Abstract
Fenretinide, a synthetic retinoid, is a promising anticancer agent based on many in vitro, animal, and chemoprevention clinical trial studies. However, cells such as HepG2 human liver cancer cells are resistant to the apoptotic effect of fenretinide. Previously, we have shown that fenretinide-induced apoptosis is Nur77 dependent, and the sensitivity of the cancer cells to fenretinide-induced apoptosis is positively associated with cytoplasmic enrichment of Nur77. The goal of current study was to identify means to modulate nuclear export of Nur77 in order to improve the efficacy of fenretinide. Fenretinide treatment deactivated ERK1/2 in Huh7 cells, but activated ERK1/2 in HepG2 cells, which was positively associated with the sensitivity of cells to the apoptotic effect of fenretinide. Neither fenretinide nor ERK1/2 inhibitor PD98059 alone could affect the survival of HepG2 cells, but the combination of both induced cell death and increased caspase 3/7 activity. In fenretinide sensitive Huh7 cells, activation of ERK1/2 by epidermal growth factor (EGF) prevented fenretinide-induced cell death and caspase 3/7 induction. In addition, modulation of ERK1/2 changed the intracellular localization of Nur77. Fenretinide/PD98059-induced cell death of HepG2 cell was positively associated with induction and cytoplasmic location as well as mitochondria enrichment of Nur77. The effect was specific for ERK1/2 because other mitogen activated protein kinases such as P38, Akt, and JNK did not have correlated changes in their phosphorylation levels. Taken together, the current study demonstrates that ERK1/2-modulated Nur77 intracellular location dictates the efficacy of fenretinide-induced apoptosis.
Keywords: fenretinide, Nur77, apoptosis, ERK1/2, ROS, HCC
1. Introduction
Retinoids are essential for development and differentiation and are used to treat and prevent various types of cancers. However, retinoids were prevented from large scale use as anti-carcinogens due to their poor clinical tolerability and toxicity [1]. Fenretinide, a synthetic retinoid with low toxicity and long-term tolerability, is emerging as a promising anticancer agent. The vast majority of studies conducted to date show that fenretinide prevents the carcinogenic process and induces apoptosis in hematological malignancy and solid tumors like breast, neuroblastoma, and bladder as well as head and neck [2, 3]. In addition to inducing apoptosis of cancer cells, fenretinide inhibits angiogenesis [4], improves insulin sensitivity[5], and prevents fibrosis[6]. Thus, fenretinide has a broad spectrum of effects in regulating liver function and disease process. However, certain cancer cells such as HepG2 are resistant to the apoptotic effect of fenretinide [7]. Thus, it is important to improve the efficacy of fenretinide.
Fenretinide-induced apoptosis of human liver cancer cells is Nur77 dependent, and the sensitivity of the cancer cells to fenretinide-induced apoptosis is positively associated with cytoplasmic enrichment of Nur77 [7]. Nur77 (also known as NR4A1, TR3 or NGFI-B), an immediate-early response gene, is rapidly induced by a diverse group of agents, including growth factors, mitogens, phorbol esters, and substances that influence cyclic AMP-dependent synthesis pathways [8]. By interacting with other nuclear receptors such as retinoid x receptor (RXRα), Nur77 can exert pleiotropic biological activities ranging from survival and differentiation to cell death in response to different extracellular stimuli [9]. Translocation of Nur77 from nucleus to mitochondria is the hallmark of the Nur77-mediated apoptotic pathway in cancer cells [10]. Once Nur77 is in the cytoplasm, it targets mitochondria through its interaction with mitochondrial Bcl-2, which in turn induces a conformational change in Bcl-2, unmasking its hidden BH-3 domain. This leads to the conversion of Bcl-2 from an anti-apoptotic to a pro-apoptotic molecule [11].
ERK1/2 plays a crucial role in cell fate ranging from proliferation, migration and differentiation to cell death [12, 13]. ERK1/2 signaling is up regulated in hepatocellular carcinoma (HCC). Molecular targeting of ERK1/2 has a therapeutic effect for HCC treatment [14, 15]. Activation of ERK1/2 signaling is frequently associated with chemotherapeutic drug resistance in HCC and hematopoietic tumors [16, 17]. Our previous study shows that HepG2 cells are resistant to fenretinide-induced apoptosis, but the underlying mechanism is not clear [7]. Thus, the current study examines whether ERK1/2 signaling is involved in the resistance of HCC cells to fenretinide-induced apoptosis.
Our data for the first time provide direct evidence that ERK1/2 deactivation enhances cytoplasmic Nur77 expression level and improves the apoptotic effect of fenretinide in human liver cancer cells. Thus, ERK1/2-modulated Nur77 intracellular location dictates the efficacy of fenretinide-induced apoptosis.
