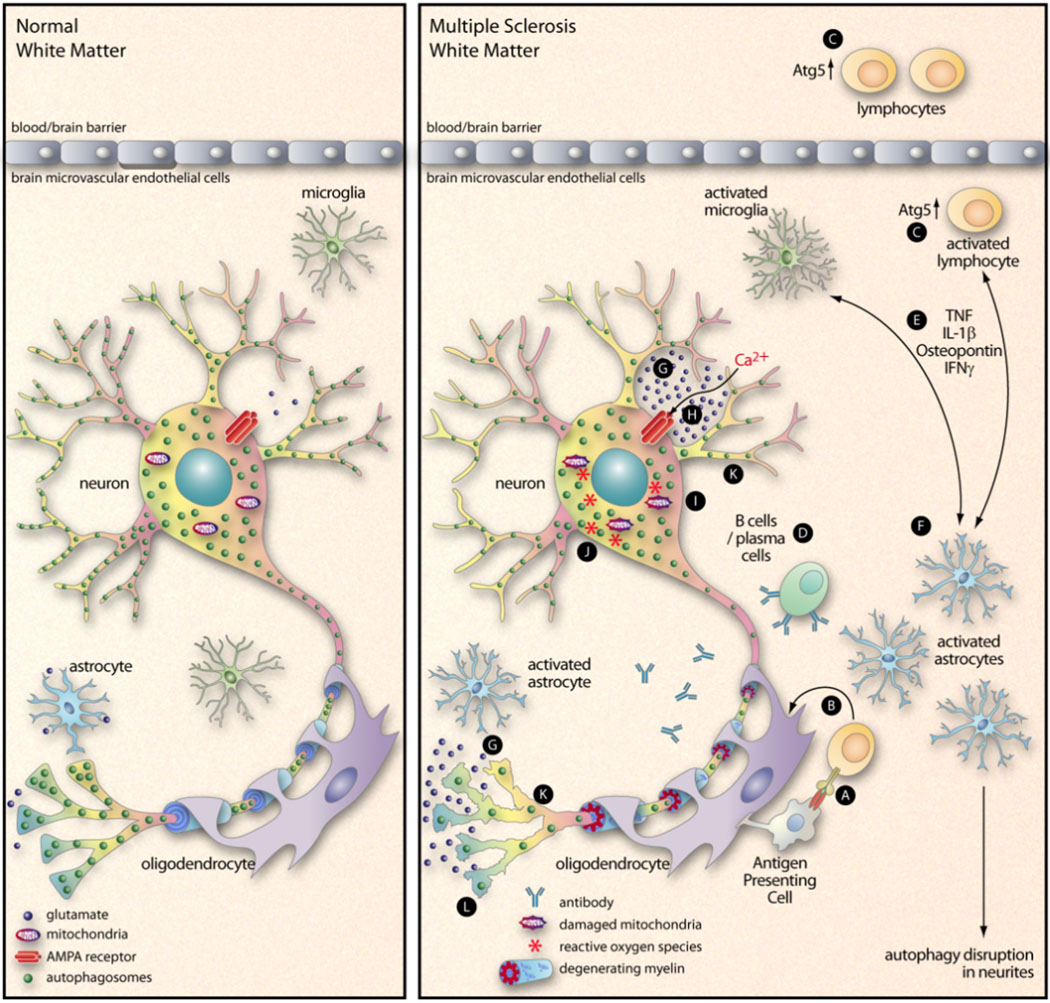
MS is a clinically diverse disease that most commonly presents as an episodic disorder, with phases of clinical disease followed by recovery. This form of MS, called relapsing-remitting MS (RRMS), is observed in ~85% of new patients (Heard, 2007). The etiology of MS is uncertain, and infections may trigger disease exacerbations, but the disease is generally considered to be autoimmune (Bennett and Stuve, 2009). MS is a demyelinating disease in which the myelin sheath, a membranous layer that is produced by oligodendrocytes and surrounds and insulates nerve fibers in the CNS, is destroyed. The resulting white matter lesions can progress toward permanent tissue injury associated with neuronal loss and consequent clinical disability (Su et al., 2009). These MS plaques are characterized by perivascular infiltration of inflammatory mononuclear cells. Activated neuroantigen-reactive T cells infiltrate the CNS and initiate a local inflammatory response, leading to glial cell activation with further recruitment of mononuclear cells through production of chemokines and an increase in blood-brain-barrier permeability. These events are accompanied by demyelination, axonal injury and cortical neuronal loss (Trapp et al., 1998; Sospedra and Martin, 2005; Steinman et al., 2002). Innate immune mechanisms involving microglia, macrophages and astrocytes also have been implicated as pivotal contributors to the disease process (Noorbakhsh et al., 2006; Tsutsui et al., 2005; Tsutsui et al., 2004). In addition, recent studies have pointed to active roles for neurons, themselves, in coordinating CD4+ regulatory T cells in the CNS, and thereby modulating neuroinflammation; crosstalk takes place between neurons and T cells, increasing T cell proliferation and altering cytokine production (Liu et al., 2006). One of the most widely-used animal models of MS is experimental autoimmune encephalomyelitis (EAE) and, in both EAE and MS, lymphocytes, microglia and macrophages release excessive amounts of glutamate (Steinman, 2000; Werner et al., 2001; Shijie et al., 2009) which, as noted above, can be neurotoxic. Glutamate can activate the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors that are present on both neurons and oligodendrocytes; AMPA receptor activation increases the level of intracytosolic Ca2+ which, in turn, can be highly toxic to both neurons (Piani et al., 1991) and oligodendrocytes (McDonald et al., 1998). Blockade of AMPA receptors using competitive antagonists such as NBQX (2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione) can ameliorate the neurological sequelae of autoimmune encephalomyelitis and, if treatment is begun after the onset of paralysis, can prevent the clinical relapses that would otherwise occur; this beneficial effect is not observed using NMDA blockers, suggesting that AMPA receptors play the key role in this neurological disorder (Smith et al., 2000; Steinman, 2000; Pitt et al., 2000; Steinman, 1999; Steinman et al., 2002; Steinman and Zamvil, 