Abstract
Purpose of review
Mendelian disorders that affect cognition provide a unique opportunity to study the mechanisms of neurodevelopmental disorders through the examination of genetic defects in animals and development of hypotheses that can be tested in human subjects. Tuberous sclerosis complex (TSC) is a genetic disease that presents with epilepsy, autism and intellectual disability. Here we review recent advances in our understanding of TSC pathogenesis and signaling pathways that may be modulated to treat the neurological symptoms.
Recent findings
Accumulating evidence suggests that TSC patients have non-tuber abnormalities that contribute to the development of the neurological phenotype – in particular, disorganization of axon tracts and deficient myelination. TSC mouse models have failed to replicate the human neuropathology entirely, but have shed light on the cellular abnormalities and the neurobehavioral phenotypes. Most importantly, cell culture and animal models have identified the mTORC1 pathway as a therapeutic target in this disease.
Summary
Preclinical data strongly suggest that TSC is a disease of abnormal neuronal connectivity. The high incidence of neurodevelopmental deficits, early detection of the disease in very young ages, and availability of mTORC1 inhibitors make TSC a model for other Mendelian disorders of neurocognition and an avenue for the mechanism-based treatment trials of neurodevelopmental disorders.
Keywords: mTOR, autism, translation, DTI
Introduction
TSC is an autosomal dominant disorder characterized by hamartomas in most organ systems including the brain. Patients with TSC are frequently diagnosed with comorbid neurological disorders, including epilepsy, intellectual disability, behavioral dysregulation, sleep disorders, and autism spectrum disorders (ASD). The genes responsible for the disorder are TSC1 and TSC2, which encode for the proteins TSC1 (hamartin) and TSC2 (tuberin), respectively. Together these proteins regulate the protein complex, mTORC1, constituting a key cellular pathway important for protein synthesis and cell size regulation (Figure 1)[1]. mTORC1 is directly controlled by Rheb, a small GTPase. TSC1 and TSC2 proteins together act to negatively regulate Rheb, thereby inhibiting protein synthesis. In patients with TSC, inactivation of either TSC1 or TSC2 leads to the overactivation of Rheb and mTORC1 with a subsequent increase in protein translation.
Figure 1.
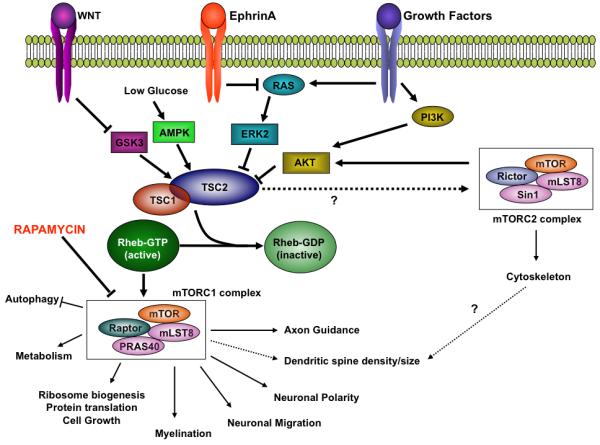
TSC mediated signaling in the central nervous system. This cartoon of TSC mediated signaling has been simplified to highlight the demonstrated biologic roles for TSC mediated mTOR signaling in the nervous system. Among the upstream signaling pathways, only the growth factors and ephrins have been shown to modulate TSC-mTOR pathway in neurons (growth factors, ephrins) while others (e.g. Wnts) have been implicated, but not proven to regulate TSC signaling in the nervous system.
1. Neuroimaging correlates of TSC manifestations
To investigate the etiologies of the neurocognitive phenotypes found in TSC patients, anatomic studies have been performed, and reveal characteristic pathological abnormalities: hamartomatous tubers and subependymal nodules that may undergo neoplastic change to form subependymal giant-cell astrocytomas (SEGAs)[2]. Many studies have correlated neurological symptoms – epilepsy, intellectual disability, and ASD – with the number and location of cortical tubers. Intellectual disability has been associated with increased tuber number[3] and frontal/occipital location[4]. However, recent studies have shown that total tuber volume, not number per se, is associated with poorer cognitive outcome[5]. In addition, ASD have been associated with temporal lobe tubers or temporal lobe epileptiform discharges[6]; however, additional studies also correlate ASD with cerebellar lesions, especially with right cerebellar involvement[7,8].
Although neuropsychiatric phenotypes can be associated with tubers, many patients without significant tuber load have disabling symptoms while patients with large tuber burdens may have few neurologic symptoms, suggesting that other abnormalities are responsible for these phenotypes. Patients with TSC have in fact been found to have pathology in other brain regions implicated in neuropsychiatric disorders. Mesial temporal sclerosis and hippocampal malrotation were described in 16% of TSC patients, associated with increased tuber number and a history of febrile seizures in the first year of life[9]. Furthermore, cerebellar abnormalities were detected in approximately 30% of TSC patients (in the absence of “cerebellar” symptoms)[10]. PET studies demonstrate hyperactivation of deep cerebellar nuclei in TSC patients with ASD, consistent with cerebellar dysfunction and decreased Purkinje cell inhibitory output[11]. Combined with the fact that ASD in TSC patients correlate with cerebellar WM abnormalities[7], these data suggest that dysfunction of cerebellar connections may contribute to neuropsychiatric symptoms found in TSC. Further studies into the cerebellar contribution to neuropsychiatric dysfunction in patients with TSC is an important area of future study.
In addition, investigators have also found aberrant connectivity in patients with TSC by using diffusion tensor imaging (DTI) to study myelination and white matter (WM) integrity. With DTI, at least three parameters which reflect the integrity of white matter may be obtained: apparent diffusion coefficient (ADC) – a reflection of total diffusion, fractional anisotropy (FA) – a measure of the directionality of diffusion, and radial diffusivity (RD) – an average of diffusion perpendicular to the direction of the axonal tracts.
