Abstract
The pontomesencephalic tegmentum (PMT) provides cholinergic input to the inferior colliculus (IC) and the medial geniculate body (MG). PMT cells are often characterized as projecting to more than one target. The purpose of this study was to determine whether individual PMT cholinergic cells, 1) innervate the auditory pathways bilaterally via collateral projections to left and right auditory thalamus; or, 2) innervate multiple levels of the auditory pathways via collateral projections to the auditory thalamus and inferior colliculus. We used multiple retrograde tracers to identify individual PMT cells that project to more than one target. We combined the retrograde tracer studies with immunohistochemistry for choline acetyltransferase to determine whether the projecting cells were cholinergic. We found that individual PMT cells send branching axonal projections to two or more auditory targets in the midbrain and thalamus. The collateral projection pattern that we observed most frequently was to the ipsilateral IC and ipsilateral MG. Cells projecting to both MGs were somewhat less common, followed by cells projecting to the contralateral IC and ipsilateral MG. Both cholinergic and non-cholinergic cells contribute to each of these projection patterns. Less often, we found cells that project to one IC and both MGs; there was no evidence for non-cholinergic cells in this projection pattern. It is likely that collateral projections from PMT cells could have coordinated effects bilaterally and at multiple levels of the ascending auditory pathways.
Keywords: acetylcholine, pedunculopontine tegmental nucleus, laterodorsal tegmental nucleus, sensorimotor gating, acoustic startle, prepulse inhibition
Cholinergic cells of the midbrain tegmentum are involved in a wide range of functions, including arousal and the sleep-wake cycle, sensory gating, attention, novelty detection, motor control, and reward prediction (Koyama et al., 1994; Reese et al., 1995b; Rye, 1997; Swerdlow et al., 2001; Kozak et al., 2005; Pan and Hyland, 2005; Chen et al., 2006; Jones, 2008; Rostron et al., 2008; Jenkinson et al., 2009). The cholinergic cells occupy two nuclei - the laterodorsal tegmental nucleus (LDT) and the pedunculopontine tegmental nucleus (PPT) – that can be referred to collectively as the pontomesencephalic tegmentum (PMT). The cholinergic cells of the PMT accomplish their wide-ranging functions through broad projections that extend to the spinal cord and to nuclei throughout the brainstem and into the diencephalon (Woolf and Butcher, 1986; Rye et al., 1988; Woolf and Butcher, 1989; Cornwall et al., 1990; Losier and Semba, 1993).
The widespread projections of the cholinergic nuclei arise from a relatively modest number of cholinergic cells (there are approximately 20,000 cholinergic cells/ side in humans and 3000/ side in rats; Jones, 1990; Manaye et al., 1999), leading to the expectation that many of the cholinergic axons must branch to innervate multiple targets. Evidence for such branching has come from a variety of approaches, including juxtacellular labeling of individual PMT cells (Mena-Segovia et al., 2008), antidromic activation from electrical stimulation of multiple brain areas (Kayama and Ogawa, 1987) and from studies with dual retrograde tracers. The retrograde tracer studies have identified projections to two thalamic nuclei (lateral geniculate nucleus and central-lateral nucleus, Shiromani et al., 1990; ventroposteromedial nucleus and ventrolateral nucleus, Beak et al., 2010; left and right dorsal lateral geniculate nuclei, Turlejski et al., 1994), or to nuclei in different areas but with shared functions (visual thalamus and superior colliculus, Billet et al., 1999; principle sensory trigeminal nucleus and facial motor nucleus, Beak et al., 2010).
There is growing evidence for a close and extensive relationship between the PMT and the auditory system. First, approximately half of the neurons in PMT respond to auditory stimuli (Reese et al., 1995a). Second, the cholinergic cells of the PMT are essential for prepulse inhibition of the acoustic startle response (Koch et al., 1993). This function relates to sensory gating and has been tied to PMT connections with the inferior and superior colliculi (Yeomans et al., 2006). Third, the PMT projects to several auditory nuclei, including the cochlear nucleus (Motts and Schofield, 2005), the inferior colliculus (IC; Motts and Schofield, 2009), and the medial geniculate body (MG; Hallanger et al., 1987; Steriade et al., 1988; Motts and Schofield, 2010). Such projections could affect the auditory pathway throughout its subcortical extent. In an earlier study, we identified collateral projections from PMT cholinergic cells to the left and right inferior colliculi (Motts and Schofield, 2009). These results were reminiscent of the earlier studies (cited above) reporting cholinergic collaterals in other systems, and suggested further that individual PMT cells exert effects bilaterally on the auditory pathways. The purpose of the present study was to determine whether PMT cholinergic cells innervate the auditory thalamus bilaterally (as described for the visual thalamus, Turlejski et al., 1994) and/ or innervate multiple levels of the auditory pathways via collateral projections to the auditory thalamus and inferior colliculus.
Experimental Procedures
All procedures were performed in accordance with the Institutional Animal Care and Use Committee and the National Institutes of Health guidelines on the ethical use of animals. Efforts were made to minimize suffering and the number of animals used for all experiments. Pigmented guinea pigs (Elm Hill; Chelmsford, MA, USA) of either gender weighing 250-875 grams were used. Data were collected from experiments in nine guinea pigs. Data from some of these animals were described in previous studies focused on projections to the IC (Motts and Schofield, 2009) or the MG (Motts and Schofield, 2010). The present study was focused on cells that send branched axonal projections to two or more targets (one IC and one or both MGs).
Surgery
Each animal was anesthetized with isoflurane (4% for induction, 1.5-2.5% for maintenance) in oxygen. The animal's head was shaved and cleansed with Betadine (Purdue Products L.P., Stamford, CT, Rochester, N Y, USA). Ophthalmic ointment (Moisture Eyes PM, Bausch & Lomb, Rochester, NY, USA) was applied to each eye. Atropine sulfate (0.05 mg/ kg, i.m.) was administered to decrease respiratory secretions during surgery, and Ketofen (ketoprofen 3 mg/ kg, i.m.; Henry Schein, Melville, N Y, USA) was given for post-operative analgesia. The animal was positioned with its head in a stereotaxic frame. A feedback-controlled heating pad was used for maintaining the animal's body temperature. Sterile instruments and aseptic technique were used for all surgical procedures.
An incision was made in the scalp and the wound edges were injected with Marcaine (0.25% bupivacaine with epinephrine 1:200,000; Hospira, Inc., Lake Forest, IL, USA). A dental drill was used to make a small hole in the skull and the tracer was injected (see details below). The opening in the skull was covered with Gelfoam (Harvard Apparatus, Holliston, MA, USA) and the scalp was sutured. Anesthesia was then discontinued and the animal was removed from the stereotaxic frame and placed in a clean cage. The animal was monitored in its cage until it was ambulatory and able to eat and drink.
Retrograde tracers
Retrograde tracers were injected into two or three different nuclei (left IC; left and/ or right MG; Table 1) in each animal. Injections into the IC were made with 1 μl Hamilton microsyringes, which were used to deposit tracer at up to four sites within each IC (injection volumes are shown in Table 1). Injections into the MG were made with 1 μl Hamilton microsyringes or with a Nanoliter Injector (World Precision Instruments, Sarasota, FL, USA) attached to a micropipette with a 25-30 μm inside tip diameter. Deposits of tracer were made at 1-4 sites within each target. We made large injections in the MG in some cases to maximize labeling and small injections in the MG in other cases to minimize tissue damage.
Table 1.
