Abstract
Purpose
To evaluate the safety and feasibility of the addition of pamidronate to chemotherapy for treatment of osteosarcoma.
Patients and Methods
We treated 40 patients with osteosarcoma with cisplatin, doxorubicin, and methotrexate with the addition of pamidronate 2 mg/kg/dose (max dose 90 mg) monthly for 12 doses. We evaluated survival, event-free survival (EFS), and durability of orthopedic reconstruction.
Results
For patients with localized disease, EFS at 5 years was 72% and survival 93%. For patients with metastatic disease, EFS at 5 years was 45% and survival 64%. Ototoxicity and nephrotoxicity were not significantly different from patients treated with chemotherapy without pamidronate. 13 of 14 uncemented implants demonstrated successful osteointegration. Among allograft reconstructions we observed 2 graft failures, 4 delayed unions and 6 successful grafts. Overall, 5 of 33 reconstructions failed. We observed no stress fractures or growth disturbances.
Conclusions
We can safely incorporate pamidronate with chemotherapy for the treatment of osteosarcoma. It does not impair the efficacy of chemotherapy. Pamidronate may improve the durability of limb reconstruction.
INTRODUCTION
The identification of effective chemotherapy for the treatment of osteosarcoma (OS) has led to significant improvement in patient outcome [1–2]. There are only four chemotherapy agents widely accepted to have efficacy against osteosarcoma: doxorubicin, cisplatin, high dose methotrexate (HDMTX), and ifosfamide [3–7]. The Children’s Oncology Group (COG) performed a randomized multi-institution cooperative trial to test the benefit of the addition of ifosfamide to a regimen of cisplatin, doxorubicin, and HDMTX [8]. The addition of ifosfamide did not result in improved event free survival (EFS) or survival. There is a continued need for new therapeutic approaches.
Bisphosphonates are analogues of endogenous pyrophosphates. In vivo, bisphosphonates bind strongly to hydroxyapatite on bone surfaces and are delivered to sites of increased bone formation or resorption. They are widely used to treat hypercalcemia of malignancy. Initially, it was felt that the mechanism of action of bisphosphonates was exclusively to stabilize bone. More recently it has become apparent that bisphosphonates have direct effects on tumor cells [9]. In vitro, bisphosphonate treatment of myeloma cells leads to growth inhibition and induction of apoptosis [10]. Bisphosphonates induce apoptosis in human breast cancer cell lines [11]. Bisphosphonates appear to inhibit adhesion of tumor cells to bone matrix [12]. Bisphosphonates may also inhibit matrix metalloproteinases which are used by tumor cells to invade tissue and establish metastases [13].
Numerous in vitro and xenograft studies support the concept that bisphosphonates have activity against osteosarcoma, alone or in combination with chemotherapy. Investigators have studied human and animal cell lines and spontaneous osteosarcoma arising in murine models. They have studied alendronate, clodronate, minodronate, pamidronate, and zoledronate [14–35] (Table 1). Oligonucleotide microarray assays of human osteosarcoma offer additional support for the concept of using bisphosphonates in osteosarcoma. Expression profiling of 30 osteosarcoma tumors from patients identified 104 genes differentially expressed between favorable and unfavorable responses to chemotherapy [36]. A striking finding was the significant decrease in osteoprotegerin, an osteoclastogenesis inhibitory factor. Additional genes involved in osteoclastogenesis and bone resorption, which were statistically different, include annexin 2, SMAD, PLA2G2A, and TGFbeta1. ECM remodeling genes include desmoplakin, SPARCL1, biglycan, and PECAM. Overexpression of desmoplakin (p=0.008), PECAM (p=0.028) and SPARCL1 (p=0.00098) were associated with a favorable chemotherapy response whereas overexpression of annexin2 (p=0.05), biglycan (p=0.025) and PLA2G2A (p=0.025) were associated with an unfavorable chemotherapy response. This suggests the interaction of the tumor with the microenvironment is a determinant of response to chemotherapy. Bisphosphonates may have activity through disruption of these interactions.
Table 1.
Preclinical investigation of bisphosphonates in osteosarcoma
| Author | Year | Bisphosphonate | Chemotherapy | Model system | Reference |
|---|---|---|---|---|---|
| Cheng | 2004 | aledronate | human lines in vitro | [16] | |
| Farese | 2004 | aledronate | dog lines in vitro | [19] | |
| Molinuevo | 2007 | aledronate | rat cell line in vitro | [29] | |
| Heikkila | 2003 | clodronate | human lines in vitro | [20] | |
| Kubo | 2006 | minodronate | human in vitro/xenograft | [27] | |
| Kubo | 2008 | minodronate | doxo | human in xenograft | [26] |
| Mackie | 2001 | pamidronate | rat cell line in vitro | [28] | |
| Sonnemann | 2001 | pamidronate | human lines in vitro | [35] | |
| Ashton | 2005 | pamidronate | dog lines in vitro | [14] | |
| Murayama | 2008 | risedronate | carbo doxo vcr etop | human lines in vitro | [31] |
| Evdokiou | 2003 | zoledronate | human lines in vitro | [18] | |
| Heymann | 2005 | zoledronate | ifosfamide | rat cell line in vivo | [21] |
| Ory | 2005 | zoledronate | mouse cell line in vivo | [33] | |
| Horie | 2006 | zoledronate | mouse cell line in vitro | [23] | |
| Kubista | 2006 | zoledronate | human lines in vitro | [25] | |
| Benassi | 2007 | zoledronate | cisplatin | human lines in vitro | [15] |
| Dass | 2007 | zoledronate | human lines in xenograft | [17] | |
| Horie | 2007 | zoledronate | doxo paclitaxel gem | mouse cell line in vitro | [22] |
| Iguchi | 2007 | zoledronate | human lines in vitro | [24] | |
| Muraro | 2007 | zoledronate | human lines in vitro | [30] | |
| Ory | 2007 | zoledronate | human/rat lines in vitro | [32] | |
| Ory | 2008 | zoledronate | human/rat lines in vitro | [34] | |
| Koto | 2009 | zoledronate | mouse spontaneous os | [62] |
Osteosarcoma is most common in the second decade of life. Pamidronate had been the most widely used bisphosphonate in children and young adults (Table 2). Pamidronate has been used to treat cancer-related hypercalcemia in children [37–39] Pamidronate has been used to treat fibrous dysplasia in children with the McCune-Albright syndrome [40]. Pamidronate has been used to treat a teenager with multifocal Langerhans cell histiocytosis (27). Pamidronate has been used to treat osteogenesis imperfecta, including some very young children [41–42]. Pamidronate has been given to children with osteoporosis resulting from a variety of etiologies [43]. Pamidronate has been safely given simultaneously with chemotherapy to children with acute lymphocytic leukemia (ALL) [44]. Although other bisphosphonates were in clinical use, this drove our selection of this agent for this clinical trial.