2. Materials and methods
2.1. Reagents
All reagents and chemicals used were from Sigma-Aldrich (St. Louis, MO) unless noted otherwise. CellTiter-Glo® Luminescent Cell Viability Assay Kit and Caspase-Glo® 3/7 Assay Kit were purchased from Promega (Madison, WI). VECTASHIELD Mounting Medium with DAPI was purchased from Vector Laboratories (Burlingame, CA). MitoSOX™ Red and NP40 Cell Lysis Buffer were purchased from Molecular Probes, Invitrogen (Carlsbad, CA). Rabbit polyclonal antibodies for Nur77, rabbit polyclonal anti-PARP and FITC labeled secondary antibody were purchased from Santa Cruz (Santa Cruz, CA). Rabbit polyclonal antibody for mouse monoclonal antibody for Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) and rabbit polyclonal anti-Porin were purchased from Abcam (Cambridge, MA). Rabbit polyclonal antibody specific for p-ERK, p-AKT (Thr308), p-JNK, p-P38, Bax, Bcl-xL, and Bcl-2, and mouse polyclonal antibody specific for Bid were purchased from Cell Signaling (Beverly, MA). Protease and phosphatase inhibitors were purchased from Roche Applied Science (Indianapolis, IN). Fenretinide were dissolved in DMSO at 10 mM as the stock solution and stored at −20°C. PD98059 were dissolved in DMSO at 20 mM as the stock solution and stored at −20°C. Epidermal Growth Factor human (EGF) were dissolved in medium at 0.1mg/ml and stored at −20°C.
2.2. Cell culture and treatment
Huh7 and HepG2 cells were maintained in Dulbecco’s Modification of Eagle’s Medium (DMEM) (Mediatech, Herndon, VA) and Minimum Essential Medium (Mediatech, Herndon, VA), respectively, and supplemented with 10% fetal calf serum (Atlanta Biologicals, Lawrenceville, GA). Cells were plated with approximately 1×106 cells per T-25 flask or 5×104 per well of 24-well plates/4-well chamber slides overnight prior to the treatments. HepG2 Cells were treated by PD98059 (20 μM) and fenretinide (10 μM) for indicated time. Huh7 Cells were treated by EGF (0.2 μg/ml) and fenretinide (10 μM). For combination treatment, HCC cells were treated with PD98059 or EGF for 2 hrs before adding fenretinide. The final concentration of DMSO in the culture medium was 0.1% in all treatments. Fresh medium plus chemical(s) were provided every 24 hrs.
2.3. Apoptosis assay
Caspase 3/7 activity and cell viability was studied by Caspase-Glo® 3/7 kit and CellTiter-Glo® Luminescent Cell Viability assay, respectively (Promega, Madison, WI).
2.4. Western blotting and antibodies
Cells were lysed with NP40 Cell Lysis Buffer (Invitrogen, Carlsbad, CA) including protease and phosphatase inhibitors (Roche Applied Science, Indianapolis, IN). Equal amounts of lysates (50 μg total protein) were electrophoresed on SDS-PAGE and blotted onto PVDF membrane (Bio-Rad, Hercules, CA). The membranes were first incubated with PBS supplemented with 0.1% Tween 20 and 5% nonfat dry milk (PBST-milk) for 1 hr at room temperature to block nonspecific binding. Immunostaining was performed by incubating the membranes with primary antibodies for Nur77, p-ERK, p-AKT, p-JNK, p-P38, Bax, Bcl-xL, Bcl-2, and GAPDH in PBST-milk overnight at 4°C. After three washes, membranes were incubated with appropriate secondary antibody for 1 hr in PBST-milk. The signal was detected using the ECL system SuperSignal West Pico Chemiluminescent Substrates (Pierce, Rockford, IL).
2.5. Confocal microscopy and Western blotting
Huh7 and HepG2 cells were grown in Chamber BD FalconTM Culture slides (BD Biosciences, Bedford MA, USA). Immunofluorescence staining and confocal microscopy was described previously [7]. Preparation of subcellular fraction and performance of western blotting was described previously [7].
2.6. ROS assay
ROS (reactive oxygen species) was detected by flow cytometry using MitoSOX™ Red mitochondrial superoxide indicator (Invitrogen, Carlsbad, CA). Following treatment, medium was replaced with 2.5 μM MitoSOX™ reagent working solution. The cells were incubated for 15 minutes at 37°C in the dark. After the staining, the cells were collected by trypsin and analyzed for MitoSOX™ Red according to the manufacturer’s instruction using a Fluorescence Activated Cell Sorter Calibur (FACSCalibur) (BD Biosciences, San Jose, CA).
2.7. Statistical analysis
Data are presented as mean ± SD. Statistical analysis was performed using Student’s t-test for two-group comparison. Significance was defined by p < 0.05.
