Abstract
We have used the Xenopus laevis egg extract system to study the roles of vertebrate Dna2 in DNA replication and double-strand-break (DSB) repair. We first establish that Xenopus Dna2 is a helicase, as well as a nuclease. We further show that Dna2 is a nuclear protein that is actively recruited to DNA only after replication origin licensing. Dna2 co-localizes in foci with RPA and is found in a complex with replication fork components And-1 and Mcm10. Dna2 interacts with the DSB repair and checkpoint proteins Nbs1 and ATM. We also determine the order of arrival of ATM, MRN, Dna2, TopBP1 and RPA to duplex DNA ends and show that it is the same both in S phase and M phase extracts. Interestingly, Dna2 can bind to DNA ends independently of MRN, but efficient nucleolytic resection, as measured by RPA recruitment, requires both MRN and Dna2. The nuclease activity of Mre11 is required, since its inhibition delays both full Dna2 recruitment and resection. Dna2 depletion inhibits but does not block resection, and Chk1 and Chk2 induction occurs in the absence of Dna2.
Keywords: And-1, Ctf4, Mcm10, ATM, MRN, Mre11, homologous recombination, lagging strand
Introduction
Yeast Dna2 is an essential helicase/nuclease involved in removing RNA/DNA primers during Okazaki fragment processing (OFP). It is also required for mitochondrial genome stability. Recently, yeast Dna2 has been recognized as a nuclease critical for resection of 5’ ends during the early steps of homology dependent repair of double-strand breaks (DSBs).1 Dna2 provides a previously missing link in the early steps of DSB repair, and the discovery of its participation now allows biochemical analysis of this stage of repair. Functional genomic screens indicate additional but to date poorly characterized roles for Dna2 in maintenance of chromatin, nuclear structure, and telomere biogenesis.2 Thus, Dna2 is a major contributor to genomic stability in yeast.
While the Dna2 nuclease and helicase active sites are well conserved in evolution, the regulatory sequences are divergent between yeast and vertebrates. Furthermore, there is controversy as to whether Dna2 from Xenopus laevis and human cells contain functional helicase activity.3-6 Finally, the extent to which the physiological functions are conserved is a matter of debate. In C. elegans, Dna2 is dispensable for embryogenesis; and in human cells, it has been proposed that Dna2 is exclusively mitochondrial.5,7 Recently, nuclear localization has been established in human cells and shRNA knockdown of Dna2 has been shown to lead to cell cycle delay and aberrant cell division.8 However, many fundamental questions remain about the nuclear function of Dna2 in vertebrates.
Xenopus egg extracts offer an attractive system in which to determine the steps of nuclear DNA replication and DSB repair that require Dna2 in vertebrates. Egg extracts contain abundant maternal replication protein stores, providing a rich source of these activities compared to normal S phase extracts. Xenopus extracts are prepared from eggs arrested in metaphase of meiosis II, which resemble M phase extracts. Addition of calcium stimulates the extract to enter interphase, or S phase, and further addition of chromosomal DNA and an ATP-regeneration system results in the formation of nuclei that initiate replication. This DNA replication is both regulated and synchronized, occurring at replication origins spaced almost every 4 kb along the DNA. In addition, cellular responses to DNA damage can be successfully recapitulated in this extract.9,10 Furthermore, the powerful immunodepletion technique allows biochemical analysis of these pathways. We previously showed that depletion of Xenopus Dna2 from such extracts led to a reduction in replication of sperm chromatin, but not a complete absence of DNA synthesis.3 In addition, recent elegant studies demonstrated that Dna2 is required for single-strand annealing (SSA), a reaction that can occur in extracts and that mimics the early steps of homology-dependent repair of a DSB.11-13 In Xenopus, SSA is a two step process in which the duplex DNA ends are unwound by a helicase, primarily WRN/FFA-1, and then the free 5’ single-stranded DNA (ssDNA) is degraded by a nuclease, primarily Dna2, revealing a free 3’ strand that can anneal to a complementary sequence.11 Thus, Xenopus extracts offers a versatile system in which to study both the DNA replication and DSB functions of Dna2.
In this work, we use Xenopus to explore in further depth the S phase activities of nuclear Dna2 in vertebrates. We first establish biochemically that Xenopus Dna2 is a helicase as well as a nuclease. We then show that Dna2 fails to bind to undamaged chromatin in S phase if replication initiation is inhibited. Interestingly, proteomic analysis reveals that a major Dna2-interacting protein detectable in S phase is And-1, a protein involved in stabilizing lagging strand replication complexes and in establishing sister chromatid cohesion. In addition we show that Dna2 interacts with DSB checkpoint and repair proteins ATM and Nbs1, a component of the MRN complex. Dna2 is recruited to DSBs after ATM and MRN, and with similar timing to RPA. Depletion of Dna2 from the extracts leads to a reduction in resection. We specifically analyze the interplay between Dna2 and MRN in the resection reaction. Finally, we find that depletion of Dna2 from extracts does not prevent induction of the DNA damage checkpoint response. Together, our results suggest that the main roles of Dna2 are in DNA replication and repair machinery rather than in signaling.
Results
Xenopus Dna2 has both helicase and nuclease activities
Due to robust nuclease activity, previous attempts to detect helicase activity in purified wild-type Xenopus Dna2 protein were unsuccessful. We reinvestigated helicase activity by changing a key aspartate residue, D278, in the putative Dna2 nuclease active site to alanine. We expressed and purified the mutant protein from insect cells, as previously described for the wildtype protein,3 and demonstrated loss of nuclease activity (Fig. 1). We measured the ability of the D278A mutant to unwind a labeled oligonucleotide with 22 bp of complementary sequence and a non-complementary 30 nt 5’ tail annealed to M13mp18. As shown in Figure 1, there is significant accumulation of the free 52 nucleotide product. Thus, like yeast and human Dna2, Xenopus Dna2 is a combined helicase-nuclease.
Figure 1.
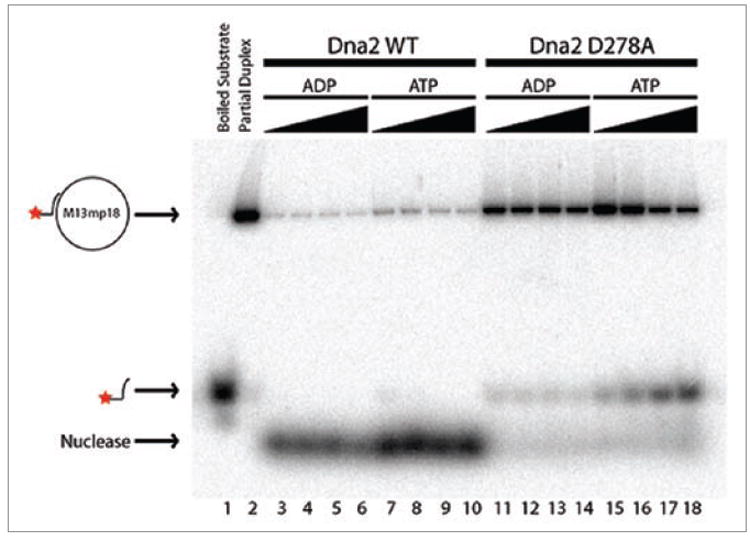
Helicase activity of Xenopus Dna2. Wild-type Xenopus Dna2 (lanes 3 to 10) and nuclease deficient Xenopus Dna2 D278A (lanes 11 to 18) were incubated in helicase assay conditions with approximately 1 fmol of annealed M13mp18 substrate at 37°C for 15 min, either in the presence of ADP (lanes 3 to 6 and 11 to 14) or ATP (lanes 7 to 10 and 15 to 18). Solid triangles represent increasing amount of Dna2 protein: 43 (lanes 3 and 7), 86 (lanes 4 and 8), 172 (lanes 5 and 9), and 344 fmol (lanes 6 and 10) of wild-type Xenopus Dna2 and 43 (lanes 3 and 7), 86 (lanes 4 and 8), 172 (lanes 5 and 9), and 344 fmol (lanes 6 and 10) of Xenopus Dna2 D278A were used. No protein was added in lanes 1 and 2. The reaction in lane 1 was boiled for 4 min. All products were separated using native gel electrophoresis and detected by autoradiography. Positions of the substrate, helicase products and nuclease products are indicated on the left of the figure.
Dna2 associates with S-phase chromatin in a regulated manner
In this text, interphase extract refers to S phase extract with no DNA added. CSF extract refers to extract that has not been stimulated by addition of calcium, a state resembling M phase extracts. For DNA replication, Xenopus demembranated sperm chromatin was used as the source of chromosomal DNA. We first examined the association of Dna2 with chromatin in S phase extract. We see that Dna2 does bind chromatin and accumulates on chromatin during DNA replication with a time course consistent with the dynamics of proteins that participate in DNA replication, such as RPA70, the large subunit of the replicative single-stranded DNA binding protein, and Cdc45, a protein required for activation of the MCM helicase complex (Fig. 2A).
Figure 2.
