Abstract
The morphology and development of flowers and pseudanthia of Calycopeplus paucifolius are described in detail in the context of recent molecular phylogenies of the tribe Euphorbieae and a recent comparative developmental analysis of other taxa within this tribe. Calycopeplus resembles subtribes Neoguillauminiinae and Anthosteminae in some respects (dichasial formation of male flowers within male partial inflorescences, late formation of a constriction in male and female flowers and early formation of a female perianth), but resembles Dichostemma (subtribe Anthosteminae) in possessing only four male partial inflorescences. Calycopeplus and all other Euphorbieae possess only three carpels, except Dichostemma, which has four carpels per female flower. The studied species differs from the closely related Neoguillauminia cleopatra (subtribe Neoguillauminiinae) in that only four nectaries are formed, situated on the rim of the cuplike involucre (in Neoguillauminia 8–10 nectaries arise directly from the base of the pseudanthium). In contrast to all other studied Euphorbieae with trimerous gynoecia, the unpaired carpel of C. paucifolius is oriented in an upper/adaxial position (it lies in the lower/abaxial position in all other studied taxa). On the basis of these results we discuss possible pathways of cyathium evolution and the role of the cyathium as a possible key innovation within Euphorbieae.
‘Calycopeplus is as perfect an example of a connecting link as a morphologist may wish for.’ (Croizat 1937, p. 404)
Introduction
In the genus Euphorbia and its close relatives in subtribe Euphorbiinae (tribe Euphorbieae), the reproductive unit – termed a cyathium – closely resembles a bisexual flower. The cyathium bears clusters of stamens, which are widely interpreted as highly reduced male flowers, surrounding a single terminal gynoecium, which is widely interpreted as a female flower. In a recent paper (Prenner and Rudall 2007) we addressed the problem of the ‘fuzzy’ organ–flower–inflorescence boundaries within the pseudanthia of Euphorbieae, by using comparative morphological and ontogenetic data from the related subtribes Anthosteminae and Neoguillauminiinae. We identified some apparent bauplan shifts in reproductive morphology during the evolution of Euphorbieae (see also Prenner et al. 2008). However, our final conclusions on the evolution of these structures were hampered by the lack of material of the critical Australian genus Calycopeplus (Neoguillauminiinae). Since then, one of us (SDH) obtained material of C. paucifolius which allowed us to complete the comparative ontogenetic analysis of pseudanthia in Euphorbieae. Following our earlier results, we focus particularly on bauplan shifts within this group, in order to address questions about the evolution of the cyathium in Euphorbieae.
Systematic background
The genus Calycopeplus Planch. was described by Planchon (1861), based on C. ephedroides Planch. (=C. paucifolius [Klotsch] Baill.), but was soon sunk into Euphorbia L. by Boissier (1862). Subsequently, Baillon (1866) and Bentham (1873) favoured retaining a distinct genus Calycopeplus and Bentham (1873) added a new species, C. marginatus Benth. Interestingly in the present context, Baillon's (1866) decision to retain a distinct genus Calycopeplus was based on his opinion that the reproductive unit in Calycopeplus is an inflorescence, whereas he regarded that of Euphorbia as a true flower. Bentham (1873) did not agree with this interpretation, and treated both genera as having inflorescences/pseudanthia (see also Bentham 1880).
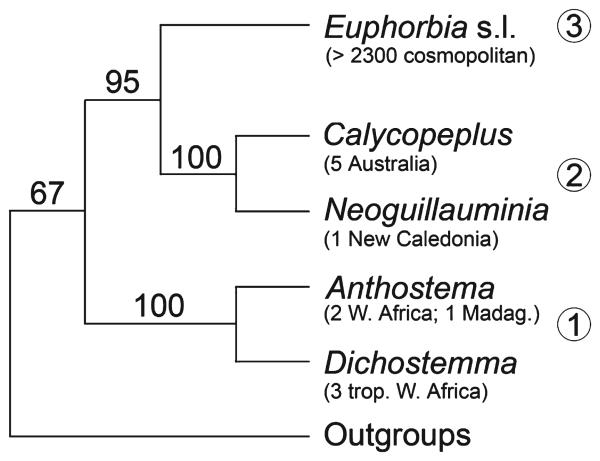
Croizat (1937) considered the genus Calycopeplus to be ‘very near Euphorbia and may be accepted for an archetype of it.’ However, he hesitated about the systematic value of the presence v. absence of a male perianth and finally placed Dichostemma Pierre with Neoguillauminia Croizat in the new subtribe Neoguillauminiinae. Subsequently, Webster's (Webster 1975, 1994b) reclassification transferred Calycopeplus to Neoguillauminiinae and placed Anthostema A.Juss. and Dichostemma together in subtribe Anthosteminae. Webster's proposed close relationships between the New Caledonian Neoguillauminia and the Australian Calycopeplus on the one hand, and between the African and Madagascan genera Anthostema and Dichostemma on the other, were later confirmed by molecular analyses (Fig. 1) (Steinmann and Porter 2002; Wurdack et al. 2005).