DTI abnormalities are reported in TSC in normal appearing (NA) WM, suggesting microstructural abnormalities beyond conventional MRI resolution[12-18]. Initially, subcortical NAWM adjacent to cortical tubers was shown to have abnormal ADC and FA values compared to the contralateral side[14]. Ictal activity may also contribute to WM disruption since NAWM and cortical tubers within epileptic zones (documented by magnetoencephalography) display decreased FA and increased RD values[12]. However, there is mounting evidence of increased ADC values in NAWM remote from areas of tubers, indicating diffuse abnormality of the white matter[13]. Reduced FA and increased RD in the corpus callosum and internal capsule have also been reported[19]. Recently, abnormalities in WM ADC values have also been found to be age and location dependent[18]. Taken together, these data support a contribution of abnormal connectivity to the neuropsychiatric phenotypes in TSC patients. However, as these studies generally involved small patient cohorts, significantly larger studies are required to validate these findings and explore their correlation with neurological symptoms.
2. Cellular genetics of TSC: interplay between haploinsufficiency vs loss of heterozygosity
TSC exhibits an autosomal dominant inheritance pattern with a high spontaneous mutation rate. About 2/3 of TSC cases are sporadic while 1/3 are familial. In agreement with the two-hit tumor-suppressor gene model, inactivation of both alleles of either TSC1 or TSC2 is necessary for some of TSC’s clinical manifestations. Most second-hit mutations are large genomic deletions, referred to as loss of heterozygosity (LOH). LOH involving the TSC1 or TSC2 has been observed in angiomyolipomas, rhabdomyomas, SEGAs and lymphangioleiomyomatosis (LAM)[20]. However, LOH has not been consistently observed in cerebral cortical tubers, suggesting that tuber pathogenesis does not require inactivation of both alleles, that only some cells within a tuber have both alleles affected or that the second hit mutations do not involve LOH[21]. Recent genetic studies examining two-hit inactivation of TSC1 or TSC2 in tubers have been inconsistent, with one report suggesting that complete inactivation of TSC1 or TSC2 occurs in tuber giant cells through second hit point mutations[22]. In this study, the investigators evaluated phospho-S6 positive neurons by laser capture microscopy from tuber samples and found a somatic mutation in both alleles in 5 of 6 cases. In contrast, another group used deep sequencing to search for small mutations in TSC1 or TSC2 in macroscopic tuber samples. Although one TSC patient had a second allele point mutation in multiple brain samples, the remaining patients studied had no second hit mutations found[23]. Thus, questions remain regarding the precise nature and timing of the genetic events underlying tuber formation. Furthermore, if second hits occur, it is unclear whether different tubers in the same patient would carry the same somatic mutation. Unfortunately, TSC animal models failed thus far to generate cortical tubers to help answer this question.
3. TSC rodent models
To further investigate the contribution of the TSC/mTORC1 pathway to neuropsychiatric phenotypes, investigators have used a naturally occurring TSC rat strain (Eker) and have generated genetic mouse models of the disorder (Table 1). No rodent model, however, has replicated the characteristic neuroanatomical findings of TSC patients, i.e. cortical tubers, subependymal nodules, and SEGAs.
Table 1.
Rodent Models of TSC in the Nervous System
| Species | Gene | Constitutive/ Conditional (Cre promoter) |
Anatomy | Neuropsychiatric phenotypes |
References |
|---|---|---|---|---|---|
| Rat | Tsc2 | Constitutive, heterozygous; IAP element insertion into codon 1272 (Eker Rat) |
Normal | Decreased novel object and social exploration; defects exacerbated by seizures |
[24,25] |
| Mouse | Tsc1 | Constitutive, heterozygous; deletion of exons 6-8 |
Normal | Deficient spatial learning and contextual fear conditioning; Impaired social approach |
[26] |
| Mouse | Tsc2 | Constitutive, heterozygous; neomycin cassette inserted into exon 2, deletion of exons 2-5 |
Normal, except axon guidance defects |
Impaired spatial learning and context discrimination; Impaired mother- pup social interaction |
[27-30] |
| Mouse | Tsc2 | Constitutive, overexpression of “dominant negative” allele |
Subpial collections of granule cells |
Increased anxiety | [31,32] |
| Mouse | Tsc1 | Conditional (Glial Fibrillary Acidic Protein – Cre); deletion of exons 17- 18 |
Astroglial hypertrophy and gliosis |
FTT, seizures, early mortality |
[33-37] |
| Mouse | Tsc1 | Conditional (Synapsin – Cre); deletion of exons 17-18 |
Dysplastic neuronal hypertrophy, abnormal myelination |
FTT, seizures, early mortality, pathological hindlimb clasping |
[38] |
| Mouse | Tsc1 | Conditional (CamKII – Cre); deletion of exons 17-18 |
Megalencephaly, neuronal hypertrophy, astrogliosis |
FTT, early mortality, pathological hindlimb clasping |
[28] |
| Mouse | Tsc2 | Conditional (human Glial Fibrillary Acidic Protein – Cre); deletion of exons 2-4 |
Megalencephaly, neuronal and glial hypertrophy, abnormal migration, astrocytosis, abnormal myelination |
FTT, seizures, early mortality |
[39] |
Germline homozygous Tsc1 or Tsc2 knockout animals are invariably lethal in early embryonic stages[27,40,41]. Heterozygous models survive and display cognitive and behavioral abnormalities. Although, Tsc2+/− (Eker) rat mutants have no deficits in learning and memory or anxiety[24,25], these animals have decreased novel object and social exploration[25], consistent with ASD in TSC patients. Unlike rat models, heterozygous TSC mouse models demonstrate cognitive impairments in addition to behavioral abnormalities – suggesting a background and species dependent contribution to neuropsychiatric phenotypes. Both Tsc1+/− and Tsc2+/− mice have impaired hippocampal dependent learning and memory with deficits in spatial learning and contextual fear conditioning[26,28]. Tsc1+/− mice also demonstrate reduced social interaction[26] while a dominant negative Tsc2 mutant displays increased anxiety [31,32]. In addition, pups born to Tsc2+/− mothers display increased isolation calls, suggesting impairments in mother-pup social interaction[29]. While heterozygous TSC mouse models display no obvious neuropathological abnormalities[26], Tsc2+/− neurons display abnormal axon guidance in vivo[30], suggesting that aberrant neuronal connectivity in the Tsc2 haploinsufficient state may underlie neurobehavioral phenotypes.
