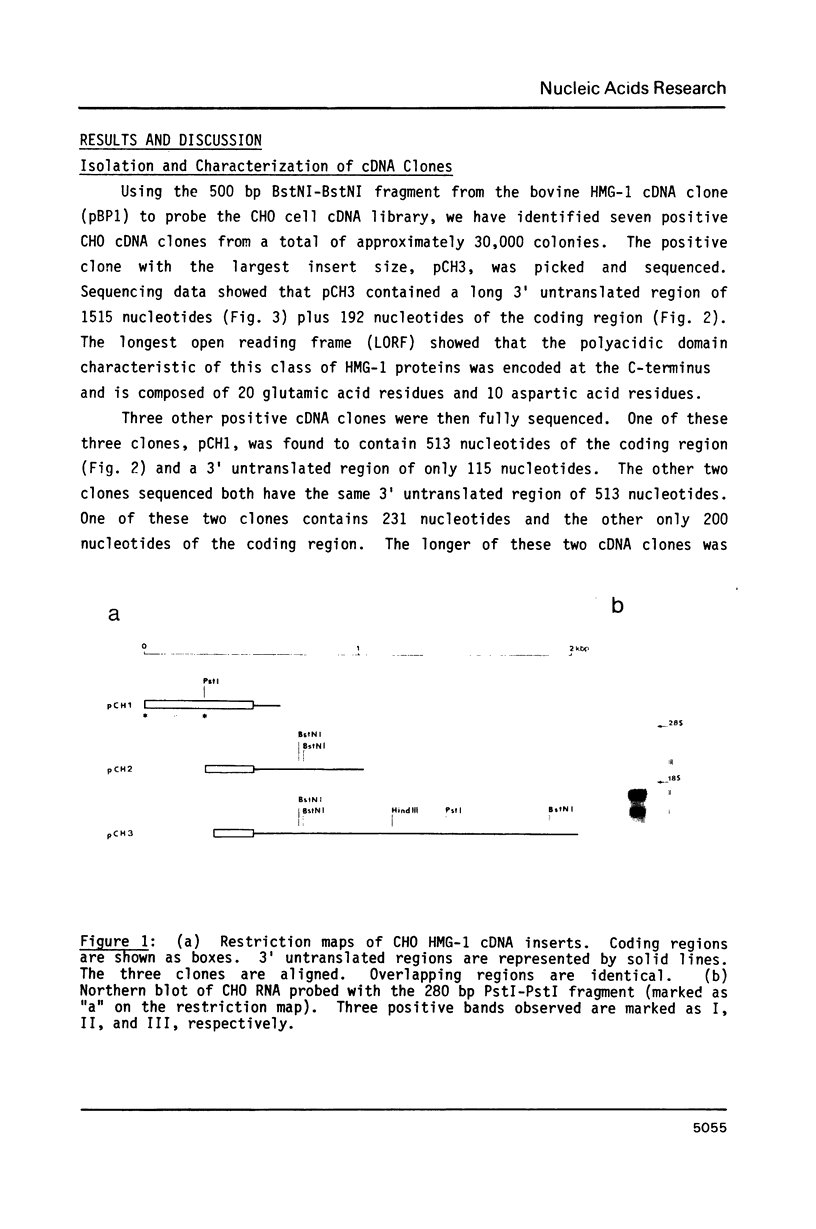
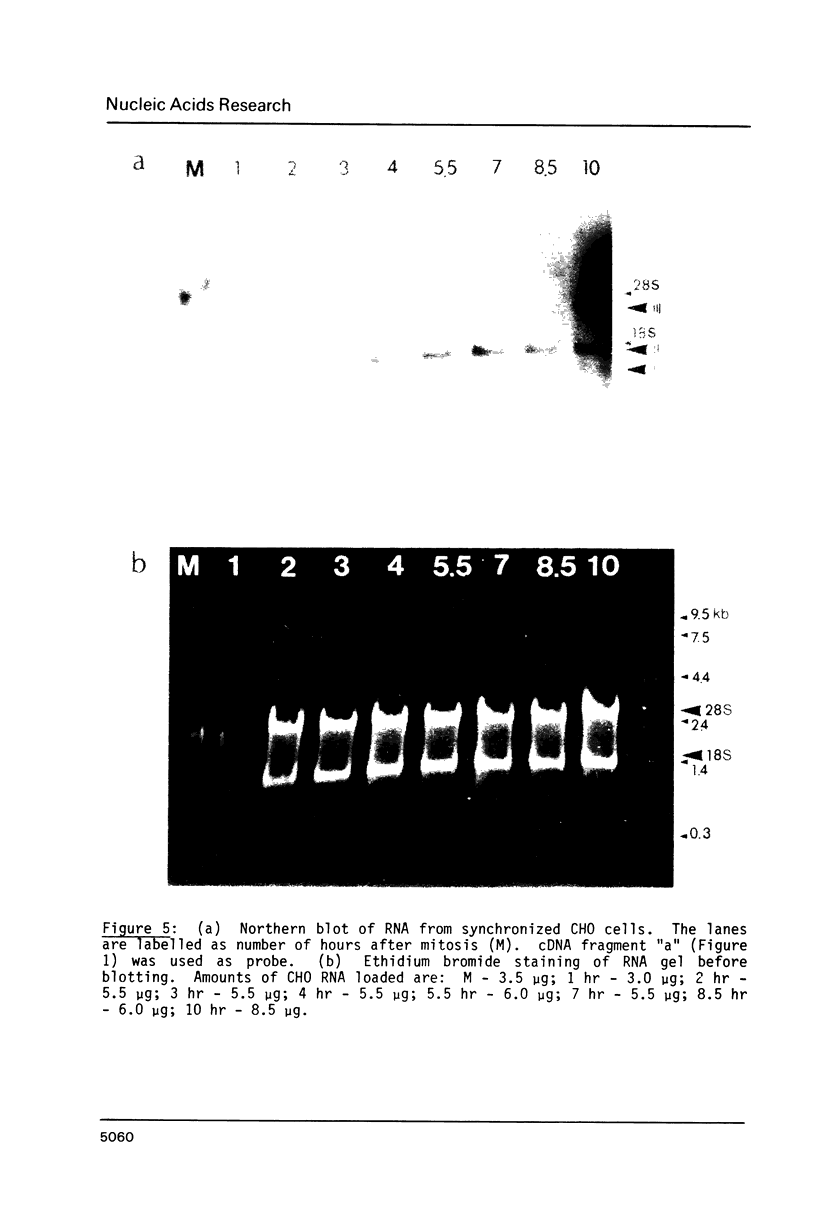
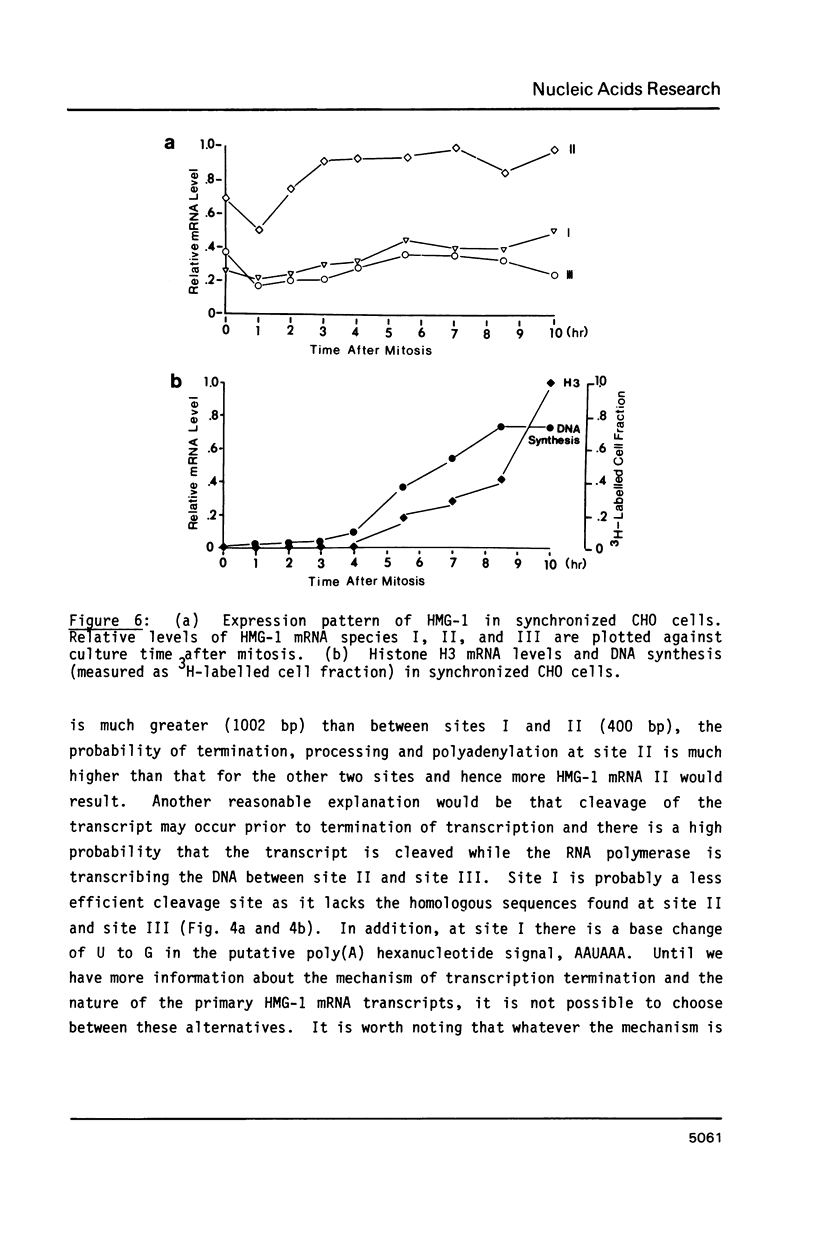
Abstract
We have isolated cDNA clones encoding the high mobility group (HMG) protein HMG-1 in line CHO Chinese hamster cells. The cDNA clones correspond to the three HMG-1 mRNA species detected on Northern blots. Three different polyadenylation sites are found to be used. The three mRNA species of sizes 1.05, 1.45 and 2.45 kb are generated by differential polyadenylation at sites 115 nucleotides, 513 nucleotides and 1515 nucleotides downstream from the stop codon. A perfectly conserved putative poly(A) signal AAUAAA is present upstream of only one of the three poly(A) sites. Two homologous but imperfect sequences exist upstream from the other two poly(A) sites. All three HMG-1 mRNA species maintain significant levels throughout the M, G1 and S phases of the cell cycle and the rate of large HMG protein (HMG-1 and HMG-2) synthesis increases approximately two-fold from G1 to S phase.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alterman R. B., Ganguly S., Schulze D. H., Marzluff W. F., Schildkraut C. L., Skoultchi A. I. Cell cycle regulation of mouse H3 histone mRNA metabolism. Mol Cell Biol. 1984 Jan;4(1):123–132. doi: 10.1128/mcb.4.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco J., States J. C., Dixon G. H. General method for isolation of DNA sequences that interact with specific nuclear proteins in chromosomes: binding of the high mobility group protein HMG-T to a subset of the protamine gene family. Biochemistry. 1985 Dec 31;24(27):8021–8028. doi: 10.1021/bi00348a028. [DOI] [PubMed] [Google Scholar]
