Abstract
The synthesis and photophysical properties of 7-(dimethylamino)-3,4-dihydrophenanthren-1(2H)-one (7) and 3-(dimethylamino)-8,9,10,11-tetrahydro-7H-cyclohepta[a]naphthalen-7-one (8) are reported. These compounds possess a cycloalkanone substructure that controls the extent of twisting of the carbonyl group. The six-membered ring in 7 forces the carbonyl group to be coplanar with the naphthalene ring, whereas the seven-membered ring in 8 induces a significant twist. Both have the substructure of PRODAN (6-propionyl-2-(dimethylamino)naphthalene, 1). Comparing the photophysical behavior of these compounds with that of PRODAN and 2,2-dimethyl-1-(4-methyl-1,2,3,4-tetrahydrobenzo[f]quinolin-8-yl)propan-1-one (3) indicates that PRODAN likely emits from a PICT excited state rather than from an O-TICT excited state.
Keywords: PRODAN, PICT, TICT, solvatochroism
I. Introduction
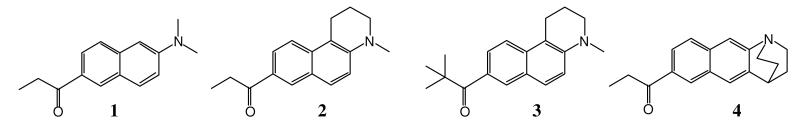
PRODAN (6-propionyl-2-dimethyaminonaphthalene, 1, Figure 1) is well known as a fluorescent probe of micropolarity. It was prepared in 1979 by Weber and Farris who wanted a donor and acceptor group separated by the greatest distance across the naphthalene nucleus.1 The sensing ability of PRODAN lies in the position of the emission wavelength, which becomes progressively longer with increasing solvent polarity. Recent work has shown that care must be exercised in using 1 as a probe of micropolarity.2, 3 The polarity-dependence behavior is attributed to an intramolecular charge transfer (ICT) excited state. In this state the carbonyl group gains electron density at the expense of the dimethylamino group resulting in an increased molecular dipole moment. However, the geometry of the excited state is still debated. Decoupling the donor and acceptor units by orthogonal twisting can lead to complete electron transfer. Such a twisted intramolecular charge transfer (TICT) state is thought to occur in the structurally similar compound DMABN (4-dimethylaminobenzonitrile).4-6 For PRODAN both the dimethylamino group and the propionyl group are capable of orthogonal twisting, and there is theoretical support for both N-TICT and O-TICT excited states, respectively.7, 8
Figure 1.
PRODAN and various geometrically constrained derivatives.
Experimental support for or against TICT states can be made through geometrically constrained model compounds. In the case of DMABN, the twisted model behaves like DMABN whereas the planar model does not.9, 10 We have shown the opposite behavior with PRODAN. The planar model 2 (Figure 1) behaves like PRODAN, while the twisted model 4 does not.11, 12 In particular, fluorescence of the twisted model compound is significantly deactivated in polar solvents. These results speak against the involvement of an N-TICT state in PRODAN. However, an O-TICT state is still possible in these systems. Indeed, we have shown that the solvatochromic behavior of compound 3, where the t-butyl group forces the carbonyl group to twist out-of-plane, is just like that of PRODAN.11
In this paper we report the preparation and photophysical behavior of two model compounds 7 and 8. The six-membered ring in 7 forces the coplanarity of the carbonyl group with the naphthalene unit, whereas the seven-membered ring in 8 induces some twist. The photophysical behavior of these compounds is compared with that of 1 and 3.
II. Experimental Section
NMR spectra were obtained with a Varian Mercury VX-400 spectrometer. High resolution ESI-MS were acquired with a Bruker Apex-Qe instrument. UV/VIS absorbance measurements were performed with an Ocean Optics HR2000 spectrometer. Fluorescence excitation/emission data were collected using a SLM-Aminco SPF-500 spectrometer as the excitation source and the Ocean Optics system as the detector. Solvents used for photophysical characterization were spectrophotometric grade from Acros except water, ethanol and 2,2,2-trifluoroethanol, which were distilled. Quantum yield measurements were made using anthracene as a reference (Φ = 0.27 in abs. EtOH) using the method of standard additions. The absorption of solutions for quantum yield determinations was kept below 0.05. AM1/SM5C semiempirical calculations were performed using AMPAC 9.0 from Semichem, Inc. Calculations incorporated the following keywords: AM1; SDC.I. = 8; singlet; qcscf; sm5c; solvnt = ‘solvent’; tight; truste; micros = 0; root = 1 (or 2); scfcrt = 0.