Table 2.
Pamidronate experience in pediatrics
| Year | Indication | Age Range | Dose | Schedule | Reference |
|---|---|---|---|---|---|
| 1997 | Hypercalcemia | 4 | [37] | ||
| 1998 | Hypercalcemia | 2 – 15 | 1 mg/kg | [39] | |
| 2001 | Hypercalcemia | 5 | 1 mg/kg | [38] | |
| 2000 | Fibrous dysplasia | 0.5–1 mg/kg qd ×3 days |
q6months | [40] | |
| 2001 | Langerhans cell histiocytosis | 14 | 2 mg/kg qd × 3 days |
q1month | [63] |
| 2002 | Osteogenesis imperfecta | 1 – 16 | 0.25–1 mg/kg qd × 3 days |
q2-4months | [41] |
| 2001 | ALL | 3 – 16 | 1 mg/kg qd × 3 days |
q3months | [44] |
The purpose of this trial was to determine if pamidronate can safely be given in conjunction with chemotherapy to young patients with osteosarcoma. We wished to determine the feasibility of this approach and to determine if the combination of chemotherapy with pamidronate results in increased toxicity, or decreased tumor efficacy. We wished to assess the ability of pamidronate to improve the survival of endoprosthetic reconstruction.
PATIENTS AND METHODS
Patients
We offered participation in the clinical trial to all patients presenting with newly diagnosed previously untreated high grade osteosarcoma. This clinical trial was approved by the Memorial Hospital Institutional Review Board. Patients were eligible if they had adequate renal, hepatic, hematopoietic, and cardiac function. Exclusion criteria included prior treatment for any cancer, prior history of Paget’s disease, prior history of pericarditis, myocarditis, or cardiac conduction abnormalities, and pregnancy or lactation. All patients or their guardians were required to provide written informed consent. Since the primary aim of the study was to evaluate the safety and feasibility of contemporaneous administration of pamidronate and chemotherapy, patients with both localized and metastatic osteosarcoma were eligible for participation.
We enrolled 40 patients on study (Table 3). 29 patients presented without clinically detectable metastatic disease and 11 patients had clinically detectable metastatic disease at initial presentation. Patients ranged in age from 7 to 36 with a median age of 15. There were 20 males and 20 females. Two patients had a pathological fracture, each successfully treated with limb preservation.
Table 3.
Patient characteristics
| Age | Gender | Primary site | Site group | Histology | Stage | Pathologic fracture |
|---|---|---|---|---|---|---|
| 7 | male | distal femur | lower | osteoblastic | Non Metastatic | no |
| 7 | female | distal femur | lower | chondroblastic | Non Metastatic | no |
| 9 | female | proximal tibia | lower | osteoblastic | Metastatic | yes |
| 10 | female | distal femur | lower | osteoblastic chondroblastic |
Non Metastatic | no |
| 10 | female | distal femur | lower | small cell | Metastatic | no |
| 11 | male | proximal tibia | lower | osteoblastic chondroblatic |
Non Metastatic | no |
| 11 | female | distal femur | lower | osteoblastic | Non Metastatic | no |
| 12 | female | distal femur | lower | osteoblastic chondroblastic |
Metastatic | no |
| 12 | male | sacrum | axial | chondroblastic | Non Metastatic | no |
| 12 | female | proximal tibia | lower | osteoblastic chondroblastic fibroblastic |
Non Metastatic | no |
| 12 | female | distal femur | lower | osteoblastic chondroblastic |
Non Metastatic | no |
| 13 | female | distal femur | lower | ossteoblastic | Non Metastatic | no |
| 13 | male | distal femur | lower | osteoblastic chondroblastic |
Non Metastatic | no |
| 13 | male | proximal tibia | lower | osteoblastic chondroblastic |
Non Metastatic | no |
| 13 | female | distal femur | lower | chondroblastic | Non Metastatic | no |
| 14 | male | distal femur | lower | osteoblastic | Non Metastatic | no |
| 14 | male | distal femur | lower | chondroblastic | Metastatic | no |
| 15 | male | proximal femur | lower | osteoblastic chondroblastic fibroblastic |
Metastatic | no |
| 15 | male | distal femur | lower | osteoblastic | Non Metastatic | no |
| 15 | male | proximal humerus | upper | osteoblastic | Non Metastatic | no |
| 15 | female | chest wall/rib | axial | osteoblastic chondroblastic |
Non Metastatic | no |
| 15 | female | proximal tibia | lower | osteoblastic chondroblastic |
Non Metastatic | no |
| 15 | female | sacrum | axial | osteoblastic | Non Metastatic | no |
| 15 | male | distal femur | lower | osteoblastic | Metastatic | no |
| 15 | male | proximal tibia | lower | osteoblastic | Non Metastatic | no |
| 16 | female | distal tibia | lower | osteoblastic chondroblastic |
Non Metastatic | no |
| 16 | female | distal tibia | lower | osteoblastic | Non Metastatic | no |
| 16 | male | spine | axial | osteoblastic | Non Metastatic | no |
| 18 | male | proximal tibia | lower | osteoblastic fibroblastic |
Non Metastatic | no |
| 18 | male | ilium | axial | chondroblastic | Metastatic | no |
| 18 | male | distal femur | lower | osteoblastic fibroblastic telangiectatic |
Metastatic | no |
| 20 | female | distal femur | lower | osteoblastic | Metastatic | no |
| 21 | female | ilium | axial | chondroblastic | Metastatic | no |
| 24 | female | maxilla | H&N | osteoblastic chondroblastic fibroblastic |
Non Metastatic | no |
| 26 | female | distal femur | lower | osteoblastic | Non Metastatic | no |
| 29 | male | distal femur | lower | osteoblastic chondroblastic |
Metastatic | yes |
| 29 | male | femoral diaphysis | lower | osteoblastic | Non Metastatic | no |
| 29 | male | distal femur | lower | osteoblastic | Non Metastatic | no |
| 35 | female | proximal humerus | upper | fibroblastic | Non Metastatic | no |
