Abstract
Light has been used to noninvasively alter the excitability of both neural and cardiac tissue 1–10. Recently, pulsed laser light has been shown to be capable of eliciting action potentials in peripheral nerves and in cultured cardiomyocytes 7–10. Here, we demonstrate for the first time optical pacing (OP) of an intact heart in vivo. Pulsed 1.875 μm infrared laser light was employed to lock the heart rate to the pulse frequency of the laser. A laser Doppler velocimetry (LDV) signal was used to verify the pacing. At low radiant exposures, embryonic quail hearts were reliably paced in vivo without detectable damage to the tissue, indicating that OP has great potential as a tool to study embryonic cardiac dynamics and development. In particular, OP can be utilized to control the heart rate, and thereby alter stresses and mechanically transduced signaling.
The avian embryo is an important model for studying the mechanisms driving normal development and the abnormalities leading to congenital defects. The embryonic heart is one of the first organs to develop and in avian models begins beating at around 40 hours of incubation. The progression of a single heart tube into a four-chambered heart (cardiac looping) is shaped by mechanical stimuli from the flowing blood and pumping heart which influence molecular and cellular responses that regulate development 11–14. Much remains unknown about the mechanisms of cardiac looping, in part because of a lack of suitable non-destructive tools with which to study the minute (< 2 mm) organ. In the adult heart, the exquisite control achievable using electrical pacing has been critical to advancing our understanding of cardiac electrophysiology. Unfortunately, electrical pacing of the embryonic heart is invasive and difficult to achieve consistently and without tissue damage. A simple noninvasive technique to control heart rate would allow one to manipulate forces applied to cells in early developing embryos, enabling a new class of experiments exploring the roles of mechanotransduction and electrical activation.
It has been previously shown that ultraviolet and visible light can noninvasively increase the excitability of neuronal and myocardial cells 3–4,6. In particular, Gimeno et al demonstrated that exposure to visible light accelerated the heart rate in the early chick embryo 4. More recently, Smith et al employed focused femtosecond pulses (780 nm) from a Ti:Sapphire laser to induce paced contractions in individual and small groups of cardiomyocytes with success rates up to 60% 7. Unfortunately, the high-powered pulses produce reactive oxygen species that can damage the cell, and optically pacing an entire heart is not currently possible with this method. In 2005, Wells et al demonstrated that pulsed infrared light could reliably elicit compound action potentials in mammalian peripheral nerves in a one-to-one fashion at radiant exposures well below the damage threshold 8,10,15. The stimulation threshold paralleled the inverse water absorption curve with ~2 μm light producing the highest damage-to-stimulation threshold ratio. Although the mechanism is not well understood, it has been suggested that temperature gradients caused by infrared light absorption open ion channels 9. To date, comparable pulsed infrared laser stimulation of cardiomyocytes or cardiac tissue has not been demonstrated. Here, we describe the use of pulsed infrared light to pace hearts of intact quail embryos, suggesting a promising new tool for studying embryonic cardiac dynamics and development.
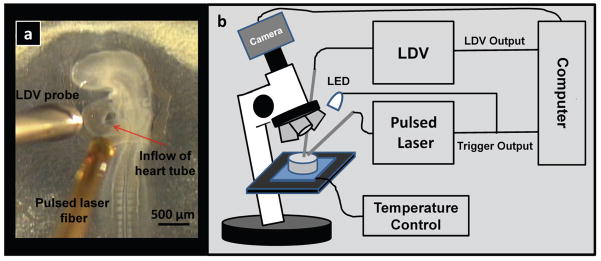
Figure 1 shows the experimental setup. A pulsed infrared diode laser (λ=1.875 μm; Capella™, Lockheed Martin Aculight) centered at 1875 nm coupled light into a 400 μm core multimode fiber. A micromanipulator positioned the fiber in close proximity to (500 μm), but not in contact with the embryo (see Figure 1A), illuminating an area of approximately 0.3 mm2 on the inflow region of the heart tube. The stimulation laser employed a red HeNe laser coupled into the same fiber for aiming, which allowed precise positioning of the stimulation pulses. The optical power of the HeNe laser light illuminating the sample was low (0.9 mW) and the sample was illuminated only briefly (< 10 s) in order to position the fiber. The stimulation laser trigger signal was recorded to document the timing and duration of the stimulation pulses. A laser Doppler velocimeter (LDV, Moor Instruments Ltd.) was used to monitor the heartbeat of the embryos. The LDV probe was positioned approximately 1 mm from the heart with a micromanipulator (see Figure 1A). The LDV signal was only intended for heartbeat detection; therefore, the LDV probe was not oriented identically with every heart, so no significance should be drawn from the shape of the LDV signals. The trigger from the laser was also directed to a white light emitting diode (LED), which flashed on and off with the pulsing of the laser and was visible under a video microscope. The output of the video microscope was recorded by a laptop computer for real-time guidance and documentation (see movie in supplementary material).
