Abstract
Heat shock protein 27 kDa (Hsp27) functions as a molecular chaperon to prevent apoptosis as well as to contribute to the regulation of cell proliferation and differentiation during development. In the present study, the localization of Hsp27 in the oral epithelium of rats and its expression change during formation of the gingiva with the tooth eruption were examined immunohistochemically to elucidate the roles of Hsp27 in the oral mucosa.
In adult rats, Hsp27-immunoreactivity was localized in the prickle and granular layers but absent in the basal and horny layers of the oral epithelium. On the other hand, in the outer and sulcular epithelia of the free gingival, Hsp27-immunoreactivity was detected in the whole layers, while it was not found in the proliferation zone of the junctional epithelium immunoreactive for Ki67. In immature rats on 10th postnatal day, Hsp27-immunoreactivity was intense in the prickle and granular layers of the oral epithelium, but was not detected in its basal layer. In rats at the eruptive phase on 15th postnatal day, Hsp27-immunoreactivity was detected in sites of the basal layer adjacent to where the dental cusps penetrated through the oral epithelium. Although the immunoreactivity for Ki67 was found in the basal layer of the oral epithelium, it was not localized in the Hsp27-immunopositive sites of tooth-penetration in the basal layer. Just after the tooth-eruption on 20th postnatal day, Hsp27-immunoreactivity was not found in the stratified squamous epithelium at the dentogingival junction, whereas it was intense in a single layer of cuboidal epithelial cells attached to the tooth neck. Ki67-positive cells were scattered in the stratified squamous epithelium at the dentogingival junction, whereas no positive cells were found in the portion of a single layer of cuboidal epithelial cells.
These findings suggest that the outer and sulcular epithelia of the free gingiva have a relatively slower rate of proliferation than other gingival and oral epithelia, and that Hsp27 might inhibit the proliferation of the basal cells. Such specific phenomenon in the free gingiva occurred immediately after the dental cusps were exposed to the oral cavity.
Keywords: heat shock protein, gingiva, proliferation, tooth eruption
I. Introduction
The entire surface of the oral cavity is lined by the stratified squamous epithelium with/without keratinization [11, 13]. The main function of the epithelial lining is to protect the subepithelial and further internal environments [13]. Peculiarly specialized structures of the oral epithelial lining are teeth. The surface of the tooth-crown is covered by the enamel, a specialized material formed by ameloblasts of ectodermal origin. The enamel of erupted teeth lacks ameloblasts and any other cells which should play essential roles to regulate biological activities including the regeneration in response/compensation to attacks by cariogenic bacteria and daily strong masticatory forces. Accordingly, the junctional tissues between the oral epithelium and the tooth enamel should be responsible for guards against the biohazard and mechanical damages.
The gingiva is specially differentiated oral mucosa located at the mucosa-tooth junction and covers the alveolar bone and the cervical neck of the tooth. The gingiva is covered with keratinized stratified squamous epithelium and shows morphological variations reflecting the tissue adaptation to the tooth and alveolar bones (Fig. 1). The variations include the attachment gingival epithelium (AGE), oral gingival (or outer) epithelium (OE), oral sulcular epithelium (SE) and junctional epithelium (JE) [26, 27]. The gingival epithelia, especially SE and JE, are easily affected chemically and damaged physically by food debris, dental plaques and calculi including a variety of pathogenic microorganisms. To correspond to such conditions, the oral mucosal epithelium has biophylaxis mechanisms such as rapid renewal and regeneration in addition to the mucosal immune system [7, 10, 28]. Proliferation and differentiation of epithelial cells are known to be regulated by a variety of growth factors and cytokines [8]. However, little is known about the molecular mechanism regulating the conversion of cellular conditions such as proliferation, differentiation or cell death. In recent studies a family of heat shock proteins (HSP) has been suggested to regulate or switch the proliferation vs. differentiation or the proliferation vs. cell death [17, 31].
