Abstract
A convergent synthesis of a unimolecular pentavalent-MUC1 glycopeptide has been accomplished. A tandem repeat of unglycosylated human tumor-associated MUC1, a potential target for cancer immunotherapy, was incorporated into the known unimolecular pentavalent carbohydrate construct (5). This is an important step towards the development of a new fully synthetic anti-cancer vaccine candidate (1).
Our laboratory has been exploring the possibility of harnessing the power of the human immune system as an anticancer treatment course. In particular, we focus on the development of carbohydrate-based anticancer vaccines elaborated through chemical synthesis.1 Our research efforts are based on the observation that tumor cells display abnormal levels and types of cell surface carbohydrates, anchored to the cancer cell either through a lipid tail or a protein domain.2 This distinguishing feature of malignantly transformed cells could potentially be exploited to induce the immune system to selectively recognize and eradicate circulating cancer cells and micrometastases.
In this context, we have developed a range of fully synthetic, carbohydrate-based anticancer vaccine conjugates. To date a series of monomeric3 and clustered4 vaccines have been prepared and evaluated in preclinical and clinical settings. These synthetic constructs are typically attached through a linker domain to an immunogenic carrier protein, such as Keyhole Limpet Hemocyanin (KLH). Preclinical and clinical trials have confirmed the capacity of such constructs to induce antibodies which selectively bind to the carbohydrate-bearing tumor cells in question.
We recently turned our attention to the development of multiantigenic vaccines, in which several different cancer-associated carbohydrates are presented on a single peptide backbone and conjugated to KLH carrier protein. By combining multiple carbohydrate antigens associated with a single cancer type, it will hopefully be possible to generate a diverse range antibodies, increasing the percentage of tumor cells targeted by the immune system.5 Several unimolecular multiantigenic vaccines have now been synthesized and evaluated in preclinical settings.6 Of particular interest is a KLH-conjugated pentavalent construct, which incorporates five breast and prostate cancer related antigens (Globo-H, GM2, sTn, TF, and Tn). This conjugate has shown very promising results in preclinical studies.
We sought to introduce an additional immunogenic component to our multiantigenic cancer vaccine constructs. Thus, most carbohydrate epitopes are able to induce only weak T-cell independent B-cell responses. In contrast, peptide domains may be much more immunogenic, as they have the capacity to bind to major histocompatibility complex (MHC) molecules, and, in favorable cases, to trigger the desired T-cell mediated reaction.7 This fact has led us to focus our attention on the human-tumor associated epithelial mucin, MUC1.8 Over-expressed on the tumor cell surface as a high molecular weight glycoprotein, MUC1 contains numerous repeating units of a 20-amino acid sequence (HGVTSAPDTRPAPGSTAPPA) in the extracellular portion of the molecule. Monoclonal antibody studies focused on tumor MUC1-induced antibodies reveal the PDTRP9 amino acid segment within the repeating units to be the most antigenic epitope. Importantly, expression of MUC1 on normal tissues is largely restricted to the apical surface of secretory cells, a site with minimal access to the immune system.10 The over-expression of MUC1 is correlated with the progression of breast,11 ovarian,12 and colon13 carcinoma, and MUC1 has long been used as a marker for monitoring recurrence of breast cancer.14 Animal studies and clinical trials show that the MUC1 antigen is capable of inducing a T-helper type I response.15 MUC1 could thus presumably serve as a key synergic component in the development of carbohydrate vaccines, in that the robust formation of antibodies depends on the cooperative interactions between T- and B-cells.
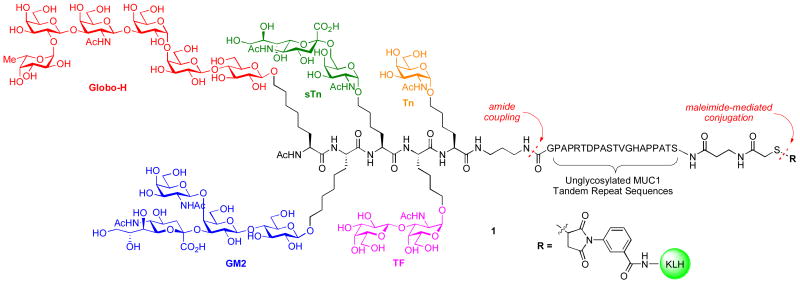
As shown in Figure 1, we envision the preparation of a hybrid vaccine construct (1) incorporating the previously synthesized unimolecular pentavalent carbohydrate domain as well as the MUC1 peptide. Accomplishment of this goal is not a trivial matter in that it requires considerable forethought in the orchestration of the orthogonal protections of the carbohydrate and peptidic domains. In addition to its intrinsic interest, we viewed this challenge as part of our broader goal of arriving at biologic level structures through chemical synthesis. Needless to say, if this goal is feasible, it would enable considerably more room for structural changes than would be imaginable by total reliance on biological pathways to generate biologics.