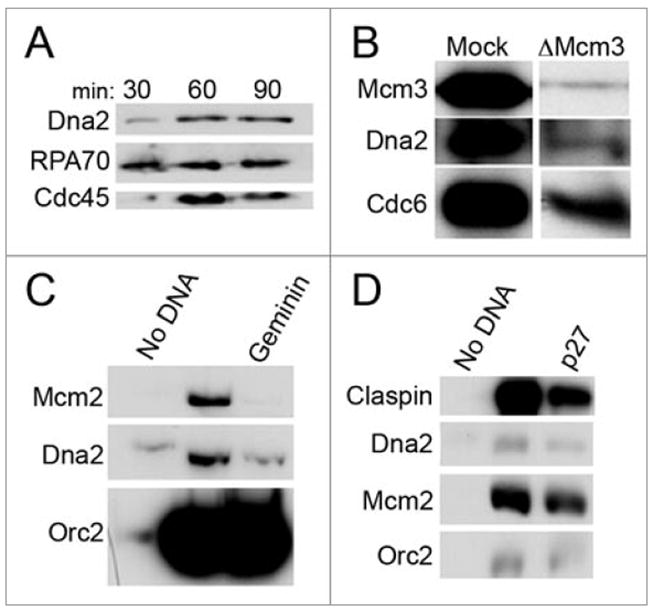
Dna2 associates with S-phase chromatin. (A) Dna2 accumulation on sperm chromatin throughout S phase. Sperm chromatin was incubated in cell-free extract at 3,000 sperm/μl, isolated at indicated time-points throughout DNA replication as previously described30 and analyzed by immunblotting. DNA replication begins at 30 min; 60 min is representative of mid-S phase; and DNA is fully replicated by 90 min. Cdc45 and RPA both associate with replicating chromatin. (B) Dna2 accumulation on chromatin requires the MCM helicase complex. Extracts were mock-depleted or Mcm3-depleted, preventing formation of the MCM helicase complex, and sperm chromatin was incubated in these extracts. Chromatin was then isolated as described,30 and protein association with chromatin was assayed by immunoblotting. (C) Pre-RC formation is necessary for Dna2 binding to chromatin. Sperm chromatin was incubated without or with 300 nM geminin, which prevents formation of the pre-RC, in extract for 100 min., and a sample containing no sperm chromatin was used as a negative control. Chromatin was then isolated and analyzed by immunoblotting as described.30 (D) Pre-IC formation is not required for Dna2 binding to chromatin. Chromatin was incubated in untreated extracts or extracts containing 0.1 mM p27, an inhibitor of pre-IC formation, for 100 min. The negative control was a sample containing no chromatin. Chromatin fractions were isolated and analyzed by immunoblotting as described.30
We next determined whether DNA replication initiation was required for the association of Dna2 with chromatin. Initiation of DNA replication requires the formation of a pre-replication complex (pre-RC), which consists of ORC, the MCM2-7 helicase complex, Cdc6 and Cdt1. The pre-RC is thought to serve as a “landing pad” for the remaining components of the replisome, or as a “licensing” complex for the initiation of DNA replication. As shown in Figure 2B, depletion of Mcm3, a component of the MCM2-7 helicase, reduced Dna2 binding to chromatin, suggesting that licensing of the replication fork is required for Dna2 association with chromatin in S phase and indicating that the binding we see is specific to replicating chromatin. To reinforce this conclusion, we took advantage of the fact that Cdt1 is required for stable association of the MCM helicase with pre-RC components, and that geminin, an inhibitor of Cdt1, inhibits pre-RC formation and thus initiation of DNA replication. As shown in Figure 2C, geminin inhibits the accumulation of Dna2 on chromatin. Thus, we conclude that pre-RC formation is required for Dna2 loading. Some residual binding is observed in the presence of geminin, which may be due to a low level of insoluble Dna2 in the extract, since the same amount is seen in control extracts to which no DNA was added. Alternatively, if Xenopus Dna2 is also both a nuclear and mitochondrial protein, as in yeast and human, this assay could also be detecting small amounts of residual mitochondrial Dna2 contaminating the chromatin fraction.
The pre-RC is activated for replication by binding of additional proteins, some of which require the active Cdk2 cyclin dependent kinase, to form the pre-IC (preinitiation complex). The binding of Cdc45, which is dependent on active Cdk2, marks the transition from the pre-RC to pre-IC. p27 is an inhibitor of Cdk2 and inhibits origin firing by preventing the loading of some replication proteins, including Cdc45, in Xenopus extracts. As shown in Figure 2D, Dna2 still associates with chromatin in the presence of p27. The recruitment of Dna2 to chromatin after pre-RC formation but in the presence of p27 is similar to loading of integral replication fork proteins such as Mcm10 and distinguishes Dna2 from pre-IC proteins such as Cdc45.14 We conclude that pre-RC formation is specifically required for the loading of Dna2 at the beginning of S phase, but Cdk2 activity is not required.
Immunofluorescence was used to track the localization of Dna2 during DNA replication. As sperm chromatin is being replicated in the extract, Dna2 co-localizes with RPA in numerous foci within the single nucleus shown in Figure 3. Co-localization of Dna2 and RPA in the absence of DNA damaging agents suggests that the foci observed are due to DNA replication complexes. Although a small fraction of the RPA foci may represent DNA repair at sites of endogenous damage, since checkpoint induction is a sensitive measure of the presence of DNA damage, the large number of foci we observe in the absence of an exogenous source of DNA damage is most consistent with DNA replication foci. This conclusion is supported further by our investigation of the status of the DNA damage checkpoint in extracts, described below, in which we consistently do not detect significant checkpoint activation during DNA replication without a source of exogenous DNA damage.
Figure 3.
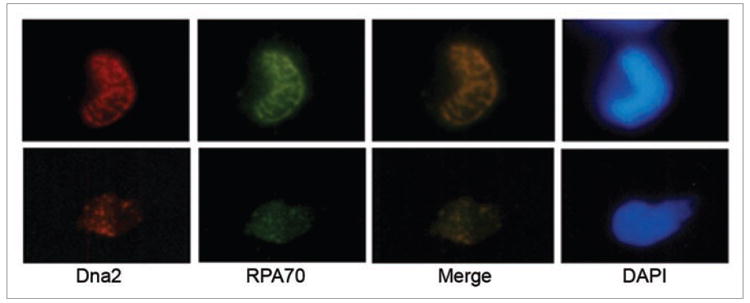
Dna2 foci during DNA replication. Sperm chromatin was incubated in extract, fixed, centrifuged onto coverslips, and subjected to immunofluorescence as previously described.57 Antibodies for immunfluorescence recognized Dna2 and RPA, and DNA was stained with DAPI.
Dna2 interacts with And-1 and Mcm10
In yeast, Dna2 has been shown to interact physically with RPA and FEN1. Because of the abundance of replication proteins in egg extracts, Xenopus seemed a good system in which to extend our knowledge of the subassemblies of replication proteins with which Dna2 interacts during the vertebrate S phase. We wished to use an unbiased approach to identify such proteins, and therefore Dna2 was immunoprecipitated from Xenopus extracts, individual bands larger than 80 kD were isolated from a protein preparative gel, and the proteins in these bands were identified by tandem mass spectrometry. A summary of results is provided in Figure 4A. Unexpectedly, the major DNA replication protein identified was the replication and sister chromatid cohesion protein And-1, the Xenopus ortholog of yeast Ctf4 (chromosome transmission fidelity 4), found in the 130–140 kDa band. A variety of additional interesting proteins were identified, including importin beta and nucleoporin 205. Importin beta is likely responsible for the nuclear import of Dna2, and nucleoporin 205 may also affect the nuclear localization of the Dna2 protein. We consider this significant because Loeillet and colleagues have shown synthetic-lethal interactions with nuclear pore proteins and DNA replication and repair proteins.15 TPR (Translocated Promoter Region) is also a constitutive component of the nuclear pore complex.16 Pcm-1 (pericentriolar material 1) is essential for the radial organization of microtubules and recruitment of proteins to the centrosome,17,18 and is necessary for preventing cells from exiting the cell cycle.19,20
Figure 4.