2005). Glutamate-driven neurotoxicity can be amplified by the inflammatory molecules that are released by activated macrophages/microglia, further increasing the influx of Ca2+ in neurons. As described above, the elevated intracellular Ca2+ causes mitochondrial damage; this releases ROS which, in turn, activates caspases and calpains, thereby disrupting autophagy, which may result in neuronal damage (Manev et al., 1989). In otherwise-healthy cells, damaged mitochondria are rapidly removed by autophagy, in a process termed mitophagy; mitophagy, therefore, extinguishes the production of ROS. However, ongoing mitochondrial damage in combination with a disruption in autophagy / mitophagy may lead to the accumulation of damaged mitochondria and, therefore, ongoing production of ROS. The affected neuron finds itself locked in a deleterious loop, in which ROS concentrations increase, further paralyzing the pathway that normally removes their source, defective mitochondria. Dysfunctional mitochondria have been identified in MS lesions (as well as in the normal-appearing white and grey matter) and may be an important determinant of the axonal dysfunction and degeneration that occurs in this disease (Mahad et al., 2008); it has been reported that an increased number of damaged mitochondria in demyelinated axons renders them more vulnerable than myelinated axons to energy deficits (Mahad et al., 2008). A diagrammatic representation of these changes is presented in Figure 2, using MS as an example.
Figure 2. Proposed interactions between inflammation, Ca2+ excitotoxicity, and autophagy in a non-infectious neurodegenerative disease.
During MS, lymphocytes infiltrate into the brain and recognize their antigen, usually myelin-derived antigen presented by an APC (A). These activated T cells then release pro-inflammatory cytokines that act on oligodendrocytes (B), leading to the demyelination that is a hallmark of the disease. This diagram focuses mainly on other events that may be involved in MS neurodegeneration. Atg5 is an important component of the autophagy pathway, and its cleavage can switch a cell’s fate from autophagy (and survival) to apoptosis (and death) (Yousefi et al., 2006). In our recent paper (Alirezaei et al., 2009) we showed that peripheral blood CD4+ T cells of some MS patients contained an elevated level of Atg5, leading us to speculate that these potentially-pathogenic cells may have prolonged lifespans (C). B cells and plasma cells, too, have been implicated in MS (D). The pro-inflammatory mediators that are released by activated lymphocytes and / or microglia (E) can activate astrocytes (F), reducing their capacity of resorb glutamate (G). The resulting increase in extracellular glutamate triggers AMPA receptors (H) which leads to an influx of Ca2+ into the neurons (Williams et al., 2008). This initiates a cascade of events including damage to mitochondria (I), an increase in ROS (J), activation of caspases and calpain, and a decrease in number of neurite autophagosomes (K). The ultimate outcome is neurodegeneration (L).