Figure 1.
Unimolecular KLH-Conjugated Pentavalent-MUC1 Construct (1).
The strategy for the synthesis of the KLH-conjugated unimolecular pentavalent-MUC1 construct (1), outlined in Figure 1, is highly convergent. The synthetic methods to be employed must be compatible with both the carbohydrate and peptide domains. The three main synthetic components are: (1) the fully protected unimolecular pentavalent glycopeptide, equipped with a C-terminal diaminopropyl spacer; (2) the fully protected unglycosylated MUC1 tandem sequence, possessing a β-alanine spacer and a terminal thiol functionality as a handle for late-stage conjugation; and (3) the KLH carrier protein. The protected glycopeptide and MUC1 peptide domains are to be assembled through direct amide coupling or chemical ligation. Subsequent global deprotection and, finally, conjugation to the carrier protein should yield construct (1).
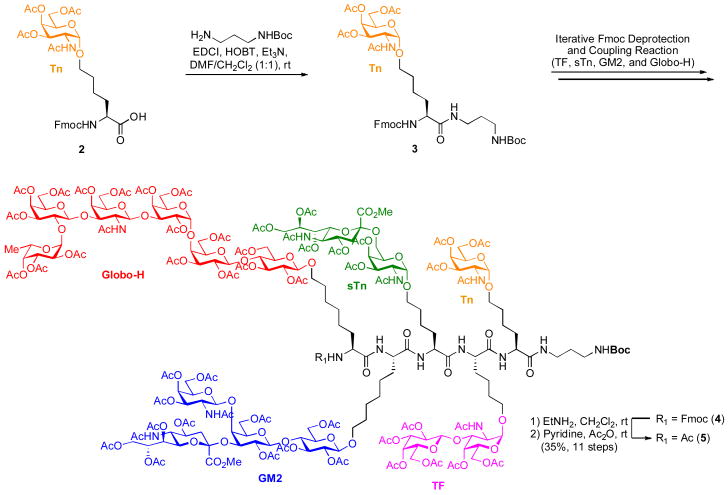
The unimolecular pentavalent carbohydrate domain (5)6d was prepared though the “cassette” approach, developed by our laboratory some time ago (Scheme 1).16 Thus, the pre-assembled, protected glycosylamino acids were coupled via iterative Fmoc deprotection and coupling reactions, as described previously, leading to the fully glycosylated polypeptide backbone. Each deprotection and coupling step proceeded in >75% yield, and our coupling conditions allowed us to access 5 in 35% overall yield for eleven transformations.
Scheme 1.
Preparation of the Unimolecular Pentavalent Carbohydrate Domain (5)
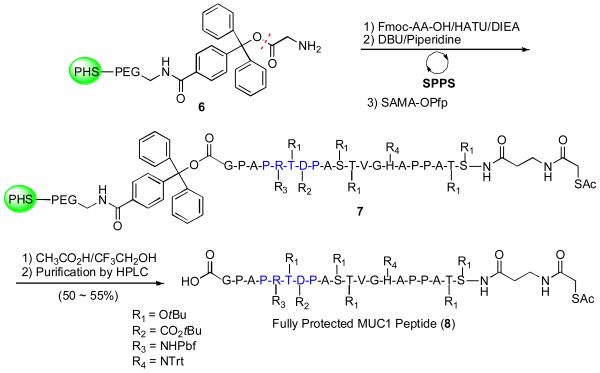
We next turned to the preparation of the peptide domain (8) (Scheme 2). In designing the unglycosylated MUC1 peptide, it was decided to position a glycine on the C-terminus, to avoid the possibility of α-epimerization during the subsequent coupling phase. We also elected to install a protected thiol functionality, to be utilized in the late-stage conjugation to KLH, at the N-terminus during the solid phase peptide synthesis. The fully protected unglycosylated MUC1 tandem repeat (8) was thus prepared by automated solid-phase peptide synthesis on a commercially available trityl resin (6) using Fmoc amino acid derivatives, according to standard procedures.17 Cleavage from the resin using TFA/TFE furnished the desired MUC1 peptide (8)18 as a carboxylic acid.