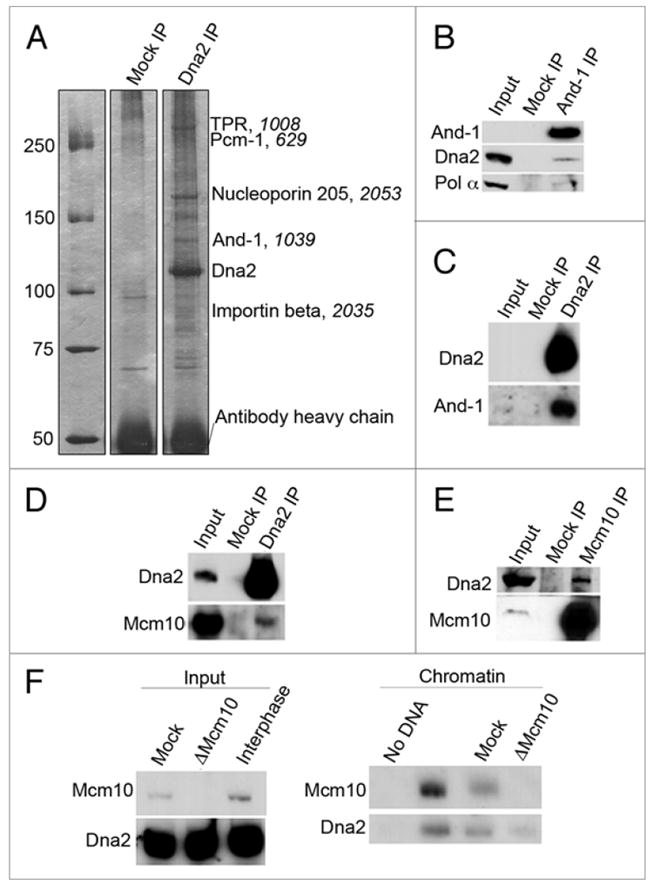
Dna2 interacts with DNA replication fork proteins. (A) Mock or Dna2 immunoprecipitates were isolated from interphase extracts, analyzed on an SDS-PAGE gel, and detected by silver stain. Results from electrospray ionization tandem mass spectrometry analysis of Dna2 immunoprecipitates are labeled, with the score from mass spectrometry analysis in italics. Only hits with an ion score above 500 are listed. (B) Immunoprecipitations from interphase extract were performed with control or anti-And-1 antibodies, and immunoprecipitates were analyzed by immunoblotting. (C) Control IgG (Mock) and anti-Dna2 antibodies were used for immunoprecipitations from interphase extracts, and samples were analyzed by immunoblotting. (D) Dna2 was immunoprecipitated in interphase extract using anti-Dna2 antibodies, and isolates were analyzed by immunoblotting. (E) Control and anti-Mcm10 antibodies were used to immunoprecipitate proteins from interphase extract. Reactions were analyzed by immunoblotting. (F) The ability of Dna2 to bind chromatin was assessed in the presence and absence of Mcm10. Interphase extracts were mock or Mcm10-depleted; 0.5 μl of this extract was analyzed by immunoblotting to confirm depletion of Mcm10. Sperm chromatin was added to the mock or Mcm10-depleted extracts, incubated for 100 min., chromatin fractions were isolated as previously described,27 and chromatin-associated proteins were analyzed by immunoblotting.
To rule out that the putative And-1 interaction was due to cross-reaction with the Dna2 antibody, rather than to interaction with Dna2, we carried out the reciprocal immunoprecipitation; we immunoprecipitated And-1 from interphase extracts. Western blotting of the And-1 immunoprecipitate showed that Dna2 co-immunoprecipitates with And-1, confirming that And-1 and Dna2 are in the same complex (Fig. 4B). Conversely, And-1 was also sufficiently abundant to be easily detectable in western blots of Dna2 immunoprecipitates (Fig. 4C). Since And-1 is known to interact with the lagging strand DNA polymerase, DNA polymerase α, the And-1 immunoprecipitate was also probed for pol α. Consistent with published results, pol α was also found in the And-1 immunoprecipitate with Dna2 (Fig. 4B). Note that sperm chromatin was not added to these extracts, and therefore And-1 interacts with Dna2 and pol α even in the absence of DNA.
Yeast Ctf4 is the most abundant pol α binding protein and a component of the RPC (Replication Progression Complex, consisting of Mcm2-7, Cdc45, GINS, Mcm10, Mrc1, Tof1-Csm3 and FACT) and therefore it plays an important role at replication forks.21-24 Similar interactions have been recently documented in human cells and Xenopus extracts.25-28 However, yeast and Xenopus Ctf4 also participate in recombination and establishment of sister chromatid cohesion, and the mechanistic contribution of Ctf4 to DNA replication is not yet clear. Xenopus Mcm10, a well-studied replication initiation and elongation protein, has been shown to interact with Xenopus And-1 and together with And-1 may be required for recruitment of pol α and lagging strand DNA replication.27 If Dna2 is interacting with And-1 during DNA replication in Xenopus extracts, we would expect to be able to detect complexes containing both Dna2 and Mcm10. As shown in Figure 4D and E, we find that Mcm10 and Dna2 co-immunoprecipitate. Taken together, these interaction studies suggest that Dna2, And-1 and Mcm10 are present in the same DNA replication complex.
In Xenopus, Mcm10 is required for chromatin loading of And-1 in S phase, and And-1 is required for stable loading of pol α.27 We wished to determine if the Dna2 interactions with And-1 and Mcm10 are required for the association of Dna2 with chromatin in S phase. Zhu et al. elegantly demonstrated that addition of anti-And-1 antibodies to the extract block pol α recruitment to chromatin.27 Unfortunately, we were not able to replicate inhibition of pol α loading using And-1 antibody (unpublished data). Instead, we were able to deplete Mcm10 from the extracts,14 and to ask if Dna2 was still able to associate with chromatin. Interestingly, the association of Dna2 with chromatin does not appear to be dependent on Mcm10 (Fig. 4F). Taken together, our results indicate that Dna2 associates with chromatin after pre-RC formation, independently of Cdk2 activation, and independently of Mcm10. This could occur if Dna2 and Mcm10 interact with a common scaffolding protein or common intermediate structure.
Dna2 interacts with DSB response proteins and with DNA ends in both S phase and M phase
Biochemical experiments have recently shown that Dna2 is a major 5’-3’ nuclease in Xenopus extract and that depletion of Dna2 results in inhibition of the completion of SSA in Xenopus egg extracts.11 However, the mechanism of Dna2 participation has not been fully investigated. To further characterize the participation of Dna2 in events at DSBs, we investigated the interaction of Dna2 with other proteins involved in DSB repair and checkpoint pathways in S phase extracts. We speculate that Dna2 acts in the early steps of recombination, so we asked if Dna2 interacts with ATM or the MRN (Mre11/Rad50/Nbs1) complex. As shown in Figure 5A, Dna2 co-immunoprecipitates both ATM and Nbs1. Thus, we conclude that Dna2 interacts with proteins that participate in the early events of DSB signaling and repair. These interactions are observed in egg extract and thus do not require DNA damage and are not mediated by DNA.
Figure 5.
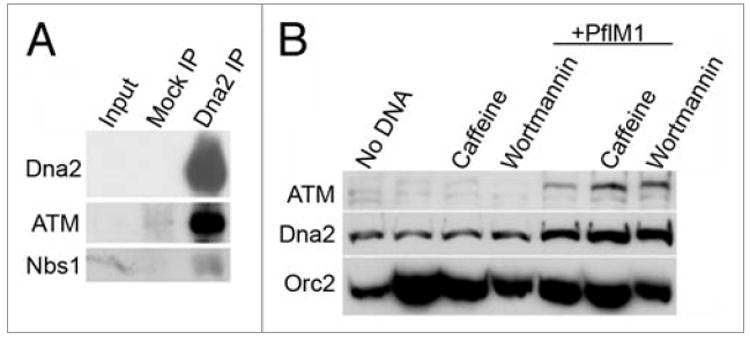
Dna2 and double-strand breaks. (A) Dna2 interacts with DSB proteins. Anti-Dna2 antibodies were used to immunoprecipitate Dna2 from interphase extract, and immunblots were performed for Dna2, ATM and Nbs1. (B) Dna2 accumulates on damaged chromatin. Sperm chromatin was added to interphase extracts in the presence or absence of 0.1 units/μl PflM1 and/or chekpoint inhibitors (5 mM caffeine or 0.1 mM wortmannin) as indicated. After a 100 min. incubation, chromatin fractions were isolated from extract as previously described30 and protein levels on chromatin were analyzed by immunoblotting.
When DSBs are induced, proteins involved in checkpoint signaling and DNA repair accumulate transiently on chromatin.29-31 To address this, PflM1, a restriction enzyme that produces a 3’ overhang, was used to induce DSBs in sperm chromatin in S phase extracts, and the amount of Dna2 on chromatin was analyzed. While some insoluble Dna2 is detected in samples that do not contain DNA, Dna2 levels on PflM1 treated chromatin are clearly increased compared to undamaged chromatin. We also observed further accumulation of Dna2 on chromatin containing DSBs when caffeine or wortmannin, inhibitors of checkpoint kinases, were present (Fig. 5B). As expected, we see a corresponding increase in ATM on damaged chromatin in the presence of checkpoint inhibitors. Similar results were seen when using EcoRI, which cleaves to reveal a 5’ overhang, to induce DSBs (unpublished data). Inhibition of the checkpoint may retard the release of Dna2 or may lead to the formation of inactive complexes on chromatin.