3. Results
3.1. Differential effect of fenretinide on ERK1/2 activation in Huh7 and HepG2 cells
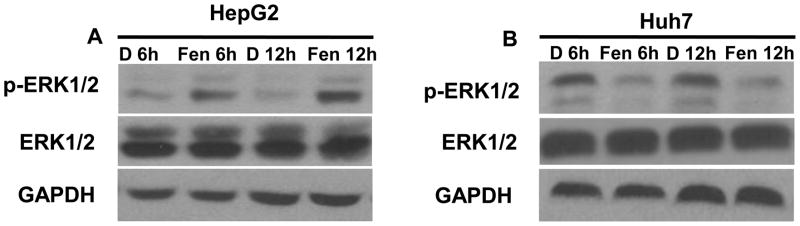
To study the effect of fenretinide on ERK1/2 activation in HCC cells, Huh7 and HepG2 cells were treated with fenretinide (10 μM) for 6 and 12 hrs. Phosphorylation of ERK1/2 was evaluated by Western blotting using antibody against the phosphorylated ERK1/2. The effect of fenretinide on ERK1/2 activation in HCC cells was cell line specific. Fenretinide increased phosphorylated ERK1/2 in the fenretinide resistant HepG2 cells, but decreased phosphorylated ERK1/2 in the fenretinide sensitive Huh7 cells (Figure 1). Thus, the status of ERK1/2 is positively associated with the sensitivity of the cell to fenretinide-induced apoptosis.
Figure 1.
Differential effect of fenretinide on ERK1/2 activation in Huh7 and HepG2 cells. Huh7 and HepG2 cells were treated with fenretinide (10 μM) for 6 and 12 hrs. Phosphorylation of ERK1/2 was analyzed by Western blotting using antibody specific for phosphorylated ERK1/2. Fenretinide increased phosphorylated ERK1/2 in HepG2 cells (A), but decreased phosphorylated ERK1/2 in Huh7 cells (B).
3.2. Modulation of ERK1/2 activity changes apoptotic effect of fenretinide in HCC cells
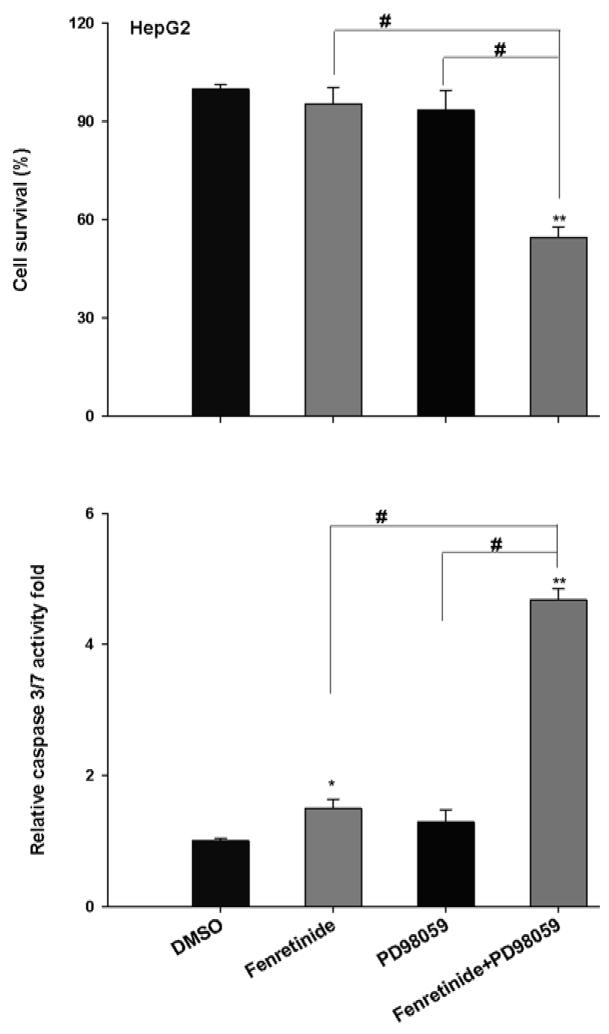
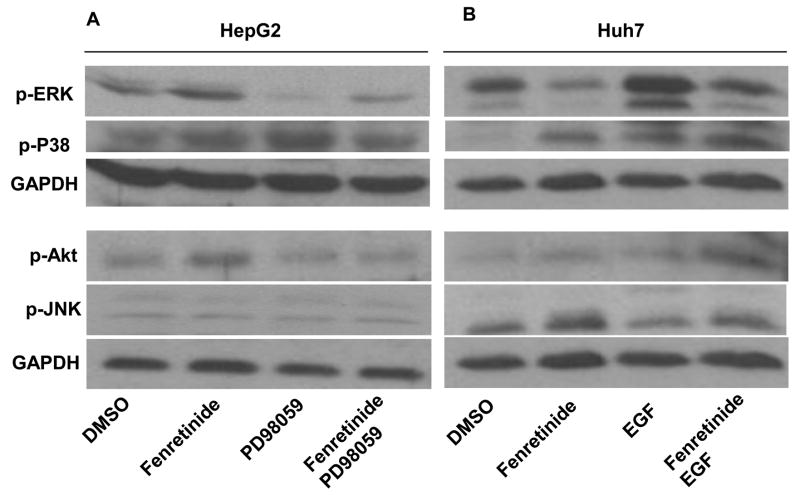
To assess the effect of ERK1/2 on fenretinide-induced apoptosis, MEK inhibitor PD98059 was used in conjunction with fenretinide to treat HepG2 cells. Apoptosis was evaluated by cell survival and caspase 3/7 activity. Neither fenretinide nor PD98059 could induce the death of HepG2 cells. The reduction of viability was only observed in the combination treatment group (Figure 2). Thus, inhibition of ERK1/2 sensitizes HepG2 cells to the apoptotic effect of fenretinide. EGF is a mitogen and can activate ERK1/2 [18, 19]. EGF alone had no effect in regulating Huh7 cell survival. However, fenretinide-induced apoptosis of Huh7 cells was significantly reduced by EGF (Figure 3). Western blots showed that PD98059 and EGF specifically inhibited and activated ERK1/2 activation in HepG2 and Huh7 cells, respectively (Figure 4). p-Akt levels were modestly increased in the conditions where treatment with fenretinide does not induce cell death i.e. fenretinide-treated HepG2 cells and fenretinide/EGF-treated Huh7 cells. The activation status of other mitogen activated protein kinases including P38 and JNK was associated with neither the sensitivity of the cells to fenretinide-induced apoptosis nor PD98059/EGF-modulated effect of fenretinide (Figure 4).