Figure 1. OP setup.
a, Photograph of a 53 hour quail embryo in the New culture. b, Block diagram of the setup of the optical pacing experiment. Embryos in the Petri dish under the microscope were stimulated by a pulsed infrared laser, while a laser Doppler velocimeter probe measured blood flow. The LDV output and trigger pulse from the laser were recorded to verify pacing. Also, the trigger pulse from the laser activated a white light LED which was observable in the video. The video output from the microscope camera was recorded at video rate (29.97 fps) by a laptop computer.
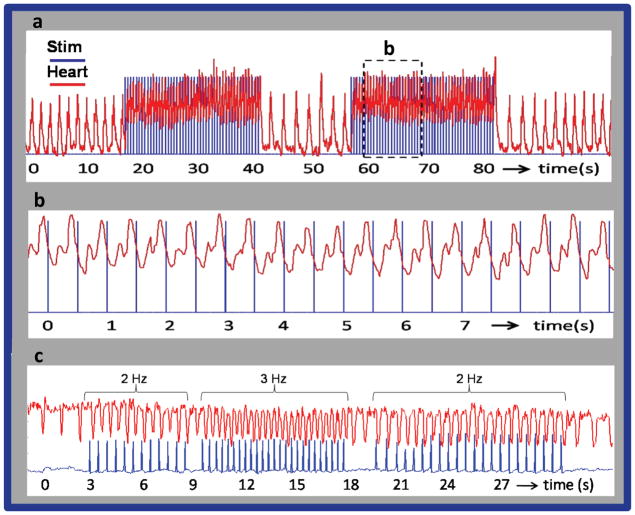
Figure 2 demonstrates optical pacing of an embryonic heart using a pulsed laser. The spikes in the blue traces represent when the laser was emitting light, while the red traces show the heart rate of the embryo. Figure 2a presents a recording from a stage 17 quail embryo in a New culture paced at 2 Hz. The dashed box in 2a is expanded for a close up view in 2b. Clearly the laser pulse and heart rate are synchronized with each laser pulse eliciting a heartbeat. In Fig. 2a, the time interval between successive heartbeats before laser stimulation was 1.58±0.038 s (0.634 Hz) and the interval decreased to 0.4996±0.017 s (2.004 Hz) during pacing. After the stimulation pulses ceased and the heart rate stabilized the time interval between heartbeats increased to 1.44±0.042 s (0.693 Hz). At this point it is not known why the heart rate was slightly higher after pacing than before, but a similar increase occurred in most of the embryos tested. Although we cannot rule out damage, a physiological explanation for the post-pacing rate increase could be enhanced calcium ion influx during pacing. Figure 2c exhibits the heart beating in synchrony with the laser pulses as the frequency of the pulses is changed between 2 and 3 Hz.
Figure 2. Pacing of the embryonic quail heart.
a,b, The trigger pulse (blue) from the pulsed laser is superimposed on the LDV recordings (red) of heart rate. (a) presents a recording from a stage 17 (59 hour) quail embryo in a New culture paced at 2 Hz. The laser pulse duration was 1 ms and the radiant exposure was 0.92 J/cm2 per pulse. The laser pulses were directed toward the inflow region of the heart tube. The laser pulses were turned on and off several times to demonstrate the robustness of optical pacing. The heart rate increased from 0.634 to 2 Hz when the stimulation laser pulses were started and decreased to 0.693 Hz by the end of the trace in 2a. (b) The dashed box in 2a is expanded for a close up view in 2b. Clearly the trigger pulse and LDV signal were synchronized with each laser pulse eliciting a heartbeat. (c) presents a recording from a stage 14 (53 hour) quail embryo in a New culture. The laser pulse duration was 2 ms and the radiant exposure was 0.84 mJ/cm2 per pulse. The frequency of the laser pulses were varied from 2 Hz to 3 Hz and back to 2 Hz to demonstrate the ability of the embryo heart to follow the pulse frequency. Laser stimulation (blue) and heart rate (red) traces were calculated from the video by plotting the pixel intensity of LED flashes triggered by the laser and intensity at the edge of the heart wall.