Dna2 likely associates with and dissociates from DNA ends to allow for downstream processing events. In yeast and human, ATM and the MRN complex are among the first proteins to be detected at a DSB, and RPA is subsequently recruited, presumably through the production of ssDNA by 5’ resection.12,13,29,32-34 ATR is recruited after the generation of RPA-coated ssDNA, and TopBP1 is involved in the ATM-dependent activation of ATR.32,35 To investigate the likely transient association of Dna2 with DSBs, we compared the kinetics of association of Dna2, MRN, ATM, and other proteins with DNA ends. To look directly at DNA ends, we examined the binding of Dna2 to linear DNA in a manner similar to previous experiments to examine Ku binding to DNA ends.36 For these experiments, we biotinylated either 1 or 2 ends of linear pBluescript with a fill-in reaction to produce blunt ends, and bound the biotinylated DNA to streptavidin beads, generating beads with DNA resembling either unbroken DNA (2X, both ends of pBluescript bound to beads) or DNA with a DSB (1X, one free end) (Fig. 6A). Protein binding to the DNA ends was then monitored over time in interphase extract, with 3 × 1011 DNA ends/μl of extract (Fig. 6B). Controls show that there is background binding of DSB-response proteins to the “unbroken” (2X biotin) DNA control beads, which we propose derives from incomplete binding of the biotinylated DNA ends to the beads. However, binding to the “broken” DNA beads (1X) is clearly greater than to controls, especially at the earlier time points. With the “broken” DNA beads (1X), MRN and ATM associate at the earliest time points, and Dna2 associates with a slight delay compared to MRN. Dna2 accumulates to peak levels with similar timing to RPA, consistent with the role of Dna2 in producing single-stranded DNA overhangs that may recruit RPA. ATR then accumulates on the RPA-coated DNA ends, consistent with the requirement of RPA-coated ssDNA for the switch from ATM to ATR in human DNA end resection.32 The kinetics of binding and release of Dna2 at the DNA ends is consistent with a role in DSB resection, since Dna2 accumulates after ATM and with similar timing to RPA70. MRX, ortholog of MRN, appears to dissociate from the DNA ends slightly before Dna2 in yeast as well, consistent with our results.13,29,33
Figure 6.
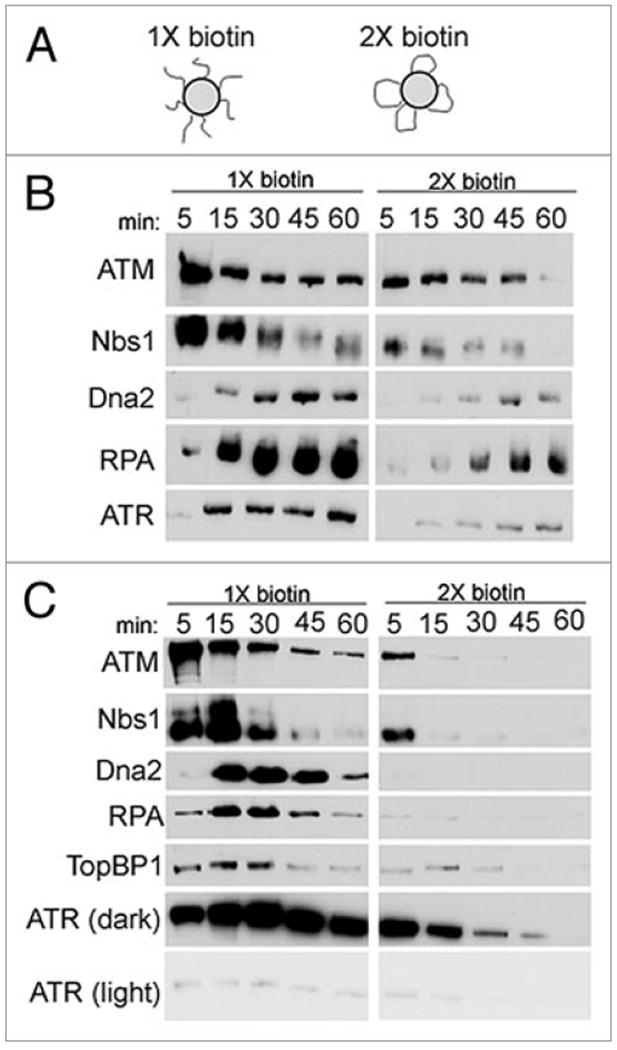
Dna2 at DNA ends. (A) Schematic of beads used for experiments. pBluescriptIIKS-was linearized and biotinylated on one or both ends, and bound to streptavidin beads. These beads simulated unbroken DNA or DNA with a DSB. (B) Time-course of binding of DSB repair and checkpoint proteins to DNA ends. Beads were incubated in interphase extract, isolated at indicated time-points, and the relative amounts of Dna2, ATM, Nbs1, RPA70 and ATR bound to the beads were analyzed by immunoblotting. (C) Time-course of binding of DSB proteins to DNA ends in CSF extract. Experiment was performed as described for (B) of this figure, except in CSF, not interphase, extract.
The role of Dna2 in DSB processing may be cell cycle regulated. Since Dna2 is required in S phase for DNA replication, the role of Dna2 in DSB repair may be limited to S phase, with a main function potentially in the response to collapsed replication forks. To determine the effect of cell cycle stage on association of Dna2 at DNA ends, we performed the same DNA end binding assay in M phase, CSF, extracts (Fig. 6C). Under these conditions, Dna2 associates with DNA ends after ATM and Nbs1, and with similar timing to RPA, consistent with results from interphase extract. We also monitored TopBP1, required for activation of ATR, and found that it associated with similar timing to RPA. While the timing of each step varies between interphase and CSF extracts, the general temporal program of binding to DNA ends is consistent. Therefore, we conclude that the role of Dna2 in DNA end resection is not limited to one phase of the cell cycle.
Interplay of nucleases: Dna2 and MRN
To establish that Dna2 participates in DNA end resection/processing in these extracts, we used accumulation of RPA at DNA ends in the bead-based assay as a measure of successful resection/processing of a DSB. We depleted Dna2 from interphase extracts by immunodepletion and monitored RPA accumulation on DNA ends. When Dna2 is lacking, both ATM and Nbs1 can associate with DNA ends. However, RPA does not accumulate on DNA ends to the same level as it does in the presence of Dna2 (Fig. 7A), indicating that Dna2 is necessary for efficient DNA end processing, though some residual processing may occur in its absence. In yeast, Dna2 can compensate for the loss of Mre11 nuclease activity, but not for complete lack of Mre11 protein, in repair of X-ray-induced DNA damage in vivo.12 This raises the question of what the additional role of the MRN complex may be. One plausible role for the MRN complex, given the Dna2/Nbs1 interaction shown in Figure 5 and the fact that Dna2 binds duplex ends after Nbs1 as shown in Figure 6, is to recruit Dna2 to breaks. To test this idea, extracts were depleted of Nbs1, which efficiently depletes the MRN complex,35 and association of repair proteins was reassessed. Dna2 still accumulates on DNA ends in the absence of Nbs1, though there is a reproducibly lower accumulation than in the presence of MRN (Fig. 7B). Without MRN, however, RPA does not accumulate on DNA ends. Therefore, although Dna2 is recruited, there is not enough ssDNA generated to recruit RPA. We conclude that MRN is not absolutely required for the recruitment of Dna2 to DNA ends, but is required for DNA end processing and resection.
Figure 7.
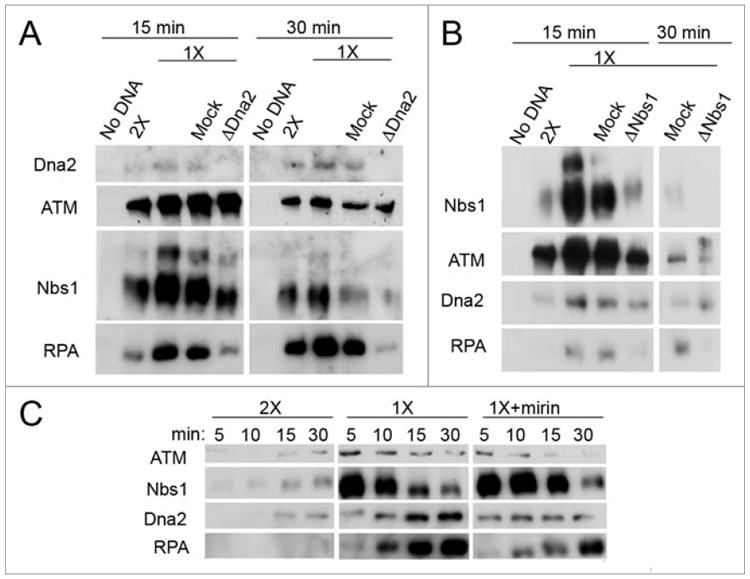
Dna2 and MRN at DNA ends. (A) Effect of Dna2 depletion on processing of DNA ends. Interphase extracts were untreated, mock or Dna2-depleted, and incubated with the appropriate beads for 15 or 30 min. Beads were isolated and protein binding was assessed by immunoblotting. (B) DNA end binding of proteins in Nbs1-depleted extract. Extracts were untreated, mock-depleted, or Nbs1-depleted, which depletes the whole MRN complex, and incubated with the appropriate beads for 15 or 30 min. Beads were isolated, and protein binding to the beads was analyzed by immunoblotting. (C) Mirin was used to inhibit the nuclease activity of Mre11. Mirin or DMSO was incubated in extracts with the appropriate beads. Beads were isolated at the indicated times and protein levels were analyzed by immunoblotting.
To investigate if Mre11 nuclease activity is required for resection, Dna2 and RPA recruitment to DNA ends was monitored in the presence and absence of Mre11 nuclease activity. Mirin, a small molecule inhibitor of the Mre11 nuclease, was used to block the nuclease activity of endogenous Mre11 in extracts,37,38 and the association of Dna2 and RPA with DNA ends was again assessed. Care was taken to use functionally validated mirin (see Materials and Methods). In the presence of mirin, Dna2 binds DNA ends, although binding is reduced compared to extracts without mirin (Fig. 7C). RPA accumulation is retarded but not abolished in the presence of mirin. We propose that RPA accumulation on chromatin is delayed upon inhibition of Mre11 nuclease, implying a partial defect in resection. We conclude that either mirin only partially inhibits Mre11 nuclease, or other nucleases can compensate when Mre11 nuclease activity is compromised. Dna2 may be one of these nucleases, since it binds to DNA ends in the absence of Mre11 nuclease activity (Fig. 7B and C).