Fig. 1.
Systematic position of Calycopeplus shown on summary tree of relationships in Euphorbieae, based on Steinmann and Porter's (2002) weighted maximum parsimony analysis of the ITS region (bootstrap percentages >50% above branches). Clade 1 = Anthosteminae, 2 = Neoguillauminiinae, 3 = Euphorbiinae. Number and distribution of species are shown in parentheses below taxon name (Modified from Prenner and Rudall 2007).
According to Forster (1995), Calycopeplus consists of five species from Queensland (C. casuarinoides L.S.Sm.), Northern Territory (C. collinus P.I.Forst.) and Western Australia (C. paucifolia, C. marginatus and C. oligandrus P.I.Forst.). Our study species, C. paucifolius, is a shrub or subshrub, with stems round in cross-section and longitudinally cylindrical. It has male flowers in clusters of 3–7 flowers and the female flower is sessile or shortly pedicellate with pedicels up to 1.8 mm.
Materials and methods
Calycopeplus paucifolius was collected by one of us (SDH, No. 8687) in Western Australia, NE of Merredin, western foot of Yanneymooning Hill. The shrubs (2–3 m high) formed a dense thicket flanking a massive granite bornhardt in shallow sandy soil. Herbarium material was deposited at Royal Botanic Gardens, Kew, and Perth. Details of material of other Euphorbiaceae included for comparison (Fig. 5) are given in Prenner and Rudall (2007).
Fig. 5.
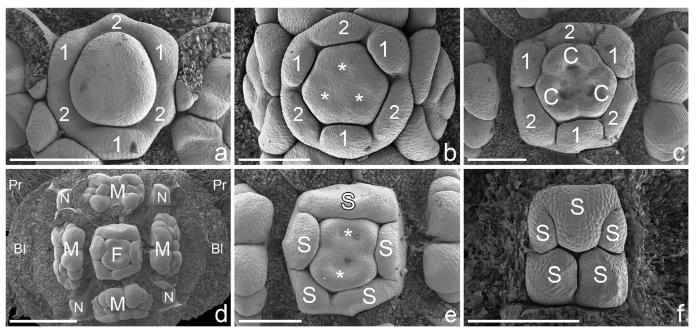
Bauplan shifts in pseudanthia of (a, b) Neoguillauminiinae, (c, f ) Euphorbiinae and (d, e) Anthosteminae. All figures in natural orientation with the adaxial side (main axis) uppermost (scanning electron micrographs). (a) Calycopeplus paucifolius: pseudanthium preceded by two lateral bracteoles and two lateral prophylls (removed). Female flower with one carpel in adaxial/median position and two in lateral/abaxial position (asterisks; for details see also Figs 1 and 2). (b) Neoguillauminia cleopatra (Baill.) Croizat: pseudanthium preceded by two lateral bracteoles (not shown), female flower with two carpels in adaxial position and one in abaxial/median position (asterisks). (c) Euphorbia ammak Schweinf.: pseudanthium preceded by two lateral bracteoles; orientation of female flower as in (b). (d) Dichostemma glaucescens Pierre: pseudanthium preceded by two bracteoles in median position and one unpaired prophyll in lateral position. Tetramerous female flower, with two carpels in median and two in lateral position (asterisks). (e) Anthostema madagascariense Baill.: pseudanthium preceded by two bracteoles in median position and two prophylls in lateral position. Female flower with two carpels in adaxial and one in abaxial position (asterisks). ( f ) Monadenium torrei L.C.Leach: pseudanthium preceded by two lateral bracteoles. Orientation of female flower as in (b, c and e). Bl, bracteole; C, carpel; M, male partial inflorescence; M′, position of fifth male partial inflorescence; N, nectaries (not shown in all figures); Pr, prophyll. Scale bars = 500 μm (a–e), 300 μm (f).