PRODAN13 and 311 were available from previous studies and were sublimed under vacuum prior to use.
5-Bromo-N,N-dimethylnaphthalen-2-amine (6)
5-Bromo-2-naphthol (8.15 g, 36.5 mmol, 5), sodium bisulfite (13.44 g, 129 mmol), aq. NaOH (7.33 g, 183 mmol, 40 mL water), and CH3NH2 • HCl (12.18 g, 180 mmol) are combined in a stainless steel autoclave, and the mixture is heated to 140°C overnight. The reaction is cooled to 90°C, and the contents are removed. The vessel is rinsed with hot water, hot ethanol and acetone until the washings are colorless. The washing are combined with the original contents, and the volatile solvents are allowed to evaporate overnight with stirring. The precipitated solid is collected by suction filtration. The filtrated is saturated with NaCl giving more solid material. The combined solids are dried in the open giving 5.11 g. This solid is combined with K2CO3 (7.0 g, 51 mmol) and CH3I (8.0 g, 56 mmol) in DMF (30 mL) and stirred overnight. The resulting precipitate is collected with suction and air-dried giving 6.99 g. This solid is added to ethanolamine (15 mL), and the mixture is heated to 150°C under Ar for 1 hr. After cooling, the reaction is poured into ice-water, and the mixture is stirred overnight. The precipitate is collected by suction filtration, air-dried, then sublimed under vacuum (0.1 Torr) giving 6 (3.75 g, 15.0 mmol, 41% over 3 steps) as a white solid, m.p. 66-67°C
1H NMR (CDCl3) δ= 8.07 (d, J=9.4 Hz, 1H), 7.59 (d, J=8.0 Hz, 1H), 7.46 (d, J=7.7 Hz, 1H), 7.22 (dd, J=2.4, 9.4 Hz, 1H), 7.17 (dd, J=7.7, 8.0 Hz, 1H), 6.87 (d, J=2.4 Hz, 1H), 3.06 (s, 6H); 13C NMR δ 149.12, 136.48, 128.17, 126.73, 126.31, 125.93, 125.23, 122.80, 117.43, 106.42, 40.85.
Found [M+H]+ 250.02211. C12H13BrN+ requires 250.02259.
General procedure for cycloalkanone preparation
Zinc dust is sifted successively though 60, 100, and 200 mesh sieves. It is washed successively with 1N HCl (3x), H2O (2x), EtOH (1x), and Et2O (1x), then dried in vacuo overnight. Some of this zinc (1.0 g, 15 mmol) is slurried in DMAC (20 mL) and activated with I2 (250 mg, 1 mmol) under Ar. When the red color of I2 has dissipated, the ethyl ω-bromoalkanoate (11 mmol) is added. The slurry is heated to 80°C for 3 hrs, then cooled to room temperature. 5-Bromo-2-dimethylaminonaphthalene (1.0 g, 4.0 mmol, 6) and Ni(PPh)3Cl2 (160 mg, 0.3 mmol) are added. The reaction is stirred under Ar for 1 day. Water (1 mL) and acetone (50 mL) are added, and the mixture is filtered through Celite. The filtrate is concentrated in vacuo, then the remaining DMAC is removed by vacuum distillation (0.1 Torr). The residue is saponified with KOH (2.0 g, 36 mmol) in 80% aq. EtOH (12 mL) at room temperature overnight. The mixture is diluted with water (200 mL). Acetic acid (25 mL) is added, and the resulting precipitate is collected by filtration. The solid is dried overnight in vacuo. The solid is covered completely with polyphosphoric acid (~4 mL), and the mixture is heated to 120°C for 20 min. The reaction is cooled, and water (100 mL) is added. The solution is extracted with CH2Cl2 (2 × 75 mL). The combined extracts are washed with water (1 × 100 mL), dried over CaCl2, and concentrated in vacuo. The crude product is purified by liquid chromatography (SiO2) using a gradient elution with ethyl acetate and hexane. The isolated product is sublimed under vacuum (0.1 Torr).
7-(Dimethylamino)-3,4-dihydrophenanthren-1(2H)-one (7) is produced in 26% yield (230 mg, 1.04 mmol) as a yellow solid, m.p.165-167°C.