Dna2 is not required for induction or signaling of checkpoints
In the DSB checkpoint, the ATM kinase is first activated, and active ATM subsequently activates the ATR kinase.39 Recognition of RPA-ssDNA complexes is thought to be part of the ATR-activation process.32,34 Since Dna2 is involved in resection to produce ssDNA, we asked if Dna2 is also involved in activation or signaling of the DSB checkpoint. To do this, we depleted Dna2, added known checkpoint inducers, and monitored phosphorylation of Chk1, an effector kinase and target of ATR, and Chk2, an effector kinase and target of ATM, as indicators of checkpoint activation and signaling. First, the checkpoint was induced with pA/T70 oligonucleotides, which activate both ATM and ATR, resulting in phosphorylation of Chk1. In Dna2-depleted extract with added pA/T70 oligonucleotides, Chk1 was efficiently phosphorylated (Fig. 8A). Since the pA/T70 oligonucleotides can form a variety of structures, we also examined the checkpoint response to linear DNA in the Dna2-depleted extract. Linear pBluescript was added to extract to activate the DSB checkpoint response, and we see that in the absence of Dna2, Chk2 is well phosphorylated in response to linear DNA (Fig. 8B). The weaker signal for phospho-Chk2 is due to a weaker interaction with the antibody in the immunoblot.40 To eliminate the possibility that Dna2 has a specific role in the checkpoint response that was overcome in these assays with synthetic checkpoint activators, we studied the activation of checkpoints in nuclei during S phase. We observed that nuclear Chk1 was also phosphorylated in the presence of stalled replication forks induced by aphidicolin and in the presence of DSBs induced by PflM1, regardless of the presence of Dna2 (Fig. 8C). We conclude that the Dna2 protein itself is not necessary for checkpoint signaling. We have shown that Dna2 plays a role in 5’-3’ resection, as measured by RPA recruitment; activation of checkpoints in the absence of Dna2 implies that another nuclease(s) can compensate for the lack of Dna2 in producing sufficient single-stranded DNA to activate ATR.
Figure 8.
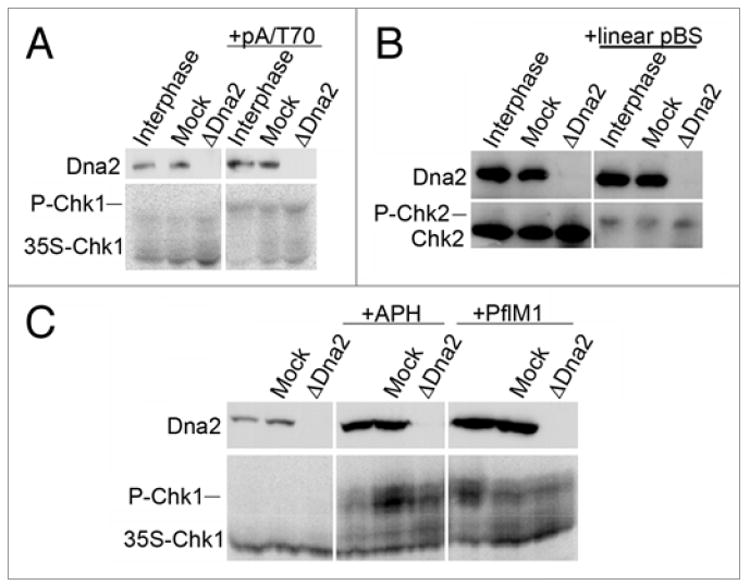
Assessment of the DNA replication checkpoint in Dna2-depleted extracts. For all panels in this figure, interphase extracts were untreated, mock, or Dna2-depleted. (A) Phospho-Chk1 in Dna2-depleted extracts. pA/T70 oligos were added to interphase extract to elicit a checkpoint response, as previously described.54 The electrophoretic mobility of 35S-Chk1 was monitored by autoradiography after a 100 min incubation in extract containing pA/T70, and 35S-Chk1 is well phosphorylated, as indicated by the arrow. (B) Phospho-Chk2 in extracts lacking Dna2. Linear pBluescript was added to extract to elicit the DSB checkpoint response, as previously described,40 and immunoblotting was used to assess activation of the checkpoint by monitoring Chk2 phosphorylation after 100 min in extract containing linear pBluescript. (C) The checkpoint response to stalled replication forks and DSBs was assessed in nuclei using aphidicolin (APH) and PflM1, respectively. Sperm chromatin was incubated in extracts for 100 minutes without or with APH, or without or with PflM1 to induce replication fork stalling or DSBs, respectively. Nuclei were isolated as previously described.30 Dna2 levels in nuclei were assessed by immunoblotting, while 35S-Chk1 electrophoretic mobility was assessed by SDS-PAGE and autoradiography.
Discussion
Dna2 is a DNA replication protein in vertebrates
In this work, we present evidence supporting our previous results suggesting that Xenopus Dna2 participates in chromosomal DNA replication. Xenopus Dna2 is recruited to chromatin in a regulated manner and binds chromatin with similar timing to other DNA replication proteins. Efficient Dna2 recruitment to chromatin requires formation of the pre-RC and origin licensing, as Dna2 is not efficiently recruited to chromatin in the absence of the MCM replicative helicase or in the presence of geminin. Dna2 appears to participate in the elongation of replicating DNA; Dna2 and RPA co-localize during replication, consistent with Dna2 being present at DNA replication forks and traveling with forks throughout DNA replication. Interestingly, And-1 and Mcm10, components of the replication fork, associate with Dna2 in interphase extracts, indicating that Dna2, like And-1 and Mcm10, may function on the lagging strand.27,41,42
Although formation of the pre-RC is a pre-requisite for Xenopus Dna2 binding to chromatin in S phase, Dna2 binding does not require activation of the pre-RC by Cdk2 activity, as is also the case for the Mcm10 protein (Figs. 1 and 3). Like Dna2, Mcm10 binds chromatin after the MCM2-7 helicase complex and independently of Cdk2 activity.14 Mcm10 is, in turn, required for the binding of Cdc45, which allows unwinding of the origin of replication. Despite their similar requirements for chromatin binding, the association of Dna2 with chromatin is independent of Mcm10. Therefore, Dna2 associates with chromatin early in the formation of the replication fork, after the MCM helicase complex but independent of the binding of Mcm10 and Cdc45. These findings may indicate that Xenopus Mcm10 and Dna2 interact with a similar intermediate in the formation of the replisome. Since this stage of replication, the conversion of the pre-RC into an active replication fork, is poorly understood in eukaryotic DNA replication, the exact role of these proteins in the transition is difficult to predict at the moment.
Dna2 is likely involved in lagging strand replication
The interaction of Dna2 with And-1 and Mcm10 correlate with genetic interactions seen in yeast, but for which no underlying mechanism had been discovered. Ctf4, the yeast ortholog of human And-1, is the most abundant pol α-interacting protein in yeast, and dna2-2 shows synthetic lethality with ctf4Δ.21,43 Additionally, yeast dna2 is synthetically lethal with mcm10, and the same mcm10 mutant is synthetically lethal with both dna2 and ctf4.2,44 The presence of these three proteins in the same complex could account for the observed synthetic lethality, since mutation of either protein might destabilize complexes containing them. The direct protein-protein contacts in the putative complex, if any, remain to be determined, however.
The accepted role of Dna2 in yeast DNA replication is to assist the major Okazaki fragment processing nuclease, Fen1, in removal of RNA/DNA primers on the lagging strand.1,45 The best single piece of evidence for this is that DNA2 is an essential gene, yet deletion of DNA2 can be complemented by overproduction of FEN1.46 Numerous biochemical and genetic interactions support this model.2,47 In both yeast and Xenopus, And-1, Mcm10 and pol α are all implicated in replication of the lagging strand. Pol α is necessary for RNA/DNA primer synthesis, and Mcm10 is responsible for preventing premature degradation of pol α in both yeast and human cells.48,49 Yeast Ctf4, Mcm10 and pol α are part the replication progression complex along with the MCM helicase, and it has been proposed that Ctf4 and Mcm10 serve to couple the lagging strand polymerase with the replicative MCM helicase.24,27,49 The occurrence of these proteins in complexes that also contain Dna2 is both consistent with the idea that Dna2 is involved in lagging strand events in Xenopus and, in turn, supports the previous findings suggesting lagging strand roles Mcm10 and And-1.