Fresh material of Calycopeplus paucifolius was fixed in formalin-acetic acid alcohol (FAA) in the field and transferred to 70% ethanol before examination. For scanning electron microscope (SEM) examination, material was dissected in 70% ethanol, dehydrated through an alcohol series to absolute ethanol, and critical-point-dried with a Balzers CPD 020 (BALT-TEC AG, Lichtenstein). Dried material was further dissected and mounted onto specimen stubs with nail polish, coated with platinum by using an Emitech K550 sputter coater (Emitech, Ashford, UK), and examined with a Hitachi cold field emission SEM S–4700–II (Hitachi High Technologies Corp., Tokyo, Japan).
Results
Earliest ontogenetic stages (Fig. 2)
Fig. 2.
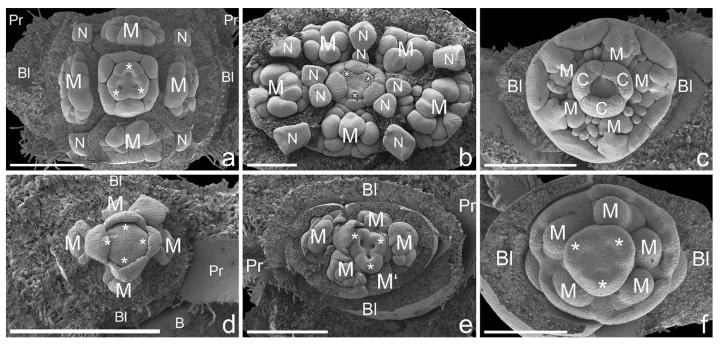
Calycopeplus paucifolius, early pseudanthium formation. (a–e) Scanning electron micrographs, (f) macrophotograph. (a) Pseudanthium initial in the axil of a bract and preceded by two lateral prophylls. (b) Lateral prophylls enlarge and enclose the young pseudanthium. (c) Same as (b), lateral prophylls removed. Young pseudanthium preceded by two lateral bracteoles (i.e. inner prophylls). Formation of first abaxial male bract. (d) Somewhat older developmental stage. Lateral prophylls enlarge and begin to enclose the young pseudanthium. (e) Lateral prophylls and bracteoles removed to show young pseudanthium with abaxial male bract removed and three remaining male bracts just formed (asterisks). (f) Side views of two pseudanthia in the axils of bracts. Note the small prophylls (asterisks). The pseudanthia are entirely enclosed by the bracteoles. B, bract; Bl, bracteole; MA, main axis; MB, male bract; Pr, prophyll; Ps, pseudanthium. Scale bars = 200 μm (a–e) and 1 mm (f).
The pseudanthium of Calycopeplus paucifolius is formed in the axil of a bract and preceded by two transversal prophylls and two bracteoles (i.e. inner prophylls) that are also formed in a transversal/lateral position (Fig. 2a–c). The prophylls enlarge and, together with the bract, contribute to bud protection during these earliest developmental stages (Fig. 2b). Later the growth of these organs ceases, and bud protection is taken over by the two bracteoles (Fig. 2c–e). In older buds, the prophylls are found as small scales flanking the pseudanthial buds (Fig. 2f). Shortly after the formation of the two lateral bracteoles, the first male bract is formed in an abaxial position (Fig. 2c, d). The remaining three male bracts are initiated more or less simultaneously (Fig. 2e). Each of the four male bracts subtends one male partial inflorescence (Fig. 3a–c). Four nectaries are formed at the positions where adjacent male bracts fuse (Fig. 3a–j).