Figure 2.
Inhibition of ERK1/2 sensitizes HepG2 cells to the apoptotic effect of fenretinide. HepG2 cells were seeded onto a 96-well plate and treated with fenretinide (10 μM) or PD98059 (20 uM). For the combination treatment, HepG2 cells were exposed to PD98059 for 2 hrs and then co-exposed to fenretinide and PD98059 for additional 24 hrs. Caspase 3/7 activity and cell survival were determined by Caspase-Glo® 3/7 and CellTiter-Glo® Luminescent Cell Viability Assay (Promega), respectively as described in the Materials and Methods. Data were expressed as mean ± SD from three independent experiments, * *p<0.01, vs. DMSO; #p <0.05.
Figure 3.
Activation of ERK1/2 by EGF protects Huh7 cells from fenretinide-induced apoptosis. Cells were seeded onto a 96-well plate and treated with fenretinide (10 μM) or EGF (0.2 μg/ml) for 24 hrs. For the combination treatment, Huh7 cells were treated with EGF for 2 hrs followed by EGF and fenretinide combination treatment for additional 24 hrs. Caspase 3/7 activity and cell survival were determined by Caspase-Glo® 3/7 and CellTiter-Glo® Luminescent Cell Viability Assay (Promega), respectively as described in the Materials and Methods. Data were expressed as mean ± SD from three independent experiments, **p<0.01, vs. DMSO; #p<0.05.
Figure 4.
Fenretinide differentially regulates ERK1/2 activation in HepG2 (A) and Huh7 (B) cells.
HCC cells were treated as described in figure legends 2 and 3. Proteins were extracted for western blot using specific antibodies as described in the Materials and Methods. GAPDH level was used for loading control. Representative data are shown from three independent experiments.
3.3. ERK1/2 modulates the intracellular localization of Nur77 in HCC cells
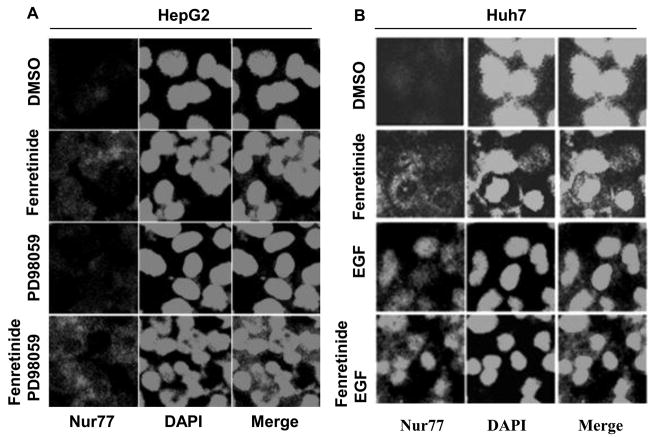
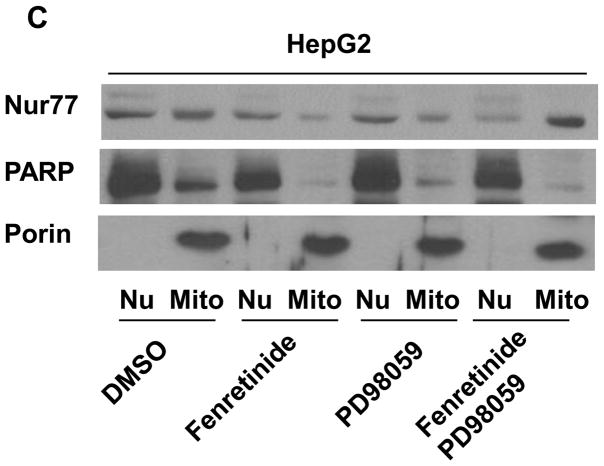
To examine whether fenretinide regulates Nur77 translocation through ERK1/2 pathway in HCC cells, PD98059 and EGF were used to modulate ERK1/2 activity. In fenretinide-resistant HepG2 cells, fenretinide modestly induced Nur77 expression. The expression pattern was diffuse and Nur77 could be detected in nucleus and cytosol. PD98059 had no effect in inducing Nur77 in HepG2 cells. Combination treatment significantly induced cytoplasmic Nur77 in HepG2 cells (Figure 5A). In fenretinide sensitive Huh7 cells, fenretinide alone strikingly induced cytoplasmic Nur77 expression. In contrast to fenretinide, EGF induced nuclear Nur77 expression in Huh7 cells. Addition of fenretinide plus PD98059 induced Nur77 expression in the nucleus as well as the cytosol of Huh7 cells (Figure 5B). To determine the subcellular