1H NMR (CDCl3) δ= 8.00 (d, J=9.1 Hz, 1H), 7.98 (d, J=8.5 Hz, 1H), 7.53 (d, J=8.5 Hz, 1H), 7.17 (dd, J=2.6, 9.1 Hz, 1H), 6.88 (d, J=2.6 Hz, 1H), 3.30 (t, J=6.2 Hz, 2H), 3.11 (s, 6H), 2.69 (t, J=6.7 Hz, 2H), 2.25 (tt, J=6.2, 6.7 Hz, 2H); 13C NMR δ= 198.52, 150.20, 143.53, 138.01, 137.97, 126.31, 125.35, 123.59, 123.49, 115.86, 106.75, 40.57, 38.53, 25.77, 23.11; UV (EtOH) λ (log ε) 369 nm (4.34).
Found [M+Na]+ 262.11968. C16H17NONa+ requires 262.12024.
3-(Dimethylamino)-8,9,10,11-tetrahydro-7H-cyclohepta[a]naphthalen-7-one (8) is produced in 17% yield (170 mg, 0.67 mmol) as a yellow solid, m.p.119-120°C.
1H NMR (CDCl3) δ= 8.08 (d, J=9.3 Hz, 1H), 7.66 (d, J=8.7 Hz, 1H), 7.53 (d, J=8.7 Hz, 1H), 7.19 (dd, J=2.8, 9.3 Hz, 1H), 6.03 (d, J=2.8 Hz, 1H), 3.31 (t, J=6.4 Hz, 2H), 3.09 (s, 6H), 2.76 (t, J=6.0 Hz, 2H), 1.93 (tt, J=6.4, 6.5 Hz, 2H), 1.81 (tt, J=6.0, 6.5 Hz, 2H); 13C NMR δ= 207.46, 149.57, 139.50, 137.67, 132.77, 125.86, 125.53, 125.16, 123.92, 116.38, 106.69, 41.08, 40.65, 26.03, 24.90, 21.03; UV (EtOH) λ (log ε) 372 nm (3.67).
Found [M+Na]+ 276.13545. C17H19NONa+ requires 276.13589.
III. Results
Synthesis
The syntheses of planar and twisted carbonyl derivatives of PRODAN are shown in Scheme 1. The bromonaphthol 5 starting material is prepared by our published method.14 The dimethylamino group is created by three step sequence that first makes the N-methyl derivative by a Bucherer reaction. Exhaustive methylation followed by selective mono-demethylation gives net mono-methylation. Negishi coupling tethers the alkanoate chain to the 5-position of the naphthalene ring. Cyclization at the 6-position is accomplished by electrophilic aromatic substitution in polyphosphoric acid with the terminal carboxylic acid that results from alkaline hydrolysis of the ester.
Scheme 1.
Synthesis of PRODAN model compounds 7 and 8
Photophysical Studies
The absorption and emission maxima of 7 and 8 in a range of solvents are shown in Table 1 together with PRODAN (1) and 3. The solvatochromic behavior for the three derivatives is similar to that of PRODAN. The absorption spectra are centered around 370 nm in all but the non-polar solvents. The absorption bands are broad and lack sharp maxima. As with PRODAN, the cyclic derivatives are efficient fluorophores. The quantum yield for 7 in ethanol is 0.76 (±0.08), while that for 8 in isopropanol is 0.82 (±0.08). All show large polarity-dependent Stokes shifts.
Table 1.
Absorption and Emission Maxima (nm) and Relative Integrated Emission Intensity for 0.02 mM solutions of 1, 3, 7 and, 8 in a variety of solvents.