No Tsc heterozygous model develops seizures or pathologic abnormalities, further suggesting that neither structural abnormalities nor seizures are exclusively responsible for the neuropsychiatric phenotypes associated with TSC. Nonetheless, in Tsc2+/− rats, anxiety and social impairment worsen with seizure induction consistent with the association between epilepsy and worsened outcome seen in TSC patients[5,25]. Similarly, exposure to immune activators during embryonic development may worsen social approach behavior in adult Tsc2+/− mice[42]. Etiology of this seizure- or immunity-induced worsening at the cellular and/or circuit level remains to be explored.
Conditional knockout models of TSC have also shed light on the role of TSC in specific cell types. Homozygous deletion of Tsc1 in astroglia produces megalencephaly, neuronal death, and astroglial hypertrophy. These mice develop seizures and have reduced survival[33]. Subsequent studies revealed increased extracellular glutamate in these animals, secondary to a deficit of the glutamate transporter Glt-1[34,37]. In addition, these animals have decreased Connexin43 expression and subsequent decreased gap junction coupling in astrocytes[35], likely lowering the seizure threshold in these animals. Interestingly, treatment of these mice with ceftriaxone (which increases glutamate transporter expression) prevents seizures if given prior to the onset of seizures, suggesting that abnormal glutamate transport is critical for epileptogenesis[36] and offering a potential therapy for TSC patients.
Neuronal specific models have also been generated with Cre recombinase expression driven by Synapsin (Syn) or CamKII promoters[28,38]. Tsc1flox/−SynI-Cre+ mice are viable perinatally, but have neuronal hypertrophy and neurofilamentous inclusions with epilepsy, tremor, hyperactivity, and mortality between 4-8 weeks postnatally[38]. Gambello and colleagues[39] also deleted Tsc2 in radial glia, which results in ectopic neurons, megalencephaly, and astrocytosis with failure to thrive, early seizures, and mortality by 3-4 weeks postnatally. Both these models also have myelination defects, consistent with the DTI abnormalities in TSC patients. However, the severe phenotype and early mortality in all of these models have limited behavioral evaluation.
4. Mechanisms underlying abnormal neuronal connections in TSC
Mouse models have also been vital to understanding the cellular mechanisms that are deranged with loss of Tsc1 or Tsc2. Over-activation of mTOR affects virtually every step of neuronal development (Figure 1). While loss of Tsc2 does not affect the proliferation of neuronal precursors in null embryos[43], Tsc1−/− astrocytes have a growth advantage[33]. Furthermore, neuronal migration is affected in Tsc2-deficient cells, in the setting of abnormal neuronal orientation in the cortex[38,44], suggesting a possible contribution for abnormal neuronal polarity to abnormal migration.
Several recent observations support the hypothesis that TSC1/2 proteins play important roles in neuronal connectivity. First, TSC1/2 regulate both dendritic spine density and morphology as well as AMPAR mediated synaptic currents[45], consistent with previously known modulation of synaptic function by pathways regulating protein synthesis (Reviewed in[46]). Furthermore, TSC1/2 are found within growth cones[47]. Most CNS neurons have a single axon and multiple dendrites, and establishing this unique polarized structure is critical for proper neuronal functioning. TSC/mTORC1 pathway components are predominantly expressed in axons and regulate neuronal polarity and axon specification[44,48,49]. This particular phenotype results from the local translation of TSC/mTORC1 pathway dependent proteins in nascent axons. The full repertoire of proteins regulated by TSC/mTORC1 in neurites remains to be identified. The abnormal neuronal polarity seen in TSC mutants requires the loss of both alleles of either Tsc1 or Tsc2. However, as discussed earlier biallelic inactivation occurs in only a minority of cells in the TSC patient’s brain and most neurons are heterozygous for the mutation[22,23]. Thus, one would predict that the downstream networks regulated by the TSC proteins would be dysregulated in the haploinsufficent state as well. This prediction has, in fact, been confirmed by the observation that retinal neurons from Tsc2+/− mice project aberrantly to their CNS targets in vivo[30], secondary to an abnormal response to ephrin/Eph receptor signaling, an important pathway for axonal pathfinding and outgrowth (Figure 1). These experiments provide a cellular substrate for abnormal neuronal connectivity in the Tsc2+/− neurons. While this study investigated the role of TSC only in the visual system, ephrins and Eph receptors play roles in the establishment of many axon tracts in the brain, including those that connect brain regions involved in language and social cognition. Evaluation of other axonal pathways will be important in future studies.
Lastly, TSC may play a role in neuronal connectivity through its role in myelination. As demonstrated in neuroimaging studies and mutant models, CNS myelination is markedly reduced in the TSC mutant background. Importantly, there is no detectable change in oligodendrocyte number or differentiation[38]. Rather it appears that loss of Tsc1 in neurons inhibits the induction of myelination, consistent with the crucial developmental interaction between neurons and oligodendrocytes. Abnormal myelination seen in TSC mouse models could potentially provide a mechanism underlying the radiologic differences in WM found in TSC patients. Parallel studies using DTI and histology in mouse models could help shed light on this possibility.