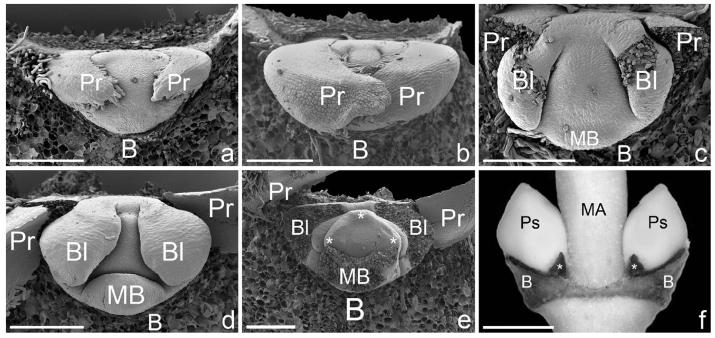
Fig. 3.
Calycopeplus paucifolius, pseudanthium development (scanning electron micrographs). (a) Young pseudanthium (frontal view) in the axil of a bract and preceded by lateral prophylls and bracteoles (removed). Four male partial inflorescences (two in transversal and two in median position) surrounding central female flower. Each of the male partial inflorescences is subtended by a bract (arrow, bract removed) and flanked by nectaries (asterisks). (b) Abaxial view of (a), showing the massive bract and lateral bracteoles (removed). (c) Abaxial male partial inflorescence flanked by two nectaries. The bract of the male partial inflorescences also becomes the bract of the first-formed male flower (1). Two lateral phyllomes will become the bracts of two lateral flowers (see e, f ). (d) Similar to (a), but with central female flower somewhat younger (see Fig. 4 for details) and male partial inflorescences somewhat older. (e) Right male partial inflorescence of (d), flanked by two nectaries. Three male flowers (1–3), of which each is subtended by a bract (arrows; bracts removed). Two adaxial organs arise (asterisks). (f) Abaxial male partial inflorescence, similar to (e) but with only one adaxial organ (asterisk). (g) Somewhat older pseudanthium, overview. (h) Abaxial male partial inflorescence of (g). Adaxial bracts enlarged and appear to be ‘empty’ bracts (asterisks) that do not subtend further male flowers. Two more male flowers are formed in abaxial position (4, 5). (i) Adaxial male partial inflorescence of (g). Only one adaxial bract is formed (asterisk). Male flowers still undifferentiated. (j) Pseudanthium with distinctly enlarged nectaries and male partial inflorescences which cover the reduced(?) female flower in the centre (asterisk). Male bracts removed in all but the right male partial inflorescence. (k) Male partial inflorescence (frontal view) with five male flowers, of which the first three already show distinct anthers. (l) Tilted side view of (k), showing the central male flower (1) without constriction, and two lateral male flowers (4, 5) in which anther formation starts. Arrows in (a–i) denote bracts and point from the bract towards the structure which they are subtending; B, bract; Bl, bracteole; F, female flower; M, male partial inflorescence; N, nectary; Pr, prophyll. Scale bars = 500 μm (a, b, d, g, j), 200 μm (c, e, f) and 300 μm (h, i, k, l).
Male partial inflorescences (Fig. 3)
Male partial inflorescences are initiated more or less simultaneously with the central female flower (Fig. 3a). A central male flower develops first, associated with the male bract (Fig. 3c, e, f). Two later-formed lateral phyllomes (Fig. 3c) represent the subtending bracts for two lateral male flowers (Fig. 3e, f). Next, either a pair of phyllomes or a single phyllome is initiated in an adaxial position (Fig. 3e, f, h, i); these structures appear to be empty, since no male flowers are formed in their axils. Two later-formed abaxial phyllomes again subtend two male flowers, which develop in rapid succession (Fig. 3h, i). Formation of anthers and maturation of male flowers follows the sequence in which the flowers were originally initiated (Fig. 3k, l). Formation of a constriction on the filament is late; in our material, the oldest male flower still lacked a constriction (Fig. 3l).