Summary of injection sites and immunolabel. List of tracers injected in each case and the fluorescent label used to visualize the ChAT immunoreactivity. Values in parentheses indicate the total volume of tracer injected into the indicated structure. “x” indicates that no injections were made at that location in that animal. * denotes a Nanoliter injection; all other injections were made with a microsyringe. AF 488 – AlexaFluor 488 [green]; AF 647 – AlexaFluor 647 [near-infrared]; FB – Fast Blue (EMS-Chemi GmbH, Gross Umstadt, Germany); FG -FluoroGold (FluoroChrome, Inc., Englewood, CO, USA); GB - green beads (Luma-Fluor, Inc., Naples, FL, USA), RB – red beads (Luma-Fluor, Inc.).
| Case | Tracer in left IC (total volume) | Tracer in left MG (total volume) | Tracer in right MG (total volume) | Immunolabel |
|---|---|---|---|---|
| GP 481 | RB (0.6 μl) | FG (0.05 μl) | GB (0.4 μl) | AF 647 |
| GP 482 | RB (0.6 μl) | FG (0.05 μl) | GB (0.4 μl) | AF 647 |
| GP 484 | RB (0.6 μl) | FG (0.05 μl) | GB (0.2 μl) | AF 647 |
| GP 585 | FB (0.6 μl) | GB* (69 nl) | RB* (69 nl) | AF 647 |
| GP 586 | FB (0.6 μl) | GB* (69 nl) | RB* (69 nl) | AF 647 |
| GP 587 | FB (0.6 μl) | GB* (69 nl) | RB* (69 nl) | AF 647 |
| GP 595 | FG (0.3 μl) | GB* (69 nl) | RB* (69 nl) | AF 647 |
| GP 604 | GB (0.6 μl) | FG (0.05 μl) | x | AF 647 |
| GP 633 | x | RB (0.4 μl) | FG (0.05 μl) | AF 488 |
Perfusion and sectioning
After 5-25 days the animal was given an overdose of either sodium pentobarbital (440 mg/ kg, i.p.) or isoflurane (5% in oxygen, inhaled). After cessation of breathing and loss of the withdrawal reflex, the animal was perfused through the vascular system with Tyrode's solution, then 250 ml of 4% paraformaldehyde in 0.1 M phosphate buffer, (pH 7.4) followed by 250 ml of the same fixative with 10% sucrose added. The brain was removed and placed in fixative with 25-30% sucrose and stored at 4° C. The following day the cerebellum and cortex were removed. The brainstem was frozen and cut in the transverse plane on a sliding microtome into 50 μm thick sections. The sections were collected serially into six sets.
Histology
For some cases, in one tissue set (containing every sixth section), the tissue sections that contained the MG were reacted with cytochrome oxidase (CO) for the identification of MG subdivisions (Anderson et al., 2007). In some cases, one set was stained with thionin for identification of cytoarchitectural borders and landmarks. The remaining sets of tissue were used for immunohistochemistry.
Choline acetyltransferase (ChAT) immunohistochemistry was used to identify putative cholinergic cells. For methodological details, including the results of control experiments, see Motts et al. (2008). Briefly, sections were incubated for 1 day at 4° C with goat anti-ChAT antibody (Chemicon AB 144P, diluted 1:25 - 1:100 [Millipore, Billerica, MA, USA]). The sections were then treated with 1% biotinylated rabbit anti-goat antibody (BA-5000, Vector Laboratories, Burlingame, CA, USA) and labeled with streptavidin conjugated to a fluorescent marker (AlexaFluor 488 [green] or AlexaFluor 647 [near-infrared]; Invitrogen, Carlsbad, CA, USA) that could be distinguished from the tracers used in the case (Table 1). The sections were mounted on gelatin-coated slides, air dried, and coverslipped with DPX (Sigma).
Data analysis
We used tissue stained with cytochrome oxidase to visualize borders of the MG subdivisions (Anderson et al., 2007). Because some of the borders of the PPT and the LDT are indistinct in a thionin stain, we used the distribution of ChAT-immunopositive cells to define the extent of the nuclei (Leonard et al., 1995; Motts et al., 2008).
Every cell in PMT was examined for the presence of two (or more) retrograde tracers. Such cells were interpreted as having branched axonal projections to the areas that received the corresponding tracer injections. Each cell was also examined for the presence of ChAT immunoreactivity. Immunopositive cells were interpreted as cholinergic.
A Neurolucida reconstruction system (version 9; MBF Bioscience, Williston, VT, USA) attached to a Zeiss Axioplan II microscope (Carl Zeiss, Inc., Thornwood, N Y, USA) was used to plot labeled cells, nuclear borders and injection sites. Adobe Illustrator CS2 (Adobe Systems, Inc., San Jose, CA, USA) was used to create line drawings.
A Zeiss Imager Z1 fluorescence microscope with AxioCam HRm (monochrome) and HRc (color) cameras (Zeiss) was used to take photomicrographs. Monochrome images, including all images of AlexaFluor 647, which fluoresces at infrared wavelengths, were obtained and pseudocolor was applied either with the camera software (AxioVision 4.6, Zeiss) or with Photoshop CS2 (Adobe Systems, Inc., San Jose, CA, USA). Photoshop CS2 or CS3 was used to add scale bars and labels, size and crop images, erase background around tissue sections, and adjust intensity levels in photomicrographs.
In order to assess the relative prominence of collateral branching, the double-retrograde cells were counted and expressed as a percentage of the cells retrogradely labeled by either single tracer. This analysis was based on a subset of cases in which 1) the MG injections were largely or completely confined to the MG; and, 2) for each projection to be analyzed, there were at least 50 retrogradely labeled cells per set (every sixth section) in the PPT and the LDT (left and right sides combined). For quantitative analysis of projections to the IC, we excluded any injection that spread beyond the IC, so the selection for quantitative analysis focused on the number of labeled cells.
Results
We combined retrograde tracing and immunohistochemistry to identify collateral projections from PMT cells to 1) the left and right MG; or, 2) an IC and one or both MGs. As described above (Table 1), we varied the tracer injected into a particular target in different animals. The purpose of this was to avoid missing parts of a pathway that may be better labeled by a particular tracer (e.g., an injection of red or green beads often labels more cells than a similarly-sized injection of FluoroGold or fast blue; see discussions in Schofield et al., 2007; Schofield, 2008). While there were differences in the overall number of cells labeled by different tracers, there were no apparent qualitative differences in the results from the various tracers. We begin by describing the results from injections of tracers into both MGs. We then describe results from injections into one IC and one MG. Finally, we describe results from cases with injections into one IC and both MGs.