5. Potential Treatment Options
Recent studies not only have revealed mechanisms underlying neurological dysfunction in TSC patients but also has shed light on potential therapies. Indeed, suppressing the activity of Rheb or mTORC1 in TSC patients could result in clinical benefit. Both preclinical and clinical trials with mTORC1 inhibitors in TSC appear promising.
In rodent models, mTORC1 inhibitors can improve both anatomic (e.g. myelination) and neurological phenotypes. In mice with neuronal specific deletion of Tsc1, rapamycin (sirolimus) or RAD001 (everolimus) treatment normalize both survival and growth, with rescue of seizures and anatomic abnormalities[28,50]. In addition, treatment of astroglial specific Tsc1 mutants with rapamycin prevents seizure development by increasing both Glt-1 and Connexin 43 levels[35,51], Finally, rapamycin treatment results in the reversal of spatial learning and contextual fear conditioning deficits, even when treatment is initiated in adulthood[28].
As a result of these preclinical data, clinical investigations have been initiated. Franz and colleagues[52] showed that rapamycin therapy induced regression of SEGAs. In this pilot study, five individuals with TSC were treated with rapamycin at standard immunosuppressive doses (serum levels 5-15ng/ml), and all lesions regressed. SEGA regrew in one patient whose therapy was interrupted, but regressed again when therapy was resumed. Subsequently, a larger (28 patients) open-label RAD001 treatment study confirmed efficacy of this compound in SEGAs, with 32% of patients having greater than 50% reduction in tumor volume at 6 months[53]. Based on these results, RAD001 was FDA-approved in November 2010 for treatment of SEGAs in TSC patients, who are not candidates for surgical resection.
Several questions remain: What are the long-term sequelae of mTORC1 inhibitor treatment in TSC patients? Since the SEGAs appear to grow when the treatment is stopped, how long would patients with SEGAs need to be on mTORC1 inhibitors? Can mTORC1 inhibitors improve epilepsy and prevent epileptogenesis clinically? Can they improve neurocognitive deficits and neurobehavioral difficulties? Is there a critical period during which treatment with mTORC1 inhibitors result in long-term improvement without requiring chronic treatment? Since there is growing evidence that TSC and Rheb may have mTORC1-independent functions[54], are there clinical aspects of TSC that are rapamycin insensitive?
Since rapamycin family mTOR inhibitors have significant side-effects, chronic therapy with these inhibitors may not be practical in many patients. However, perhaps using these inhibitors only during critical periods of pathogenesis or in patients refractory to other treatments. Another alternative is the intermittent use of these drugs, which has been effective in one animal model[55] and may be less toxic. Several other mTOR inhibitors recently have been developed although it remains unclear if they will have a better efficacy or side-effect profile[56-61].
Conclusions
Emerging evidence – from abnormal white matter on neuroimaging to deficits in axonal integrity in animal models – supports the hypothesis that abnormal neuronal wiring contributes to the neuropsychiatric symptoms in TSC patients. Further studies using functional imaging and electrophysiology will be important to delineate the timing and specific circuits underlying these abnormalities. While important areas of future study remain and have been highlighted throughout this review, several additional questions remain. What causes the remarkable variability of neurological phenotype in patients with the same TSC gene mutations? What are the genetic and non-genetic modifiers of TSC disease? Will it be possible to generate animal models that fully replicate the human neuropathology? Finally, findings from TSC may also have implications for other diseases in which the mTOR pathway is hyperactive – such as PTEN hamartoma syndrome, Fragile X Syndrome (FXS), and Neurofibromatosis 1 (NF1), all of which have been associated with ASD, behavioral dysregulation, or intellectual disability[62-66]. It will be crucial to compare and contrast the neuronal dysfunction in each of these conditions to determine whether modulation of mTORC1 function will have therapeutic or detrimental effects. Such information should shed light on the mechanisms underlying TSC and on the development of future therapeutic modalities.
Bullet points.
Accumulating evidence suggests that TSC patients have non-tuber abnormalities that contribute to the development of the neurological phenotype.
While TSC mouse models have failed to replicate the human neuropathology in its entirety, they have shed light on the cellular abnormalities and the neurobehavioral phenotypes.
Preclinical data strongly suggest that TSC is a disease of abnormal neuronal connectivity.
Cell culture and animal models have identified the mTORC1 pathway as a therapeutic target in TSC, and clinical trials are in progress.
The high incidence of neurodevelopmental deficits, early detection of the disease in very young ages, and availability of potential therapeutic targets and drugs make TSC a model for other Mendelian disorders of neurocognition.
Acknowledgements
We would like to thank all members of the TSC community for many helpful discussions. We are also grateful to Drs. , Elizabeth Henske and Joseph Volpe for critical reading of the manuscript. Owing to limited space we have not quoted all literature in the field, and we apologize to those whose articles are not referenced. Dr. Sahin served as a consultant and site-PI for Novartis, received honoraria for two talks from Athena Diagnostics. Research in Dr. Sahin’s laboratory is funded by the NIH R01NS058956, Tuberous Sclerosis Alliance, Autism Speaks, John Merck Fund, Nancy Lurie Marks Family Foundation, Children’s Hospital Boston Translational Research Program and the Manton Family Foundation. Peter Tsai is funded by Developmental Neurology training grant T32NS007473.