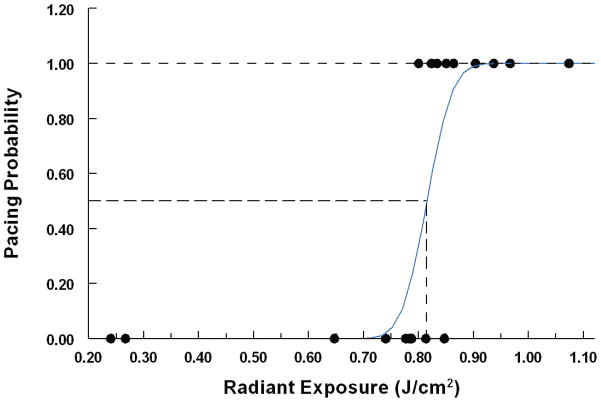
The radiant exposure threshold required to pace a day 2 embryonic quail heart in a New culture was determined by subjecting each embryo to a different radiant exposure level and noting whether pacing had occurred. Successful pacing was defined as one to one stimulation, laser pulse to heartbeat, over a twenty second interval. 25 embryos were tested and the results were fit to a normal cumulative distribution function as shown in Figure 3. The threshold (50% probability point) was 0.81+/−0.01 J/cm2 with a t-value of 79.9 and 95% confidence interval between 0.794 and 0.836 J/cm2. The standard deviation of the distribution was 0.036+/−0.016 with a t-value of 2.32. In a shorter study we demonstrated that OP is feasible in embryos cultured on the yolk (see supplemental material). This method of embryo culture is compatible with longer viability of the embryos and would be useful for following long term effects of OP.
Figure 3. Threshold measurement.
25 embryos in New cultures were exposed to varying radiant exposures and the results were plotted in terms of pacing probability (successful pacing = 1, unsuccessful pacing = 0). The data were fit to a normal cumulative distribution function (blue line). The threshold (50% probability point) was 0.81+/−0.01 J/cm2 with a t-value of 79.9 and 95% confidence interval between 0.794 and 0.836 J/cm2. The standard deviation of the distribution was 0.036+/−0.016 with a t-value of 2.32.
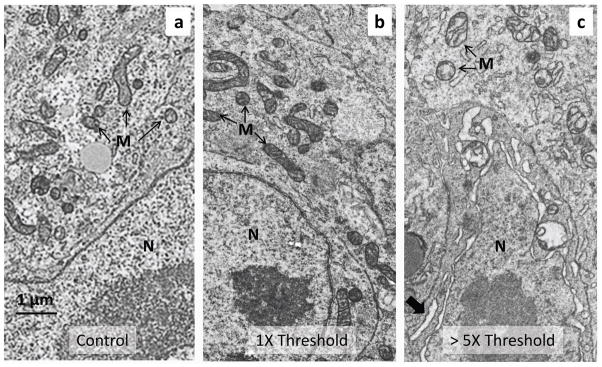
Several approaches were used to determine if optical pacing damaged the embryo. Standard transmission electron microscopy (TEM) was employed to examine the ultrastructural detail of cardiomyocytes in the inflow region of the heart tube where the laser was aimed. Figs. 4a-c represent a control embryo (same experimental protocol, but no light exposure), an embryo paced slightly above threshold (0.88 J/cm2) and an embryo paced well above threshold (4.33 J/cm2). In hearts paced well above threshold, the mitochondria are vacuolated and the nuclear envelope and rough endoplasmic reticulum are slightly expanded, whereas the ultrastructure of the cardiomyocytes of embryos paced slightly above threshold looked similar to that of the controls. In all three examples several regions of the heart tube were examined in detail to look for anomalies and no clear indications of cellular damage were identified in the embryo paced slightly above threshold. Some subtle abnormalities in the mitochondrial cristae, small spaces within the cristae, are present in the control and threshold embryo and are likely the result of necessary embryo manipulations during harvest prior to fixation. Other damage assays showed no structural damage to embryo heart cardiomyocytes paced at threshold levels (supplemental material). At this point we did not detect evidence of damage resulting from optical pacing, but further study is warranted.