localization of Nur77 in response to the treatments in HepG2 cells, nuclear- and mitochondria-enriched fractions were isolated. Porin and PARP (Poly (ADP-ribose) polymerase) were used as mitochondrial and nuclear markers, respectively. The data showed that Nur77 was mainly located in the mitochondria-enriched fraction in fenretinide and PD98059 combination treated cells (Figure 5C). In addition, nuclear localization of Nur77 was associated with the survival of HepG2 cells (Fig. 5C). Taken together, the intracellular location of Nur77 is positively associated with the apoptotic effect caused by fenretinide in the presence or absence PD98059 or EGF.
Figure 5.
Modulation of ERK1/2 activation changes the intracellular localization of Nur77. HepG2 (A) and Huh7 (B) cells were treated as described in figure legends 2 and 3, respectively for 24 hrs. Immunofluorescence staining was performed using anti-Nur77 antibody and nuclear counterstaining with DAPI and viewed by confocal microscopy. Representative images of three independent experiments are shown. (C) Nuclear (Nu) and Mitochondria (Mit) enriched fractions were isolated from treated HepG2 cells. Proteins were fractionated followed by western blot using antibodies specific to Nur77, PARP, and Porin.
3.4. The expression levels of anti-apoptotic and pro-apoptotic protein were not associated with the apoptotic effect induced by fenretinide and/or PD98059/EGF treatment
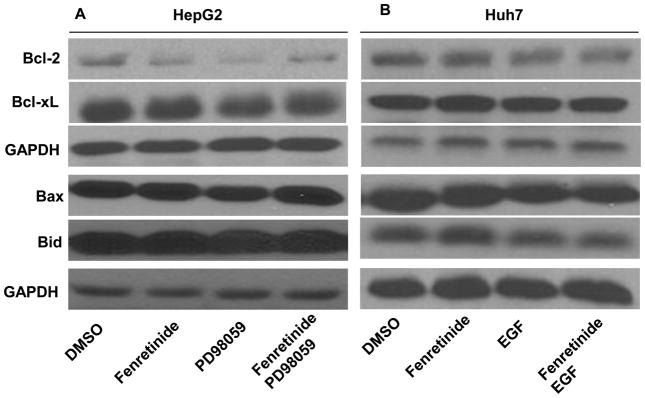
Study was performed to investigate the effect of fenretinide, PD98059, or EGF on the expression of anti-apoptotic (Bcl-2 and Bcl-xL) and pro-apoptotic (Bax and Bid) protein. Western blot analysis showed that Bcl-2 was modestly reduced, but the levels of Bcl-xL, Bax as well as Bid were not changed in HepG2 cells after fenretinide or PD98059 treatments (Figure 6A). In addition, neither fenretinide nor EGF regulated Bcl-2, Bcl-xL, Bax, and Bid in Huh7 cells (Figure 6B). Thus, the expression levels of anti-apoptotic (Bcl-2 and Bcl-xL) and pro-apoptotic (Bax and Bid) proteins were not associated with the apoptotic effect of fenretinide and/or PD98059 and EGF treatments in HCC cells.
Figure 6.
The expression levels of Bcl-2, Bcl-xL, Bax, and Bid are not associated with the apoptotic effect induced by fenretinide, PD98059, or EGF treatments. HepG2 (A) and Huh7 (B) cells were treated as described in figure legends 2 and 3, respectively. Proteins were extracted for western blot using specific antibodies as described in the Materials and Methods. GAPDH level was used for loading control. Representative data are shown from three independent experiments.