References
Annotated references in yellow highlight: Red letters indicate ••; black letters indicate •
- 1.Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asato MR, Hardan AY. Neuropsychiatric problems in tuberous sclerosis complex. J Child Neurol. 2004;19:241–249. doi: 10.1177/088307380401900401. [DOI] [PubMed] [Google Scholar]
- 3.Goodman M, Lamm SH, Engel A, Shepherd CW, Houser OW, Gomez MR. Cortical tuber count: a biomarker indicating neurologic severity of tuberous sclerosis complex. J Child Neurol. 1997;12:85–90. doi: 10.1177/088307389701200203. [DOI] [PubMed] [Google Scholar]
- 4.Jambaque I, Cusmai R, Curatolo P, Cortesi F, Perrot C, Dulac O. Neuropsychological aspects of tuberous sclerosis in relation to epilepsy and MRI findings. Dev Med Child Neurol. 1991;33:698–705. doi: 10.1111/j.1469-8749.1991.tb14947.x. [DOI] [PubMed] [Google Scholar]
- 5.Jansen FE, Vincken KL, Algra A, Anbeek P, Braams O, Nellist M, Zonnenberg BA, Jennekens-Schinkel A, van den Ouweland A, Halley D, et al. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology. 2008;70:916–923. doi: 10.1212/01.wnl.0000280579.04974.c0. [DOI] [PubMed] [Google Scholar]
- 6.Bolton PF, Park RJ, Higgins JN, Griffiths PD, Pickles A. Neuro-epileptic determinants of autism spectrum disorders in tuberous sclerosis complex. Brain. 2002;125:1247–1255. doi: 10.1093/brain/awf124. [DOI] [PubMed] [Google Scholar]
- 7.Eluvathingal TJ, Behen ME, Chugani HT, Janisse J, Bernardi B, Chakraborty P, Juhasz C, Muzik O, Chugani DC. Cerebellar lesions in tuberous sclerosis complex: neurobehavioral and neuroimaging correlates. J Child Neurol. 2006;21:846–851. doi: 10.1177/08830738060210100301. [DOI] [PubMed] [Google Scholar]
- 8.Weber AM, Egelhoff JC, McKellop JM, Franz DN. Autism and the cerebellum: evidence from tuberous sclerosis. J Autism Dev Disord. 2000;30:511–517. doi: 10.1023/a:1005679108529. [DOI] [PubMed] [Google Scholar]
- 9.Gama HP, da Rocha AJ, Valerio RM, da Silva CJ, Garcia LA. Hippocampal abnormalities in an MR imaging series of patients with tuberous sclerosis. AJNR Am J Neuroradiol. 2010;31:1059–1062. doi: 10.3174/ajnr.A1972. Gama 2010: The authors find hippocampal abnormalities in patients with TSC that are highly correlated with a history of febrile seizures.
- 10.Ertan G, Arulrajah S, Tekes A, Jordan L, Huisman TA. Cerebellar abnormality in children and young adults with tuberous sclerosis complex: MR and diffusion weighted imaging findings. J Neuroradiol. 2010;37:231–238. doi: 10.1016/j.neurad.2009.12.006. Ertan 2010: The authors report cerebellar abnormalities in a third of patients with TSC, even in the absence of classic cerebellar symptoms.
- 11.Asano E, Chugani DC, Muzik O, Behen M, Janisse J, Rothermel R, Mangner TJ, Chakraborty PK, Chugani HT. Autism in tuberous sclerosis complex is related to both cortical and subcortical dysfunction. Neurology. 2001;57:1269–1277. doi: 10.1212/wnl.57.7.1269. [DOI] [PubMed] [Google Scholar]
- 12.Widjaja E, Simao G, Mahmoodabadi SZ, Ochi A, Snead OC, Rutka J, Otsubo H. Diffusion tensor imaging identifies changes in normal-appearing white matter within the epileptogenic zone in tuberous sclerosis complex. Epilepsy Res. 2010;89:246–253. doi: 10.1016/j.eplepsyres.2010.01.008. Widjaja 2010: The authors demonstrate WM disruption in epileptic zones as compared to non epileptic zones in both normal appearing WM and in areas with tubers, suggesting an ictal contribution to white matter abnormalities in TSC.
- 13.Garaci FG, Floris R, Bozzao A, Manenti G, Simonetti A, Lupattelli T, Curatolo P, Simonetti G. Increased brain apparent diffusion coefficient in tuberous sclerosis. Radiology. 2004;232:461–465. doi: 10.1148/radiol.2322030198. [DOI] [PubMed] [Google Scholar]
- 14.Peng SS, Lee WT, Wang YH, Huang KM. Cerebral diffusion tensor images in children with tuberous sclerosis: a preliminary report. Pediatr Radiol. 2004;34:387–392. doi: 10.1007/s00247-004-1162-3. [DOI] [PubMed] [Google Scholar]
- 15.Krishnan ML, Commowick O, Jeste SS, Weisenfeld N, Hans A, Gregas MC, Sahin M, Warfield SK. Diffusion features of white matter in tuberous sclerosis with tractography. Pediatr Neurol. 2010;42:101–106. doi: 10.1016/j.pediatrneurol.2009.08.001. Krishnan 2010: The authors find evidence of structurally compromised axon tracts and abnormal myelination in TSC patients.
- 16.Simao G, Raybaud C, Chuang S, Go C, Snead OC, Widjaja E. Diffusion tensor imaging of commissural and projection white matter in tuberous sclerosis complex and correlation with tuber load. AJNR Am J Neuroradiol. 2010;31:1273–1277. doi: 10.3174/ajnr.A2033. Simao 2010: Abnormalities in white matter indices of the corpus callosum and internal capsule are found in patients with TSC and are directly correlated with cortical tuber volume.