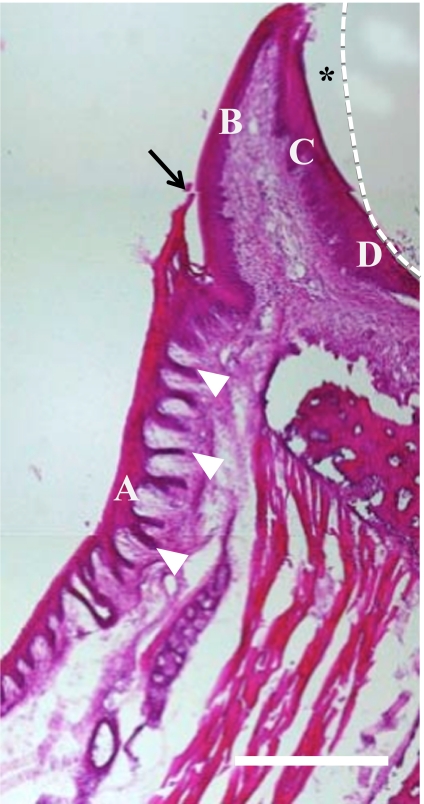
Fig. 1.
Histology of the gingiva of adult rats. The enamel is completely decalcified and diminished (dotted line). A, attached gingival epithelium (AGE); B, oral (outer) gingival epithelium (OE), C, oral sulcular epithelium (SE) facing the gingival sulcus (*), D, junctional epithelium (JE). In the present study, the outer gingiva in general is divided by the free gingival junction (arrow) into OE and AGE. AGE possesses abundant epithelial legs (arrowheads). Bars=100 µm.
HSPs are induced by heat-shocks and other non-physiological stimuli, and serve to protect against the cell death as molecular chaperons [3, 19]. HSPs are also expressed in cells under non-stress states, and are considered to play a variety of roles in addition to their anti-apoptotic roles [14]. HSPs are divided into major groups based on their respective molecular weights, among which Hsp27, a low molecular-mass species, plays an essential role in the regulation of cell proliferation, differentiation, and apoptosis [2, 4, 12, 20].
In developing epidermis and oral epithelium consisting of simple cuboidal epithelium, Hsp27 is strongly expressed in the basal cuboidal cells [30]. After development of the flattened suprabasal layer, the occurrence of Hsp27-immunoreactivity gradually shifts from the basal to suprabasal cells. Such a shift in the expression pattern of Hsp27 in developing stratified squamous epithelium from the basal cells to suprabasal cells representing cells of the mature prickle and granular layers, is the case widely in mature stratified squamous epithelium [15, 18, 30, 31]. On the other hand, because the transitional expression of Hsp27-immunoreactivity in the basal cells is also the case in specialized epithelial derivatives such as developing teeth, hair follicles, and taste buds [1, 30], Hsp27 has been proposed to regulate the keratinocyte proliferation and differentiation [15, 18].
In the present study, we examined the immunolocalization of Hsp27 in the gingival epithelium of rats, with focus on the regulation of keratinocyte proliferation with the histological changes of gingival epithelium during tooth eruption.
II. Materials and Methods
Laboratory animals
Wistar-strain male rats on postnatal 10th day (P10), P15, P16, P17, P18, P19, P20 and P45 (Japan SLC, Hamamatsu, Japan) were used in the present study. Standard chow and water were supplied ad libitum throughout the experimental period. Rats were maintained and used in accordance with the Guidelines for Care and Use of Laboratory Animals of Meikai University School of Dentistry, and the present experimental plan was approved by Meikai University Animal Ethics Committee (C0717).
Immunohistochemistry
Rats, after the anesthesia by the intraperitoneal injection with 1 ml/kg pentobarbital sodium (Somnopentyl, Kyoritsu Seiyaku, Tokyo, Japan), were perfused through the heart first with saline and subsequently with 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.2. Then the heads were extirpated and immersed in the same fixative overnight at 4°C. After fixation, the specimens were immersed in 0.1 M EDTA, pH 7.4, for 3 weeks at 4°C and then immersed overnight in 30% sucrose/phosphate buffer, pH 7.2. Sections of 10 µm thickness were cut on a cryostat and used for immunohistochemistry and hematoxylin-eosin (H-E) staining.
Cryosections on glass slides were treated for 60 min in 0.3% Triton X-100/phosphate-buffered saline (PBS), pH 7.4, and then in 10% normal goat serum (Nichirei, Tokyo, Japan) for 30 min.
After rinsing with PBS, the sections were treated overnight at room temperature with rabbit anti-mouse Hsp27 polyclonal antibody (1:500, Stressgen Bioreagents, Victoria, Canada) or goat anti-mouse Ki67 polyclonal antibody (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA). After rinsing with PBS, they were reacted for 60 min with the secondary antibody, Cy2-labeled goat anti-rabbit IgG (1:500, Chemicon, Temecula CA, USA) or Cy3-labeled donkey anti-goat IgG (1:500, Chemicon). As a control, some cryosections were treated with PBS in the absence of the primary antibody, and stained in the same way as above.