Autophagy in immune cells may indirectly modulate neurodegenerative diseases
Above we describe how neuronal viability may be directly altered by autophagy; that is, by changes in autophagy within the neurons themselves. However, changes in autophagy in non-neuronal cells also may affect neuronal viability, in this case indirectly. For example, it is now clear that autophagy has a dramatic effect on the development, proliferation and maintenance of T and B lymphocytes (Li et al., 2006; Miller et al., 2008; Pua et al., 2007; Pua and He, 2007), and autophagic signaling can alter a T cell’s apoptotic status thereby effecting its viability (Yousefi et al., 2006). Lymphocytes, of course, play a key role in protecting against infections, so one can imagine how changes in lymphocyte autophagy could impact CNS infection and, thereby, the neurodegenerative consequences thereof. Furthermore, these cells are central to autoimmune diseases such as MS, and preliminary studies have shown a correlation between MS disease status and a marker of autophagy in peripheral T cells. One of many genes involved in autophagy is Atg5, the deletion of which abrogates autophagy (Kuma et al., 2004). The Atg5 protein also has been implicated in lymphocyte homeostasis (Miller et al., 2008; Pua et al., 2007; Pua and He, 2007; Yousefi et al., 2006) and, therefore, we chose to focus on this gene and its product in a recent study of EAE and of MS tissues (Alirezaei et al., 2009). We found that, in mice suffering from EAE, there was a strong correlation between Atg5 mRNA levels in peripheral blood cells and the degree of clinical disability; in addition, increased post-translational modifications of the Atg5 protein were present in EAE mice (Alirezaei et al., 2009). Furthermore, our studies suggested a possible role for Atg5 in RRMS. When T cells from patients with active RRMS (i.e., experiencing a disease exacerbation) were compared to those from RRMS patients in the quiescent phase, we found that Atg5 mRNA was substantially higher in the former group. Furthermore, protein lysates from the lesion areas of RRMS brains were analyzed and, when compared to non-diseased brains, Atg5 protein conjugates were more readily detected, and immunohistochemical analysis of the RMSS lesions suggested that the increased Atg5 protein was present in infiltrating CD3+ T lymphocytes (Alirezaei et al., 2009). Taken together, our data suggest that autophagy may be increased in T cells of MS patients, possibly extending the survival of the potentially-harmful cells and thereby contributing to disease, as diagrammed in Figure 2, C. Not only T cells, but also B cells and/or plasma cells, may be involved in MS pathogenesis (Figure 2, D). In the context of autoimmunity and MS, B cells function as sensors and regulators of the immune response, which has strengthened the view that B cells and autoantibodies are fundamental for activating T cells and/or mediating tissue injury (Browning, 2006; Dalakas, 2008a; Dalakas, 2008b; Hasler and Zouali, 2006; Owens et al., 2006; Shlomchik et al., 2001). Recent data reveal an important role for autophagy in B cell homeostasis; Atg5 is required for the development and the maintenance of B cells (Miller et al., 2008). Therefore, autophagy genes might have an impact on the survival of B cells, as well as T cells, in MS or EAE. Studies have reported an antigen-driven B cell response in the cerebro-spinal fluid (CSF) of MS patients, which could contribute to the ongoing production of oligoclonal immunoglobulin in MS CSF, and to the development of secondary lymphoid tissue in brains of patients with secondary progressive MS (Harp et al., 2007).
We do not argue, herein, that disruption of autophagy is the sole, or even necessarily the major, pathway that leads to neurodegeneration in infectious and/or autoimmune diseases of the CNS. Rather, our intent is to highlight the fact that changes in autophagy may contribute to CNS neurodegeneration and disease, and to demonstrate that this may occur in two general ways. 1. Autophagy may act independently; failure of autophagy can, in and of itself, lead to neurodegeneration. 2. Alternatively, autophagy may act in concert with other potential neurotoxins; this, too, can be divided mechanistically into two subgroups. 2(a). In some cases, autophagy may be directly involved in the neurodegeneration; e.g., the neurotoxic effects of a molecule (e.g., a viral product) may be mediated, in part or in whole, by its capacity to inhibit autophagy. 2(b). In other cases, autophagy may be indirectly involved in the neurodegenerative process; e.g., the effects of a potential toxin on a neuron may be limited or ameliorated by active autophagy within the cell, but any reduction in autophagy will allow the neurotoxin to drive neurodegenerative change.