- Bonne-Andrea C., Harper F., Sobczak J., De Recondo A. M. Rat liver HMG1: a physiological nucleosome assembly factor. EMBO J. 1984 May;3(5):1193–1199. doi: 10.1002/j.1460-2075.1984.tb01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary P. D., Turner C. H., Leung I., Mayes E., Crane-Robinson C. Conformation and domain structure of the non-histone chromosomal proteins HMG 1 and 2. Domain interactions. Eur J Biochem. 1984 Sep 3;143(2):323–330. doi: 10.1111/j.1432-1033.1984.tb08375.x. [DOI] [PubMed] [Google Scholar]
- Chandler P. M., Rimkus D., Davidson N. Gel electrophoretic fractionation of RNAs by partial denaturation with methylmercuric hydroxide. Anal Biochem. 1979 Oct 15;99(1):200–206. doi: 10.1016/0003-2697(79)90063-0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Citron B., Falck-Pedersen E., Salditt-Georgieff M., Darnell J. E., Jr Transcription termination occurs within a 1000 base pair region downstream from the poly(A) site of the mouse beta-globin (major) gene. Nucleic Acids Res. 1984 Nov 26;12(22):8723–8731. doi: 10.1093/nar/12.22.8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Anna J. A., Becker R. R., Tobey R. A., Gurley L. R. Composition and synthesis during G1 and S phase of a high mobility group-E/G component from Chinese hamster ovary cells. Biochim Biophys Acta. 1983 Mar 10;739(2):197–206. doi: 10.1016/0167-4781(83)90030-1. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Gurley L. R., Tobey R. A. Syntheses and modulations in the chromatin contents of histones H1 degrees and H1 during G1 and S phases in Chinese hamster cells. Biochemistry. 1982 Aug 17;21(17):3991–4001. doi: 10.1021/bi00260a014. [DOI] [PubMed] [Google Scholar]
- D'Anna J. A., Thayer M. M., Tobey R. A., Gurley L. R. G1- and S-phase syntheses of histones H1 and H1o in mitotically selected CHO cells: utilization of high-performance liquid chromatography. Biochemistry. 1985 Apr 9;24(8):2005–2010. doi: 10.1021/bi00329a031. [DOI] [PubMed] [Google Scholar]