Collateral projections to the left and right MG
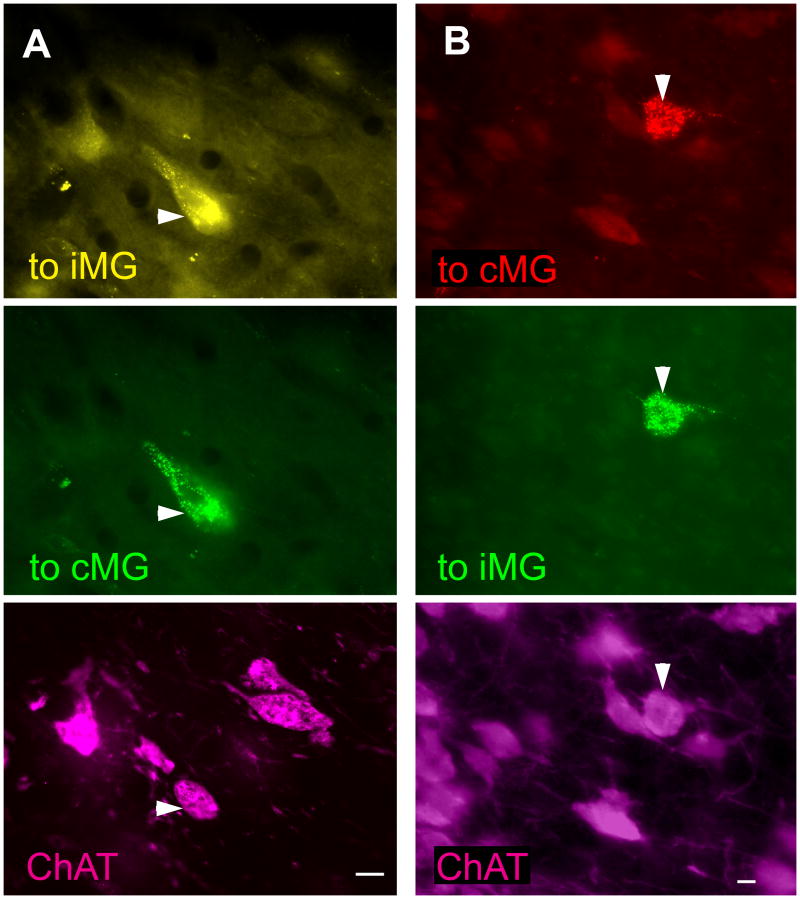
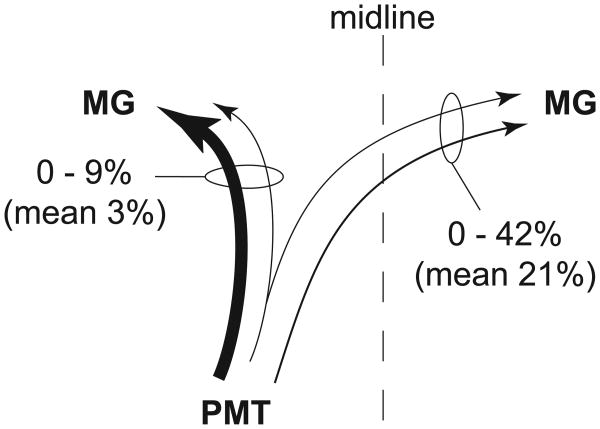
The larger injection sites typically filled most of the MG, and extended to varying degrees into neighboring nuclei, such as the dorsal lateral geniculate nucleus or lateral posterior nucleus (Fig. 1 A and B). The smaller injections were largely or completely confined to the MG, but did not fill its entire extent (Figure 1 C and D). Given that the PMT projects to the surrounding nuclei (Steriade et al., 1988), for quantitative analysis we included only those cases in which the injections were largely or completely confined to the MG (three cases). In each of these three cases, we found PMT cells that project to both MGs (Fig. 2). The majority of such cells (68%; 25 of 37 cells) were also immunopositive for ChAT, identifying them as cholinergic cells. Among the immunopositive cells, the double-retrograde cells constituted up to 9% of the cells that were labeled from the ipsilateral MG and up to 42% of the cells that were labeled from the contralateral MG (Fig. 3). Overall, fewer cells project contralaterally than ipsilaterally, so the double-retrograde cells make up a larger proportion of the contralateral projection than the ipsilateral projection.
Figure 1.
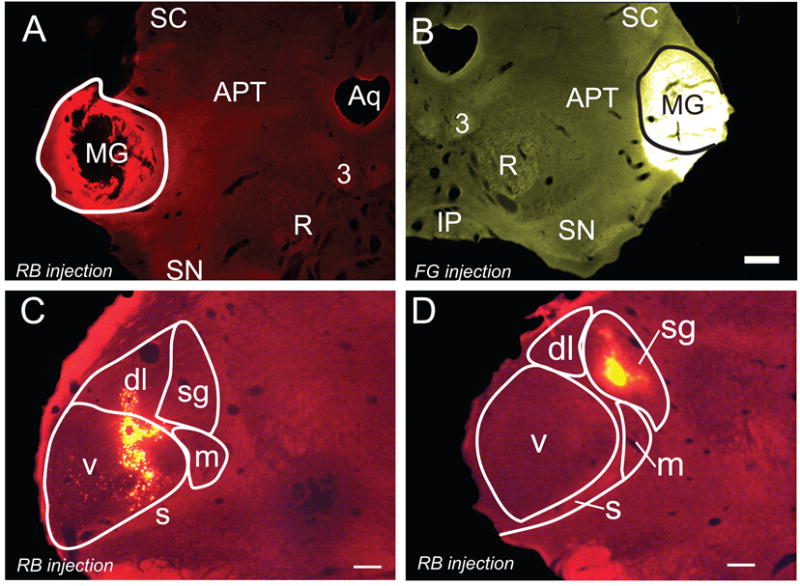
Photomicrographs of representative large and small tracer injections into the MG. A) Large red bead (RB) injection that was confined to the left MG (solid outline). GP 633. The black area in the center of the injection represents an area in which the tissue fell loose during processing. B) A large FluoroGold injection that spread ventrally beyond the borders of the right MG. GP 633. Solid line - approximate borders of the MG. Scale bar applies to A and B (500 μm). C) Smaller injection of red beads that is centered in the ventral MG (v) and extends into the dorsolateral subdivision (dl). GP 595R. D) A small injection of red beads within the suprageniculate subdivision (sg). GP 586R. Transverse sections; dorsal is up; lateral is to the left in A, C-D and to the right in B. Scale bars = 500 μm. 3, oculomotor nucleus; APT, anterior pretectal nucleus; Aq, aqueduct; dl, dorsolateral; m, medial; s, shell; SC, superior colliculus; sg, suprageniculate; SN, substantia nigra; v, ventral. C and D adapted from Motts and Schofield, 2010, with permission.
Figure 2.
Photomicrographs of immunopositive PMT cells that project to both MGs. For each column, the top panel represents labeling from an injection in the left MG, the middle panel represents labeling from an injection in the right MG, and the bottom panel represents immunolabeling for ChAT. In each column all three panels show the same field of view using different filters to visualize the different fluorescent labels. A) FluoroGold (FG) was injected in the left MG and green beads (GB) were injected in the right MG. Arrowhead - cell in left PPT labeled for FG (top panel), GB (middle panel), and ChAT-immunoreactivity (bottom panel). GP 484. B) Green beads were injected in the left MG and red beads were injected into the right MG. Arrowhead - cell in the left LDT labeled for GB (top panel), RB (middle panel), and ChAT-immunoreactivity (bottom panel). GP 585. Scale bars = 10 μm.
Figure 3.
Schematic summary of cholinergic collateral projections from PMT cells to the ipsilateral and contralateral MG. Three cases were used for quantitative analysis of this projection pattern. The numbers express the range of percentages of cells with collateral projections compared with the cells that project to only one of the two nuclei. In other words, the bilaterally-projecting cells (those that contained both retrograde tracers) constituted up to 9% of the cells that projected to the ipsilateral MG (indicated by the associated oval, and representing cells that contained only the ipsilateral tracer + cells that contained both tracers). The same population of bilaterally-projecting cells constituted up to 42% of the cells that projected to the contralateral MG. Line thickness of arrows in this and subsequent drawings reflects the relative size of the projection (measured as the number of cells found in each projection). Note that this (and subsequent) schematics illustrate the immunopositive [i.e., cholinergic) cells; immunonegative cells were also labeled but are not included in the schematic summaries.
Collateral projections to one MG and one IC
Figure 4 shows a representative tracer injection in the IC. Tracer deposits into the IC typically involved parts of all three major IC subdivisions (central nucleus, dorsal cortex, external cortex). It is common, particularly with the large injections used here, to observe an area of tissue damage or necrosis in the center of the injection site (Schofield et al., 2007; Schofield, 2008). Whether the necrosis occurs after the tracer is taken up, or damage to axons in the necrotic zone allows uptake of the tracer, it appears that cells that project to the necrotic area (as well as the surrounding, intact injection area) are routinely labeled. We excluded any case in which the tracer extended medially into the periaqueductal gray, ventrally into the tegmentum or rostrally into the superior colliculus. None of the injections extended across the midline into the contralateral IC.
Figure 4.