| 36 | male | proximal humerus | upper | fibroblastic | Non Metastatic | no |
Regimen
The treatment plan called for a period of induction, followed by definitive surgical resection of the primary tumor, followed by resection of any pulmonary metastases, followed by a period of maintenance chemotherapy. Chemotherapy consisted of cisplatin 120 mg/m2 administered as a four hour infusion four times with doxorubicin, twice during induction at weeks 0 and 5, and twice during maintenance at weeks 0 and 5. Doxorubicin was administered as a 15–30 minute infusion at a dose of 37.5 mg/m2/day for 2 consecutive days six times, twice during induction at weeks 0 and 5, and four times during maintenance at weeks 0, 5, 10, and 15. We administered dexrazoxane 375 mg/m2 as a 15–30 minute infusion 15 minutes prior to each dose of doxorubicin. The first four courses were administered with cisplatin, twice during induction and twice during maintenance. High dose methotrexate (HDMTX) 12 g/m2 with a maximum dose of 20 g was administered as a four hour infusion followed by leucovorin administration at a dose of 10 mg (not adjusted to body surface area) beginning 24 hours from the initiation of the methotrexate infusion and continuing until the serum methotrexate level was less than 1 × 10−7M (100 nanomolar). Serum methotrexate levels and renal function were monitored daily and hydration, alkalinization, and leucovorin doses were specified in the event of delayed methotrexate excretion [45]. HDMTX was administered twelve times, four times during induction at weeks 3, 4, 8, and 9; and eight times during maintenance at weeks 3,4,8,9,13,14,18, and 19. In an effort to maintain the dose intensity of doxorubicin, the protocol specified that if there were a delay greater than one week between the first and the second of each pair of HDMTX administrations, the second of the pair was to be omitted.
We administered bisphosphonate with chemotherapy agents with potential nephrotoxicity. We chose a dose of 2 mg/kg (maximum dose 90 mg) given in one day as a safe dose. We specified that administration of pamidronate would be separated from administration of cisplatin or high dose methotrexate by at least 72 hours. Pamidronate was administered once each month for a total of 12 doses. The first dose of pamidronate was given during the first cycle of chemotherapy. Pamidronate was administered as a two hour infusion.
Orthopedic reconstruction
Surgery was of curative intent and achieved a wide margin in all but one sacral case where the margin was positive on final pathological review. Reconstructions varied based on the location and circumstances, including patient growth potential, and the presence of metastatic disease. There were 3 amputations and 5 patients who didn’t have any reconstruction. There were 23 prosthetic reconstructions, including 19 pure implants (1 pressfit, 13 Compress, 4 cemented stems) and 4 allograft prosthetic composites (APC) where the stem was cemented into the allograft, and the remaining stem pas press fit into the host bone (3 patients) or cemented into the host bone (one patient). There were 11 allografts, including the 4 APC’s. Two of the intercalary grafts were coupled with vascularized fibular transplants. We fixed the allografts with plates in all cases, and the APC’s also had intramedullary stem transfixation. A vascularized fibula alone was used for one intercalary reconstruction.
Mobilization was based on a standard protocol. Cemented implants were allowed immediate weightbearing. Uncemented implants, including the Compress stems, were protected by toe touch weight-bearing for 6 weeks then half weight- bearing for 6 weeks. APC’s were protected by half weight-bearing for 12 weeks. Allografts and vascularized grafts were protected with half weight-bearing until there was painless radiographic union. The vascularized fibula was then braced for an additional 2 years until graft hypertrophy. Five of the 23 prosthetic reconstructions also included extensible shaft segments. These were lengthened as needed to keep the limb length inequality under one cm. Limb lengths were monitored by physical examination using blocks under foot to level the pelvis and tape measurements from the anterior iliac spine to the medial malleolus. We rarely used scanograms.
Graft failure was defined by removal for any reason, persistent nonunion of 18 months after surgery or 12 months after the conclusion of chemotherapy. Prosthetic failure was defined as the removal of an implant for any reason, or pain with progressive radiolucency. Successful osteointegration was defined by retention of the prosthesis, no pain, no radiolucency, and progressive bone hypertrophy around the implant.
Followup
Patients had followup including height, weight, and growth chart monthly for the first year, every other month for the second year, every third month for the third year, every six months for the fourth and fifth year following completion of chemotherapy and annually thereafter. Followup included chest Xray at every visit, bone scan every three months for the first year and thereafter as clinically indicated. Patients with pulmonary metastasis at initial presentation had CT scans every three months for the first year. Orthopedic followup was performed every three months for the first year, every six months in years 2–5, then annually, and also when clinically indicated. Orthopedic evaluation included plain film of the reconstruction and cross sectional imaging as indicated. We did not perform routine scanograms.