Speculation: Might food restriction represent a novel, safe, simple and inexpensive approach to the prevention and treatment of CNS neurodegenerative disease
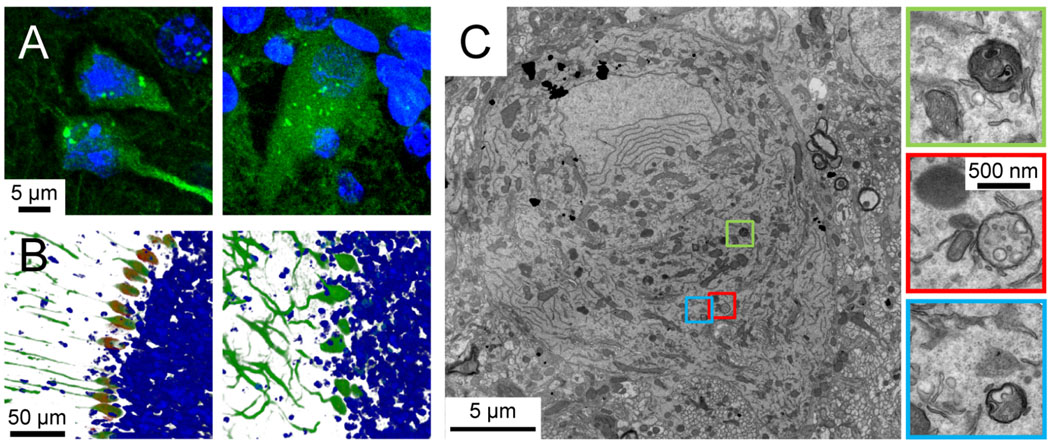
We have argued herein that disruption of the autophagy pathway may cause or exacerbate the neuronal damage that accrues in infectious and non-infectious neurodegenerative diseases. As we noted at the outset of this brief review, therapeutic up-regulation of neuronal autophagy may be beneficial. Consequently, researchers and pharmaceutical companies are actively pursuing different approaches to induce CNS neuronal autophagy, but these studies face at least two hurdles: first, the blood brain barrier, which is impervious to many drugs; and, second, ensuring that any CNS-permeable autophagy-inducing small molecules are specific for neurons. These concerns may not be insuperable, because some recent studies have shown that neuronal autophagy can be enhanced using small molecules (Sarkar et al., 2009; Williams et al., 2008), but another possible problem exists; as is true for all drugs, there may be undesirable side-effects. Thus, a simple, safe and inexpensive method to up-regulate CNS neuronal autophagy may be of therapeutic value in the treatment of neurodegenerative disease. Undoubtedly the safest and simplest means by which to up-regulate autophagy is food restriction, which is known to induce autophagy in most tissues, including the peripheral nervous system (PNS). Intuitively, it may seem unlikely that mere fasting could have any beneficial effect on degenerative neurological disease, but a recent study in a mouse model of Charcot-Marie-Tooth disease showed that intermittent fasting increases autophagy in Schwann cells in the PNS, reducing protein aggregation in these cells and increasing the thickness of myelin sheath (Madorsky et al., 2009). Remarkably, the mice also showed improved locomotor performance, indicating that fasting can confer clinically-evident benefits in a genetic neurodegenerative disorder (Madorsky et al., 2009). Thus, unlikely as it may appear at first blush, we speculate that the same simple approach may delay, ameliorate, or even prevent some of the CNS neurodegenerative diseases that we describe above. However, there are two objections to this proposal. First, if intermittent fasting increases autophagy in autoreactive T cells, this might worsen the related diseases; to our knowledge, the effects of sporadic fasting on T cells have not yet been evaluated. The second, and perhaps more important, objection is that autophagy pathways in the brain have long been considered unresponsive to food restriction (Mizushima et al., 2004). Thus, current dogma holds that the CNS is an exception to the general rule that fasting induces autophagy. However, those conclusions were derived from biochemical analyses of extracts from whole brains; this approach, although valuable, is too broad to identify fasting-induced changes in autophagy that might occur in a limited number of CNS cells. Recently, we have used a new approach to challenge this dogma, and have demonstrated that brief (24–48 hour) food restriction can induce neuronal autophagy in the brain; compared to normal-fed mice, the abundance of autophagosomes increases by 3- to 4-fold after 48 hours of food restriction (Alirezaei et al., 2010). We employed laser scanning confocal microscopy of 80–100 µm vibratome sections of the brains of mice that express a GFP-tagged version of LC3 (Atg8), a protein that accumulates on the membranes of autophagosomes and is considered the best marker of these vesicles (Mizushima et al., 2004; Mizushima and Kuma, 2008). As shown in Figure 3A, autophagosomes are abundant in cortical neurons and Purkinje cells of mice that have been food-restricted for 48 hours. Furthermore, the activity of mTOR, an inhibitor of autophagy, is dramatically reduced in Purkinje cells following food restriction (Figure 3B). Finally, transmission electron microscopy confirmed the presence of numerous autophagosomes in Purkinje cells (Figure 3C) from food-restricted mice.