3.5. PD98059 or EGF did not affect fenretinide-generated ROS in HCC cells
To evaluate whether ROS generation was involved in fenretinide and/or PD98059 and EGF-regulated apoptosis of liver cancer cells, ROS production was evaluated by flow cytometry using MitoSOX™ Red mitochondrial superoxide indicator (Invitrogen). In HepG2 cells, increased production of ROS was not detected in any treatment groups (Figure 7A). In Huh7 cells, the mitochondrial ROS generation was modestly increased (Figure 7B). Although EGF prevented fenretinide-induced apoptosis, it did not prevent fenretinide-induced ROS production. Thus, there was no correlation between ROS production and apoptosis. Taken together, ROS generation does not seem to have an important role in fenretinide/PD98059 or EGF regulated apoptosis of HCC cells; instead, other pathways such as induction and nuclear export of Nur77 may play a more significant role.
Figure 7.
Modulation ERK1/2 activity does not affect fenretinide-generated ROS in HCC cells. HepG2 (A) and HuH7 (B) cells were treated as described in figure legends 2 and 3 for 6 hrs followed by staining using MitoSOX™ Red mitochondrial superoxide indicator. Cells were analyzed by flow cytometry. The results were generated from three independent experiments. *p<0.05 vs. DMSO.
4. Discussion
Our previously published data and current findings indicate that Nur77 nuclear export is essential for fenretinide-induced apoptosis of HCC cells. In the present study, we show that ERK1/2 activation modulates Nur77 intracellular location, which in turns dictates the efficacy of fenretinide in inducing apoptosis of liver cancer cells. Surprisingly, ROS production, which is the key apoptogenic feature of fenretinide-induced apoptosis [2], does not seem to play an important role in fenretinide/ERK1/2-modulated liver cancer cell death. Modulation of ERK1/2 activity does not affect ROS production, but can change the effect of fenretinide in inducing apoptosis, further suggesting that Nur77 targeting of the mitochondria is a more important mechanism by which fenretinide induces apoptosis. Whether fenretinide-generated ROS, which leads to apoptosis, is cell type-specific remains to be investigated. Nur77 nuclear export is also involved in N-butylidenephthalide-induced apoptosis in HepG2 and J5 hepatocellular carcinoma cells using a xenograft model [20]. In addition, induction of nuclear Nur77 expression level by hepatitis B virus X protein may play a role in viral carcinogenesis [21]. Interrupting Nur77 intracellular translocation-mediated apoptosis sustain the survival of HCC cells [22]. These findings point to the importance of Nur77 in liver carcinogenesis and can be used as a target for liver cancer treatment.
The apoptosis-associated nuclear export of Nur77 is regulated by its heterodimerization with RXRα observed in many types of cancer cells [23]. In PC12 phaeochromocytoma cells, the RXRα translocates with Nur77 from nucleus to cytoplasm in response to nerve growth factor treatment [24] and apoptotic stimuli such as TPA [25]. The RXRα is also required for Nur77 nuclear export in response to IGFBP-3 [26]. In addition, RXRα prevents EGF-induced nuclear accumulation of Nur77 [27]. RXRα-modulated Nur77 subcellular localization is ligand-dependent as the activation of RXRα nuclear export signaling is conformationally regulated by ligand binding [25]. However, regardless of the sensitivity of the cell to the apoptotic effect of fenretinide, fenretinide consistently decreases RXRα protein level in both Huh7 and HepG2 cells (our unpublished data). Thus, RXRα may not be responsible for Nur77 nuclear export in these two HCC cell lines. Our previous publication shows that fenretinide can activate RARβ-mediated pathway and fenretinide-induced apoptosis is RARβ dependent [28]. It has been shown that PD153035, which is a tyrosine kinase inhibitor and an upstream of ERK1/2 pathway, can inhibit the growth of ovarian carcinoma cells through inhibiting EGF receptor activation and inducing RARβ [29, 30]. It is a possibility that ERK1/2 is involved in RARβ mediated pathway to exert the apoptotic effect of fenretinide in HCC cells. Additional experiment is needed to prove this.
ERK1/2 pathway plays an important role in regulating Nur77 function through phosphorylation of Nur77 [31]. ERK1/2 activation and deactivation both contribute to cell death, activated ERK1/2 pathway contributes to phosphorylation of Nur77 and apoptosis in human T cells and embryonic kidney FLP 293 cells [32, 33]. EGF inhibits glutamate-induced neuronal cell death by blocking nuclear export of Nur77 through activating ERK1/2 pathway [27, 34]. Consistent with the findings, our data also show that deactivation of ERK1/2 sensitizes fenretinide-resistant HepG2 cells to become susceptible to fenretinide-induced apoptosis. In addition, the increased sensitivity is due to Nur77 nuclear export. Taken together, activation of ERK1/2 retains the transcriptional function exerted by fenretinide, which is due to nuclear localization of the receptors and thus apoptosis does not occur; whereas deactivation of ERK1/2, which results in nuclear export of Nur77, leads to apoptosis. Furthermore, because the expression levels of apoptotic and anti-apoptotic protein were not associated with the apoptotic effect of fenretinide and/or PD98059 or EGF treatments, the deactivation of ERK1/2 and Nur77-induced apoptosis was most likely due to conformational change of apoptotic-related protein such as Bcl2. Taken together, the efficacy of fenretinide can be improved by ERK1/2 deactivation and this is due to increased Nur77 nuclear export. Combination treatment using fenretinide and ERK1/2 inhibitor may be an effective way to induce the death of liver cancer cell.