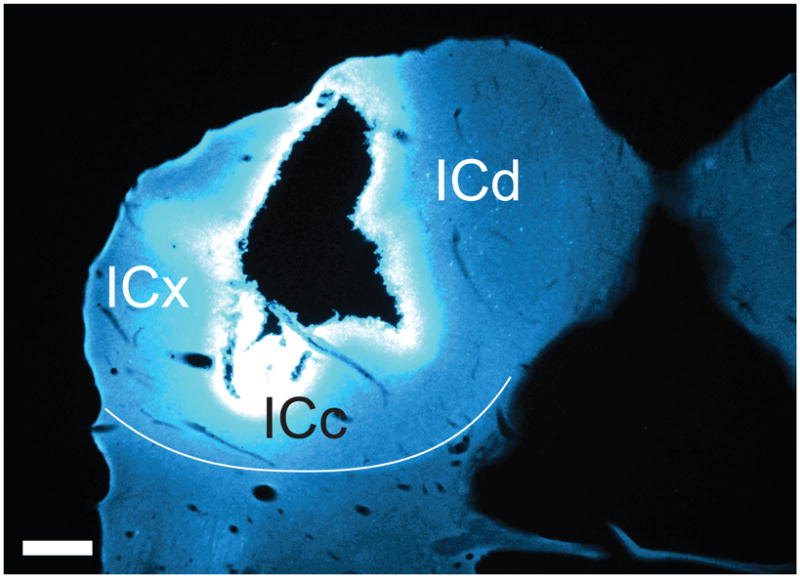
Photomicrograph through the center of a Fast Blue injection site in the left IC. The black area in the center of the injection represents an area in which the tissue disintegrated during processing. GP 587. Transverse section; dorsal is up; lateral is to the left. Scale bar = 500 μm. ICc, central nucleus of the inferior colliculus; ICd, dorsal cortex of the inferior colliculus; ICx, external cortex of the inferior colliculus.
Projections to an MG and an IC located on the same side
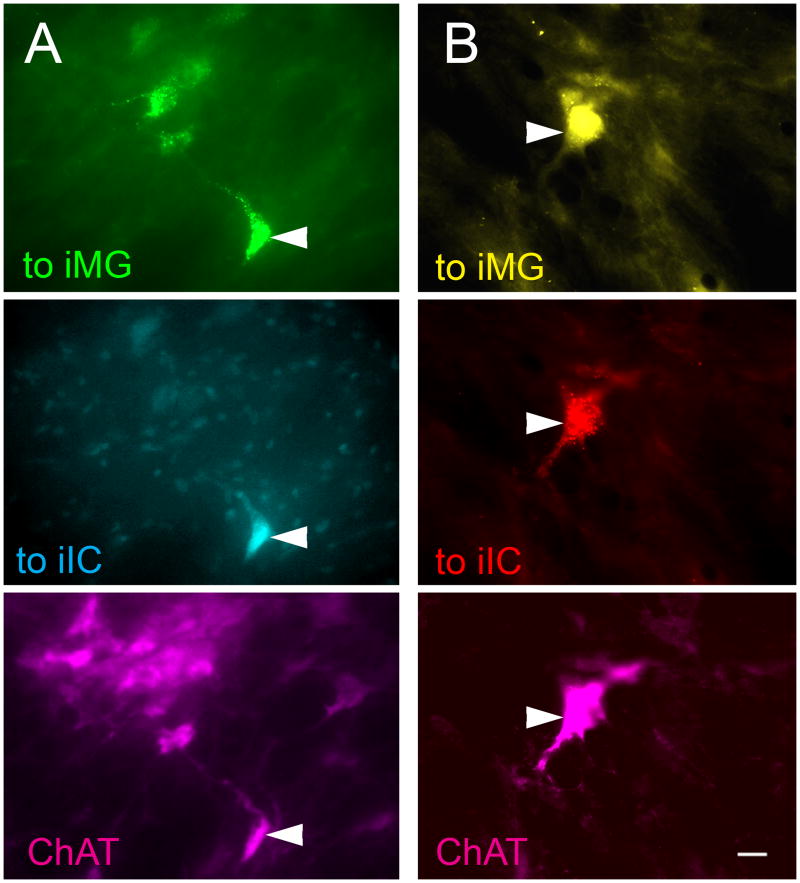
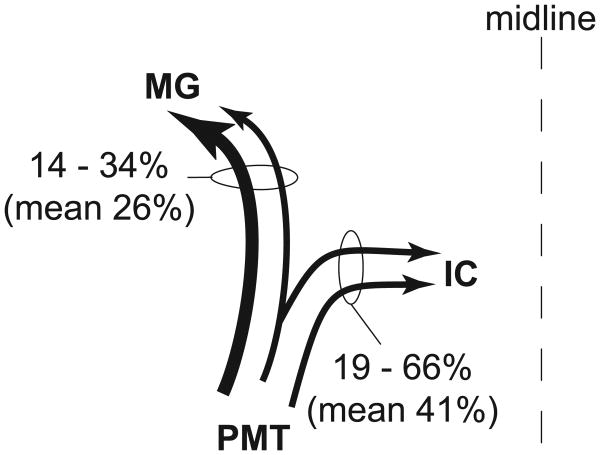
Figure 5 shows representative examples of cells in the PMT that were labeled by both retrograde tracers. This pattern of injections produced many more double-retrograde cells than any of the other patterns we tested. Most, or in some cases all, of the double-retrograde cells in PMT were located ipsilateral to the injections. The vast majority of cells projecting to both the ipsilateral IC and the ipsilateral MG (85%; 95 of 112 cells; n = four cases analyzed) were immunolabeled for ChAT. Among the immunopositive cells projecting to the two ipsilateral targets, the double-retrograde cells constituted up to 66% of the cells that were labeled from the ipsilateral IC and up to 34% of the cells that were labeled from the ipsilateral MG (Fig. 6).
Figure 5.
Photomicrographs of immunopositive PMT cells that project to both the ipsilateral IC and the ipsilateral MG. For each column, the top panel represents labeling from the injection in the ipsilateral MG, the middle panel represents labeling from the injection in the ipsilateral IC, and the bottom panel represents immunolabeling for ChAT. In each column all three panels show the same field of view using different filters to visualize the different fluorescent labels. A) Green beads were injected into the left MG and Fast Blue was injected into the left IC. Arrowhead - cell in the left PPT labeled for GB (top panel), FB (middle panel), and ChAT-immunoreactivity (bottom panel). GP 587. B) FG was injected into the left MG and RB was injected into the left IC. Arrowhead - cell in the left PPT labeled for FG (top panel), RB (middle panel), and ChAT-immunoreactivity (bottom panel). Scale bars = 10 μm. iIC, ipsilateral IC; iMG, ipsilateral MG.
Figure 6.
Schematic summary of cholinergic collateral projections from PMT cells to the ipsilateral IC and ipsilateral MG. Four cases were used for quantitative analysis of this projection pattern. The numbers express the percentages of cells with collateral projections compared with the cells that project to only one of the two nuclei. Line thickness for this and other arrow drawings reflects the relative size of the projection (measured as the number of cells found in each projection).
Double-retrograde cells in the PMT contralateral to the IC and MG injections were observed in two of the four cases used for quantitative analysis of this injection pattern. Two cells were observed in one case and four cells in the other, suggesting that this projection pattern is much less common than that to ipsilateral MG and ipsilateral IC. Despite the small number of labeled cells, the population included both immunopositive and immunonegative cells.