End Points and Statistical Methods
End points were event free survival, survival, toxicity, and success rates for endoprosthetic reconstruction following definitive resection of primary tumor. Actuarial curves were estimated using the Kaplan-Meier method for EFS and survival. Toxicity was monitored with NCI common toxicity criteria, with special attention to hepatotoxicity, ototoxicity, nephrotoxicity, and incidence of osteonecrosis of the jaw. We compared the incidence of grade III and IV adverse events for these selected toxicities in this regimen to the incidence in 390 patients who received the identical chemotherapy regimen without pamidronate in the prospective randomized trial performed by the pediatric cooperative groups [8].
RESULTS
Data were analyzed as of April, 2010. Median followup for the entire cohort of 40 patients, including patients who experienced an event, was 53 months.
EFS and survival
EFS for 29 patients who presented with localized disease was 72% five years from study enrollment (Figure 1). EFS for the 11 patients who presented with clinically detectable metastatic disease was 45% at the same time point. Overall survival for the localized patients at five years was 93%; for patients who presented with metastatic disease overall survival at five years was 64% (Figure 2). We did not observe local recurrence. Two patients developed myelodysplastic syndrome as the first event. All other first events were metastatic recurrence.
Figure 1.
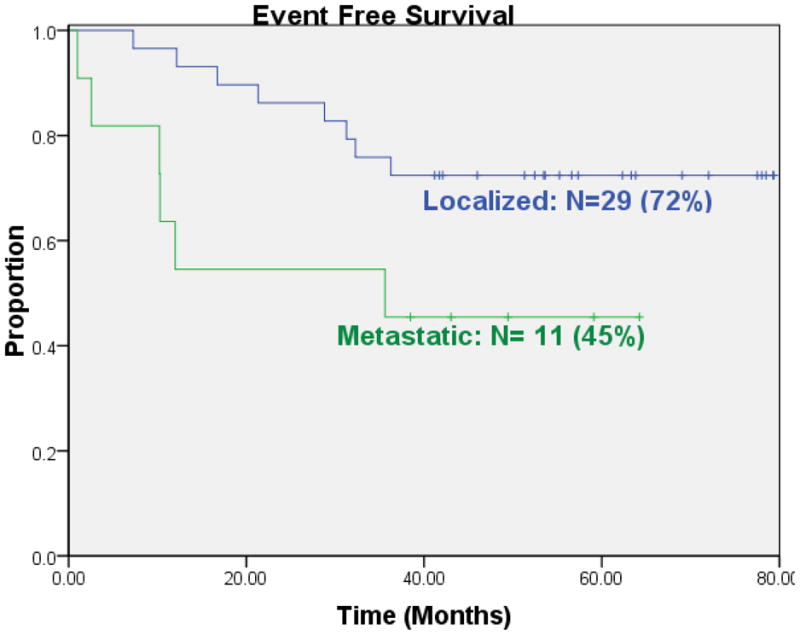
Event free survival for patients who presented with localized or metastatic osteosarcoma at initial presentation.
Figure 2.
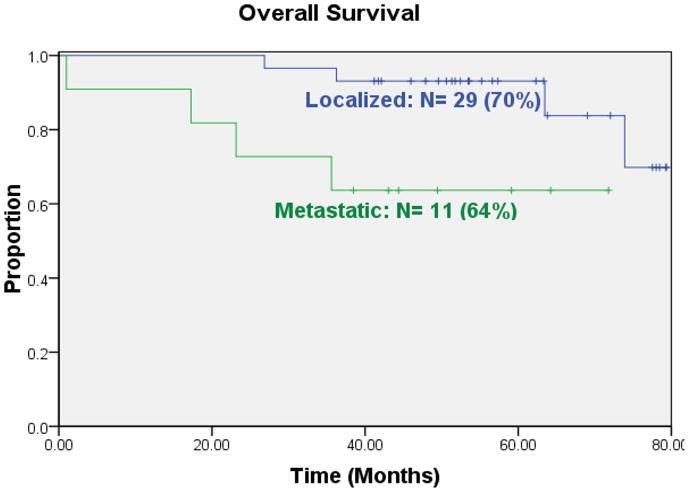
Overall survival for patients who presented with localized or metastatic osteosarcoma at initial presentation.
Toxicity
The toxicities observed in patients in this study were similar to the toxicities observed in patients treated with the same chemotherapy regimen who did not receive pamidronate. Hypocalcemia was common after administration of pamidronate, but only two patients experienced symptoms, including peri-oral numbness and paresthesias of the hands and feet [Table 4]. Symptomatic hypocalcemia responded promptly to oral calcium supplementation and subsequent administration of pamidronate preceded by oral calcium supplementation was not associated with symptoms. With subsequent administration of pamidronate the incidence of hypocalcemia decreased. We observed ototoxicity ≥ grade 3 in 6 of 40 patients (15%, 95% CI: 6%-30%). Ototoxicity ≥ grade 3 was observed in 39 of 390 patients treated with the same chemotherapy regimen without bisphosphonate in the pediatric cooperative group trial (p = 0.29, Fisher’s exact test) [Table 5]. We observed nephrotoxicity ≥ grade 3 in 1 of 40 patients (2.5%, 95% CI: 0.1%–13%). Nephrotoxicity ≥ grade 3 was observed in 8 of 390 patients treated with the same chemotherapy regimen without bisphosphonate in the pediatric cooperative group trial (p = 0.58, Fisher’s exact test)[Table 5]. There were no cases of osteonecrosis of the jaw, either during study therapy or during followup. No patients sustained atypical subtrochanteric or other long bone fractures that have been reported with bone metastasis and osteoporosis patients receiving bisphosphonate therapy for greater than five years.[46]
Table 4.