Acknowledgments
This work was supported by grants funded by NIH (CA 53596 and P20RR021940 Molecular Biology Core), the National Natural Science Foundation of China (No.81001109) and Research Scholar project from Bureau of Education of Guangzhou Municipality (10A015G). The authors thank Ms. Zoe Raglow for editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moon RC, Mehta RG. Chemoprevention of experimental carcinogenesis in animals. Prev Med. 1989;18:576–91. doi: 10.1016/0091-7435(89)90031-5. [DOI] [PubMed] [Google Scholar]
- 2.Hail N, Jr, Kim HJ, Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis. 2006;11:1677–94. doi: 10.1007/s10495-006-9289-3. [DOI] [PubMed] [Google Scholar]
- 3.Wu JM, DiPietrantonio AM, Hsieh TC. Mechanism of fenretinide (4-HPR)-induced cell death. Apoptosis. 2001;6:377–88. doi: 10.1023/a:1011342220621. [DOI] [PubMed] [Google Scholar]
- 4.Sogno I, Vene R, Ferrari N, De Censi A, Imperatori A, Noonan DM, et al. Angioprevention with fenretinide: targeting angiogenesis in prevention and therapeutic strategies. Crit Rev Oncol Hematol. 2010;75:2–14. doi: 10.1016/j.critrevonc.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Preitner F, Mody N, Graham TE, Peroni OD, Kahn BB. Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am J Physiol Endocrinol Metab. 2009;297:E1420–9. doi: 10.1152/ajpendo.00362.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian J, Zhang JS, Wang XQ, Ji JL, Mei S. Fenretinide stimulates the apoptosis of hepatic stellate cells and ameliorates hepatic fibrosis in mice. Hepatol Res. 2009;39:1229–47. doi: 10.1111/j.1872-034X.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Bushue N, Bu P, Yvonne Wan YJ. Induction and intracellular localization of Nur77 dictate fenretinide-induced apoptosis of human liver cancer cells. Biochem Pharmacol. 2010;79:948–54. doi: 10.1016/j.bcp.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsu HC, Zhou T, Mountz JD. Nur77 family of nuclear hormone receptors. Curr Drug Targets Inflamm Allergy. 2004;3:413–23. doi: 10.2174/1568010042634523. [DOI] [PubMed] [Google Scholar]
- 9.Zhang XK. Targeting Nur77 translocation. Expert Opin Ther Targets. 2007;11:69–79. doi: 10.1517/14728222.11.1.69. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–64. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 11.Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–40. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 12.von Kriegsheim A, Baiocchi D, Birtwistle M, Sumpton D, Bienvenut W, Morrice N, et al. Cell fate decisions are specified by the dynamic ERK interactome. Nat Cell Biol. 2009;11:1458–64. doi: 10.1038/ncb1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida T, Hisamoto T, Akiba J, Koga H, Nakamura K, Tokunaga Y, et al. Spreds, inhibitors of the Ras/ERK signal transduction, are dysregulated in human hepatocellular carcinoma and linked to the malignant phenotype of tumors. Oncogene. 2006;25:6056–66. doi: 10.1038/sj.onc.1209635. [DOI] [PubMed] [Google Scholar]
- 15.Yip-Schneider MT, Klein PJ, Wentz SC, Zeni A, Menze A, Schmidt CM. Resistance to mitogen-activated protein kinase kinase (MEK) inhibitors correlates with up-regulation of the MEK/extracellular signal-regulated kinase pathway in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2009;329:1063–70. doi: 10.1124/jpet.108.147306. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Zhou X, Shen H, Wang D, Wang Y. Phosphorylated ERK is a potential predictor of sensitivity to sorafenib when treating hepatocellular carcinoma: evidence from an in vitro study. BMC Med. 2009;7:41. doi: 10.1186/1741-7015-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexia C, Lasfer M, Groyer A. Role of constitutively activated and insulin-like growth factor-stimulated ERK1/2 signaling in human hepatoma cell proliferation and apoptosis: evidence for heterogeneity of tumor cell lines. Ann N Y Acad Sci. 2004;1030:219–29. doi: 10.1196/annals.1329.028. [DOI] [PubMed] [Google Scholar]