Scheme 2.
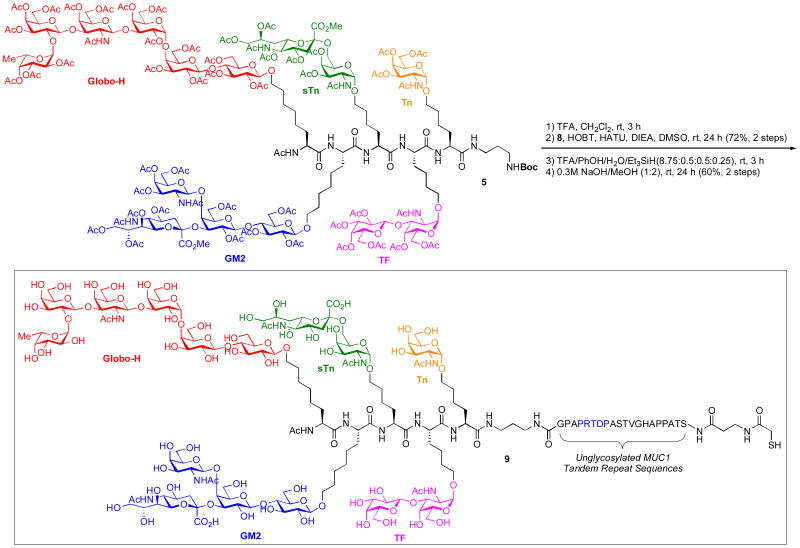
Having gained synthetic access to both the pentavalent carbohydrate domain (5) and the desired peptide (8), we now sought to merge the two domains, thus assembling the complete framework of the target vaccine. First, the C-terminal Boc group on the diaminopropyl spacer (5) was removed under acidic conditions (TFA/CH2Cl2). Next, the fully protected MUC1 peptide (8), activated with HATU/HOBT, was coupled to the carbohydrate domiain (5) in the presence of DIEA, leading to the desired fully protected glycopeptide in 72% yield over 2 steps after purification by preparative HPLC (Scheme 3). All acid-labile side chain protecting groups of the peptide were simultaneously removed by a cleavage cocktail (TFA/PhOH/H2O/Et3SiH). Finally, global deprotection of the O-acetyl and methyl groups with NaOH/MeOH afforded the desired unconjugated unimolecular pentavalent-MUC1 glycopeptide (9)19 in 60% over 2 steps after purification by preparative HPLC.
Scheme 3.
Preparation of the Unimolecular Pentavalent-MUC1 Glycopeptide Construct (9)
In summary, the pentavalent-MUC1 glycopeptide construct (9) has been synthesized in a highly convergent and efficient way. Conjugation to KLH carrier protein and immunological testing of the resultant vaccine conjugate (1) will be reported in due course.
Supplementary Material
Acknowledgments
This work was supported by Grant CA28824. DL is grateful for support from the Terri Brodeur Breast Cancer Foundation.