Hypocalcemia following administration of pamidronate
| Pamidronate Cycle | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calcium nadir mg/dL Mean ± s.d. | 7.0 ± 1.0 | 7.9 ± 0.7 | 8.1 ± 0.7 | 8.2 ± 0.6 | 8.0 ± 0.6 | 8.4 ± 0.3 | 8.4 ± 0.4 | 8.9 ± 0.5 | 8.9 ± 0.5 | 9.2 ± 0.4 | 9.2 ± 0.4 | 9.2 ± 0.5 |
| Hypocalcemia Grade 3/4 | 19 | 5 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Table 5.
Selected toxicity comparison
| Toxicity | Current trial | Intergroup trial | Fisher’s exact test |
|---|---|---|---|
| Ototoxicity ≥ Grade 3 | 6/40 (15%) | 39/390 (10%) | p = 0.29 |
| Nephrotoxicity ≥ Grade 3 | 1/40 (2.5%) | 8/390 (2%) | p = 0.58 |
Orthopedic reconstruction
11 allograft reconstructions included four osteoarticular tibial replacements (all plated, 3 with intramedullary cement), one intercalary femur replacement (plated without cement), two intercalary femoral replacements with intramedullary vascularized fibulas (plated without cement), and four alloprosthetic composites (1 proximal humerus,1 proximal femur, and 2 proximal tibias, all of which had intramedullary prosthetic stems, 2 had supplemental plates, and 1of 4 was cemented into the remaining host bone.) The number of variables among the patients is too great to allow meaningful comparisons. Due to 3–6 month variation in the intervals between extremity films, the time to union results may not be precise. Nevertheless, radiographic union was achieved in 11 of 13 osteosynthesis sites at a mean (SD) of 19.4 (7.2) months. Our impression was that the healing was at least as fast as what was historically seen for similar reconstructions during chemotherapy. Ultimate union and graft retention are more reproducible and clinically meaningful outcomes.
Five reconstructions failed. One (of one) uncemented press fit stems had aseptic loosening and was converted to a cemented stemmed implant that has a stable 2 mm radiolucent line over 1/3 of the stem length, 5years 5 months after implantation. Four allografts failed, two from infection (one exchanged for a cement intercalary spacer and one amputation) and two from nonunion, successfully treated by autogenous bone grafting and exchange from plate to rod fixation. A fifth allograft, part of an APC, had a persistent asymptomatic nonunion that had not failed nor required surgery by the time of the patient’s death of disease, 18 months postop. Of a total of 14 osteosynthesis sites, 9 united.
DISCUSSION
Successful treatment for osteosarcoma requires the combination of effective systemic therapy and surgery to remove all sites of clinically detectable disease. Different combinations of the four active chemotherapy drugs have been used. Large trials from single institutions and cooperative groups have achieved similar outcomes [1, 8, 47–49].
The bisphosphonates are good candidates to employ in the treatment of osteosarcoma. Osteosarcoma cells demonstrate upregulation of many genes whose normal functions are to participate in osteogenesis and whose functions can be inhibited by bisphosphonates. In addition, evidence is accruing that bisphosphonates may have the ability to interfere with the processes used by tumor cells to establish metastases. The treatment of osteosarcoma requires surgical resection of tumor bearing bone and reconstruction of the resected area with metal or bone graft. Osteosarcoma occurs predominantly in young patients and long term survival of the reconstruction is essential. Bisphosphonates may improve the outcome for osteosarcoma by direct anti-tumor effects, decreasing the risk of metastasis, and improving the durability of reconstruction following tumor resection.
Our experience represents a pilot study with a single bisphosphonate, pamidronate. We chose pamidronate because there was prior experience with pamidronate in children with cancer. Newer bisphosphonates such as zoledronate are significantly more potent than pamidronate. There are more data from pre-clinical studies for zoledronate in osteosarcoma than for any other bisphosphonate. Future clinical trials of bisphosphonates in osteosarcoma will almost certainly employ zoledronate. It is important to recognize that while zoledronate is more potent than pamidronate, it is also associated with a greater risk of osteonecrosis of the jaw, a significant risk associated with bisphosphonate therapy.[50] Its risk in young children with deciduous teeth is unknown.
We treated 40 patients with conventional chemotherapy for osteosarcoma and pamidronate. Given the limitations of small patient sample and limited duration of follow-up, we observed no statistically significant increase in toxicity. We saw no osteonecrosis of the jaw. We believe that pamidronate can safely be incorporated into a multi-agent chemotherapy regimen for the treatment of osteosarcoma. If zoledronate replaces pamidronate in the treatment strategy, we will need to acquire similar safety data. We observed EFS and survival for our patients very similar to our prior experience and the published experience in the literature for similar chemotherapy treatment regimens. This was a small single arm study that included both localized and metastatic patients. We cannot draw any firm conclusions about the impact of pamidronate of the efficacy of treatment for osteosarcoma, but it does not appear to have impaired the outcome.