- 17.Luat AF, Makki M, Chugani HT. Neuroimaging in tuberous sclerosis complex. Curr Opin Neurol. 2007;20:142–150. doi: 10.1097/WCO.0b013e3280895d93. [DOI] [PubMed] [Google Scholar]
- 18.Arulrajah S, Ertan G, Jordan L, Tekes A, Khaykin E, Izbudak I, Huisman TA. Magnetic resonance imaging and diffusion-weighted imaging of normal-appearing white matter in children and young adults with tuberous sclerosis complex. Neuroradiology. 2009;51:781–786. doi: 10.1007/s00234-009-0563-2. Arulrajah 2009: The authors demonstrate diffusion abnormalities in normal appearing white matter in an age and location dependent manner. No significant differences in ADC values were found in TSC children up to 8 years of age, while increased ADC values were detected in TSC patients in frontal and pontine white matter in patients aged 8-12yrs, and in the right parietal and occipital areas in children over 12 yrs of age.
- 19.Makki MI, Chugani DC, Janisse J, Chugani HT. Characteristics of abnormal diffusivity in normal-appearing white matter investigated with diffusion tensor MR imaging in tuberous sclerosis complex. AJNR Am J Neuroradiol. 2007;28:1662–1667. doi: 10.3174/ajnr.A0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niida Y, Stemmer-Rachamimov AO, Logrip M, Tapon D, Perez R, Kwiatkowski DJ, Sims K, MacCollin M, Louis DN, Ramesh V. Survey of somatic mutations in tuberous sclerosis complex (TSC) hamartomas suggests different genetic mechanisms for pathogenesis of TSC lesions. Am J Hum Genet. 2001;69:493–503. doi: 10.1086/321972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 22.Crino PB, Aronica E, Baltuch G, Nathanson KL. Biallelic TSC gene inactivation in tuberous sclerosis complex. Neurology. 2010;74:1716–1723. doi: 10.1212/WNL.0b013e3181e04325. Crino 2010: The investigators used Sanger sequencing of DNA obtained from 100 phospho-S6 positive neurons in each tuber sample. A somatic mutation was identified in 5 of 6 cases.
- 23.Qin W, Chan JA, Vinters HV, Mathern GW, Franz DN, Taillon BE, Bouffard P, Kwiatkowski DJ. Analysis of TSC cortical tubers by deep sequencing of TSC1, TSC2 and KRAS demonstrates that small second-hit mutations in these genes are rare events. Brain Pathol. 2010;20:1096–1105. doi: 10.1111/j.1750-3639.2010.00416.x. Qin 2010: The authors used ultra-deep pyrosequencing (454 sequencing) to evaluate mutations in 46 tubers samples. A second allele mutation was found at low frequency (up to 10%) in one patient, while in the vast majority of the samples, there was little evidence for this event.
- 24.Waltereit R, Welzl H, Dichgans J, Lipp HP, Schmidt WJ, Weller M. Enhanced episodic-like memory and kindling epilepsy in a rat model of tuberous sclerosis. J Neurochem. 2006;96:407–413. doi: 10.1111/j.1471-4159.2005.03538.x. [DOI] [PubMed] [Google Scholar]
- 25.Waltereit R, Japs B, Schneider M, de Vries PJ, Bartsch D. Epilepsy and Tsc2 Haploinsufficiency Lead to Autistic-Like Social Deficit Behaviors in Rats. Behav Genet. 2010 doi: 10.1007/s10519-010-9399-0. Waltereit 2010: This study demonstrates abnormal social interaction and novel exploratory behavior that is worsened by seizures in a Tsc2+/− rat model.
- 26.Goorden SM, van Woerden GM, van der Weerd L, Cheadle JP, Elgersma Y. Cognitive deficits in Tsc1+/− mice in the absence of cerebral lesions and seizures. Ann Neurol. 2007;62:648–655. doi: 10.1002/ana.21317. [DOI] [PubMed] [Google Scholar]
- 27.Onda H, Lueck A, Marks PW, Warren HB, Kwiatkowski DJ. Tsc2(+/−) mice develop tumors in multiple sites that express gelsolin and are influenced by genetic background. J Clin Invest. 1999;104:687–695. doi: 10.1172/JCI7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehninger D, Han S, Shilyansky C, Zhou Y, Li W, Kwiatkowski DJ, Ramesh V, Silva AJ. Reversal of learning deficits in a Tsc2+/− mouse model of tuberous sclerosis. Nat Med. 2008;14:843–848. doi: 10.1038/nm1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young DM, Schenk AK, Yang SB, Jan YN, Jan LY. Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proc Natl Acad Sci U S A. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. Young 2010: The authors report abnormal vocalizations in pups from Tsc2+/− mothers – a model for abnormal mother-pup social interactions in TSC.
- 30.Nie D, Di Nardo A, Han JM, Baharanyi H, Kramvis I, Huynh T, Dabora S, Codeluppi S, Pandolfi PP, Pasquale EB, et al. Tsc2-Rheb signaling regulates EphA-mediated axon guidance. Nat Neurosci. 2010;13:163–172. doi: 10.1038/nn.2477. Nie 2010: The authors demonstrate that TSC2-mTOR signaling interacts with the ephrin-Eph receptor system to regulate axon guidance.
- 31.Govindarajan B, Brat DJ, Csete M, Martin WD, Murad E, Litani K, Cohen C, Cerimele F, Nunnelley M, Lefkove B, et al. Transgenic expression of dominant negative tuberin through a strong constitutive promoter results in a tissue-specific tuberous sclerosis phenotype in the skin and brain. J Biol Chem. 2005;280:5870–5874. doi: 10.1074/jbc.M411768200. [DOI] [PubMed] [Google Scholar]
- 32.Ehninger D, Silva AJ. Increased Levels of Anxiety-related Behaviors in a Tsc2 Dominant Negative Transgenic Mouse Model of Tuberous Sclerosis. Behav Genet. 2010 doi: 10.1007/s10519-010-9398-1. Ehninger 2010: This study reports elevated anxiety behaviors in a Tsc2-dominant negative transgenic mouse model of tuberous sclerosis.