References
- 1.(a) Fung PY, Madej M, Koganty RR.Cancer Res 1996565309–5318.8968075 [Google Scholar]; (b) Ouerfelli O, Warren JD, Wilson RM, Danishefsky SJ. Expert Rev Vaccines. 2005;4:677–685. doi: 10.1586/14760584.4.5.677. [DOI] [PubMed] [Google Scholar]
- 2.Slovin SF, Keding SJ, Ragupathi G. Immunol Cell Biol. 2005;83:418–428. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- 3.(a) Ragupathi G, Park TK, Zhang SL, Kim IJ, Graber L, Adluri S, Lloyd KO, Danishefsky SJ, Livingston PO. Angew Chem Int Ed. 1997;36:125–128. [Google Scholar]; (b) Slovin SF, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann WG, Fazzari M, Dantis L, Olkiewicz K, Lloyd KO, Livingston PO, Danishefsky SJ, Scher HI. Proc Natl Acad Sci U S A. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gilewski T, Ragupathi G, Bhuta S, Williams LJ, Musselli C, Zhang XF, Bencsath KP, Panageas KS, Chin J, Hudis CA, Norton L, Houghton AN, Livingston PO, Danishefsky SJ. Proc Natl Acad Sci U S A. 2001;98:3270–3275. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Krug LM, Ragupathi G, Hood C, Kris MG, Miller VA, Allen JR, Keding SJ, Danishefsky SJ, Gomez J, Tyson L, Pizzo B, Baez V, Livingston PO. Clin Cancer Res. 2004;10:6094–6100. doi: 10.1158/1078-0432.CCR-04-0482. [DOI] [PubMed] [Google Scholar]; (e) Sabbatini PJ, Kudryashov V, Ragupathi G, Danishefsky SJ, Livingston PO, Bornmann WG, Spassova M, Zatorski A, Spriggs D, Aghajanian C, Soignet S, Peyton M, O'Flaherty C, Curtin J, Lloyd KO. Int J Cancer. 2000;87:79–85. [PubMed] [Google Scholar]
- 4.Kuduk SD, Schwarz JB, Chen XT, Glunz PW, Sames D, Ragupathi G, Livingston PO, Danishefsky SJ. J Am Chem Soc. 1998;120:12474–12485. [Google Scholar]
- 5.(a) Zhang SL, Cordon-Cardo C, Zhang HS, Reuter VE, Adluri S, Hamilton WB, Lloyd KO, Livingston PO. Int J Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]; (b) Zhang SL, Zhang HS, Cordon-Cardo C, Reuter VE, Singhal AK, Lloyd KO, Livingston PO. Int J Cancer. 1997;73:50–56. doi: 10.1002/(sici)1097-0215(19970926)73:1<50::aid-ijc9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.(a) Allen JR, Harris CR, Danishefsky SJ. J Am Chem Soc. 2001;123:1890–1897. doi: 10.1021/ja002779i. [DOI] [PubMed] [Google Scholar]; (b) Ragupathi G, Coltart DM, Williams LJ, Koide F, Kagan E, Allen J, Harris C, Glunz PW, Livingston PO, Danishefsky SJ. Proc Natl Acad Sci U S A. 2002;99:13699–13704. doi: 10.1073/pnas.202427599. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Keding SJ, Danishefsky SJ. Proc Natl Acad Sci U S A. 2004;101:11937–11942. doi: 10.1073/pnas.0401894101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ragupathi G, Koide F, Livingston PO, Cho YS, Endo A, Wan Q, Spassova MK, Keding SJ, Allen J, Ouerfelli O, Wilson RM, Danishefsky SJ. J Am Chem Soc. 2006;128:2715–2725. doi: 10.1021/ja057244+. [DOI] [PubMed] [Google Scholar]
- 7.(a) Deck B, Elofsson K, Kihlberg J, Unanue EE. J Immunol. 1995;155:1074–1078. [PubMed] [Google Scholar]; (b) Haurum JS, Arsequell G, Lellouch AC, Wong SYC, Dwek RA, McMichael AJ, Elliot T. J Exp Med. 1994;180:739–744. doi: 10.1084/jem.180.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mouritsen S, Meldal M, Christiansen-Brams I, Elsner H, Werdelin O. Eur J Immunol. 1994;24:1066–1072. doi: 10.1002/eji.1830240509. [DOI] [PubMed] [Google Scholar]; (d) Sieling PA, Chatterjee D, Porcelli SA, Prigozy TL, Mazzaccaro RJ, Soriano T, Bloom BR, Brenner MB, Kronenberg M, Brennan PJ. Science. 1995;269:227–230. doi: 10.1126/science.7542404. [DOI] [PubMed] [Google Scholar]