In a series of 108 patients with primary bone sarcoma treated at Memorial Sloan-Kettering Cancer Center (MSKCC), 15 patients (14%) suffered fractures during treatment [51]. These children remain at risk for osteoporosis and insufficiency fractures throughout their lifetimes. Treatment related osteopenia is an under-recognized problem in young patients receiving chemotherapy [52]. In adults with osteoporosis, treatments with bisphosphonates resulted in a 50% decrease in fracture rates after one year of treatment. These adults achieved bone mass gains of 2 – 4% per year during the first four years of treatment. Even more devastating for these patients, pathologic fractures through a tumor can affect survival and local recurrence rates. In a multicenter retrospective matched control review, patients with OS and a pathologic fracture had a 55% 5 year survival rate compared to a survival rate of 77% for patients without fracture. Local recurrence at five years was also increased in the group with pathologic fractures (25% compared to 4%) [53]
Most patients with OS undergo limb preservation surgical resection with insertion of an endoprosthesis. During chemotherapy, ingrowth into the surface of these prostheses is delayed. Aseptic loosening, with poor bone/implant interface contact remains one of the major factors leading to the necessity for implant revision surgery. At MSKCC, 15.8% of prosthetic knee reconstructions require revision for aseptic loosening. Overall prosthesis failure free rates were 82%, 71%, and 50% at 3, 5, and 10 years respectively following initial implantation [54]. At UCLA, 11.6% of hip prostheses required revision for aseptic loosening or fatigue failure, and failure free survival at 7 years was 69% [55]. Even recent uncemented prostheses and implants with novel compression fixation have 12% early failure by 5 years [56] Currently young patients who survive OS can anticipate multiple revisions of their prostheses during their lifetime. Bisphosphonate therapy may contribute to improved prosthetic longevity by several mechanisms including 1. improved bone density and strength, 2. promoting more robust bone ingrowth into porous surfaces of uncemented prostheses, and 3. stabilization of the bone-prosthesis or bone-cement interface retarding osteoclastic bone resorption stimulated by particulate wear debris.[57–61] Bisphosphonates improve the fixation interface and would be predicted to improve failure free implant survival. None of our implants suffered mechanical failure or fracture, and the only aseptic loosening in this series was in an uncemented press fit stem. The 13 Compress stems and the 4 cemented stems experienced no mechanical failure or aseptic loosening.
We observed a high rate of successful osteointegration of endoprosthetic reconstructions and union following allografts. Overall durability of reconstructions has been good. We did not formally evaluate bone density in patients receiving pamidronate; such prospective evaluation in future trials of more potent bisphosphonates such as Zoledronate may provide additional information on the utility of bisphosphonates in patients with sarcomas receiving therapy associated with an increased risk of osteopenia. Again, the limitations of a small single arm study preclude firm conclusions, but these outcomes compares favorably with our prior institutional experience.
Our experience with pamidronate and chemotherapy for the treatment of osteosarcoma suggests that we can safely incorporate pamidronate with chemotherapy. It suggests the efficacy of therapy is comparable to our prior experience. It suggests that bisphosphonates may improve the durability of reconstruction. These results provide feasibility data, support, and justification for a prospective randomized trial of the addition of bisphosphonates to chemotherapy for the treatment of osteosarcoma. It seems appropriate that such a trial should utilize a newer, more potent bisphosphonate than pamidronate, such as zoledronate. The use of zoledronate will require vigilant monitoring
BIBLIOGRAPHY
- 1.Meyers PA, et al. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol. 1992;10(1):5–15. doi: 10.1200/JCO.1992.10.1.5. [DOI] [PubMed] [Google Scholar]
- 2.Link MP, et al. The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosarcoma of the extremity. N Engl J Med. 1986;314(25):1600–6. doi: 10.1056/NEJM198606193142502. [DOI] [PubMed] [Google Scholar]
- 3.Baum ES, et al. Phase II study of cis-dichlorodiammineplatinum(II) in childhood osteosarcoma: Children’s Cancer Study Group Report. Cancer Treat Rep. 1979;63(9–10):1621–7. [PubMed] [Google Scholar]
- 4.Cortes EP, Holland JF, Wang JJ, Sinks LF. Doxorubicin in disseminated osteosarcoma. JAMA. 1972;3144(25):1132–1138. doi: 10.1001/jama.221.10.1132. [DOI] [PubMed] [Google Scholar]
- 5.Jaffe N, et al. Favorable response of metastatic osteogenic sarcoma to pulse high-dose methotrexate with citrovorum rescue and radiation therapy. Cancer. 1973;31(6):1367–73. doi: 10.1002/1097-0142(197306)31:6<1367::aid-cncr2820310611>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Marti C, et al. High-dose ifosfamide in advanced osteosarcoma. Cancer Treat Rep. 1985;69(1):115–7. [PubMed] [Google Scholar]
- 7.Nitschke R, et al. Cis-diamminedichloroplatinum (NSC-119875) in childhood malignancies: a Southwest Oncology Group study. Med Pediatr Oncol. 1978;4(2):127–32. doi: 10.1002/mpo.2950040208. [DOI] [PubMed] [Google Scholar]
- 8.Meyers PA, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival--a report from the Children’s Oncology Group. J Clin Oncol. 2008;26(4):633–8. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 9.Senaratne SG, Colston KW. Direct effects of bisphosphonates on breast cancer cells. Breast Cancer Res. 2002;4(1):18–23. doi: 10.1186/bcr412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shipman CM, et al. Bisphosphonates induce apoptosis in human myeloma cell lines: a novel anti-tumour activity. Br J Haematol. 1997;98(3):665–72. doi: 10.1046/j.1365-2141.1997.2713086.x. [DOI] [PubMed] [Google Scholar]