For double immunostaining, sections were treated overnight at room temperature with the mixture solution of rabbit anti-mouse Hsp27 polyclonal antibody (1:500, Stressgen Bioreagents) plus goat anti-mouse Ki67 polyclonal antibody (1:500, Santa Cruz Biotechnology), rabbit anti-mouse Hsp27 polyclonal antibody (1:500, Stressgen Bioreagents) plus goat anti-human keratin polyclonal antibody (1:500, Abcam plc, Cambridge, UK), or rabbit anti-mouse Hsp27 polyclonal antibody (1:500, Stressgen Bioreagents) plus mouse anti-mouse Hsp47 monoclonal antibody (1:500, Stressgen Bioreagents). After rinsing with PBS, they were reacted for 60 min with the mixture of secondary antibodies, Cy2 labeled goat anti-rabbit IgG (1:500, Chemicon) plus Cy3 labeled donkey anti-goat IgG (1:500, Chemicon) or Cy2 labeled goat anti-rabbit IgG (1:500, Chemicon) plus Cy3 labeled donkey anti-mouse IgG (1:500, Chemicon).
III. Results
Hsp27 localization in adult rat gingiva
Intense immunoreactivity for Hsp27 was localized in the prickle and granular layers of the stratified squamous epithelium throughout the gingiva, while the immunoreactivity in the basal layer was confined to OE and SE (Fig. 2a, b). No immunoreactivity was found in the horny layer throughout the gingival epithelium.
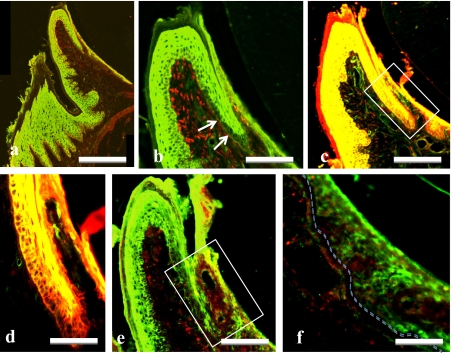
Fig. 2.
Double immunostaining for Hsp27 (green, a–f) plus Hsp47 (red, a, b), keratin (red, c, d), or Ki67 (red, e, f) in the gingiva of adult rats. Higher magnification of a, and solid frames in c and e were showed in b, d, f, respectively. Note that Hsp27-immunoreacitivity is localized in basal and suprabasal layer of OE and SE except for the transitional area (arrows) adjacent to JE. Basal layer of the transitional area (arrows in b and frames in c, e) is absent from Hsp27-immunoreacitivity and shows the presence of keratin and Ki67 immunoreactivities. Basal membrane is indicated by dotted line (f). Bars=100 µm (a); 50 µm (b, c, e); 10 µm (d, f).
In double immunostaining for Hsp27 and keratin, a marker of epithelial cells, co-localization for the two antigens was found the prickle and granular layers throughout the gingival epithelium (Fig. 2c). On the other hand, co-localization was also found in the basal layer of only OE and SE (Fig. 2d).
In double immunostaining for Hsp27 and Ki67, a proliferating cell marker, no co-localization for the two antigens was found throughout the gingival epithelia (Fig. 2e). Immunoreactivity for Ki67 was localized in nuclei of cells in the basal layer throughout the gingival and alveolar epithelia except for OE and SE in which Hsp27-immunoreactivity was detected in the basal layer. In JE, Ki67-immunopositive basal layer and Hsp27-immunopositive suprabasal layer were recognized (Fig. 2e, f).
Cells immunoreactive for Hsp47, a marker of fibroblasts producing collagen fibers, was localized subjacent to the epithelium and deeply in the lamina propria (Fig. 3a). The immunoreactivity for Hsp47 was also found in the basal layer of long epithelial legs throughout the gingival epithelia although it was not detected in the basal layer of short legs in the alveolar mucosal epithelium (Fig. 3b).