| solvent | 1 |
3 |
7 |
8 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| abs | em | I/Imax | abs | em | I/Imax | abs | em | I/Imax | abs | em | I/Imax | |
| cyclohexane | 343 | 392 | 0.01 | 353 | 407 | 0.14 | 347 | 407 | 0.01 | 341 | 414 | 0.02 |
| toluene | 349 | 416 | 0.32 | 361 | 432 | 0.57 | 353 | 417 | 0.15 | 347 | 428 | 0.40 |
| CH2Cl2 | 354 | 440 | 0.75 | 364 | 458 | 0.91 | 358 | 440 | 0.65 | 354 | 448 | 0.85 |
| CH3CN | 352 | 456 | 0.85 | 362 | 465 | 0.99 | 356 | 448 | 0.79 | 350 | 482 | 0.97 |
| DMSO | 358 | 464 | 0.87 | 369 | 472 | 0.97 | 363 | 453 | 0.84 | 357 | 485 | 1.00 |
| iPrOH | 367 | 480 | 0.99 | 368 | 494 | 1.00 | 367 | 482 | 1.00 | 365 | 495 | 0.96 |
| nBuOH | 368 | 490 | 0.98 | 369 | 503 | 0.71 | 369 | 485 | 0.87 | 370 | 499 | 0.86 |
| EtOH | 369 | 494 | 1.00 | 369 | 510 | 0.35 | 369 | 489 | 0.82 | 369 | 506 | 0.64 |
| MeOH | 369 | 503 | 0.77 | 367 | 516 | 0.10 | 367 | 495 | 0.75 | 367 | 513 | 0.16 |
| CF3CH2OH | 349 | 515 | 0.19 | 353 | 518 | 0.01 | 349 | 507 | 0.34 | 356 | 521 | 0.00 |
| H2O | 363 | 520 | 0.14 | 365 | 522 | 0.01 | 362 | 512 | 0.15 | 359 | 527 | 0.01 |
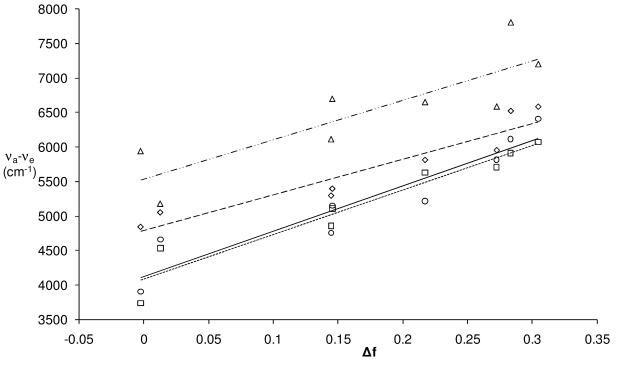
The relative magnitude of the solvatochroism is revealed by the Lippert-Mataga equation (1).15 The differences in the dipole moments between the ground and excited states (μ* − μ) are related to the slopes of the best-fit lines in the plot of the Stokes shifts vs. the solvent polarity factor Δf. The slopes determined for all of the compounds are nearly the same: 5200, 5700, 6400 and 6600 and cm−1 for 7, 8, 3 and 1, respectively (Figure 2).
| (1) |
Figure 2.
Lippert-Mataga plots for 7 (△, –··–), 8 (◇,– – –), 3 (□,----) and 1 (○, ——).
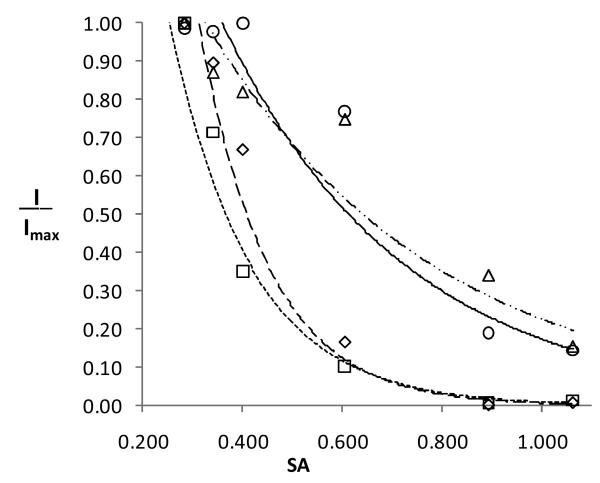
The fluorescence intensity shows similar solvent dependence for all four compounds. The fluorescence is very weak in cyclohexane and increases greatly with increasing solvent polarity. The intensity reaches a maximum in DMSO. With protic solvents the maximum intensity occurs in isopropanol. Compared with the other alcohol solvents, isopropanol is intermediate in polarity, but weakest in H-bond donating ability. With the other alcohols the intensity decreases as the H-bond donating ability increases. The fluorescence is weakest in water and trifluoroethanol. However, for 1 and 7 the fluorescence in water is an order of magnitude greater than that of the 3 and 8. This behavior is shown graphically in Figure 3 using Catalan's solvent acidity parameter to characterize the H-bond donating ability of the solvent.16 It should be noted that the effect of H-bonding solvents on the fluorescence of 1 continues to be an active area of investigation.3, 17-20
Figure 3.
Fluorescence intensity (I/Imax) vs. solvent acidity (SA) for compounds 7 (△, –··–), 8 (◇,– – –), 3 (□,----) and 1 (○, ——) in several protic solvents.