Figure 4. Transmission electron microscopy (TEM) after the OP procedure.
a-c, The TEM images show a typical cardiomyocyte in the inflow (sinoatrial) region of the heart tube of three different quail embryos, where laser light was directed. Each embryo was between 52 to 58 hours of development. The torso of each embryo was excised and fixed immediately after OP. a, Control embryo. The embryo was exposed to the OP experimental procedure, except that the pacing laser was not turned on during the experiment. b, Embryo paced slightly above threshold. The embryo heart was paced for approximately 20 seconds with 2 ms pulses (2.64 mJ/pulse) at 2 Hz. c, Embryo paced well above threshold. The embryo heart was paced for approximately 20 seconds with 4 ms pulses (13 mJ/pulse) at 2 Hz. The micrograph image was taken from a border region separating severely damaged tissue (partially ablated) from apparently healthy tissue. The mitochondria are vacuolated and the nuclear envelope is swollen, as are elements of the rough ER (large arrow). The cells and mitochondria in 4a and b have a similar appearance and show no signs of damage from the pulsed laser light. Some subtle abnormalities in the cristae present in cells from both threshold paced and unpaced control hearts are due to the unavoidable delay in excising the embryonic torso. N -nucleus, M - mitochondria.
The mechanism of the observed phenomenon is at this point unclear. However, given the laser parameters used (wavelength, pulse duration and peak power), some inferences can be drawn. Gimeno et al used continuous visible light to increase the heart rate with maximum effect at 475 nm (photon energy = 2.61 eV) 4. Proposed mechanisms included acetylcholine sensitivity to light and inhibition of ATPase. In a different study, Smith et al showed that a focused near-infrared femtosecond laser caused contraction in cultured neonatal rat cardiomyocytes 7. A window for this effect was found to occur between 15 and 30 mW average power for an 80 fs, 82 MHz pulse train of 780 nm, using 8 ms exposures applied periodically at 1 to 2 Hz. Mechanistically this effect was attributed to the laser-induced release of intracellular calcium which has been shown in various cell types for these laser parameters. In contrast, our study used near infrared pulses (λ = 1,870 nm) and relatively long (ms) pulses of continuous laser light. Thus the photon energy (0.66 eV) is insufficient to directly drive photochemistry as was the case in Gimeno’s work and the irradiance (W/cm2) is too low for multiphoton effects as in Smith’s work (irradiance from the fs pulses and diffraction limited spot size is roughly 8 orders of magnitude higher than we used). It is more likely that the mechanisms responsible for the effects seen in our study align with those described by Wells et al, who used identical laser parameters in the rat sciatic nerve and concluded that the laser-induced spatio-temporal temperature gradient was responsible for the induction of action potentials (APs) in excitable tissues 8–9. It has been shown that non-absorbing wavelengths with otherwise similar parameters do not result in APs in the peripheral nerve and pulse durations that violate the conditions of thermal confinement are similarly unable to induce APs. We have shown here and in previous studies that optical stimulation with these laser parameters is feasible without inducing thermal damage 15. The question of how the laser-induced thermal gradient ultimately results in the opening of ion channels remains unanswered although several hypotheses are currently under investigation.
Here, we have demonstrated a noninvasive method to pace embryonic hearts with pulsed infrared light. Compared to electrical pacing, OP does not require contact, has high spatial precision and avoids stimulation artifacts in electrode recordings. OP was consistently achieved with quail embryos ranging from stage 12 to stage 18 (looped heart stages prior to septation) in both New cultures and cultured on the yolk at radiant exposures well below levels at which damage was apparent. In the future we will make use of OP together with advanced imaging to study mechanotransduction (i.e. shear stress on the endocardium) by controlling the heart rate of the embryo, thereby manipulating mechanical stress 16–19. OP will not only enable a new class of experiments in developmental cardiology, but also may become a useful tool for investigating cardiac electrophysiology, single-cell (cardiomyocyte) dynamics, and cardiac tissue engineering. Furthermore, OP may potentially be capable of pacing the adult heart, which could lead to clinical applications.
Methods
Egg preparation
Fertilized quail (Coturnix conturnix) eggs were removed between 48–60 hours post-fertilization from a humidified, forced draft incubator (38 °F). Embryos were removed from the egg and either cultured on the yolk in a sterilized 3.5 cm diameter Petri dish or cultured using the New culture in which the embryo is inverted on an egg-agar substrate 20–21. The New culture fully exposes the heart, which facilitates interventions, but decreases lifespan to a few days, whereas embryos cultured on the yolk can survive through gestation. Both techniques are routinely employed in the field. Figure 1A shows a healthy 53-hour embryo in the New culture. For pacing experiments, embryos were placed upon a temperature-controlled heating plate (ATC 1000, WPI), which maintained the temperature at 37 °C.