- 11.Senaratne SG, et al. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer. 2000;82(8):1459–68. doi: 10.1054/bjoc.1999.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boissier S, et al. Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Res. 1997;57(18):3890–4. [PubMed] [Google Scholar]
- 13.Boissier S, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60(11):2949–54. [PubMed] [Google Scholar]
- 14.Ashton JA, et al. Investigation of the effect of pamidronate disodium on the in vitro viability of osteosarcoma cells from dogs. Am J Vet Res. 2005;66(5):885–91. doi: 10.2460/ajvr.2005.66.885. [DOI] [PubMed] [Google Scholar]
- 15.Benassi MS, et al. Growth inhibition and sensitization to cisplatin by zoledronic acid in osteosarcoma cells. Cancer Lett. 2007;250(2):194–205. doi: 10.1016/j.canlet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Cheng YY, et al. Alendronate regulates cell invasion and MMP-2 secretion in human osteosarcoma cell lines. Pediatr Blood Cancer. 2004;42(5):410–5. doi: 10.1002/pbc.20019. [DOI] [PubMed] [Google Scholar]
- 17.Dass CR, Choong PF. Zoledronic acid inhibits osteosarcoma growth in an orthotopic model. Mol Cancer Ther. 2007;6(12 Pt 1):3263–70. doi: 10.1158/1535-7163.MCT-07-0546. [DOI] [PubMed] [Google Scholar]
- 18.Evdokiou A, et al. Induction of cell death of human osteogenic sarcoma cells by zoledronic acid resembles anoikis. Bone. 2003;33(2):216–28. doi: 10.1016/s8756-3282(03)00223-0. [DOI] [PubMed] [Google Scholar]
- 19.Farese JP, et al. The effect of the bisphosphonate alendronate on viability of canine osteosarcoma cells in vitro. In Vitro Cell Dev Biol Anim. 2004;40(3–4):113–7. doi: 10.1290/1543-706x(2004)040<0113:teotba>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Heikkila P, et al. Inhibition of matrix metalloproteinase-14 in osteosarcoma cells by clodronate. J Surg Res. 2003;111(1):45–52. doi: 10.1016/s0022-4804(03)00086-6. [DOI] [PubMed] [Google Scholar]
- 21.Heymann D, et al. Enhanced tumor regression and tissue repair when zoledronic acid is combined with ifosfamide in rat osteosarcoma. Bone. 2005;37(1):74–86. doi: 10.1016/j.bone.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Horie N, et al. Combined effects of a third-generation bisphosphonate, zoledronic acid with other anticancer agents against murine osteosarcoma. Br J Cancer. 2007;96(2):255–61. doi: 10.1038/sj.bjc.6603548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horie N, et al. The third-generation bisphosphonates inhibit proliferation of murine osteosarcoma cells with induction of apoptosis. Cancer Lett. 2006;238(1):111–8. doi: 10.1016/j.canlet.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 24.Iguchi T, et al. Zoledronate-induced S phase arrest and apoptosis accompanied by DNA damage and activation of the ATM/Chk1/cdc25 pathway in human osteosarcoma cells. Int J Oncol. 2007;31(2):285–91. [PubMed] [Google Scholar]
- 25.Kubista B, et al. Anticancer effects of zoledronic acid against human osteosarcoma cells. J Orthop Res. 2006;24(6):1145–52. doi: 10.1002/jor.20129. [DOI] [PubMed] [Google Scholar]
- 26.Kubo T, et al. Efficacy of a nitrogen-containing bisphosphonate, minodronate, in conjunction with a p38 mitogen activated protein kinase inhibitor or doxorubicin against malignant bone tumor cells. Cancer Chemother Pharmacol. 2008;62(1):111–6. doi: 10.1007/s00280-007-0580-y. [DOI] [PubMed] [Google Scholar]
- 27.Kubo T, et al. Inhibitory effects of a new bisphosphonate, minodronate, on proliferation and invasion of a variety of malignant bone tumor cells. J Orthop Res. 2006;24(6):1138–44. doi: 10.1002/jor.20177. [DOI] [PubMed] [Google Scholar]
- 28.Mackie PS, et al. Bisphosphonates regulate cell growth and gene expression in the UMR 106–01 clonal rat osteosarcoma cell line. Br J Cancer. 2001;84(7):951–8. doi: 10.1054/bjoc.2000.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molinuevo MS, Bruzzone L, Cortizo AM. Alendronate induces anti-migratory effects and inhibition of neutral phosphatases in UMR106 osteosarcoma cells. Eur J Pharmacol. 2007;562(1–2):28–33. doi: 10.1016/j.ejphar.2007.01.054. [DOI] [PubMed] [Google Scholar]
- 30.Muraro M, et al. Osteosarcoma cell line growth inhibition by zoledronate-stimulated effector cells. Cell Immunol. 2007;249(2):63–72. doi: 10.1016/j.cellimm.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Murayama T, et al. Efficacy of the third-generation bisphosphonate risedronate alone and in combination with anticancer drugs against osteosarcoma cell lines. Anticancer Res. 2008;28(4B):2147–54. [PubMed] [Google Scholar]
- 32.Ory B, et al. Zoledronic acid activates the DNA S-phase checkpoint and induces osteosarcoma cell death characterized by apoptosis-inducing factor and endonuclease-G translocation independently of p53 and retinoblastoma status. Mol Pharmacol. 2007;71(1):333–43. doi: 10.1124/mol.106.028837. [DOI] [PubMed] [Google Scholar]
- 33.Ory B, et al. Zoledronic acid suppresses lung metastases and prolongs overall survival of osteosarcoma-bearing mice. Cancer. 2005;104(11):2522–9. doi: 10.1002/cncr.21530. [DOI] [PubMed] [Google Scholar]
- 34.Ory B, et al. Farnesyl diphosphate synthase is involved in the resistance to zoledronic acid of osteosarcoma cells. J Cell Mol Med. 2008;12(3):928–41. doi: 10.1111/j.1582-4934.2008.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonnemann J, et al. The bisphosphonate pamidronate is a potent inhibitor of human osteosarcoma cell growth in vitro. Anticancer Drugs. 2001;12(5):459–65. doi: 10.1097/00001813-200106000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Mintz MB, et al. An expression signature classifies chemotherapy-resistant pediatric osteosarcoma. Cancer Res. 2005;65(5):1748–54. doi: 10.1158/0008-5472.CAN-04-2463. [DOI] [PubMed] [Google Scholar]