Projections to an MG and an IC located on different sides
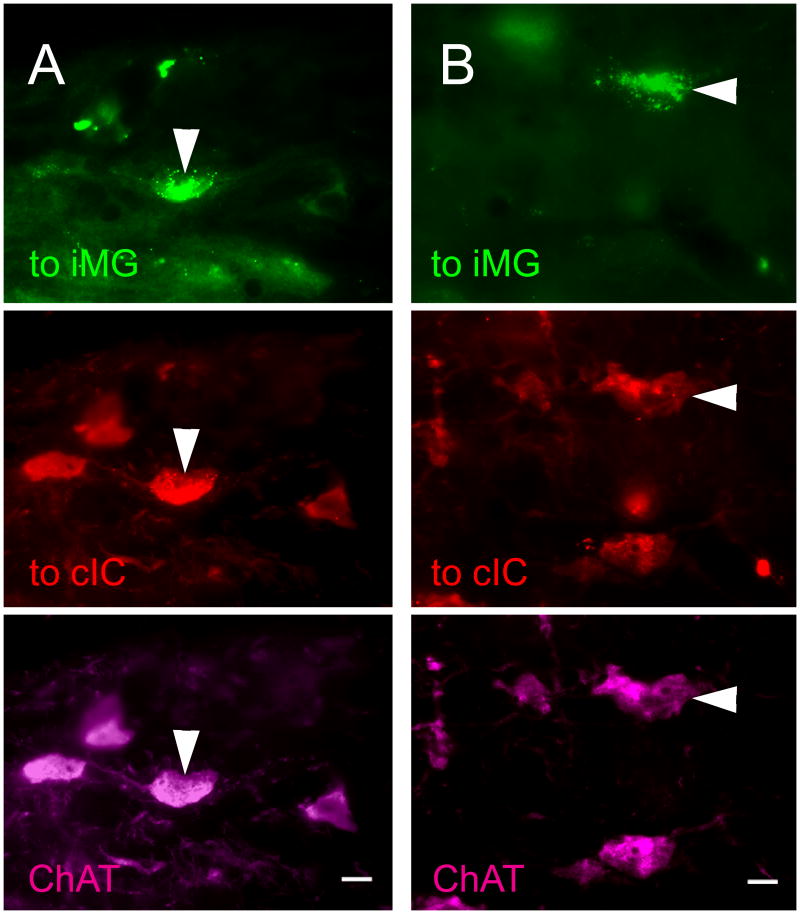
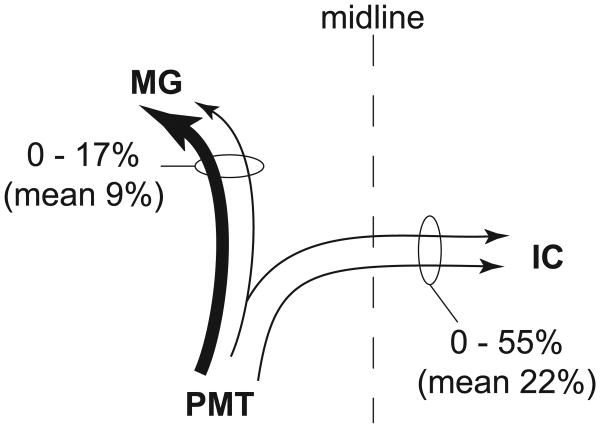
Collateral projections from the PMT to the contralateral IC and the ipsilateral MG were relatively common (Fig. 7). Six cases with injections into the left IC and the right MG met the criteria for quantitative analysis. The projection from PMT to the contralateral IC and the ipsilateral MG was seen in five of these six cases. Sixty-three percent of the cells with this projection pattern were immunopositive (26 of 41 cells). Among the immunopositive cells, the double-retrograde cells constituted up to 55% of the cells that were labeled from the contralateral IC and up to 17% of the cells that were labeled from the ipsilateral MG (Fig. 8).
Figure 7.
PMT cells that project to the contralateral IC and the ipsilateral MG. For each column, the top panel represents labeling from a GB injection in the right MG, the middle panel represents labeling from a RB injection in the left IC, and the bottom panel represents immunolabeling for ChAT. All three panels show the same field of view using different filters to visualize the different fluorescent labels. Cells in the right PPT (A) and LDT (B) labeled for GB (top panel), RB (middle panel), and ChAT immunoreactivity. Arrowheads show cells that project to the contralateral IC and ipsilateral MG and are ChAT immunopositive. GP 484. B) Cell in the right LDT that projects to the contralateral IC and ipsilateral MG and is ChAT immunopositive. GP 484. Scale bars = 10 μm. cIC, contralateral IC; cMG, contralateral MG; iIC, ipsilateral IC; iMG, ipsilateral MG.
Figure 8.
Schematic summary of cholinergic collateral projections from PMT cells to the contralateral IC and ipsilateral MG. Six cases were used for quantitative analysis of this projection pattern. The numbers express the percentages of cells with collateral projections compared with the cells that project to only one of the two nuclei. Line thickness reflects the relative size of the projection (measured as the number of cells found in each projection).
Five of the six cases in which we observed the PMT collateral projection to the contralateral IC and the ipsilateral MG also had collateral projections to the ipsilateral IC and the contralateral MG. However, fewer cells showed this projection pattern (19, vs. 41 in the previous pattern), suggesting that it is less common. Both immunopositive and immunonegative cells were observed, indicating cholinergic and non-cholinergic collateral projections.
Collateral projections to both MGs and one IC
Two animals had confined injections in the left IC and in each MG. This pattern of injections could reveal cells that have branched projections to all three targets. We observed a total of seven cells in the two animals that were triple labeled with retrograde tracers; all of these cells were immunopositive (Fig. 9).
Figure 9.
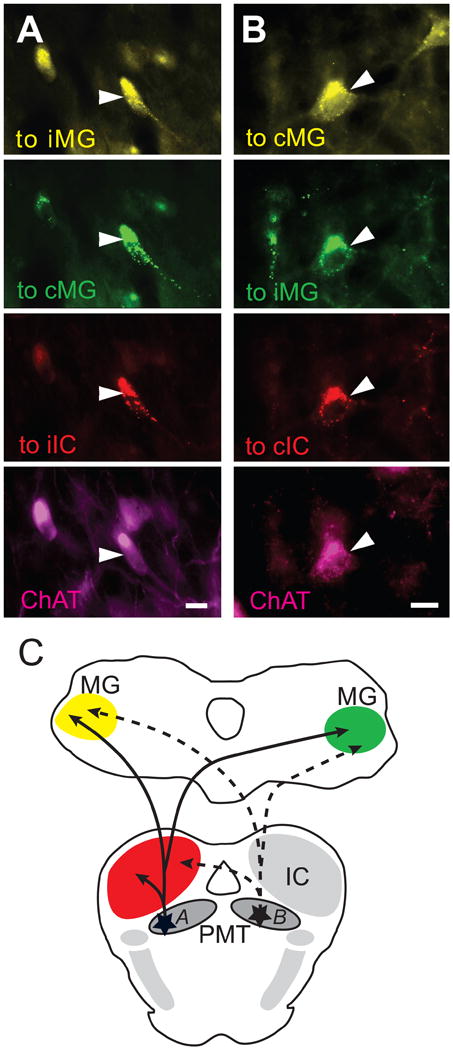
Photomicrographs of immunopositive PMT cells that project to one IC and both MGs. For each column, the top panel represents labeling after a FG injection in the left MG, the second panel represents labeling after a GB injection in the right MG, the third panel represents labeling after a RB injection in the left IC, and the bottom panel represents immunolabeling for ChAT. All four panels show the same field of view using different filters to visualize the different fluorescent labels. GP 484. A) Cells in the left LDT labeled for FG (top panel), GB (second panel), RB (third panel), and ChAT immunoreactivity (bottom panel). B) Cells in right PPT labeled for the same markers as listed in A. Arrowheads show a cell that contains all four markers. Scale bars = 10 μm. C) Schematic illustrating the two projection patterns shown in A and B. The solid lines indicate the projections of cell A, while the dotted lines indicate the projections of cell B. cIC, contralateral IC; cMG, contralateral MG; iIC, ipsilateral IC; iMG, ipsilateral MG.