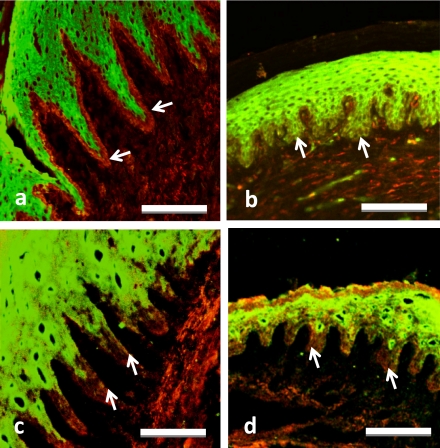
Fig. 3.
Double immunostaining for Hsp27 (green, a–d) plus Hsp47 (red, a, b) or Ki67 (red, c, d) in AGE (a, c) and alveolar mucosa (b, d) of adult rats. Arrows indicate the Hsp27-immunonegative basal layer with (a) and without (b) Hsp47-immunoreacitivity, and with Ki67-immunoreactivity (c, d). Bars=20 µm (a–d).
Cells immunoreactive for Ki67 were detected in the basal layer of attachment gingival epithelium (AGE) with long epithelial legs (Fig. 3c) and of alveolar mucosal epithelium with short epithelial legs (Fig. 3d). All epithelial cells and layers immunopositive for Ki67 were immunonegative for Hsp27. These results suggest that expression of Hsp27 is negatively regulated by cell proliferational activity.
Summary of immunohistochemical detection of Hsp27 and Ki67 is shown in Table 1.
Table 1.
Expression of Hsp27 and Ki67 in the rat gingival epithelium
| Gingival portions | AE | AGE | OE | SE | JE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Layer/Antigen | Hsp27 | Ki67 | Hsp27 | Ki67 | Hsp27 | Ki67 | Hsp27 | Ki67 | Hsp27 | Ki67 |
| basal | – | + | – | + | + | – | + | – | – | + |
| prickle/granular | + | – | + | – | + | – | + | – | + | – |
AE, alveolar epithelium; AGE, attached gingival epithelium; OE, oral (outer) gingival epithelium; SE, sulcular epithelium; JE, junctional epithelium.
Developing gingiva during tooth eruption
Before the tooth eruption (P10), molar tooth germs were covered by the oral mucosa and the enamel organ and the oral epithelium was connected by a short dental lamina (Fig. 4a, b). The oral epithelium covering the tooth germs showed the same localization pattern for Hsp27 as that of adult AGE and other types of the oral epithelium described in the previous section (Fig. 4c). The basal layer immunonegative for Hsp27 was continuous to the outer enamel epithelium via immunonegative dental lamina. Intense immunoreactivity for Hsp27 was detected in ameloblasts in the inner enamel epithelium, but was not in the stellate reticulum (Fig. 4c).
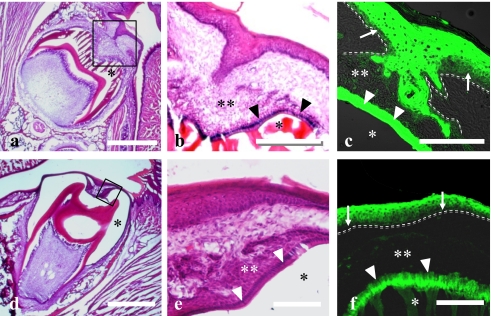
Fig. 4.
Light micrographs of the tooth germ and covering oral epithelium of rats of 10th day (a–c) and 15th (d–f) day after birth stained with H-E (a, b, d, e) or anti-Hsp27 antibody (c, f). Higher magnification of solid frames in a and d were showed in b and e, respectively. c and f represent similar areas to b and e, respectively. Basal membrane is indicated by dotted line (c, f). Hsp27-immunoreactivity was found in the prickle/granular and ameloblastic layers (arrowheads). The stellate reticulum (double asterisks in b, c) and papillary layer (double asterisks in e, f) as well as basal layer (arrows) were immunonegative. Single asterisk indicates the decalcified enamel. Bars=500 µm (a, d); 50 µm (b, c); 20 µm (e, f).
Shortly before the tooth eruption (P15), the oral mucosa accompanying a very thin lamina propria covered the molar tooth germ (Fig. 4d, e) and showed the same immunoreaction pattern as at the previous stage (Fig. 4f). Ameloblasts were immunopositive for Hsp27 (Fig. 4d). The stellate reticulum and papillary layer of the tooth germ were immunonegative for Hsp27 (Fig. 4f).