Radiant exposure calculations
Radiant exposures (J/cm2) were calculated by dividing the pulse energies by the laser spot size. Pulse energies were measured using a pyroelectric energy meter (PE50BB, Ophir) and spot size was computed using the numerical aperture, fiber diameter and fiber distance from the tissue. Laser light was delivered to the tissue via a 400+/−8 μm - diameter flat-polished multi-mode optical fiber (Ocean Optics) having a numerical aperture of 0.22 +/−0.02. The fiber-to-heart distance was held constant at approximately 500 μm for each trial. The tissue was assumed to be perpendicular to the fiber. While the fiber was oriented at an angle to the membrane of approximately 37 degrees from perpendicular, the curvature of the membrane covering the heart was assumed to offset the angle of the fiber sufficiently to make these effects negligible.
Threshold measurements
25 embryos in New cultures were tested to determine the radiant exposure level needed to induce optical pacing. To quickly find to the precise location for optimal stimulation, the laser was turned to a level slightly above threshold and the position of the fiber was optimized, which normally took less than ten seconds. The optical fiber was positioned 500 μm from the embryo. Because the LDV fiber took a significant time to accurately place, for this experiment we relied on the LED flashes and heart motion in the microscope video to determine successful pacing. After a brief waiting period (~ 1 min) with the laser off, the radiant exposure was adjusted and the laser was turned on for 20–30 seconds. Embryos were then either fixed for further experiments or discarded. For each embryo we determined whether there was successful pacing (1) or failed pacing (0) and plotted the results in figure 3. Some embryos took two or three beats to synchronize to the laser pulses, but were counted as successful pacing if the heart rate was consistent after the first couple beats. Conversely, if the heart followed the laser pulses, but skipped more than one beat it was scored unsuccessful. The difference between successful pacing and unsuccessful pacing was easy to distinguish by closely observing the videos.
TEM preparation
The torso of the embryo was excised and immediately fixed by immersion in the triple aldehyde-DMSO mixture of Kalt and Tandler22. After rinsing, the tissues were postfixed in ferrocyanide-reduced osmium tetroxide23. After again rinsing, they were soaked overnight in acidified uranyl acetate24. Thin sections were sequentially stained with acidified uranyl acetate24 followed by Sato’s triple lead stain as modified by Hanaichi et al.25 and examined in a JEOL 1200 electron microscope.
Supplementary Material
Acknowledgments
This research is supported in part by National Institutes of Health (RO1-HL083048 (AMR), RO1-HL095717 (AMR), RO1-NS052407 (EDJ) and R44-NS051926 (EDJ)). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR12463-01 from the National Center of Research Resources, National Institutes of Health.
Footnotes
Individual contributions
M.W.J. conceived the original idea, performed and designed experiments, analyzed data and wrote paper, A.R.D and S.G. performed and designed experiments and analyzed data, H.J.C designed experiments and analyzed data, H.F. supervised analysis and creation of micrographs, M.W. supervised damage studies and embryo handling. E.D.J. and A.M.R. supervised optical pacing experiments. All authors helped edit the paper. All authors except H.F. discussed the results and implications at all stages.
Additional information
Supplementary information accompanies this paper at www.nature.com/naturephotonics. Reprints and permission information is available online at http://npg.nature.com/reprintsandpermissions/. Correspondence and requests for materials should be addressed to A.M.R.
The authors appreciate the contributions of Midori Hitomi and Yong-Qiu Doughman in preparing embryos for TEM and histology.