Computational Studies
The electronic structures for the first two singlet roots of 7 and 8 are calculated using the AM1 semiempirical method.. The advantage to this method is that allows for large CAS-CI level calculations that incorporate the effect of solvent. The CI level for these calculations is set to 8 in accord with a recent study, and only singly and doubly excited microstates are considered.18
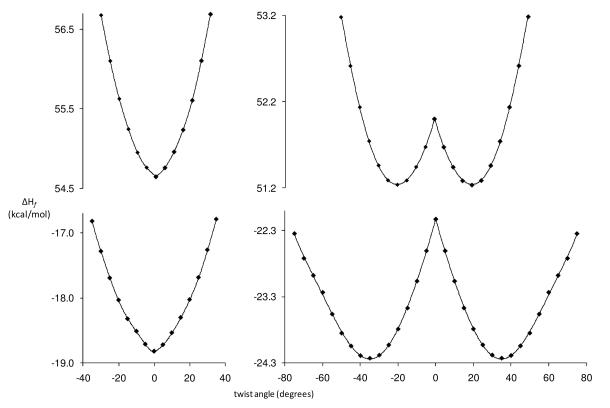
To determine the effect of the ring fusion on the dihedral angle of the carbonyl group, the heats of formation for the ground and first excited states are calculated for a series of increasing twist angles. The plots of energy vs. twist angle are shown in Figure 4. The calculations suggest that the carbonyl group in the six-membered ring structure does not twist either in the ground state or the excited state. On the other hand, the seven-membered ring is predicted to force the carbonyl group to twist significantly out-of-plane. The effect of electronic excitation in 8 is to reduce the degree of twist to just over 20°. Calculations indicate that the bond order between the carbonyl group and the aromatic ring increases 8% in the excited state for 7.
Figure 4.
Calculated ΔHf (kcal/mol) for 7 (left) and 8 (right) for the ground state (bottom) and the first singlet excited state (top) as a function of dihedral angle between the carbonyl group and the naphthalene ring.
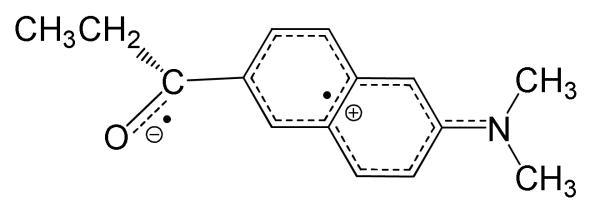
An O-TICT structure is predicted to have a negative charge localized on the twisted carbonyl group and a positive charge that is delocalized between the naphthalene ring and dimethylamino group (Figure 5). As a result, the bond order of the carbonyl group should decrease, while that of the nitrogen atom and the ipso aromatic carbon should increase. The third single root of the 90° twisted structure has these characteristics. The relevant parameters of this structure is compared to those of the planar ground state of 1 in Table 2.
Figure 5.
Predicted structure for the O-TICT state of PRODAN.
Table 2.
Calculateda structural characteristics for the planar and twisted geometries of 1.
| carbonyl geometry |
singlet root |
ΔHf (kcal/mol) |
dipole (D) |
bond order |
charge |
||
|---|---|---|---|---|---|---|---|
| CAr-N | C=O | N | O | ||||
| planarb | 1 | −21.7 | 7.8 | 1.12 | 1.80 | −0.29 | −0.42 |
| planar | 2 | 49.9 | 15.7 | 1.22 | 1.68 | −0.09 | −0.48 |
| planar | 3 | 57.3 | 12.8 | 1.18 | 1.71 | −0.16 | −0.46 |
| twistedc | 1 | −19.9 | 6.4 | 1.11 | 1.84 | −0.30 | −0.40 |
| twisted | 2 | 56.2 | 11.5 | 1.23 | 1.83 | −0.08 | −0.40 |
| twisted | 3 | 62.0 | 26.0 | 1.31 | 1.24 | −0.10 | −0.70 |
Using DMSO for the solvent.
Carbonyl constrained to 0° dihedral angle.
Carbonyl constrained to 90° dihedral angle.