Non-cholinergic projections
The results above focus on the cholinergic cells in PMT with collateral projections. However, we found immunonegative single and double-retrogradely labeled cells in every case. The immunonegative cells were interspersed among the immunopositive cells. Immunonegative and immunopositive cells were visible within the same focal plane, indicating effective immunostaining. It seems likely that these immunonegative cells were in fact non-cholinergic. Therefore, non-cholinergic cells contributed to each of the single and double-retrograde pathways that we have described. It is interesting to note however that every triple-retrograde labeled cell was immunopositive.
Discussion
We and others have described cholinergic projections from the PMT to the IC (Motts and Schofield, 2009) and to the MG (Hallanger and Wainer, 1988; Steriade et al., 1988; Motts and Schofield, 2010). In this paper we show that individual PMT cells send branching axonal projections to two or more auditory targets in the midbrain and thalamus. The collateral projection pattern that we observed most frequently was to the ipsilateral IC and ipsilateral MG. Cells projecting to both MGs were also common, as were cells projecting to the contralateral IC and ipsilateral MG. Both cholinergic and non-cholinergic cells appear to contribute to each of these projection patterns. Less often, we found cells that project to at least one IC and both MGs; there was no evidence for non-cholinergic cells in this projection pattern. We first address the technical issues of our experiments. We then explore the functional significance of the projections. Finally, we discuss implications of our results for studies that use electrical stimulation of the IC or MG.
Technical considerations
In order to identify cholinergic cells, we used an antibody against choline acetyltransferase (ChAT). ChAT is considered a specific marker for cholinergic cells (Levey and Wainer, 1982; Armstrong et al., 1983; German et al., 1985; Maley et al., 1988). We have validated this antibody in guinea pigs (Motts et al., 2008). We saw clearly labeled cells in the expected areas, such as the cranial nerve motor nuclei and PMT. We conclude that the immunolabeled cells are cholinergic.
The PPT and the LDT contain a variety of cell types, including cholinergic, GABAergic, glutamatergic, and catecholaminergic cells (Vincent et al., 1986; Lavoie and Parent, 1994; Ford et al., 1995; Leonard et al., 1995; Jia et al., 2003; Wang and Morales, 2009). Our experiments yielded immunopositive and immunonegative retrogradely-labeled cells within the same focal plane, which suggests that the antibody infiltrated the tissue adequately and that the immunonegative cells are non-cholinergic. An important area for future studies will be to characterize the non-cholinergic cell types that contribute to the PMT projections to IC and MG. This information will help in understanding the effects of the PMT projections to subcortical auditory structures.
Some of our large injections spread beyond the MG, raising the possibility that some of the labeled cells terminate outside the MG. However, we found multi-labeled cells following injections confined to the MG, suggesting that at least some of the labeled cells terminate within the MG. Injections into the IC were less problematic. We excluded any case in which the tracer injection spread beyond the confines of the IC. The remaining injections were large, but never filled the IC. We conclude that, as with any study that uses multiple retrograde tracers to identify collateral projections, our data underestimated the prevalence of collateral projections.
Another issue with the use of retrograde tracers is the possibility of labeling axons of passage. There is no evidence that PMT axons travel through the IC to another target (Shute and Lewis, 1967), so it is unlikely that IC injections labeled PMT cells via axons of passage. Injections in the MG could label PMT cells that project to more rostral thalamic (or extrathalamic) targets if their axons traverse the MG. We have made attempts to minimize the possibility that we have labeled axons of passage. We used red beads and green beads in many of our experiments, which are less likely than other tracers to be taken up by axons of passage (Katz and Iarovici, 1990). In addition, we have used micropipettes for some of our injections (Table 1). Micropipettes cause less damage than syringes, thus decreasing the likelihood of tracer uptake by axons of passage. We conclude that the majority of PMT cells labeled by our MG injections are likely to project to the MG.
We report the frequency of collateral projections as a percentage of the cells that project to either target. Because of the inherent underestimation of the frequency of collaterals, we report the range of percentages across cases and suggest that the maximum percentage seen may be a more accurate representation of the true frequency of the occurrence of collaterals than the average percentage across cases.
Our use of relatively large injections maximizes the number of labeled cells, allowing us to identify as many projection patterns as possible and minimizing the limitations associated with double-labeling with retrograde tracers (Schofield et al., 2007). This is a reasonable “first step” in identifying collateral projection patterns, but it limits conclusions regarding the finer details of the projections. It will be of particular interest in future studies to determine whether cholinergic axons terminate differentially in specific subdivisions of the IC or MG. Given the different functions attributed to these subdivisions (see discussions in Rouiller, 1997; Malmierca and Hackett, 2010), distinct patterns of cholinergic innervation could provide additional insight into the cholinergic effects on auditory processing.
Functional implications
The PMT is considered part of the ascending reticular activating system. It has been implicated in a wide array of functions, including attention, arousal, sleep and wakefulness, locomotion, memory, posture, and muscle control (Reese et al., 1995b; Rye, 1997). More recent studies have raised the possibility of a PMT contribution to stimulus-specific adaptation and novelty detection in the auditory system (Koyama et al., 1994; Schofield and Motts, 2009; Schofield, 2010). Collateral projections may be associated with many of the PMT functions. Woolf and Butcher (1986) report multiple PMT collateral projections - some to two thalamic targets, some to one thalamic target plus one other target, and some to two non-thalamic sites. They suggest that the “widespread, extensively collateralizing projections of the PMT cholinergic complex…provide neuroanatomical support for the conjecture that this system plays a role in the coordination of sensory, motor, and limbic functions” (Woolf and Butcher, 1986, page 635). In the context of the auditory system, the PMT cholinergic cells have been implicated in sensory gating. Much of the work on this issue has focused on the essential role of the PMT in prepulse inhibition of the startle reflex (Koch et al., 1993; Fendt et al., 2001; Diederich and Koch, 2005; Yeomans et al., 2006; Bosch and Schmid, 2008). It seems likely that the PMT cholinergic projections to the IC and the MG could play a similar role in gating auditory information. The existence of collateral projections to multiple auditory targets, as shown in the present study, could reflect a gating function exerted nearly simultaneously at several points along the auditory pathways. If the cholinergic cells are activated by novel stimuli, then their collateral projections could represent an efficient means for a relatively small number of cells to promote transmission of novel information to a level of conscious awareness.
There is now evidence that cholinergic effects can be exerted by bilaterally branching axonal projections in multiple sensory systems. In the visual system, Turlejski et al. (1994) demonstrated PMT collateral projections to both dorsal lateral geniculate nuclei. They surmised that “the projections from the modulatory nuclei of the pontomesencephalic tegmentum are likely to contribute to the functional synchronization of both dorsal lateral geniculate nuclei during the sleep -wakefulness cycle and saccadic eye movements” (page 533). We have now shown bilateral projections from PMT to several combinations of auditory nuclei: to both ICs (Motts and Schofield, 2009); to both MGs (present study); and to one IC and the opposite MG (present study). It is likely, then, that the cholinergic projections have coordinated effects on the ascending auditory pathways on both sides of the brain. Cholinergic cells in the PMT are known to innervate other sensory systems (e.g., vestibular: Woolf and Butcher, 1989; somatosensory: Beak et al., 2010; taste: Ruggiero et al., 1990); it would be interesting to determine whether bilateral axonal branching characterizes the cholinergic projections to these sensory systems.