References
- 1.Allegre G, Avrillier S, Albe-Fessard D. Stimulation in the rat of a nerve fiber bundle by a short UV pulse from an excimer laser. Neurosci Lett. 1994;180:261–264. doi: 10.1016/0304-3940(94)90534-7. [DOI] [PubMed] [Google Scholar]
- 2.Balaban P, et al. He-Ne laser irradiation of single identified neurons. Lasers Surg Med. 1992;12:329–337. doi: 10.1002/lsm.1900120315. [DOI] [PubMed] [Google Scholar]
- 3.Fork RL. Laser stimulation of nerve cells in Aplysia. Science. 1971;171:907–908. doi: 10.1126/science.171.3974.907. [DOI] [PubMed] [Google Scholar]
- 4.Gimeno MA, Robets CM, Webb JL. Acceleration of rate of the early chick embryo heart by visible light. Nature. 1967;214:1014–1016. doi: 10.1038/2141014a0. [DOI] [PubMed] [Google Scholar]
- 5.Hirase H, Nikolenko V, Goldberg JH, Yuste R. Multiphoton stimulation of neurons. J Neurobiol. 2002;51:237–247. doi: 10.1002/neu.10056. [DOI] [PubMed] [Google Scholar]
- 6.Nathan RD, Pooler JP, DeHaan RL. Ultraviolet-induced alterations of beat rate and electrical properties of embryonic chick heart cell aggregates. J Gen Physiol. 1976;67:27–44. doi: 10.1085/jgp.67.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith NI, et al. A femtosecond laser pacemaker for heart muscle cells. Opt Express. 2008;16:8604–8616. doi: 10.1364/oe.16.008604. [DOI] [PubMed] [Google Scholar]
- 8.Wells J, Kao C, Jansen ED, Konrad P, Mahadevan-Jansen A. Application of infrared light for in vivo neural stimulation. J Biomed Opt. 2005;10:064003. doi: 10.1117/1.2121772. [DOI] [PubMed] [Google Scholar]
- 9.Wells J, et al. Biophysical mechanisms of transient optical stimulation of peripheral nerve. Biophys J. 2007;93:2567–2580. doi: 10.1529/biophysj.107.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells J, et al. Optical stimulation of neural tissue in vivo. Opt Lett. 2005;30:504–506. doi: 10.1364/ol.30.000504. [DOI] [PubMed] [Google Scholar]
- 11.Bartman T, Hove J. Mechanics and function in heart morphogenesis. Dev Dyn. 2005;233:373–381. doi: 10.1002/dvdy.20367. [DOI] [PubMed] [Google Scholar]
- 12.North TE, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardanaud L, Eichmann A. Stem cells: The stress of forming blood cells. Nature. 2009;459:1068–1069. doi: 10.1038/4591068a. [DOI] [PubMed] [Google Scholar]
- 14.Poelmann RE, Gittenberger-de Groot AC, Hierck BP. The development of the heart and microcirculation: role of shear stress. Med Biol Eng Comput. 2008;46:479–484. doi: 10.1007/s11517-008-0304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells JD, et al. Optically mediated nerve stimulation: Identification of injury thresholds. Lasers Surg Med. 2007;39:513–526. doi: 10.1002/lsm.20522. [DOI] [PubMed] [Google Scholar]
- 16.Gargesha M, Jenkins MW, Wilson DL, Rollins AM. High temporal resolution OCT using image-based retrospective gating. Opt Express. 2009;17:10786–10799. doi: 10.1364/oe.17.010786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins MW, et al. Bios SPIE. San Jose: 2008. [Google Scholar]
- 18.Jenkins MW, et al. Ultrahigh-speed optical coherence tomography imaging and visualization of the embryonic avian heart using a buffered Fourier Domain Mode Locked laser. Opt Express. 2007;15:6251–6267. doi: 10.1364/oe.15.006251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jenkins MW, et al. Bios, SPIE. San Francisco: 2009. [Google Scholar]
- 20.Darnell DK, Schoenwolf GC. In: Methods in Molecular Biology. Tuan RS, Lo CW, editors. Vol. 135. Humana Press; 2000. pp. 31–38. Ch. 5. [DOI] [PubMed] [Google Scholar]
- 21.New DAT. A new technique for the cultivation of the chick embryo in vitro. J Embryol Exp Morphol. 1955;3:326–331. [Google Scholar]
- 22.Kalt MR, Tandler B. A study of fixation of early amphibian embryos for electron microscopy. J Ultrastruct Res. 1971;36:633–645. doi: 10.1016/s0022-5320(71)90020-7. [DOI] [PubMed] [Google Scholar]
- 23.Karnovsky MJ. 11th Annual Meeting of the American Society of Cell Biology; p. 146. [Google Scholar]
- 24.Tandler B. Improved uranyl acetate staining for electron microscopy. J Electron Microsc Tech. 1990;16:81–82. doi: 10.1002/jemt.1060160110. [DOI] [PubMed] [Google Scholar]
- 25.Hanaichi T, et al. A stable lead by modification of Sato’s method. J Electron Microsc. 1986;35:304–306. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.