- 33.Uhlmann EJ, Wong M, Baldwin RL, Bajenaru ML, Onda H, Kwiatkowski DJ, Yamada K, Gutmann DH. Astrocyte-specific TSC1 conditional knockout mice exhibit abnormal neuronal organization and seizures. Ann Neurol. 2002;52:285–296. doi: 10.1002/ana.10283. [DOI] [PubMed] [Google Scholar]
- 34.Wong M, Ess KC, Uhlmann EJ, Jansen LA, Li W, Crino PB, Mennerick S, Yamada KA, Gutmann DH. Impaired glial glutamate transport in a mouse tuberous sclerosis epilepsy model. Ann Neurol. 2003;54:251–256. doi: 10.1002/ana.10648. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Zeng LH, Wong M. Impaired astrocytic gap junction coupling and potassium buffering in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2009;34:291–299. doi: 10.1016/j.nbd.2009.01.010. Xu 2009: The authors demonstrate decreased Connexin43 and associated gap junction coupling in an astrocyte specific Tsc1 mutant model. Abnormalities are reversed with rapamycin treatment.
- 36.Zeng LH, Bero AW, Zhang B, Holtzman DM, Wong M. Modulation of astrocyte glutamate transporters decreases seizures in a mouse model of Tuberous Sclerosis Complex. Neurobiol Dis. 2010;37:764–771. doi: 10.1016/j.nbd.2009.12.020. Zeng 2010: The authors are able to prevent epilepsy in a mouse model with targeted deletion of Tsc1 in astrocytes by upregulation of glutamate transporters, suggesting that abnormal glutamate transport is critical for epileptogenesis in TSC.
- 37.Zeng LH, Ouyang Y, Gazit V, Cirrito JR, Jansen LA, Ess KC, Yamada KA, Wozniak DF, Holtzman DM, Gutmann DH, et al. Abnormal glutamate homeostasis and impaired synaptic plasticity and learning in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2007;28:184–196. doi: 10.1016/j.nbd.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meikle L, Talos DM, Onda H, Pollizzi K, Rotenberg A, Sahin M, Jensen FE, Kwiatkowski DJ. A mouse model of tuberous sclerosis: neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J Neurosci. 2007;27:5546–5558. doi: 10.1523/JNEUROSCI.5540-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Way SW, McKenna J, 3rd, Mietzsch U, Reith RM, Wu HC, Gambello MJ. Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse. Hum Mol Genet. 2009;18:1252–1265. doi: 10.1093/hmg/ddp025. Way 2009: In a radial glial specific mutant of Tsc1, the authors reproduce several of the pathologic features seen in patients with TSC, namely heterotopias, astrocytosis, dysplastic neurons, and abnormal myelination.
- 40.Kobayashi T, Minowa O, Kuno J, Mitani H, Hino O, Noda T. Renal carcinogenesis, hepatic hemangiomatosis, and embryonic lethality caused by a germ-line Tsc2 mutation in mice. Cancer Res. 1999;59:1206–1211. [PubMed] [Google Scholar]
- 41.Kobayashi T, Minowa O, Sugitani Y, Takai S, Mitani H, Kobayashi E, Noda T, Hino O. A germ-line Tsc1 mutation causes tumor development and embryonic lethality that are similar, but not identical to, those caused by Tsc2 mutation in mice. Proc Natl Acad Sci U S A. 2001;98:8762–8767. doi: 10.1073/pnas.151033798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehninger D, Sano Y, de Vries PJ, Dies K, Franz D, Geschwind DH, Kaur M, Lee YS, Li W, Lowe JK, et al. Gestational immune activation and Tsc2 haploinsufficiency cooperate to disrupt fetal survival and may perturb social behavior in adult mice. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.115. Ehninger, 2010: This study reports that exposure of Tsc2+/− mice to immune activation during the embryonic period results in abnormal social approach behavior in adulthood.
- 43.Onda H, Crino PB, Zhang H, Murphey RD, Rastelli L, Gould Rothberg BE, Kwiatkowski DJ. Tsc2 null murine neuroepithelial cells are a model for human tuber giant cells, and show activation of an mTOR pathway. Mol Cell Neurosci. 2002;21:561–574. doi: 10.1006/mcne.2002.1184. [DOI] [PubMed] [Google Scholar]
- 44.Choi YJ, Di Nardo A, Kramvis I, Meikle L, Kwiatkowski DJ, Sahin M, He X. Tuberous sclerosis complex proteins control axon formation. Genes Dev. 2008;22:2485–2495. doi: 10.1101/gad.1685008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tavazoie SF, Alvarez VA, Ridenour DA, Kwiatkowski DJ, Sabatini BL. Regulation of neuronal morphology and function by the tumor suppressors Tsc1 and Tsc2. Nat Neurosci. 2005;8:1727–1734. doi: 10.1038/nn1566. [DOI] [PubMed] [Google Scholar]
- 46.Cajigas IJ, Will T, Schuman EM. Protein homeostasis and synaptic plasticity. EMBO J. 2010;29:2746–2752. doi: 10.1038/emboj.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haddad LA, Smith N, Bowser M, Niida Y, Murthy V, Gonzalez-Agosti C, Ramesh V. The TSC1 tumor suppressor hamartin interacts with neurofilament-L and possibly functions as a novel integrator of the neuronal cytoskeleton. J Biol Chem. 2002;277:44180–44186. doi: 10.1074/jbc.M207211200. [DOI] [PubMed] [Google Scholar]
- 48.Li YH, Werner H, Puschel AW. Rheb and mTOR regulate neuronal polarity through Rap1B. J Biol Chem. 2008;283:33784–33792. doi: 10.1074/jbc.M802431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morita T, Sobue K. Specification of neuronal polarity regulated by local translation of CRMP2 and Tau via the mTOR-p70S6K pathway. J Biol Chem. 2009;284:27734–27745. doi: 10.1074/jbc.M109.008177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meikle L, Pollizzi K, Egnor A, Kramvis I, Lane H, Sahin M, Kwiatkowski DJ. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J Neurosci. 2008;28:5422–5432. doi: 10.1523/JNEUROSCI.0955-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franz DN, Leonard J, Tudor C, Chuck G, Care M, Sethuraman G, Dinopoulos A, Thomas G, Crone KR. Rapamycin causes regression of astrocytomas in tuberous sclerosis complex. Ann Neurol. 2006;59:490–498. doi: 10.1002/ana.20784. [DOI] [PubMed] [Google Scholar]
- 53.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, Wilson KA, Byars A, Sahmoud T, Franz DN. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–1811. doi: 10.1056/NEJMoa1001671. Krueger 2010: The authors demonstrate reduction in SEGA size and seizure frequency in TSC patients treated with the mTORC1 specific inhibitor everolimus.