Figure 3. Food restriction induces autophagy in cortical neurons and Purkinje cells.
Panel A. Autophagosomes (bright green punctae) in cortical neurons (left) and Purkinje cells (right) of GFP-LC3 mice after 48 hrs of food restriction. Panel B. Images of cerebellar sections stained with an antibody specific for phospho-S6RP (red), a protein whose abundance varies directly with mTOR activity, and inversely with autophagy. GFP-LC3, which is expressed at high levels in Purkinje cells, is shown in green and nuclei are stained with Hoechst dye (blue). Purkinje neurons in a normal fed animal (left) have high mTOR activity (indicating low autophagy) while cells in the 48 hrs food-restricted animal (right) have low mTOR activity (and, thus, a highly active autophagy pathway). Images were generated using Imaris software, blended mode (Bitplane, Inc). Panel C. A transmission electron microscope image of a single Purkinje cell from a 48 hour food-restricted mouse is shown. Autophagosomes are enclosed in colored boxes, and a higher-magnification image of each is provided.
Conclusion
Disruption of autophagy goes hand-in-hand with neurodegeneration, and a cause and effect relationship between the two may contribute to neuronal damage. For this reason, drug-induced up-regulation of autophagy is being evaluated in CNS neurodegenerative disorders. However, we propose that an additional avenue of research should be followed. Preliminary studies in animal models indicate that up-regulation of autophagy by food restriction leads to a demonstrable improvement in the signs and symptoms of a PNS neurodegenerative disease. Our recent demonstration that fasting also affects the CNS, causing a rapid and profound change in autophagy in cortical neurons and in Purkinje cells, leads us to suggest that the effects of food restriction should be evaluated in CNS neurodegenerative diseases. Autophagy is sometimes referred to as cellular cleansing and, as we have previously noted (Alirezaei et al., 2010), this proposed therapeutic approach provides an attractive parallel between cellular changes (neuronal “cleansing”) and the more holistic organismal benefits that are thought, by some, to derive from fasting.
ACKNOWLEDGEMENTS
We are grateful to Annette Lord for excellent secretarial support, to Malcolm R. Wood and William B. Kiosses (Scripps Microscopy core) for image acquisition, and to Janet Hightower for preparation of Figures 1 & 2. This work was supported by NIH grants AI-027028, AI-052351 and AI-042314. This is manuscript number 20705 from the Scripps Research Institute.