- 8.Gendler SJ, Lancaster CA, Taylor-Papadimitriou T, Duhig N, Peat N, Burchell J, Pemberton EN, Lalani N, Wilson D. J Biol Chem. 1990;265:15286–15293. [PubMed] [Google Scholar]
- 9.Burchell J, Taylor-Papadimitriou J, Boshell M, Gendler S, Duhig T. Int J Cancer. 1989;44:691–696. doi: 10.1002/ijc.2910440423. [DOI] [PubMed] [Google Scholar]; (b) Price MR, Hudecz F, O'Sullivan C, Baldwin RW, Edwards PM, Tendler SJB. J Mol Immunol. 1990;62:795–802. doi: 10.1016/0161-5890(90)90089-i. [DOI] [PubMed] [Google Scholar]
- 10.(a) Arklie J, Taylor-Papadimitriou J, Bodmer W, Egan M, Millis R. Int J Cancer. 1981;28:23–29. doi: 10.1002/ijc.2910280105. [DOI] [PubMed] [Google Scholar]; (b) Hollingsworth MA, Strawhecker JM, Caffrey TC, Mack DR. Int J Cancer. 1994;57:198–203. doi: 10.1002/ijc.2910570212. [DOI] [PubMed] [Google Scholar]
- 11.Tampellini M, Berruti A, Gerbino A, Buniva T, Torta M, Gorzegno G, Faggiuolo R, Cannoner R, Farris A, Destefanis M, Moro G, Deltetto F, Dogliotti L. Br J Cancer. 1997;75:698–702. doi: 10.1038/bjc.1997.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bon GG, Verheijen RHM, Zuetenhorst JM, Van Kamp GJ, Verstraeten AA, Kenemans P. Gynecol Obstet Inv. 1996;42:58–62. doi: 10.1159/000291890. [DOI] [PubMed] [Google Scholar]
- 13.Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T. Gasteroenterology. 1994;106:353–361. doi: 10.1016/0016-5085(94)90592-4. [DOI] [PubMed] [Google Scholar]
- 14.(a) Hilkens J, Buijs F, Hilgers J, Hageman P, Calafat J, Sonnenberg A, Der VanVlak M. Int J Cancer. 1984;34:197–206. doi: 10.1002/ijc.2910340210. [DOI] [PubMed] [Google Scholar]; (b) Bon GG, Von Mensdorff-Pouilly S, Kenemans P, Van Kamp GJ, Verstraeten RA, Hilgers J, Meijer S, Vermorken JB. Clin Chem. 1997;43:585–593. [PubMed] [Google Scholar]
- 15.Butts C, Murray N, Maksymiuk A, Goss G, Marshall E, Soulières D, Cormier Y, Ellis P, Price A, Sawhney R, Davis M, Mansi J, Smith C, Vergidis D, Ellis P, MacNeil M, Palmer M. J Clin Oncol. 2005;23:6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 16.(a) Allen JR, Harris CR, Danishefsky SJ. J Am Chem Soc. 2001;123:1890–1897. doi: 10.1021/ja002779i. [DOI] [PubMed] [Google Scholar]; (b) Biswas K, Coltart DM, Danishefsky SJ. Tetrahedron Lett. 2002;43:6107–6110. [Google Scholar]; (c) Keding SJ, Endo A, Biswas K, Zatorski A, Danishefsky SJ. Tetrahedron Lett. 2003;44:3413–3416. [Google Scholar]; (d) Keding SJ, Endo A, Danishefsky SJ. Tetrahedron. 2003;59:7023–7031. [Google Scholar]; (e) Cho YS, Wan Q, Danishefsky SJ. Bioorg Med Chem. 2005;13:5259–5266. doi: 10.1016/j.bmc.2005.06.018. [DOI] [PubMed] [Google Scholar]; (f) Wan Q, Cho YS, Lambert TH. J Carbohydr Chem. 2005;24:425–440. [Google Scholar]
- 17.Atherton E, Sheppard RC. Solid phase synthesis: A practical approach. IRL Press, Oxford University Press; Oxford: 1989. [Google Scholar]
- 18.LC/MS analysis showed (60-80% MeCN/H2O, Microsorb C18, 300-5, 2 × 150 mm, 0.2 mL/min) showed that the peptide (8) at 13.57 min and MS spectrum with base peaks of 1453.86 (M+2H+, [1452.76 calc]).
- 19.LC/MS analysis showed (1-10% MeCN/H2O (5 min), 20-50% MeCN/H2O (30 min), Microsorb C18, 300-5, 2 × 150 mm, 0.2 mL/min) showed that the glycopeptide (9) at 12.15 min and MS spectrum with base peaks of 1904.76 (M+3H+, [1903.84 calc]).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.