- 54.Lee PS, Tsang SW, Moses MA, Trayes-Gibson Z, Hsiao LL, Jensen R, Squillace R, Kwiatkowski DJ. Rapamycin-insensitive up-regulation of MMP2 and other genes in tuberous sclerosis complex 2-deficient lymphangioleiomyomatosis-like cells. Am J Respir Cell Mol Biol. 2010;42:227–234. doi: 10.1165/rcmb.2009-0050OC. Lee 2010: Using global gene expression analysis, the authors show that MMP-2 and multiple other genes are regulated in a rapamycin-independent manner in TSC-deficient cell.
- 55.Lee N, Woodrum CL, Nobil AM, Rauktys AE, Messina MP, Dabora SL. Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models. BMC Pharmacol. 2009;9:8. doi: 10.1186/1471-2210-9-8. Lee 2009: The authors demonstrate improved treatment efficacy with combined mTORC1 and VEGF inhibition in a TSC mouse tumor model. Furthermore, prolonged treatment with low doses of mTOR inhibitors appear to result in more complete and lasting TSC-related tumor responses.
- 56.Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. Kalender 2010: The authors report mTORC1 inhibition by metformin in an AMPK- and TSC-independent manner.
- 57.Sini P, James D, Chresta C, Guichard S. Simultaneous inhibition of mTORC1 and mTORC2 by mTOR kinase inhibitor AZD8055 induces autophagy and cell death in cancer cells. Autophagy. 2010;6:553–554. doi: 10.4161/auto.6.4.11671. Sini 2010: The authors demonstrate cell death in cancer cells through use of the novel mTORC1 and mTORC2 inhibitor, AZD8055.
- 58.Lin CJ, Robert F, Sukarieh R, Michnick S, Pelletier J. The antidepressant sertraline inhibits translation initiation by curtailing mammalian target of rapamycin signaling. Cancer Res. 2010;70:3199–3208. doi: 10.1158/0008-5472.CAN-09-4072. Lin 2010: The authors describe a novel role for the antidepressant, sertraline, in the TSC/Rheb independent inhibition of mTORC1 mediated protein synthesis and cell growth.
- 59.Liu M, Wilk SA, Wang A, Zhou L, Wang RH, Ogawa W, Deng C, Dong LQ, Liu F. Resveratrol inhibits mTOR signaling by promoting the interaction between mTOR and DEPTOR. J Biol Chem. 2010;285:36387–94. doi: 10.1074/jbc.M110.169284. Liu 2010: The authors report increased DEPTOR mediated inhibition of mTOR signaling in cells treated with the polyphenol Resveratrol.
- 60.Balgi AD, Fonseca BD, Donohue E, Tsang TC, Lajoie P, Proud CG, Nabi IR, Roberge M. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS One. 2009;4:e7124. doi: 10.1371/journal.pone.0007124. Balgi 2009: The authors discover novel roles for amiodarone, perhexiline, niclosamide, and rottlerin in the inhibition of mTORC1 signaling with irreversible mTORC1 inhibition by amiodarone and TSC2 dependent inhibition by rottlerin.
- 61.Richard DJ, Verheijen JC, Zask A. Recent advances in the development of selective, ATP-competitive inhibitors of mTOR. Curr Opin Drug Discov Devel. 2010;13:428–440. [PubMed] [Google Scholar]
- 62.Johannessen CM, Johnson BW, Williams SM, Chan AW, Reczek EE, Lynch RC, Rioth MJ, McClatchey A, Ryeom S, Cichowski K. TORC1 is essential for NF1-associated malignancies. Curr Biol. 2008;18:56–62. doi: 10.1016/j.cub.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 63.Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, Warren ST, Bassell GJ. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30:10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. Gross 2010: The authors show that FMRP (protein responsible for Fragile X Syndrome) regulates the synthesis of PI3K catalytic subunit p110beta in an mGluR dependent and independent manner. They also demonstrate reversal of several Fragile X Syndrome phenotypes with inhibition of PI3K signaling, thereby implicating PI3K as a potential therapeutic target in Fragile X Syndrome.
- 64.Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Dysregulation of mTOR signaling in fragile X syndrome. J Neurosci. 2010;30:694–702. doi: 10.1523/JNEUROSCI.3696-09.2010. Sharma 2010: The authors demonstrate a PI3K dependent increase in mTOR signaling in a mouse model of Fragile X Syndrome.
- 65.Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. Pten Regulates Neuronal Arborization and Social Interaction in Mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, Miles JH, Wang CH, Stratton R, Pilarski R, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–321. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]