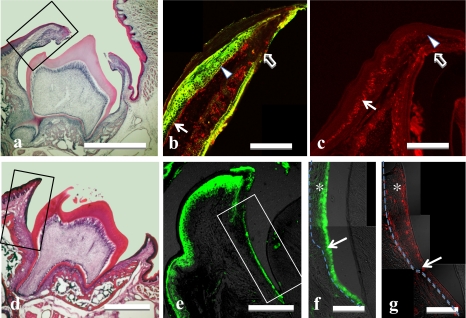
When small cracks occurred in the gingiva above the tooth germ and an apex of the cusp was first exposed to the oral cavity (P16, Fig. 5a), the epithelium covering the lateral surface of tooth crown was composed of keratinized stratified squamous cells at the oral cavity (outer) side and of simple columnar cells originating from the reduced enamel epithelium at the tooth (inner) side. The oral stratified epithelium suddenly changed to a reduced enamel epithelial layer about 1/3 from the gingival margin in the developing gingiva of tooth side (Fig. 5b). The immunoreactivity for Hsp27 was localized strongly at the prickle and granular layers of developing gingiva, and it was also found in the basal layer at a portion close to the gingival margin (Fig. 5b).
Fig. 5.
Light micrographs of rats (a–c: 16th day after birth; d–g: 20th day after birth) tooth germ and covering oral epithelium stained with H-E (a, d), double stained for Hsp27 (green; b) plus Hsp47 (red; b), or single stained for Ki67 (red; c, g), and Hsp27 (green; c, e, f). Areas of black solid frames in a and d are immunostained and shown at higher magnification in b, c, and e, respectively. Arrows (b, c) indicate the Hsp27-immunonegative basal layer in oral epithelium with Ki67. Arrowheads (b, c) indicate the Hsp27-immunopositive basal layer without Ki67. Open arrows in b and c indicate the junction of oral epithelium and reduced enamel epithelium. Area of solid frame (e) is higher magnified in f. Dotted lines (f, g) indicate the basal membrane. Arrows (f, g) indicate junction of the oral and the reduced enamel epithelia. Note that Hsp27-immunonegative epithelium (asterisk in f) contains many Ki67-immunopositive cells (asterisk in g). The single layer of cuboidal epithelium under the arrow (f) has no Ki67-immunopositive cells (g). Bars=500 µm (a, d); 50 µm (b, c, e); 20 µm (f, g).
No cell nuclei immunoreactive for Ki67 were found in the basal layer of a portion of the oral epithelium covering molar tooth germs close to the gingival margin (Fig. 5c). The basal layer without Ki67-immunoreactivity was reciprocally immunopositive for Hsp27 (Fig. 5b). In other portions of the epithelium farther away from the gingival margin though covering the still impacted molar crown, the basal layer was composed of cells Ki67-immunoreactive but Hsp27-immunonegative (Fig. 5b).
In P20, the tooth eruption advanced and the occlusal surface of the molar was almost exposed in the oral cavity (Fig. 5d). The root formation was still incomplete but the histological architecture of the gingiva was almost the same as that of adult specimens, although SE and JE were not fully differentiated in the epithelium facing the developing gingival sulcus.
The epithelium facing the gingival sulcus was composed of the reduced enamel epithelium and stratified squamous epithelium continuing to OE at the gingival margin. Hsp27-immunoreactivity was localized in whole layers of OE except for the horny layer (Fig. 5e). Hsp27 was not expressed in the basal layer of AGE while the other layers, prickle and granular, were immunopositive for Hsp27 (Fig. 5e). Only portions of stratified squamous epithelium facing the enamel of erupting crown exhibited Hsp27-immunoreactivity, and simple cuboidal epithelium formed by the reduced enamel epithelium exhibited Hsp27-immunoreactivity (Fig. 5f).
No Ki67-immunopositive nuclei were seen in simple cuboidal epithelium formed by the reduced enamel epithelium, but the immunoreactivity was localized in all layers of the stratified squamous epithelium (Fig. 5g). At this stage, no co-localization of Hsp27 and Ki67 was recognized.