REFERENCES
- Alirezaei M, Fox HS, Flynn CT, Moore CS, Hebb ALO, Frausto RF, Bhan V, Kiosses WB, Whitton JL, Robertson GS, Crocker SJ. Elevated ATG5 expression in autoimmune demyelination and multiple sclerosis. Autophagy. 2009;5:152–158. doi: 10.4161/auto.5.2.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010;6:702–710. doi: 10.4161/auto.6.6.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS. ONE. 2008a;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Kiosses WB, Fox HS. Decreased neuronal autophagy in HIV dementia: a mechanism of indirect neurotoxicity. Autophagy. 2008b;4:963–966. doi: 10.4161/auto.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alirezaei M, Watry DD, Flynn CF, Kiosses WB, Masliah E, Williams BR, Kaul M, Lipton SA, Fox HS. Human immunodeficiency virus-1/surface glycoprotein 120 induces apoptosis through RNA-activated protein kinase signaling in neurons. J. Neurosci. 2007;27:11047–11055. doi: 10.1523/JNEUROSCI.2733-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JL, Stuve O. Update on inflammation, neurodegeneration, and immunoregulation in multiple sclerosis: therapeutic implications. Clin. Neuropharmacol. 2009;32:121–132. doi: 10.1097/WNF.0b013e3181880359. [DOI] [PubMed] [Google Scholar]
- Berliocchi L, Corasaniti MT, Bagetta G, Lipton SA. Neuroinflammation in neuronal degeneration and repair. Cell Death Differ. 2007;14:883–884. doi: 10.1038/sj.cdd.4402097. [DOI] [PubMed] [Google Scholar]
- Browning JL. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat. Rev. Drug Discov. 2006;5:564–576. doi: 10.1038/nrd2085. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Chauhan A, Dimayuga FO, Gee J, Keller JN, Nath A. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J. Neurosci. 2003;23:8417–8422. doi: 10.1523/JNEUROSCI.23-23-08417.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Hamilton DJ, Barnett JL, Paskevich PA, Nixon RA. Properties of the endosomal-lysosomal system in the human central nervous system: disturbances mark most neurons in populations at risk to degenerate in Alzheimer's disease. J. Neurosci. 1996;16:186–199. doi: 10.1523/JNEUROSCI.16-01-00186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Duan F, Morsey B, Persidsky Y, Kanmogne GD. HIV-1 activates proinflammatory and interferon-inducible genes in human brain microvascular endothelial cells: putative mechanisms of blood-brain barrier dysfunction. J. Cereb. Blood Flow Metab. 2008;28:697–711. doi: 10.1038/sj.jcbfm.9600567. [DOI] [PubMed] [Google Scholar]
- Chen M, Ona VO, Li M, Ferrante RJ, Fink KB, Zhu S, Bian J, Guo L, Farrell LA, Hersch SM, Hobbs W, Vonsattel JP, Cha JH, Friedlander RM. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- Croisier E, Graeber MB. Glial degeneration and reactive gliosis in alpha-synucleinopathies: the emerging concept of primary gliodegeneration. Acta Neuropathol. 2006;112:517–530. doi: 10.1007/s00401-006-0119-z. [DOI] [PubMed] [Google Scholar]
- Custer SK, Garden GA, Gill N, Rueb U, Libby RT, Schultz C, Guyenet SJ, Deller T, Westrum LE, Sopher BL, La Spada AR. Bergmann glia expression of polyglutamine-expanded ataxin-7 produces neurodegeneration by impairing glutamate transport. Nat. Neurosci. 2006;9:1302–1311. doi: 10.1038/nn1750. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. B cells as therapeutic targets in autoimmune neurological disorders. Nat. Clin. Pract. Neurol. 2008a;4:557–567. doi: 10.1038/ncpneuro0901. [DOI] [PubMed] [Google Scholar]
- Dalakas MC. Invited article: inhibition of B cell functions: implications for neurology. Neurol. 2008b;70:2252–2260. doi: 10.1212/01.wnl.0000313840.27060.bf. [DOI] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, Scherzinger E, Wanker EE, Mangiarini L, Bates GP. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- Demarchi F, Bertoli C, Copetti T, Tanida I, Brancolini C, Eskelinen EL, Schneider C. Calpain is required for macroautophagy in mammalian cells. J. Cell Biol. 2006;175:595–605. doi: 10.1083/jcb.200601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc. Natl. Acad. Sci. U. S. A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal J, Strom AL, Kwinter DM, Kilty R, Zhang J, Shi P, Fu W, Wooten MW, Zhu H. Sequestosome 1/p62 links familial ALS mutant SOD1 to LC3 via an ubiquitin-independent mechanism. J Neurochem. 2009;111:1062–1073. doi: 10.1111/j.1471-4159.2009.06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Libby RT, Fu YH, Kinoshita Y, Huang J, Possin DE, Smith AC, Martinez RA, Fine GC, Grote SK, Ware CB, Einum DD, Morrison RS, Ptacek LJ, Sopher BL, La Spada AR. Polyglutamine-expanded ataxin-7 promotes non-cell-autonomous purkinje cell degeneration and displays proteolytic cleavage in ataxic transgenic mice. J. Neurosci. 