Female flower (Figs 3, 4)
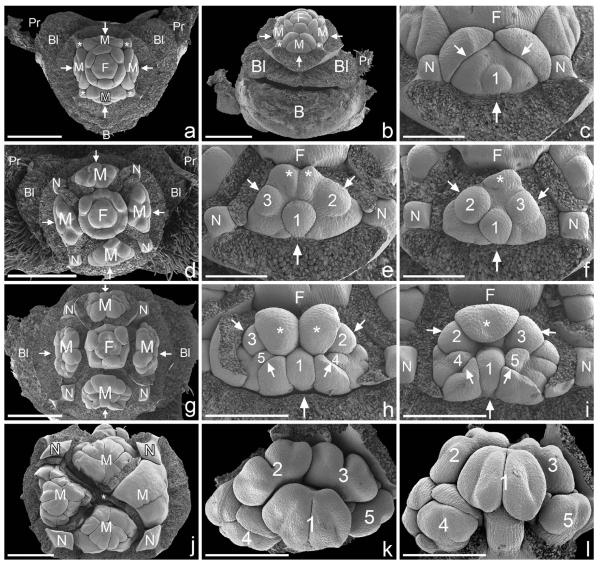
Fig. 4.
Calycopeplus paucifolius, development of female flowers in natural orientation with the adaxial side (main axis) on top (scanning electron micrographs). (a) Young female flower with early formed perianth. Three lobes are formed first (1), followed by three lobes shortly afterwards (2). The perianth appears to be fused from the very beginning. (b) Perianth lobes enlarge and three carpels begin to form (asterisks). (c) Female flower with six perianth lobes and three carpels, one in median/adaxial position, two in abaxial/transversal position. (d) Pseudanthium with dimerous female flower (overview). (e) Detail of (d). The female flower consists of five perianth lobes and two carpels in a median position (asterisks). (f) Older female flower with closed pentamerous perianth. Bl, bracteole; C, carpel; F, female flower; M, male partial inflorescence; S, perianth lobes. Scale bars = 200 μm (a-c, e, f) and 500 μm (d).
Initiation and maturation of the central female flower appears to be more or less parallel with the initiation and maturation of the male partial inflorescences (Fig. 3a, d, g). The female flower appeared to be reduced in some older pseudanthia, but at least a reduced carpel (i.e. carpellodium) was present in all pseudanthia investigated. A hexamerous perianth is formed in the female flower. Two upper/adaxial and one lower/median sepal are formed first, followed by the remaining three sepal primordia, one in an upper/adaxial position and two in a lower/abaxial position (Fig. 4a). Soon, the six sepals appear similar in size and the three carpels are initiated (Fig. 4b, c). The gynoecium is oriented with one carpel in an upper/adaxial position and two in a lower/abaxial position (Fig. 4b, c). We found one example of a young female flower consisting of only two carpels (both lying in a median position) and five sepals (Fig. 4d, e). One somewhat older female flower possessed a closed perianth consisting of five sepals (Fig. 4f ).
Comparative merism (merosity) and orientation of pseudanthia in Euphorbieae (Figs 5, 6)
Fig. 6.
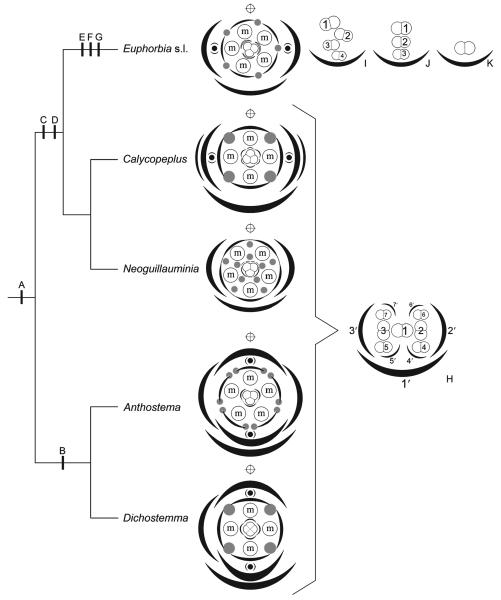
Optimisation of evolutionary transitions on summary tree of relationships in tribe Euphorbieae, and associated diagrams of pseudanthia and male partial inflorescences (H–K). Phylogeny is based on Steinmann and Porter's (2002) weighted maximum parsimony analysis of ITS region, as in Fig. 1. A = Condensation of inflorescence. B = Inner prophylls (i.e. bracteoles) in median position. C = Switch from centripetal to centrifugal formation of male organs. D = Switch of cyathial bracteoles (= inner prophylls) from median to transversal position. E = Reduction of male inflorescences from dichasial pattern (see H) to monochasial patterns (see I–K). F = Loss of male perianth. G = Switch from centripetal to centrifugal formation of female organs. Diagrams show stylised nectaries (grey dots) and stylised male partial inflorescences (encircled ‘m’). Occasional female perianth-like structures, as found in some Euphorbia and allies, are indicated as grey arcs. Pseudanthia are subtended by an abaxial bract and 2–4 preceding prophylls. All taxa except Neoguillauminia possess axial pseudanthia (i.e. accessory buds shown as black dots with preceding prophylls) either in median position (Dichostemma and Anthostema) or transversal position (Calycopeplus and Euphorbia s.l.). Main axis is indicated by a crossed circle above each diagram. (H) Diagram of dichasial male partial inflorescence as found in Anthostema, Dichostemma, Calycopeplus and Neoguillauminia. Only the first seven flowers are shown and numbered in order