- 18.Ji H, Wang J, Nika H, Hawke D, Keezer S, Ge Q, et al. EGF-induced ERK activation promotes CK2-mediated disassociation of alpha-Catenin from beta-Catenin and transactivation of beta-Catenin. Mol Cell. 2009;36:547–59. doi: 10.1016/j.molcel.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Choi JH, Lim HI, Lee SK, Kim WW, Cho S, et al. EGF-induced MMP-9 expression is mediated by the JAK3/ERK pathway, but not by the JAK3/STAT-3 pathway in a SKBR3 breast cancer cell line. Cell Signal. 2009;21:892–8. doi: 10.1016/j.cellsig.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 20.Chen YL, Jian MH, Lin CC, Kang JC, Chen SP, Lin PC, et al. The induction of orphan nuclear receptor Nur77 expression by n-butylenephthalide as pharmaceuticals on hepatocellular carcinoma cell therapy. Mol Pharmacol. 2008;74:1046–58. doi: 10.1124/mol.107.044800. [DOI] [PubMed] [Google Scholar]
- 21.Lee MO, Kang HJ, Cho H, Shin EC, Park JH, Kim SJ. Hepatitis B virus X protein induced expression of the Nur77 gene. Biochem Biophys Res Commun. 2001;288:1162–8. doi: 10.1006/bbrc.2001.5910. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Hu L, Chan TH, Tsao GS, Xie D, Huo KK, et al. Chromodomain helicase/adenosine triphosphatase DNA binding protein 1-like (CHD1l) gene suppresses the nucleus-to-mitochondria translocation of nur77 to sustain hepatocellular carcinoma cell survival. Hepatology. 2009;50:122–9. doi: 10.1002/hep.22933. [DOI] [PubMed] [Google Scholar]
- 23.Moll UM, Marchenko N, Zhang XK. p53 and Nur77/TR3 - transcription factors that directly target mitochondria for cell death induction. Oncogene. 2006;25:4725–43. doi: 10.1038/sj.onc.1209601. [DOI] [PubMed] [Google Scholar]
- 24.Katagiri Y, Takeda K, Yu ZX, Ferrans VJ, Ozato K, Guroff G. Modulation of retinoid signalling through NGF-induced nuclear export of NGFI-B. Nat Cell Biol. 2000;2:435–40. doi: 10.1038/35017072. [DOI] [PubMed] [Google Scholar]
- 25.Cao X, Liu W, Lin F, Li H, Kolluri SK, Lin B, et al. Retinoid X receptor regulates Nur77/TR3-dependent apoptosis [corrected] by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol. 2004;24:9705–25. doi: 10.1128/MCB.24.22.9705-9725.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KW, Ma L, Yan X, Liu B, Zhang XK, Cohen P. Rapid apoptosis induction by IGFBP-3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRalpha/Nur77. J Biol Chem. 2005;280:16942–8. doi: 10.1074/jbc.M412757200. [DOI] [PubMed] [Google Scholar]
- 27.Jacobs CM, Paulsen RE. Crosstalk between ERK2 and RXR regulates nuclear import of transcription factor NGFI-B. Biochem Biophys Res Commun. 2005;336:646–52. doi: 10.1016/j.bbrc.2005.08.143. [DOI] [PubMed] [Google Scholar]
- 28.Bu P, Wan YJ. Fenretinide-induced apoptosis of Huh-7 hepatocellular carcinoma is retinoic acid receptor beta dependent. BMC Cancer. 2007;7:236. doi: 10.1186/1471-2407-7-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bos M, Mendelsohn J, Kim YM, Albanell J, Fry DW, Baselga J. PD153035, a tyrosine kinase inhibitor, prevents epidermal growth factor receptor activation and inhibits growth of cancer cells in a receptor number-dependent manner. Clin Cancer Res. 1997;3:2099–106. [PubMed] [Google Scholar]
- 30.Grunt TW, Tomek K, Wagner R, Puckmair K, Kainz B, Runzler D, et al. Upregulation of retinoic acid receptor-beta by the epidermal growth factor-receptor inhibitor PD153035 is not mediated by blockade of ErbB pathways. J Cell Physiol. 2007;211:803–15. doi: 10.1002/jcp.20990. [DOI] [PubMed] [Google Scholar]
- 31.Wingate AD, Arthur JS. Post-translational control of Nur77. Biochem Soc Trans. 2006;34:1107–9. doi: 10.1042/BST0341107. [DOI] [PubMed] [Google Scholar]
- 32.Cottrell GS, Padilla BE, Amadesi S, Poole DP, Murphy JE, Hardt M, et al. Endosomal endothelin-converting enzyme-1: a regulator of beta-arrestin-dependent ERK signaling. J Biol Chem. 2009;284:22411–25. doi: 10.1074/jbc.M109.026674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang A, Rud J, Olson CM, Jr, Anguita J, Osborne BA. Phosphorylation of Nur77 by the MEK-ERK-RSK cascade induces mitochondrial translocation and apoptosis in T cells. J Immunol. 2009;183:3268–77. doi: 10.4049/jimmunol.0900894. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs CM, Boldingh KA, Slagsvold HH, Thoresen GH, Paulsen RE. ERK2 prohibits apoptosis-induced subcellular translocation of orphan nuclear receptor NGFI-B/TR3. J Biol Chem. 2004;279:50097–101. doi: 10.1074/jbc.M409145200. [DOI] [PubMed] [Google Scholar]