IV. Discussion
The major finding of the present study is that, different from the AGE as well as the oral mucosal epithelium in which the immunoreactivity for Hsp27 was confined to the spinous and granular layers of the stratified squamous epithelium, the immunoreactivity was localized in the basal layer as well as the spinous and granular layers of the OE and SE in the free gingiva. It is known that the cell proliferation by mitosis takes place in the basal layer of the stratified squamous epithelium, and that newly generated cells migrate toward the epithelial surface through the spinous and granular layers with the differentiation to keratinocytes which is intimately associated with expression and maturation of keratin protein. Considering the knowledge on the cell proliferation and migration with differentiation in the stratified squamous epithelium, the confined localization of Hsp27 to the spinous and granular layers of the general stratified epithelium suggests the possibility that Hsp27 is involved in the differentiation of keratinocytes, and/or in the inhibition of the cell proliferation [9, 20, 32].
Previous experiments using mouse ES cells have shown that Hsp27 prevents apoptosis at the initial stage of differentiation and functions as a molecular switch from proliferation to differentiation, by involvement in the transient oligomer formation, prevention of protein aggregation, and promotion of decomposition of unnecessary protein [2–4]. It has also been shown that the inhibition of Hsp27 expression induces the ES cell proliferation and apoptosis, while the enhancement of Hsp27 expression results in inhibition of cell proliferation, apoptosis and progress of differentiation [20]. Regarding the possibility of the inhibition of cell proliferation, it is known that the overexpression of Hsp27 results in marked increase in the rate of translation of p21, a cyclin-depending kinase 2-related protein, whose gene promoter contains a binding site with p53 through which p21 is involved in inhibition of p53-inducing cell proliferation [23].
On the other hand, the localization of Hsp27 in the basal layer of OE and SE suggests the possibility that Hsp27 inhibits the cell proliferation in the free gingival. In support of this possibility, the present study showed no immunoreactivity for Ki67, a marker of cells at all cell cycle stages except for G0 [16], in the Hsp27-immunopositive cells in the basal layer of the OE and SE, and there has been data that no BrdU-positive cells are detected in the basal cells in the free gingival epithelium [29].
The present study showed that the specific localization pattern of Hsp27-immunoreactivity in the free gingival epithelium was completed shortly after the tooth eruption. A similar specific pattern of Hsp27-immunoreactivity has already been reported to occur in the nail bed epithelium, in which Hsp27 is suggested to inhibit the cell proliferation [30]. These results suggest that low proliferative activity in the free gingiva becomes evident immediately after the tooth eruption and gingival differentiation. Further studies are necessary to clarify detailed molecular mechanism of inhibition of cell proliferation by Hsp27.
Origin of gingival epithelium except for the JE is still controversial, whether the gingival epithelium comes from the reduced enamel epithelium or not. Ameloblasts in the late tooth germ express Hsp27 constitutively, while earlier stages of tooth germ express Hsp27 transitionally with the downregulation of ameloblast proliferation [22, 24]. As ameloblasts after the secretion phase constitutively express Hsp27 [24], our results at least indicate that a part of OE originates from the enamel epithelium during tooth eruption.
The present study also showed intense immunoreactivity for Hsp27 in cells located in the most outer zone in the JE in matured rats. These cells adhere directly to enamel by hemidesmosomes and outer basal lamina [28]. There have been data showing that phosphorylated Hsp27 regulates the binding of actin and intermediate filaments and pemphigus [21], an autoimmune disease characterized by the blistering of the prickle layer caused by the destruction of intercellular desmosomes, induces phosphorylation of Hsp27 and p38MAPK after a binding of the pemphigus IgG to keratinocytes. While the inhibitor of p38MAPK regulates a phosphorylation of Hsp27 and suppresses a degeneration of cytoskeleton in cultured keratinocytes [5, 6], Hsp27 may act in cell adherence in the gingival epithelium.
Additionally, the expression of Hsp47 was also found in the basal layer of AGE. Hsp47 has an affinity with various types of collagen and is expressed in fibroblasts [25]. Immunoreactivity of Hsp47 in the basal cells may indicate the active production of basal membrane components such as type IV collagen. The expression of Hsp47 of the basal layer was present in AGE accompanying numerous long epithelial legs. On the other hand, it was absent in other types of gingival/oral epithelia with smooth junctional borders. Epithelial legs in AGE may be linked with an attachment of abundant gingival fibers composed of type I collagen. Therefore, it is suggested that Hsp47 gingival epithelial cells is associated with the attachment and/or production of the components of gingival fibers.
V. Acknowledgment
This work was supported by a Grant-in-Aid for Scientific Research (C) of Japan Society for the Promotion of Science for O.A. (20592146).