It has been claimed that in human cells, Dna2 is solely a mitochondrial protein.5 While other work has revealed that human Dna2 does reside in both nuclei and mitochondria,8 the role of human Dna2 in nuclei has yet to be thoroughly studied. Our results show that Xenopus Dna2 clearly participates in nuclear genomic DNA replication, and the protein-protein interactions demonstrated here with And-1 and Mcm10 indicate an important role for nuclear Dna2. It is likely that these mechanisms are conserved in human cells, where depletion of Dna2 leads to cell cycle delay in G2 and aberrant nuclear division.8
Dna2 in DSB repair
In addition to its role during lagging strand DNA replication, yeast Dna2 has been shown to play a major role in 5’ to 3’ resection during the early steps of DSB repair during G2 phase of the cell cycle.12,13 Previous evidence for a similar role in Xenopus is also strong.11 Our new findings add to this evidence. We show that Dna2 physically interacts with ATM and Nbs1 (Fig. 5A), which are both recruited to and accumulate at DSBs. We found that Dna2 hyper-loads on chromatin containing induced DSBs, similar to the hyper-loading of pol α seen on chromatin in aphidicolin-inhibited replicating chromatin.50 In our studies, Dna2 accumulates to an even greater extent on DNA ends when checkpoint kinase inhibitors such as caffeine and wortmannin are present. This may be due to either retention of DSB processing proteins on chromatin or the generation of non-functional DNA replication and repair complexes on chromatin.
DSB repair and checkpoint proteins associate with and dissociate from DSBs in a specific temporal order.32 Our finding that Dna2 accumulates slightly after ATM and Nbs1, that Dna2 peak levels of binding occur after ATM and Nbs1 have already begun to dissociate, and that RPA accumulates with similar timing to Dna2 suggests that resection may be initiated by MRN but continues due to the activity of Dna2 nuclease.11 Like MRN, Dna2 also binds transiently, presumably allowing for the downstream strand invasion events, though we did not study those events here. These kinetics are similar to the ordered binding and dissociation of MRN and Dna2 that is observed in S. cerevisiae.13,29,33 We also show that these events occur in both S phase and M phase. These data place Dna2 early in the timeline of the double-strand break response, and we speculate that the nuclease activity of Dna2 participates in DSB resection.
Resection of DSBs in yeast involves both Dna2 and the MRX complex. MRX appears to initiate strand displacement and Dna2 further degrades the 5’ strand, revealing an elongated 3’ ssDNA strand to be used for strand exchange.13 The MRX complex itself must be present for resection, but resection still occurs with a complex containing nuclease-dead Mre11.12,51 The ability to bypass the requirement for the Mre11 nuclease activity relies on compensation by Dna2 for the nuclease-dead Mre11. However, Dna2 can not compensate for the complete absence of the Mre11 protein.12,13 The non-nucleolytic role of Mre11 is matter of interest. One possible explanation is that another protein, such as Ku, may compete in the resection reaction in the absence of MRN.52 Another possibility is that the MRN protein complex is required at DSBs to recruit additional proteins necessary for DNA end resection, or perhaps the real requirement for successful DNA end resection has more to do with Rad50 or Nbs1 in the MRN complex, as opposed to the Mre11 nuclease activity. The MRN complex, regardless of Mre11 nuclease activity, may be necessary to process the DNA and create a substrate for Dna2. The Xenopus extract system used here allows us to begin to discriminate among such possibilities. We found that the MRN complex was not necessary for recruitment of Dna2, but even though Dna2 was recruited to DNA ends, resection was not efficient. Further study is warranted, however, since the level of Dna2 on DNA ends was reproducibly lower in the absence of MRN.
Mirin is an inhibitor of the Mre11 nuclease that does not prevent the binding of MRN to a DSB.37,38 Thus, mirin can be used to distinguish whether it is the presence of the MRN complex or the Mre11 nuclease activity that is required for bound Dna2 to create a substrate for RPA. Mirin, as expected, does not inhibit the recruitment of Dna2 to the DSB. Unlike the MRN depletion, however, RPA did accumulate at the DNA ends, although with a significant delay. We speculate this delayed RPA accumulation is due to other nucleases, possibly Dna2, compensating for the lack of Mre11 nuclease activity, as this appears to happen in yeast. Alternatively, we cannot rule out that mirin may not fully inhibit Mre11 nuclease activity, and we are detecting residual activity.
DNA damage checkpoint activation
The DSB checkpoint first activates the ATM kinase, which subsequently activates the ATR kinase.32,39 A possible role for Dna2 in checkpoint activation and signaling was assessed by monitoring phosphorylation and activation of Chk1 and Chk2, downstream targets of the DNA damage checkpoint pathway, in the absence of Dna2. When checkpoint inducers, pA/T70 oligonucleotides and linear DNA, are added to Dna2-depleted extracts, Chk1 and Chk2 are well phosphorylated. We also observed checkpoint activation in the absence of Dna2 in nuclei during DNA replication stress, i.e., in the presence of stalled replication forks induced by aphidicolin, or DSBs induced by the addition of PflM1 restriction endonuclease (Fig. 8). Therefore, neither the Dna2 protein itself nor the enzymatic activities of Dna2 appear to be necessary for the checkpoint response, indicating that the role of Dna2 in replication fidelity does not rest with activation of checkpoints, but with allowing efficient DNA replication and repair of damaged DNA. We speculate that another nuclease may compensate for the lack of Dna2, so ssDNA will still be generated at DSBs and the checkpoint will be functional. Redundancy in resection is consistent with the observation that processing is not completely defective in the SSA assay in Xenopus nuclear extracts.11 We observed minimal RPA binding to DNA ends in Dna2-depleted extracts in our bead-based assay (Fig. 7A), but limitations of this assay restrict its usage to early time-points. However, the checkpoint assays in which aphidicolin or PflM1 is added to induce checkpoint activation observe a much later time-point (100 minutes). Compensating nucleases may be slower than Dna2 to resect the DSB, but 100 min. may be sufficient for compensation. It is also possible that compensating nucleases are more concentrated in nuclei than in interphase extract, allowing a more efficient nuclease compensation than in interphase extract. A likely candidate nuclease is the homolog of yeast Exo1, and it will be valuable to test Xenopus Exo1.
In conclusion, our studies have used biochemistry, depletion and protein-protein interaction studies to probe the physiological roles of Dna2. This study is the first to show that Xenopus Dna2 is a helicase/nuclease, that And-1 and Mcm10 interact with Dna2, and that there is an interplay between Dna2 and MRN at DNA ends resembling double-strand breaks. At this stage of our studies, we find many similarities between yeast Dna2 and vertebrate Dna2. While future detailed studies can be expected to reveal differences between metazoan and yeast Dna2, given divergent regulatory sequences, the general roles of Dna2 in DNA transactions appear to be evolutionarily conserved.
Materials and Methods
Helicase assay
Helicase activity of recombinant Dna2 was measured using the nuclease-deficient mutant of Dna2 (human Dna2 D294A or Xenopus Dna2 D278A) in a 20 μl standard reaction mixture containing 50 mM Tris-HCl (pH 7.5), 25 mM NaCl, 2 mM DTT, 0.25 mg/ml bovine serum albumin, 4 mM MgCl2, 4 mM ATP and 32P-labeled helicase substrate. After incubation at 37°C for 1 h, reactions were stopped with 5x stop solution, which consisted of 60 mM EDTA, 40% sucrose, 0.6% SDS, 0.25% bromophenol blue and 0.25% xylene cyanole FF. Reaction products were then separated using 8% native polyacrylamide gels containing 0.1% SDS and detected using a Storm 860 PhosphorImager.
Xenopus egg extracts
Xenopus cell-free extract was prepared as described previously.53 To elicit a checkpoint response, extracts were treated with either 50 μg/ml pA/T70 oligonucleotides or 25 μg/ml linear pBluescript.40,54 For reactions involving nuclei, demembranated sperm chromatin was incubated at 3,000 sperm/μl in extract for 100 min. Inhibitors (5 mM caffeine, 0.1 mM wortmannin, 0.3 mM geminin, 0.1 mM p27) were incubated in extract for 20 min on ice before addition of sperm chromatin. Double-strand breaks were induced by addition of 0.1 units/μl PflM1. Nuclei and chromatin were isolated as described.30 Chromatin isolation in Mcm10-depleted extracts was performed as previously described.27
Antibodies and recombinant proteins
Anti-Dna2 antibodies were affinity-purified with the N-terminal 712 aa of Dna2 as previously described.3 Antibodies recognizing DNA polymerase α p70 subunit, RPA70, Cdc45, Claspin, Orc2, ATM, Nbs1, Chk2, ATR and TopBP1 were previously described.30,35,40,55,56 Anti-human BM28 monoclonal antibody, which recognizes Xenopus Mcm2, was purchased from Cell Signaling Technology (Beverly, MA), and control rabbit IgG was purchased from Zymed Laboratories (South San Francisco, CA). Anti-And-1 antibodies were a gift of A. Dutta (Univ. of Virginia), anti-Mcm10 antibodies were a gift of J. Walter (Harvard Medical School), and anti-Mcm3, anti-Cdc6 and anti-RPA70 antibodies used for immunofluorescence were a gift of P. Jackson (Genentech). Production of recombinant Xenopus Dna2 is described in Liu et al. 2000.3 35S-Labeled Chk1 was generated using the TnT system (Promega, Madison, WI).