- Frayne E. G., Leys E. J., Crouse G. F., Hook A. G., Kellems R. E. Transcription of the mouse dihydrofolate reductase gene proceeds unabated through seven polyadenylation sites and terminates near a region of repeated DNA. Mol Cell Biol. 1984 Dec;4(12):2921–2924. doi: 10.1128/mcb.4.12.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebelhaus D. H., Heikkila J. J., Schultz G. A. Changes in the quantity of histone and actin messenger RNA during the development of preimplantation mouse embryos. Dev Biol. 1983 Jul;98(1):148–154. doi: 10.1016/0012-1606(83)90343-3. [DOI] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman D., Bustin M. Chromosomal proteins HMG-14 and HMG-17. Distinct multigene families coding for similar types of transcripts. J Biol Chem. 1986 Dec 5;261(34):16087–16091. [PubMed] [Google Scholar]
- Leff S. E., Rosenfeld M. G., Evans R. M. Complex transcriptional units: diversity in gene expression by alternative RNA processing. Annu Rev Biochem. 1986;55:1091–1117. doi: 10.1146/annurev.bi.55.070186.005303. [DOI] [PubMed] [Google Scholar]
- Levy B. W., Connor W., Dixon G. H. A subset of trout testis nucleosomes enriched in transcribed DNA sequences contains high mobility group proteins as major structural components. J Biol Chem. 1979 Feb 10;254(3):609–620. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner R. J., Lai C., Nave K. A., Lenoir D., Ogata J., Sutcliffe J. G. Nucleotide sequences of two mRNAs for rat brain myelin proteolipid protein. Cell. 1985 Oct;42(3):931–939. doi: 10.1016/0092-8674(85)90289-2. [DOI] [PubMed] [Google Scholar]