2002;22:4897–4905. doi: 10.1523/JNEUROSCI.22-12-04897.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat. Rev. Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Harp C, Lee J, Lambracht-Washington D, Cameron E, Olsen G, Frohman E, Racke M, Monson N. Cerebrospinal fluid B cells from multiple sclerosis patients are subject to normal germinal center selection. J. Neuroimmunol. 2007;183:189–199. doi: 10.1016/j.jneuroim.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler P, Zouali M. B lymphocytes as therapeutic targets in systemic lupus erythematosus. Expert Opin. Ther. Targets. 2006;10:803–815. doi: 10.1517/14728222.10.6.803. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Oksenberg JR. The neurobiology of multiple sclerosis: genes, inflammation, and neurodegeneration. Neuron. 2006;52:61–76. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Heard RN. The spectrum of multiple sclerosis. Curr. Allergy Asthma Rep. 2007;7:280–284. doi: 10.1007/s11882-007-0042-y. [DOI] [PubMed] [Google Scholar]
- Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12 Suppl 1:878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid. Redox. Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kuma A, Hatano M, Matsui M, Yamamoto A, Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T, Mizushima N. The role of autophagy during the early neonatal starvation period. Nature. 2004;432:1032–1036. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- Kundu M, Thompson CB. Autophagy: basic principles and relevance to disease. Annu. Rev. Pathol. 2008;3:427–455. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in aging, disease and death: the true identity of a cell death impostor. Cell Death Differ. 2009;16:1–2. doi: 10.1038/cdd.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Capan E, Zhao Y, Zhao J, Stolz D, Watkins SC, Jin S, Lu B. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J. Immunol. 2006;177:5163–5168. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- Liu Y, Teige I, Birnir B, Issazadeh-Navikas S. Neuron-mediated generation of regulatory T cells from encephalitogenic T cells suppresses EAE. Nat. Med. 2006;12:518–525. doi: 10.1038/nm1402. [DOI] [PubMed] [Google Scholar]
- Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat. Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madorsky I, Opalach K, Waber A, Verrier JD, Solmo C, Foster T, Dunn WA, Jr, Notterpek L. Intermittent fasting alleviates the neuropathic phenotype in a mouse model of Charcot-Marie-Tooth disease. Neurobiol. Dis. 2009;34:146–154. doi: 10.1016/j.nbd.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad D, Lassmann H, Turnbull D. Review: Mitochondria and disease progression in multiple sclerosis. Neuropathol. Appl. Neurobiol. 2008;34:577–589. doi: 10.1111/j.1365-2990.2008.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Favaron M, Guidotti A, Costa E. Delayed increase of Ca2+ influx elicited by glutamate: role in neuronal death. Mol. Pharmacol. 1989;36:106–112. [PubMed] [Google Scholar]
- Martinez-Vicente M, Cuervo AM. Autophagy and neurodegeneration: when the cleaning crew goes on strike. Lancet Neurol. 2007;6:352–361. doi: 10.1016/S1474-4422(07)70076-5. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Althomsons SP, Hyrc KL, Choi DW, Goldberg MP. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- Miller BC, Zhao Z, Stephenson LM, Cadwell K, Pua HH, Lee HK, Mizushima NN, Iwasaki A, He YW, Swat W, Virgin HW. The autophagy gene ATG5 plays an essential role in B lymphocyte development. Autophagy. 2008;4:309–314. doi: 10.4161/auto.5474. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Kuma A. Autophagosomes in GFP-LC3 Transgenic Mice. Methods Mol. Biol. 2008;445:119–124. doi: 10.1007/978-1-59745-157-4_7. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses AV, Stenglein SG, Nelson JA. HIV infection of the brain microvasculature and its contribution to the AIDS dementia complex. J. NeuroAIDS. 1996;1:85–99. doi: 10.1300/j128v01n01_04. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J. Neuropathol. Exp. Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F, Tsutsui S, Vergnolle N, Boven LA, Shariat N, Vodjgani M, Warren KG, Andrade-Gordon P, Hollenberg MD, Power C. Proteinase-activated receptor 2 modulates neuroinflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J. Exp. Med. 2006;203:425–435. doi: 10.1084/jem.20052148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, Levine B. Eating the enemy within: autophagy in infectious diseases. Cell Death Differ. 2009;16:57–69. doi: 10.1038/cdd.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GP, Bennett JL, Gilden DH, Burgoon MP. The B cell response in multiple sclerosis. Neurol. Res. 2006;28:236–244. doi: 10.1179/016164106X98099. [DOI] [PubMed] [Google Scholar]