Immunological methods
For immunoprecipitations, 2.5 μg antibodies were pre-incubated with 5 μl Protein A Support (BioRad) and subsequently incubated with 50 μl interphase extract for 1 hr at 4°C. Beads were washed 4 times with 10 mM HEPES-KOH [pH 7.6], 150 mM NaCl, 0.1% CHAPS, 2.5 mM EGTA and analyzed by SDS-PAGE. Mcm3 was depleted with 30 μl of antibodies per 100 μl extract, using 2 rounds of depletion that were 45 minutes each. Immunofluorescence on sperm nuclei was performed as described, using 30 μl anti-RPA70 antibodies raised in chicken and 2.5 μl anti-Dna2 antibodies raised in rabbit per sample.57 Dna2 and Nbs1 depletions were performed as described.3,35
Mass spectrometry
Dna2 interphase IPs were performed as described above but from 400 μl extract, subjected to SDS-PAGE and stained with Coomassie Blue. Bands were excised, an in-gel trypsin digest was performed, peptides were extracted and subjected to electrospray ionization tandem mass spectrometry, and samples were identified with the Xenopus Mascot Search database. Hits with an ion score >500 were used for analysis. Mass spectrometry work was done by Sonja Hess at the Proteome Exploration Laboratory at Caltech.
DNA end binding experiments
DNA binding experiments were modified from previously published assays using DNA immobilized on magnetic beads.36,58 Briefly, pBluescript II KS - was linearized using either NotI (for biotinylation of both ends) or NotI and EcoRI (for biotinylation of one end). Klenow was used for fill-in reactions in the presence of biotin-[C14]dCTP. The 2.9 kb DNA fragments were then purified and bound to M-280 Streptavidin Dynabeads (Invitrogen, Carlsbad, CA) at a concentration of 0.5 μg DNA/5 μg beads, following the manufacturer’s protocol. Beads were incubated in extract for the indicated times, washed 2 times with 5 volumes of 20 mM HEPES-KOH [pH 7.6], 80 mM KCl, 2.5 mM K-gluconate, 10 mM Mg-gluconate, 1% NP-40 and 1 mM DTT, and subjected to SDS-PAGE and immunoblotting. For experiments involving mirin, 100 μM mirin was added to extracts. Experiments were conducted using both validated mirin that was a kind gift of Dr. Alan Eastman and mirin purchased from Enzo Life Sciences (Plymouth Meeting, PA). The two mirin preparations yielded similar results, and were thus determined equivalent.
Acknowledgments
We thank Dr. A. Eastman (Dartmouth College) for the gift of validated mirin and helpful discussions, Dr. A. Dutta (University of Virginia) for the gift of antibodies recognizing Xenopus And-1, Dr. J. Walter (Harvard University) for the gift of antibodies recognizing Xenopus Mcm10, and Dr. P. Jackson for the gift of anti-Mcm3, anti-RPA70 and anti-Cdc6 antibodies. We thank Sonja Hess of the Proteome Exploration Laboratory at Caltech for performing our mass spectrometry work, and we also thank Jules Chen for the immunofluorescence work in Figure 3. This work was supported by NIH grants GM043974 and GM070891 to W.G.D. and GM078666 to J.L.C.
Abbreviations
- ssDNA
single-stranded DNA
- dsDNA
double-stranded DNA
- pre-RC
pre-replication complex
- DSB
double-strand break
- SSA
single-strand annealing
- MRN
Mre11-Rad50-Nbs1
- MRX
Mre11-Rad50-Xrs2
- And-1
acidic nucleoplasmic DNA-binding protein 1
- Ctf4
chromosome transmission fidelity 4
- ATM
ataxia telangiectasia mutated
- ATR
ATM and Rad3-related
References
- 1.Budd ME, Cox LS, Campbell JL. Coordination of Nucleases and Helicases during DNA Replication and Double-strand Break Repair. In: Cox LS, editor. Molecular Themes in DNA Replication. London: RSC Publishing; 2009. [Google Scholar]
- 2.Budd ME, Tong AHY, Polaczek P, Peng X, Boone C, Campbell JL. A network of multi-tasking proteins at the DNA replication fork preserves genome stability. Plos Genet. 2005;1:634–50. doi: 10.1371/journal.pgen.0010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu QQ, Choe WC, Campbell JL. Identification of the Xenopus laevis homolog of Saccharomyces cerevisiae DNA2 and its role in DNA replication. J Biol Chem. 2000;275:1615–24. doi: 10.1074/jbc.275.3.1615. [DOI] [PubMed] [Google Scholar]
- 4.Masuda-Sasa T, Imamura O, Campbell JL. Biochemical analysis of human Dna2. Nucleic Acids Res. 2006;34:1865–75. doi: 10.1093/nar/gkl070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng L, Zhou MA, Guo ZG, Lu HM, Qian LM, Dai HF, et al. Human DNA2 Is a Mitochondrial Nuclease/Helicase for Efficient Processing of DNA Replication and Repair Intermediates. Mol Cell. 2008;32:325–36. doi: 10.1016/j.molcel.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JH, Kim HD, Ryu GH, Kim DH, Hurwitz J, Seo YS. Isolation of human Dna2 endonuclease and characterization of its enzymatic properties. Nucleic Acids Res. 2006;34:1854–64. doi: 10.1093/nar/gkl102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee K-H, Lee M, Lee T, Han J, Park Y, Ahnn J, et al. Dna2 Requirement for Normal Reproduction of Caenorhabditis elegans Is Temperature-dependent. Mol Cells. 2003;15:81–6. [PubMed] [Google Scholar]
- 8.Duxin JP, Dao B, Martinsson P, Rajala N, Guittat L, Campbell JL, et al. Human Dna2 Is a Nuclear and Mitochondrial DNA Maintenance Protein. Mol Cell Biol. 2009;29:4274–82. doi: 10.1128/MCB.01834-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blow JJ, Laskey RA. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell. 1986;47:577–87. doi: 10.1016/0092-8674(86)90622-7. [DOI] [PubMed] [Google Scholar]
- 10.Garner E, Costanzo V. Studying the DNA damage response using in vitro model systems. DNA Repair. 2009;8:1025–37. doi: 10.1016/j.dnarep.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Liao S, Toczylowski T, Yan H. Identification of the Xenopus DNA2 protein as a major nuclease for the 5’→3’ strand-specific processing of DNA ends. Nucl Acids Res. 2008;36:6091–100. doi: 10.1093/nar/gkn616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Budd M, Campbell J. Interplay of Mre11 nuclease with Dna2 plus Sgs1 in Rad51-dependent recombinational repair. Plos One. 2009;4:4267. doi: 10.1371/journal.pone.0004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–94. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohlschlegel JA, Dhar SK, Prokhorova TA, Dutta A, Walter JC. Xenopus Mcm10 Binds to Origins of DNA Replication after Mcm2-7 and Stimulates Origin Binding of Cdc45. Mol Cell. 2002;9:233–40. doi: 10.1016/s1097-2765(02)00456-2. [DOI] [PubMed] [Google Scholar]
- 15.Loeillet S, Palancade B, Cartron M, Thierry A, Richard G-F, Dujon B, et al. Genetic network interactions among replication, repair and nuclear pore deficiencies in yeast. DNA Repair. 2005;4:459–68. doi: 10.1016/j.dnarep.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Byrd DA, Sweet DJ, Pante N, Konstantinov KN, Guan T, Saphire AC, et al. Tpr, a large coiled coil protein whose amino terminus is involved in activation of oncogenic kinases, is localized to the cytoplasmic surface of the nuclear pore complex. J Cell Biol. 1994;127:1515–26. doi: 10.1083/jcb.127.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hames RS, Crookes RE, Straatman KR, Merdes A, Hayes MJ, Faragher AJ, Fry AM. Dynamic Recruitment of Nek2 Kinase to the Centrosome Involves Microtubules, PCM-1 and Localized Proteasomal Degradation. Mol Biol Cell. 2005;16:1711–24. doi: 10.1091/mbc.E04-08-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dammermann A, Merdes A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J Cell Biol. 2002;159:255–66. doi: 10.1083/jcb.200204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srsen V, Gnadt N, Dammermann A, Merdes A. Inhibition of centrosome protein assembly leads to p53-dependent exit from the cell cycle. J Cell Biol. 2006;174:625–30. doi: 10.1083/jcb.200606051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balczon R, Simerly C, Takahashi D, Schatten G. Arrest of cell cycle progression during first interphase in murine zygotes microinjected with anti-PCM-1 antibodies. Cell Motil Cytoskeleton. 2002;52:183–92. doi: 10.1002/cm.10043. [DOI] [PubMed] [Google Scholar]
- 21.Miles J, Formosa T. Protein affinity chromatography with purified yeast DNA polymerase alpha detects proteins that bind to DNA polymerase. PNAS. 1992;89:1276–80. doi: 10.1073/pnas.89.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gambus A, Jones RC, Sanchez-Diaz A, Kanemaki M, van Deursen F, Edmondson RD, Labib K. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat Cell Biol. 2006;8:341–58. doi: 10.1038/ncb1382. [DOI] [PubMed] [Google Scholar]