Implications for electrical stimulation studies
Electrical stimulation of auditory nuclei has been a useful tool in a variety of studies (Nowak et al., 1999; Ota et al., 2004; Silva et al., 2005; Wu and Yan, 2007). Our results highlight a possibility for antidromic/ collateral activation that may not have been previously considered. Electrical stimulation of either the IC or the MG could activate cholinergic arousal (or other) mechanisms via antidromic stimulation of PMT. Given the present results, stimulation of one MG, for example, could lead to release of acetylcholine in the opposite MG or either IC. Stimulation of the IC could lead to release of acetylcholine bilaterally in the MG. In fact, we have shown previously that PMT cells send collateral projections to both ICs (Motts and Schofield, 2009) and that some PMT cells send branches to the IC and the cochlear nucleus (Motts and Schofield, 2006). It seems likely, then, that electrical stimulation at one of these sites could lead to release of acetylcholine across a wide range of the auditory pathways. The divergent projections from these cells could be important not only for the function of the cholinergic system but also for the design and interpretation of electrical stimulation studies. In this context, it is particularly noteworthy that several investigators have supplemented electrical stimulation studies with parallel studies using chemical stimulation (e.g., Dringenberg et al., 2004). The chemical stimulation relies on activation of receptors on the neurons in the stimulation area, and thus does not activate axons in the stimulation area. While there are additional limitations with chemical stimulation (e.g., compared with electrical stimulation, one has much less control over the temporal aspects of chemical stimulation), the ability to avoid antidromic/ collateral activation provides a strong benefit for interpreting the chemical stimulation studies.
Acknowledgments
Special thanks to Colleen Sowick and Megan Storey-Workley for technical assistance. Thanks also to Martha Bickford, Jeffrey Wenstrup, David Glass, Alexander Galazyuk, Jeffrey Mellott, and Kyle Nakamoto for comments on an earlier draft of the manuscript. Supported by NIH DC04391 and DC08463.
List of Abbreviations
- 3
oculomotor nucleus
- AF 488
AlexaFluor 488
- AF 647
AlexaFluor 647
- APT
anterior pretectal nucleus
- Aq
aqueduct
- ChAT
choline acetyltransferase
- cIC
contralateral inferior colliculus
- cMG
contralateral medial geniculate body
- CO
cytochrome oxidase
- dl
dorsolateral subdivision of the medial geniculate body
- FB
fast blue
- FG
FluoroGold
- GB
green beads
- IC
inferior colliculus
- ICc
central nucleus of the inferior colliculus
- ICd
dorsal cortex of the inferior colliculus
- ICx
external cortex of the inferior colliculus
- iIC
ipsilateral inferior colliculus
- iMG
ipsilateral medial geniculate body
- LDT
laterodorsal tegmental nucleus
- m
medial subdivision of the medial geniculate body
- MG
medial geniculate body
- PPT
pedunculopontine tegmental nucleus
- PMT
pontomesencephalic tegmentum
- RB
red beads
- s
shell
- SC
superior colliculus
- sg
suprageniculate subdivision of the medial geniculate body
- SN
substantia nigra
- v
ventral subdivision of the medial geniculate body
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Anderson LA, Wallace MN, Palmer AR. Identification of subdivisions in the medial geniculate body of the guinea pig. Hear Res. 2007;228:156–167. doi: 10.1016/j.heares.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Armstrong DM, Saper CB, Levey AI, Wainer BH, Terry RD. Distribution of cholinergic neurons in rat brain: demonstrated by the immunocytochemical localization of choline acetyltransferase. J Comp Neurol. 1983;216:53–68. doi: 10.1002/cne.902160106. [DOI] [PubMed] [Google Scholar]
- Beak SK, Hong EY, Lee HS. Collateral projection from the forebrain and mesopontine cholinergic neurons to whisker-related, sensory and motor regions of the rat. Brain Res. 2010;1336:30–45. doi: 10.1016/j.brainres.2010.03.100. [DOI] [PubMed] [Google Scholar]
- Billet S, Cant NB, Hall WC. Cholinergic projections to the visual thalamus and superior colliculus. Brain Res. 1999;847:121–123. doi: 10.1016/s0006-8993(99)01900-9. [DOI] [PubMed] [Google Scholar]
- Bosch D, Schmid S. Cholinergic mechanism underlying prepulse inhibition of the startle response in rats. Neuroscience. 2008;155:326–335. doi: 10.1016/j.neuroscience.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Chen J, Nakamura M, Kawamura T, Takahashi T, Nakahara D. Roles of pedunculopontine tegmental cholinergic receptors in brain stimulation reward in the rat. Psychopharmacology (Berl) 2006;184:514–522. doi: 10.1007/s00213-005-0252-8. [DOI] [PubMed] [Google Scholar]
- Cornwall J, Cooper JD, Phillipson OT. Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 1990;25:271–284. doi: 10.1016/0361-9230(90)90072-8. [DOI] [PubMed] [Google Scholar]
- Diederich K, Koch M. Role of the pedunculopontine tegmental nucleus in sensorimotor gating and reward-related behavior in rats. Psychopharmacology (Berl) 2005;179:402–408. doi: 10.1007/s00213-004-2052-y. [DOI] [PubMed] [Google Scholar]
- Dringenberg HC, Yahia N, Cirasuolo J, McKee D, Kuo MC. Neocortical activation by electrical and chemical stimulation of the rat inferior colliculus: Intra-collicular mapping and neuropharmacological characterization. Exp Brain Res. 2004;154:461–469. doi: 10.1007/s00221-003-1675-2. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology (Berl) 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- German DC, Bruce G, Hersh LB. Immunohistochemical staining of cholinergic neurons in the human brain using a polyclonal antibody to human choline acetyltransferase. Neurosci Lett. 1985;61:1–5. doi: 10.1016/0304-3940(85)90391-x. [DOI] [PubMed] [Google Scholar]
- Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH. The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol. 1987;262:105–124. doi: 10.1002/cne.902620109. [DOI] [PubMed] [Google Scholar]
- Jenkinson N, Nandi D, Muthusamy K, Ray NJ, Gregory R, Stein JF, Aziz TZ. Anatomy, physiology, and pathophysiology of the pedunculopontine nucleus. Mov Disord. 2009;24:319–328. doi: 10.1002/mds.22189. [DOI] [PubMed] [Google Scholar]
- Jia HG, Yamuy J, Sampogna S, Morales FR, Chase MH. Colocalization of gamma-aminobutyric acid and acetylcholine in neurons in the laterodorsal and pedunculopontine tegmental nuclei in the cat: a light and electron microscopic study. Brain Res. 2003;992:205–219. doi: 10.1016/j.brainres.2003.08.062. [DOI] [PubMed] [Google Scholar]
- Jones BE. Immunohistochemical study of choline acetyltransferase-immunoreactive processes and cells innervating the pontomedullary reticular formation in the rat. J Comp Neurol. 1990;295:485–514. doi: 10.1002/cne.902950311. [DOI] [PubMed] [Google Scholar]
- Jones BE. Modulation of cortical activation and behavioral arousal by cholinergic and orexinergic systems. Ann N Y Acad Sci. 2008;1129:26–34. doi: 10.1196/annals.1417.026. [DOI] [PubMed] [Google Scholar]
- Katz LC, Iarovici DM. Green fluorescent latex microspheres: a new retrograde tracer. Neuroscience. 1990;34:511–520. doi: 10.1016/0306-4522(90)90159-2. [DOI] [PubMed] [Google Scholar]
- Kayama Y, Ogawa T. Electrophysiology of ascending, possibly cholinergic neurons in the rat laterodorsal tegmental nucleus: comparison with monoamine neurons. Neurosci Lett. 1987;77:277–282. doi: 10.1016/0304-3940(87)90512-x. [DOI] [PubMed] [Google Scholar]
- Koch M, Kungel M, Herbert H. Cholinergic neurons in the pedunculopontine tegmental nucleus are involved in the mediation of prepulse inhibition of the acoustic startle response in the rat. Exp Brain Res. 1993;97:71–82. doi: 10.1007/BF00228818. [DOI] [PubMed] [Google Scholar]