VI. References
- 1.Adly M. A., Assaf H. A., Hussein M. R. Expression of the heat shock protein-27 in the adult human scalp skin and hair follicle: hair cycle-dependent changes. J. Am. Acad. Dermatol. 2006;54:811–817. doi: 10.1016/j.jaad.2005.11.1097. [DOI] [PubMed] [Google Scholar]
- 2.Arrigo A. P., Landry J. In “The Biology of Heat Shock Proteins and Molecular Chaperons”, ed. by R. I. Morimoto. Cold Spring Harbor Laboratory Press; New York: 1994. Expression and function of the low molecular weight heat shock proteins; pp. 335–374. [Google Scholar]
- 3.Arrigo A. P., Mehlen P. In “Heat Shock Proteins in the Nervous System”, ed. by R. J. Mayer and I. R. Brown. Academic Press; London: 1994. Expression, cellular localization and function of low molecular weight heat shock proteins (Hsp 20s) during development of the nervous system; pp. 145–167. [Google Scholar]
- 4.Arrigo A. P., Preville X. In “Handbook of Experimental Pharmacology. Vol. 136. Stress proteins”, ed. by D. S. Latchman. Springer; New York: 1999. Role of Hsp27 and related proteins; pp. 101–132. [Google Scholar]
- 5.Berkowitz P., Hu P., Liu Z., Diaz L. A., Enghild J. J., Chua M. P., Rubenstein D. S. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J. Biol. Chem. 2005;280:23778–23784. doi: 10.1074/jbc.M501365200. [DOI] [PubMed] [Google Scholar]
- 6.Berkowitz P., Hu P., Warren S., Liu Z., Diaz L. A., Rubenstein D. S. p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proc. Natl. Acad. Sci. U S A. 2006;103:12855–12860. doi: 10.1073/pnas.0602973103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bosshart D. D., Lang N. P. The junctional epithelium: from health to disease. J. Dent. Res. 2005;84:9–20. doi: 10.1177/154405910508400102. [DOI] [PubMed] [Google Scholar]
- 8.Byrne C., Hardman M. In “Mouse Development”, ed. by J. Rossant and P. P. L. Tam. Academic Press; San Diego: 2002. Integumentary structure; pp. 567–589. [Google Scholar]
- 9.Byrne C., Tainsky M., Fuchs E. Programming gene expression in developing epidermis. Development. 1994;120:2369–2383. doi: 10.1242/dev.120.9.2369. [DOI] [PubMed] [Google Scholar]
- 10.Cho M. I., Garant P. R. Development and general structure of the periodontium. Periodontol. 2000. 2000;24:9–27. doi: 10.1034/j.1600-0757.2000.2240102.x. [DOI] [PubMed] [Google Scholar]
- 11.Chung W. O., Dommisch H., Yin L., Dale B. A. Expression of defensins in gingiva and their role in periodontal health and disease. Curr. Pharm. Des. 2007;13:3073–3083. doi: 10.2174/138161207782110435. [DOI] [PubMed] [Google Scholar]
- 12.Ciocca D. R., Oesterreich S., Chamness G. C., McGuire W. L., Fuqua S. A. Biological and clinical implications of heat shock protein 27000 (Hsp27): a review. J. Natl. Cancer Inst. 1993;85:1558–1570. doi: 10.1093/jnci/85.19.1558. [DOI] [PubMed] [Google Scholar]
- 13.Fiorellini J. P., Kim D. M., Ishikawa S. O. In “Carramaza’s Clinical Periodontology, 10th ed.”, ed. by M. G. Newman, H. H. Takei and P. R. Klokkevold. Sanders Elsevier; St. Louis: 2006. The gingiva; pp. 46–67. [Google Scholar]
- 14.Georgopoulos C., Welch W. J. Role of the major heat shock proteins as molecular chaperones. Annu. Rev. Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 15.Hell-Pourmojib M., Neuner P., Fischer H., Rezaie S., Kindas-Mugge I., Knoler R., Trautinger F. Differential expression of a novel gene in response to hsp27 and cell differentiation in human keratinocytes. J. Invest. Dermatol. 2002;119:154–159. doi: 10.1046/j.1523-1747.2002.01793.x. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa K., Sakai H., Hosoi M., Yanai T., Masegi T. Evaluation of cell proliferation in canine tumors by the bromodeoxyuridine labeling method: Immunostaining of Ki-67 antigen and proliferating cell nuclear antigen. J. Toxicol. Pathol. 2006;19:123–127. [Google Scholar]