- 23.Gambus A, van Deursen F, Polychronopoulos D, Foltman M, Jones RC, Edmondson RD, et al. A key role for Ctf4 in coupling the MCM2-7 helicase to DNA polymerase [alpha] within the eukaryotic replisome. EMBO J. 2009 doi: 10.1038/emboj.2009.226. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka H, Katou Y, Yagura M, Saitoh K, Itoh T, Araki H, et al. Ctf4 coordinates the progression of helicase and DNA polymerase alpha. Genes Cells. 2009;14:807–20. doi: 10.1111/j.1365-2443.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- 25.Im J-S, Ki S-H, Farina A, Jung D-S, Hurwitz J, Lee J-K. Assembly of the Cdc45-Mcm2-7-GINS complex in human cells requires the Ctf4/And-1, RecQL4 and Mcm10 proteins. Proc Natl Acad Sci USA. 2009;106:15628–32. doi: 10.1073/pnas.0908039106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka H, Kubota Y, Tsujimura T, Kumano M, Masai H, Takisawa H. Replisome progression complex links DNA replication to sister chromatid cohesion in Xenopus egg extracts. Genes Cells. 2009;14:949–63. doi: 10.1111/j.1365-2443.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhu W, Ukomadu C, Jha S, Senga T, Dhar SK, Wohlschlegel JA, et al. Mcm10 and And-1/CTF4 recruit DNA polymerase alpha to chromatin for initiation of DNA replication. Genes Dev. 2007;21:2288–99. doi: 10.1101/gad.1585607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Rochette PJ, Feyissa EA, Su TV, Liu Y. MCM10 mediates RECQ4 association with MCM2-7 helicase complex during DNA replication. EMBO J. 2009;28:3005–14. doi: 10.1038/emboj.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lisby M, Barlow JH, Burgess RC, Rothstein R. Choreography of the DNA Damage Response: Spatiotemporal Relationships among Checkpoint and Repair Proteins. Cell. 2004;118:699–713. doi: 10.1016/j.cell.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Kumagai A, Dunphy WG. Claspin, a Chk1-Regulatory Protein, Monitors DNA Replication on Chromatin Independently of RPA ATR, and Rad17. Mol Cell. 2003;11:329–40. doi: 10.1016/s1097-2765(03)00045-5. [DOI] [PubMed] [Google Scholar]
- 31.Andegeko Y, Moyal L, Mittelman L, Tsarfaty I, Shiloh Y, Rotman G. Nuclear retention of ATM at sites of DNA double strand breaks. J Biol Chem. 2001;276:38224–30. doi: 10.1074/jbc.M102986200. [DOI] [PubMed] [Google Scholar]
- 32.Shiotani B, Zou L. Single-stranded DNA orchestrates an ATM-to-ATR switch at DNA breaks. Mol Cell. 2009;33:547–58. doi: 10.1016/j.molcel.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shroff R, Arbel-Eden A, Pilch D, Ira G, Bonner WM, Petrini JH, et al. Distribution and Dynamics of Chromatin Modification Induced by a Defined DNA Double-Strand Break. Curr Biol. 2004;14:1703–11. doi: 10.1016/j.cub.2004.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou L, Elledge SJ. Sensing DNA Damage Through ATRIP Recognition of RPA-ssDNA Complexes. Science. 2003;300:1542–8. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 35.Yoo HY, Kumagai A, Shevchenko A, Dunphy WG. The Mre11-Rad50-Nbs1 Complex Mediates Activation of TopBP1 by ATM. Mol Biol Cell. 2009;20:2351–60. doi: 10.1091/mbc.E08-12-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Postow L, Ghenoiu C, Woo EM, Krutchinsky AN, Chait BT, Funabiki H. Ku80 removal from DNA through double strand break-induced ubiquitylation. J Cell Biol. 2008;182:467–79. doi: 10.1083/jcb.200802146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dupre A, Boyer-Chatenet L, Sattler RM, Modi AP, Lee JH, Nicolette ML, et al. A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex. Nat Chem Biol. 2008;4:119–25. doi: 10.1038/nchembio.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garner KM, Pletnev AA, Eastman A. Corrected structure of mirin, a small-molecule inhibitor of the Mre11-Rad50-Nbs1 complex. Nat Chem Biol. 2009;5:129–30. doi: 10.1038/nchembio0309-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoo HY, Kumagai A, Shevchenko A, Dunphy WG. Ataxia-telangiectasia mutated (ATM)-dependent activation of ATR occurs through phosphorylation of TopBP1 by ATM. J Biol Chem. 2007;282:17501–6. doi: 10.1074/jbc.M701770200. [DOI] [PubMed] [Google Scholar]
- 40.Guo ZJ, Dunphy WG. Response of Xenopus Cds1 in cell-free extracts to DNA templates with double-stranded ends. Mol Biol Cell. 2000;11:1535–46. doi: 10.1091/mbc.11.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pacek M, Tutter AV, Kubota Y, Takisawa H, Walter JC. Localization of MCM2-7, Cdc45 and GINS to the Site of DNA Unwinding during Eukaryotic DNA Replication. Mol Cell. 2006;21:581–7. doi: 10.1016/j.molcel.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 42.Yoshizawa-Sugata N, Masai H. Roles of human AND-1 in chromosome transactions in S phase. J Biol Chem. 2009;284:20718–28. doi: 10.1074/jbc.M806711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Formosa T, Nittis T. Dna2 mutants reveal interactions with Dna polymerase alpha and Ctf4, a Pol alpha accessory factor, and show that full Dna2 helicase activity is not essential for growth. Genetics. 1999;151:1459–70. doi: 10.1093/genetics/151.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Araki Y, Kawasaki Y, Sasanuma H, Tye BK, Sugino A. Budding yeast mcm10/dna43 mutant requires a novel repair pathway for viability. Genes Cells. 2003;8:465–80. doi: 10.1046/j.1365-2443.2003.00648.x. [DOI] [PubMed] [Google Scholar]
- 45.Burgers PMJ. Polymerase Dynamics at the Eukaryotic DNA Replication Fork. J Biol Chem. 2009;284:4041–5. doi: 10.1074/jbc.R800062200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Budd ME, Campbell JL. The pattern of sensitivity of yeast dna2 mutants to DNA damaging agents suggests a role in DSB and postreplication repair pathways. Mutat Res-DNA Repair. 2000;459:173–86. doi: 10.1016/s0921-8777(99)00072-5. [DOI] [PubMed] [Google Scholar]
- 47.Stewart JA, Miller AS, Campbell JL, Bambara RA. Dynamic Removal of Replication Protein A by Dna2 Facilitates Primer Cleavage during Okazaki Fragment Processing in Saccharomyces cerevisiae. J Biol Chem. 2008;283:31356–65. doi: 10.1074/jbc.M805965200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chattopadhyay S, Bielinsky AK. Human Mcm10 regulates the catalytic subunit of DNA polymerase-alpha and prevents DNA damage during replication. Mol Biol Cell. 2007;18:4085–95. doi: 10.1091/mbc.E06-12-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricke RM, Bielinsky AK. Mcm10 regulates the stability and chromatin association of DNA polymerase-alpha. Mol Cell. 2004;16:173–85. doi: 10.1016/j.molcel.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Yan S, Michael WM. TopBP1 and DNA polymerase-alpha directly recruit the 9-1-1 complex to stalled DNA replication forks. J Cell Biol. 2009;184:793–804. doi: 10.1083/jcb.200810185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Llorente B, Symington LS. The Mre11 Nuclease Is Not Required for 5’ to 3’ Resection at Multiple HO-Induced Double-Strand Breaks. Mol Cell Biol. 2004;24:9682–94. doi: 10.1128/MCB.24.21.9682-9694.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wasko BM, Holland CL, Resnick MA, Lewis LK. Inhibition of DNA double-strand break repair by the Ku heterodimer in mrx mutants of Saccharomyces cerevisiae. DNA Repair. 2009;8:162–9. doi: 10.1016/j.dnarep.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- 54.Kumagai A, Dunphy WG. Claspin, a novel protein required for the activation of Chk1 during a DNA replication checkpoint response in Xenopus egg extracts. Mol Cell. 2000;6:839–49. doi: 10.1016/s1097-2765(05)00092-4. [DOI] [PubMed] [Google Scholar]
- 55.Kumagai A, Lee J, Yoo HY, Dunphy WG. TopBP1 activates the ATR-ATRIP complex. Cell. 2006;124:943–55. doi: 10.1016/j.cell.2005.12.041. [DOI] [PubMed] [Google Scholar]
- 56.Yoo HY, Shevchenko A, Shevchenko A, Dunphy WG. Mcm2 Is a Direct Substrate of ATM and ATR during DNA Damage and DNA Replication Checkpoint Responses. J Biol Chem. 2004;279:53353–64. doi: 10.1074/jbc.M408026200. [DOI] [PubMed] [Google Scholar]
- 57.Carpenter PB, Mueller PR, Dunphy WG. Role for a Xenopus Orc2-related protein in controlling DNA replication. Nature. 1996;379:357–60. doi: 10.1038/379357a0. [DOI] [PubMed] [Google Scholar]
- 58.Nishiyama A, Muraki K, Saito M, Ohsumi K, Kishimoto T, Ishikawa F. Cell cycle-dependent Xenopus TRF1 recruitment to telomere chromatin regulated by Polo-like kinase. EMBO J. 2006;25:575–84. doi: 10.1038/sj.emboj.7600964. [DOI] [PMC free article] [PubMed] [Google Scholar]