- Piani D, Frei K, Do KQ, Cuenod M, Fontana A. Murine brain macrophages induced NMDA receptor mediated neurotoxicity in vitro by secreting glutamate. Neurosci Lett. 1991;133:159–162. doi: 10.1016/0304-3940(91)90559-c. [DOI] [PubMed] [Google Scholar]
- Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat. Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J. Exp. Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pua HH, He YW. Maintaining T lymphocyte homeostasis: another duty of autophagy. Autophagy. 2007;3:266–267. doi: 10.4161/auto.3908. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Ravikumar B, Floto RA, Rubinsztein DC. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies. Cell Death Differ. 2009;16:46–56. doi: 10.1038/cdd.2008.110. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Scherzinger E, Lurz R, Turmaine M, Mangiarini L, Hollenbach B, Hasenbank R, Bates GP, Davies SW, Lehrach H, Wanker EE. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90:549–558. doi: 10.1016/s0092-8674(00)80514-0. [DOI] [PubMed] [Google Scholar]
- Shen W, Ganetzky B. Autophagy promotes synapse development in Drosophila. J. Cell Biol. 2009;187:71–79. doi: 10.1083/jcb.200907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shijie J, Takeuchi H, Yawata I, Harada Y, Sonobe Y, Doi Y, Liang J, Hua L, Yasuoka S, Zhou Y, Noda M, Kawanokuchi J, Mizuno T, Suzumura A. Blockade of glutamate release from microglia attenuates experimental autoimmune encephalomyelitis in mice. Tohoku J. Exp. Med. 2009;217:87–92. doi: 10.1620/tjem.217.87. [DOI] [PubMed] [Google Scholar]
- Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ. Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J. Cell Biol. 2005;171:1001–1012. doi: 10.1083/jcb.200508072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat. Rev. Immunol. 2001;1:147–153. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- Siciliano G, Carlesi C, Pasquali L, Piazza S, Pietracupa S, Fornai F, Ruggieri S, Murri L. Clinical Trials for Neuroprotection in ALS. CNS. Neurol. Disord. Drug Targets. 2010;9:305–313. doi: 10.2174/187152710791292648. [DOI] [PubMed] [Google Scholar]
- Smith T, Groom A, Zhu B, Turski L. Autoimmune encephalomyelitis ameliorated by AMPA antagonists. Nat. Med. 2000;6:62–66. doi: 10.1038/71548. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 1999;24:511–514. doi: 10.1016/s0896-6273(00)81107-1. [DOI] [PubMed] [Google Scholar]
- Steinman L. Multiple approaches to multiple sclerosis. Nat. Med. 2000;6:15–16. doi: 10.1038/71466. [DOI] [PubMed] [Google Scholar]
- Steinman L, Martin R, Bernard C, Conlon P, Oksenberg JR. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu. Rev. Neurosci. 2002;25:491–505. doi: 10.1146/annurev.neuro.25.112701.142913. [DOI] [PubMed] [Google Scholar]
- Steinman L, Zamvil SS. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005;26:565–571. doi: 10.1016/j.it.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Su KG, Banker G, Bourdette D, Forte M. Axonal degeneration in multiple sclerosis: the mitochondrial hypothesis. Curr. Neurol. Neurosci. Rep. 2009;9:411–417. doi: 10.1007/s11910-009-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Noorbakhsh F, Sullivan A, Henderson AJ, Warren K, Toney-Earley K, Waltz SE, Power C. RON-regulated innate immunity is protective in an animal model of multiple sclerosis. Ann. Neurol. 2005;57:883–895. doi: 10.1002/ana.20502. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Schnermann J, Noorbakhsh F, Henry S, Yong VW, Winston BW, Warren K, Power C. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J. Neurosci. 2004;24:1521–1529. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner P, Pitt D, Raine CS. Multiple sclerosis: altered glutamate homeostasis in lesions correlates with oligodendrocyte and axonal damage. Ann. Neurol. 2001;50:169–180. doi: 10.1002/ana.1077. [DOI] [PubMed] [Google Scholar]
- Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, O'Kane CJ, Floto RA, Rubinsztein DC. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nat. Chem. Biol. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L, Brunner T, Simon HU. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- Zhang K, McQuibban GA, Silva C, Butler GS, Johnston JB, Holden J, Clark-Lewis I, Overall CM, Power C. HIV-induced metalloproteinase processing of the chemokine stromal cell derived factor-1 causes neurodegeneration. Nat. Neurosci. 2003;6:1064–1071. doi: 10.1038/nn1127. [DOI] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]