- 17.Kiriyama M. T., Oka M., Takehana M., Kobayashi S. Expression of a small heat shock protein 27 (HSP27) in mouse skin tumors induced by UVB-irradiation. Biol. Pharm. Bull. 2001;24:197–200. doi: 10.1248/bpb.24.197. [DOI] [PubMed] [Google Scholar]
- 18.Laplante A. F., Moulin V., Auger F. A., Landry J., Li H., Morrow G., Tanguay R. M., Germain L. Expression of heat shock proteins in mouse skin during wound healing. J. Histochem. Cytochem. 1998;46:1291–1301. doi: 10.1177/002215549804601109. [DOI] [PubMed] [Google Scholar]
- 19.Lindquist S., Craig E. A. The heat-shock proteins. Ann. Rev. Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 20.Mehlen P., Mehlen A., Godet J., Arrigo A. P. hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J. Biol. Chem. 1997;272:31657–31665. doi: 10.1074/jbc.272.50.31657. [DOI] [PubMed] [Google Scholar]
- 21.Miron T., Vancompernolle K., Vandekerckhove J., Wilchek M., Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J. Cell. Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakasone N., Yoshie H., Ohshima H. An immunohistochemical study of the expression of heat-shock protein-25 and cell proliferation in the dental pulp and enamel organ during odontogenesis in rat molars. Arch. Oral Biol. 2006;51:378–386. doi: 10.1016/j.archoralbio.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Park S. H., Lee Y. S., Osawa Y., Hachiya M., Akashi M. Hsp 25 regulates the expression of p21(Waf1/Cip1/Sdi1) through multiple mechanisms. J. Biochem. 2002;131:869–875. doi: 10.1093/oxfordjournals.jbchem.a003177. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki A., Yamada T., Doi T., Okayasu M., Tokunaga H., Kanegae H., Amano O. Localization of 27 kDa heat-shock protein (Hsp27) in molar tooth germ of mouse embryos. J. Meikai Dent. Med. 2009;38:58–66. [Google Scholar]
- 25.Sauk J. J., Norris K., Moehring J., Foster R. A., Somerman M. J. The expression of colligin/hsp47 after stress in human periodontal fibroblasts in vitro. Arch. Oral Biol. 1990;35:645–651. doi: 10.1016/0003-9969(90)90031-5. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder H. E. In “Handbook of Microscopic Anatomy, Vol. 5, The Periodontium”, ed. by A. Oksche and L. Vollrath. Springer-Verlag; Berlin: 1986. Gingiva; pp. 233–323. [Google Scholar]
- 27.Schroeder H. E. Georg Thime Medical Publisher; New York: 1991. Oral Structural Biology. [Google Scholar]
- 28.Shimono M., Ishikawa T., Enokiya Y., Muramatsu T., Ichi K., Inoue T., Abiko Y., Yamaza T., Kido M., Tanaka T., Hashimato S. Biological characteristics of junctional epithelium. J. Electron Microsc. 2003;52:627–639. doi: 10.1093/jmicro/52.6.627. [DOI] [PubMed] [Google Scholar]
- 29.Takano S., Kiyoshima T., Kobayashi I., Moroi R., Ibuki T., Nagadome M., Terada Y., Sakai H. Age-dependent changes in the distribution of BrdU-and TUNEL-positive cells in the murine gingival tissue. J. Periodontol. 1999;70:973–981. doi: 10.1902/jop.1999.70.9.973. [DOI] [PubMed] [Google Scholar]
- 30.Tani Y., Yamada T., Horiuchi Y. Immunolocalization of heat shock protein 25 kDa (Hsp25) in the development of mouse oral epithelium. J. Meikai Dent. Med. 2006;35:76–85. [Google Scholar]
- 31.Wakayama T., Iseki S. Expression and cellular localization of the mRNA for the 25-kDa heat shock protein in the mouse. Cell Biol. Int. 1998;22:295–304. doi: 10.1006/cbir.1998.0252. [DOI] [PubMed] [Google Scholar]
- 32.Yuspa S. H., Hennings H., Tucker R. W., Jaken S., Kilkenny A. E., Roop D. R. Signal transduction for proliferation and differentiation in keratinocytes. Ann. N. Y. Acad. Sci. 1988;548:191–196. doi: 10.1111/j.1749-6632.1988.tb18806.x. [DOI] [PubMed] [Google Scholar]