- Pentecost B. T., Wright J. M., Dixon G. H. Isolation and sequence of cDNA clones coding for a member of the family of high mobility group proteins (HMG-T) in trout and analysis of HMG-T-mRNA's in trout tissues. Nucleic Acids Res. 1985 Jul 11;13(13):4871–4888. doi: 10.1093/nar/13.13.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentecost B., Dixon G. H. Isolation and partial sequence of bovine cDNA clones for the high-mobility-group protein (HMG-1). Biosci Rep. 1984 Jan;4(1):49–57. doi: 10.1007/BF01120823. [DOI] [PubMed] [Google Scholar]
- Queen C., Korn L. J. A comprehensive sequence analysis program for the IBM personal computer. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):581–599. doi: 10.1093/nar/12.1part2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Stein A., Whitlock J. P., Jr, Bina M. Acidic polypeptides can assemble both histones and chromatin in vitro at physiological ionic strength. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5000–5004. doi: 10.1073/pnas.76.10.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey R. A., Anderson E. C., Petersen D. F. Properties of mitotic cells prepared by mechanically shaking monolayer cultures of Chinese hamster cells. J Cell Physiol. 1967 Aug;70(1):63–68. doi: 10.1002/jcp.1040700109. [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Ley K. D. Isoleucine-mediated regulation of genome repliction in various mammalian cell lines. Cancer Res. 1971 Jan;31(1):46–51. [PubMed] [Google Scholar]
- Yoshida M. High glutamic and aspartic region in nonhistone protein HMG(1+2) unwinds DNA double helical structure. J Biochem. 1987 Jan;101(1):175–180. doi: 10.1093/oxfordjournals.jbchem.a121888. [DOI] [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Characterization of the chicken vimentin gene: single copy gene producing multiple mRNAs. Proc Natl Acad Sci U S A. 1983 Feb;80(4):911–915. doi: 10.1073/pnas.80.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]