- 37.Kutluk MT, et al. Childhood cancer and hypercalcemia: report of a case treated with pamidronate. J Pediatr. 1997;130(5):828–31. doi: 10.1016/s0022-3476(97)80030-3. [DOI] [PubMed] [Google Scholar]
- 38.Schmid I, et al. Pamidronate and calcitonin as therapy of acute cancer-related hypercalcemia in children. Klin Padiatr. 2001;213(1):30–4. doi: 10.1055/s-2001-11271. [DOI] [PubMed] [Google Scholar]
- 39.Young G, Shende A. Use of pamidronate in the management of acute cancer-related hypercalcemia in children. Med Pediatr Oncol. 1998;30(2):117–21. doi: 10.1002/(sici)1096-911x(199802)30:2<117::aid-mpo9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Lala R, et al. Pamidronate treatment of bone fibrous dysplasia in nine children with McCune-Albright syndrome. Acta Paediatr. 2000;89(2):188–93. doi: 10.1080/080352500750028816. [DOI] [PubMed] [Google Scholar]
- 41.Rauch F, et al. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. J Clin Invest. 2002;110(9):1293–9. doi: 10.1172/JCI15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glorieux FH, et al. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339(14):947–52. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 43.Shaw NJ, Boivin CM, Crabtree NJ. Intravenous pamidronate in juvenile osteoporosis. Arch Dis Child. 2000;83(2):143–5. doi: 10.1136/adc.83.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barr RD, et al. Osteopenia in children with acute lymphoblastic leukemia: a pilot study of amelioration with Pamidronate. Med Pediatr Oncol. 2002;39(1):44–6. doi: 10.1002/mpo.10057. [DOI] [PubMed] [Google Scholar]
- 45.Zelcer S, et al. The Memorial Sloan Kettering Cancer Center experience with outpatient administration of high dose methotrexate with leucovorin rescue. Pediatr Blood Cancer. 2008;50(6):1176–80. doi: 10.1002/pbc.21419. [DOI] [PubMed] [Google Scholar]
- 46.Black DM, et al. Bisphosphonates and Fractures of the Subtrochanteric or Diaphyseal Femur. N Engl J Med. 2010 doi: 10.1056/NEJMoa1001086. [DOI] [PubMed] [Google Scholar]
- 47.Goorin AM, et al. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J Clin Oncol. 2003;21(8):1574–80. doi: 10.1200/JCO.2003.08.165. [DOI] [PubMed] [Google Scholar]
- 48.Picci P, et al. The treatment of localized osteosarcoma of the extremities: the Italian experience. Studies of the National Research Council. Ann Oncol. 1992;3(Suppl 2):S13–7. doi: 10.1093/annonc/3.suppl_2.s13. [DOI] [PubMed] [Google Scholar]
- 49.Winkler K, et al. Treatment of osteosarcoma: experience of the Cooperative Osteosarcoma Study Group (COSS) Cancer Treat Res. 1993;62:269–77. doi: 10.1007/978-1-4615-3518-8_32. [DOI] [PubMed] [Google Scholar]
- 50.Healey JH. Bisphosphonate-Related Necrosis of the Jaw. Invited Commentary. Radiographics. 2009;29(7):1984–1986. doi: 10.1148/radiographics.29.7.0291984. [DOI] [PubMed] [Google Scholar]
- 51.Healey JH. The epidemic of chemotherapy-related osteoporosis. Current Opinion in Orthopedics. 1999;10(5):331–333. [Google Scholar]
- 52.Wasilewski-Masker K, et al. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics. 2008;121(3):e705–13. doi: 10.1542/peds.2007-1396. [DOI] [PubMed] [Google Scholar]
- 53.Scully SP, et al. Pathologic fracture in osteosarcoma: prognostic importance and treatment implications. J Bone Joint Surg Am. 2002;84-A(1):49–57. [PubMed] [Google Scholar]
- 54.Kawai A, et al. Relationship between magnitude of resection, complication, and prosthetic survival after prosthetic knee reconstructions for distal femoral tumors. J Surg Oncol. 1999;70(2):109–15. doi: 10.1002/(sici)1096-9098(199902)70:2<109::aid-jso9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 55.Wirganowicz PZ, et al. Etiology and results of tumor endoprosthesis revision surgery in 64 patients. Clin Orthop Relat Res. 1999;(358):64–74. [PubMed] [Google Scholar]
- 56.Falfalli G, Boland PB, Morris CD, Athanasian EA, Healey JH. Equivalence of uncemented and Compress fixation of megaprostheses. Clin Orthop Rel Res. 2009 doi: 10.1007/s11999-009-0912-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garbuz DS, et al. Enhanced gap filling and osteoconduction associated with alendronate-calcium phosphate-coated porous tantalum. J Bone Joint Surg Am. 2008;90(5):1090–100. doi: 10.2106/JBJS.G.00415. [DOI] [PubMed] [Google Scholar]
- 58.Bobyn JD, et al. Zoledronic acid causes enhancement of bone growth into porous implants. J Bone Joint Surg Br. 2005;87(3):416–20. doi: 10.1302/0301-620x.87b3.14665. [DOI] [PubMed] [Google Scholar]
- 59.Tanzer M, et al. The Otto Aufranc Award: bone augmentation around and within porous implants by local bisphosphonate elution. Clin Orthop Relat Res. 2005;441:30–9. doi: 10.1097/01.blo.0000194728.62996.2d. [DOI] [PubMed] [Google Scholar]
- 60.Shanbhag AS. Use of bisphosphonates to improve the durability of total joint replacements. J Am Acad Orthop Surg. 2006;14(4):215–25. doi: 10.5435/00124635-200604000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Jensen TB, et al. Systemic alendronate treatment improves fixation of press-fit implants: a canine study using nonloaded implants. J Orthop Res. 2007;25(6):772–8. doi: 10.1002/jor.20272. [DOI] [PubMed] [Google Scholar]
- 62.Koto K, et al. Clinically relevant dose of zoledronic acid inhibits spontaneous lung metastasis in a murine osteosarcoma model. Cancer Lett. 2009;274(2):271–8. doi: 10.1016/j.canlet.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 63.Farran RP, Zaretski E, Egeler RM. Treatment of Langerhans cell histiocytosis with pamidronate. J Pediatr Hematol Oncol. 2001;23(1):54–6. doi: 10.1097/00043426-200101000-00013. [DOI] [PubMed] [Google Scholar]