IV. Discussion
The solvatochroism for all derivatives is remarkably similar. This observation is quantified by the similar slopes in the Lippert-Mataga plots. Since the slope is related to the square of the difference in dipole moments between the excited and ground states, and since the ground state dipole moments for all of the derivatives is around 6 D in nonpolar solvents, then all of the compounds should have similar excited-state dipole moments (~12 D). The similar excited-state dipole moments occur in spite of the fact that the t-butyl derivative 3 and the seven-membered ring compound 8 have twisted carbonyl structures, while in the six-membered ring 7 the carbonyl is planar. Thus, twisting has very little effect on the excited-state structure. An O-TICT structure should have a much larger excited state dipole moment than the planar structure. Such a larger excited state dipole moment is not observed for PRODAN, and we conclude that the O-TICT state is not consistent with the photophysical behavior. On the other hand, the results here indicate that the photophysical behavior is insensitive to twisting. The similar solvatochroism in these compounds leaves open the question as to whether the carbonyl group in PRODAN twists. The quenching behavior with protic solvents suggests that it does not. The twisted derivatives are very sensitive to protic solvents, and their fluorescence is efficiently quenched as the H-bond donating ability in the solvent increases. PRODAN behaves like 7 in that there is significant residual fluorescence even in water. Since PRODAN behaves like the planar derivative and not like the slightly twisted derivative, we suggest that twisting of the carbonyl group in the excited state of PRODAN is not significant.
V. Conclusions
The ICT excited state of PRODAN is planar (PICT). Twisting about the carbonyl group gives rise to efficient quenching of the excited state by protic solvents.
Acknowledgment
This research was supported by Grant 1R15 089925-01 from the NIH/NHLBI
References
- 1.Weber G, Farris FJ. Biochemistry. 1979;18:3075–3078. doi: 10.1021/bi00581a025. [DOI] [PubMed] [Google Scholar]
- 2.Adhikary R, Barnes CA, Petrich JW. J. Phys. Chem. B. 2009;113:11999–12004. doi: 10.1021/jp905139n. [DOI] [PubMed] [Google Scholar]
- 3.Rowe BA, Roach CA, Lin J, Asiago V, Dmitrenko O, Neal SL. J. Phys. Chem. A. 2008;112:13402–13412. doi: 10.1021/jp802260y. [DOI] [PubMed] [Google Scholar]
- 4.Lippert E, Rettig W, Bonacic-Koutecky V, Heisel F, Miehe JA. Adv. Chem. Phys. 1987;68:1–173. [Google Scholar]
- 5.Rettig W. Charge Angew. Chem. 1986;98:969–986. [Google Scholar]
- 6.Grabowski ZR, Rotkiewicz K, Rettig W. Chem. Rev. 2003;103:3899–4031. doi: 10.1021/cr940745l. [DOI] [PubMed] [Google Scholar]
- 7.Parusel ABJ, Nowak W, Grimme S, Kohler G. J. Phys. Chem. A. 1998;102:7149–7156. [Google Scholar]
- 8.Parusel ABJ, Schneider FW, Köhler G. J. Mol. Struct. THEOCHEM. 1997;398:341–346. [Google Scholar]
- 9.Okada T, Uesugi M, Köhler G, Rechthaler K, Rotkiewicz K, Rettig W, Grabner G. Chem. Phys. 1999;241:327–337. [Google Scholar]
- 10.Wermuth G, Rettig W. J. Phys. Chem. 1984;88:2729–2735. [Google Scholar]
- 11.Lobo BC, Abelt CJ. J. Phys. Chem. A. 2003;107:10938–10943. [Google Scholar]
- 12.Davis BN, Abelt CJ. J. Phys. Chem. A. 2005;109:1295–1298. doi: 10.1021/jp046050y. [DOI] [PubMed] [Google Scholar]
- 13.Silvonek SS, Giller CB, Abelt CJ. Org. Prep. Proced. Int. 2005;37:589. [Google Scholar]
- 14.Everett R, Hamilton J, Abelt C. Molbank. 2009 doi: 10.3390/M602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mataga N. Pure Appl. Chem. 1997;69:729–734. [Google Scholar]
- 16.Catalán J. J. Phys. Chem. B. 2009;113:5951–5960. doi: 10.1021/jp8095727. [DOI] [PubMed] [Google Scholar]
- 17.Artukhov VY, Zharkova OM, Morozova JP. Spectrochim. Acta A. 2007;68:36–42. doi: 10.1016/j.saa.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 18.Bakalova SM, Kaneti J. Spectrochim. Acta A. 2009;72:36–40. doi: 10.1016/j.saa.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Mennucci B, Caricato M, Ingrosso F, Cappelli C, Cammi R, Tomasi J, Scalmani G, Frisch MJ. J. Phys. Chem. B. 2008;112:414–423. doi: 10.1021/jp076138m. [DOI] [PubMed] [Google Scholar]
- 20.Moyano F, Biasutti MA, Silber JJ, Correa NM. J Phys Chem B. 2006;110:11838–11846. doi: 10.1021/jp057208x. [DOI] [PubMed] [Google Scholar]