- Koyama Y, Jodo E, Kayama Y. Sensory responsiveness of “Broad-spike” Neurons in the laterodorsal tegmental nucleus, locus coeruleus and dorsal raphe of awake rats: Implications for cholinergic and monoaminergic neuron-specific responses. Neuroscience. 1994;63:1021–1031. doi: 10.1016/0306-4522(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Kozak R, Bowman EM, Latimer MP, Rostron CL, Winn P. Excitotoxic lesions of the pedunculopontine tegmental nucleus in rats impair performance on a test of sustained attention. Exp Brain Res. 2005;162:257–264. doi: 10.1007/s00221-004-2143-3. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons. J Comp Neurol. 1994;344:190–209. doi: 10.1002/cne.903440203. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Kerman I, Blaha G, Taveras E, Taylor B. Interdigitation of nitric oxide synthase-, tyrosine hydroxylase-, and serotonin-containing neurons in and around the laterodorsal and pedunculopontine tegmental nuclei of the guinea pig. J Comp Neurol. 1995;362:411–432. doi: 10.1002/cne.903620309. [DOI] [PubMed] [Google Scholar]
- Levey AI, Wainer BH. Cross-species and intraspecies reactivities of monoclonal antibodies against choline acetyltransferase. Brain Res. 1982;234:469–473. doi: 10.1016/0006-8993(82)90889-7. [DOI] [PubMed] [Google Scholar]
- Losier BJ, Semba K. Dual projections of single cholinergic and aminergic brainstem neurons to the thalamus and basal forebrain in the rat. Brain Res. 1993;604:41–52. doi: 10.1016/0006-8993(93)90350-v. [DOI] [PubMed] [Google Scholar]
- Maley BE, Frick ML, Levey AI, Wainer BH, Elde RP. Immunohistochemistry of choline acetyltransferase in the guinea pig brain. Neurosci Lett. 1988;84:137–142. doi: 10.1016/0304-3940(88)90397-7. [DOI] [PubMed] [Google Scholar]
- Malmierca E, Hackett TA. Structural organization of the ascending auditory pathway. In: Rees A, Palmer AR, editors. The oxford handbook of auditory neuroscience vol 2: The auditory brain. Oxford: Oxford University Press; 2010. pp. 9–41. [Google Scholar]
- Manaye KF, Zweig R, Wu D, Hersh LB, De Lacalle S, Saper CB, German DC. Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain. Neuroscience. 1999;89:759–770. doi: 10.1016/s0306-4522(98)00380-7. [DOI] [PubMed] [Google Scholar]
- Mena-Segovia J, Sims HM, Magill PJ, Bolam JP. Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J Physiol. 2008;586:2947–2960. doi: 10.1113/jphysiol.2008.153874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motts SD, Schofield BR. Olivary and extra-olivary sources of cholinergic input to the cochlear nucleus. Assoc Res Otolaryngol Abs. 2005;27:242. [Google Scholar]
- Motts SD, Schofield BR. Cholinergic cells have branching projections to multiple brainstem auditory nuclei in giunea pigs. Assoc Res Otolaryngol Abs. 2006;42 [Google Scholar]
- Motts SD, Slusarczyk AS, Sowick CS, Schofield BR. Distribution of cholinergic cells in guinea pig brainstem. Neuroscience. 2008;154:186–95. doi: 10.1016/j.neuroscience.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motts SD, Schofield BR. Sources of cholinergic input to the inferior colliculus. Neuroscience. 2009;160:103–114. doi: 10.1016/j.neuroscience.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motts SD, Schofield BR. Cholinergic and non-cholinergic projections from the pedunculopontine and laterodorsal tegmental nuclei to the medial geniculate body in guinea pigs. Front Neuroanat. 2010;4:137. doi: 10.3389/ fnana.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak AJ, Kehoe EJ, Macrae M, Gormezano I. Conditioning and reflex modification of the rabbit nictitating membrane response using electrical stimulation in auditory nuclei. Behav Brain Res. 1999;105:189–198. doi: 10.1016/s0166-4328(99)00073-x. [DOI] [PubMed] [Google Scholar]
- Ota Y, Oliver DL, Dolan DF. Frequency-specific effects on cochlear responses during activation of the inferior colliculus in the guinea pig. J Neurophysiol. 2004;91:2185–2193. doi: 10.1152/jn.01155.2003. [DOI] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese NB, Garcia-Rill E, Skinner RD. Auditory input to the pedunculopontine nucleus: II. Unit responses. Brain Res Bull. 1995a;37:265–273. doi: 10.1016/0361-9230(95)00001-u. [DOI] [PubMed] [Google Scholar]
- Reese NB, Garcia-Rill E, Skinner RD. The pedunculopontine nucleus--auditory input, arousal and pathophysiology. Prog Neurobiol. 1995b;47:105–133. doi: 10.1016/0301-0082(95)00023-o. [DOI] [PubMed] [Google Scholar]
- Rostron CL, Farquhar MJ, Latimer MP, Winn P. The pedunculopontine tegmental nucleus and the nucleus basalis magnocellularis: Do both have a role in sustained attention? BMC Neurosci. 2008;9:16. doi: 10.1186/1471-2202-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller EM. Functional organization of the auditory pathways. In: Ehret G, Romand R, editors. The central auditory system. New York: Oxford University Press; 1997. pp. 3–96. [Google Scholar]
- Ruggiero DA, Giuliano R, Anwar M, Stornetta R, Reis DJ. Anatomical substrates of cholinergic-autonomic regulation in the rat. J Comp Neurol. 1990;292:1–53. doi: 10.1002/cne.902920102. [DOI] [PubMed] [Google Scholar]
- Rye DB. Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep. 1997;20:757–788. doi: 10.1093/sleep/20.9.757. [DOI] [PubMed] [Google Scholar]
- Rye DB, Lee HJ, Saper CB, Wainer BH. Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J Comp Neurol. 1988;269:315–341. doi: 10.1002/cne.902690302. [DOI] [PubMed] [Google Scholar]
- Shiromani PJ, Floyd C, Velazquez-Moctezuma J. Pontine cholinergic neurons simultaneously innervate two thalamic targets. Brain Res. 1990;532:317–322. doi: 10.1016/0006-8993(90)91774-b. [DOI] [PubMed] [Google Scholar]
- Shute CC, Lewis PR. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967;90:497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- Silva RC, Sandner G, Brandao ML. Unilateral electrical stimulation of the inferior colliculus of rats modifies the prepulse modulation of the startle response (PPI): effects of ketamine and diazepam. Behav Brain Res. 2005;160:323–330. doi: 10.1016/j.bbr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Steriade M, Paré D, Parent A, Smith Y. Projections of cholinergic and non-cholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey. Neuroscience. 1988;25:47–67. doi: 10.1016/0306-4522(88)90006-1. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: Current knowledge and future challenges. Psychopharmacology (Ber) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Turlejski K, Djavadian RL, Dreher B. Extent of bilateral collateralization among pontomesencephalic tegmental afferents to dorsal lateral geniculate nuclei of pigmented and albino rats. Neuroscience. 1994;60:521–535. doi: 10.1016/0306-4522(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Satoh K, Fibiger HC. The localization of central cholinergic neurons. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:637–656. doi: 10.1016/0278-5846(86)90033-3. [DOI] [PubMed] [Google Scholar]
- Wang HL, Morales M. Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: IV. Descending projections of the pontomesencephalic tegmentum. Brain Res Bull. 1989;23:519–540. doi: 10.1016/0361-9230(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Wu Y, Yan J. Modulation of the receptive fields of midbrain neurons elicited by thalamic electrical stimulation through corticofugal feedback. J Neurosci. 2007;27:10651–10658. doi: 10.1523/JNEUROSCI.1320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Lee J, Yeomans MH, Steidl S, Li L. Midbrain pathways for prepulse inhibition and startle activation in rat. Neuroscience. 2006;142:921–929. doi: 10.1016/j.neuroscience.2006.06.025. [DOI] [PubMed] [Google Scholar]