of their initiation (1–7), as are their subtending bracts (1′–7′). Male perianth omitted. (I) Diagram of monochasial male partial inflorescence (cincinnus) as found in most Euphorbiinae (Euphorbia s.l.). Occasional prophyll-like structures and frequent other filamentous structures omitted for clarity. (J) Diagram of monochasial male partial inflorescences with male flowers formed one above the other. Prophyll-like structures omitted. (K) Highly reduced single-flowered male partial ‘inflorescence’ as found in some species of Chamaesyce. (Modified from Prenner and Rudall 2007).
Comparative observations on other Euphorbieae are drawn from Prenner and Rudall (2007), and shown in Figs 5 and 6. Both Calycopeplus (Neoguillauminiinae) and Dichostemma (Anthosteminae) possess four male partial inflorescences (Fig. 5a, d). The abaxial pseudanthium-subtending bract is similar in both taxa, but Calycopeplus possesses two prophylls and two bracteoles in a lateral position (Fig. 5a), whereas in Dichostemma there is only one unpaired lateral prophyll and the pseudanthium-preceding bracteoles occur in a median position (Fig. 5d). The position of the male inflorescences is similar in both genera, with two in a median position and two in a transversal position. In contrast, female flowers differ strongly between the two genera; Calycopeplus possesses a trimerous gynoecium with the unpaired carpel in an upper/adaxial position and a hexamerous perianth (Fig. 5a), whereas the female flower of Dichostemma is tetramerous with four carpels and four adjacent sepals (Fig. 5d).
Both Neoguillauminia cleopatra (Neoguillauminiinae) and Anthostema madagascariense (Anthosteminae) possess four to five male partial inflorescences. In both species, the pseudanthium is formed in the axil of an abaxial/lowermost bract. The pseudanthium of Neoguillauminia is preceded by two lateral bracteoles (not shown) and the pseudanthium of Anthostema possesses two lateral prophylls and two pseudanthium-preceding bracteoles in a median position (Fig. 5e). In both species, the female flower is trimerous and the unpaired carpel is oriented in the lowermost/abaxial position. The female perianth of Neoguillauminia appears to be more or less irregularly lobed, with two larger lobes in a lateral position, whereas the perianth of Anthostema is strictly trimerous, with the three young sepals differing in size (Fig. 5b–e).
In subtribe Euphorbiinae, the pseudanthia are mainly pentamerous (though tetramerous pseudanthia occur occasionally), with five male partial inflorescences surrounding a trimerous naked female flower. In addition to the abaxial bract, two lateral pseudanthium-preceding bracteoles are present. The female flower is oriented with the unpaired carpel in a lower/abaxial position (Fig. 5c–f).
Discussion
In general, this investigation of Calycopeplus emphasises that there is considerable flexibility in the pseudanthium of the relatively small subtribe Neoguillauminiinae, as also in Anthosteminae (described by Prenner and Rudall 2007). In contrast, the bauplan of the true cyathium of the species-rich subtribe Euphorbiinae – the sister to Neoguillauminiinae – is relatively fixed and canalised.
Orientation of the pseudanthium and bauplan shifts
The pseudanthium of Calycopeplus is preceded by two pairs of prophylls, both transversely oriented; the inner prophylls (i.e. the bracteoles) subtend accessory buds (Fig. 6). The two outer prophylls remain small, and superficially appear to be stipules of the pseudanthium-subtending bract (Fig. 2). However, our ontogenetic study does not show strong evidence for a stipulate nature for these organs. The presence of four prophylls preceding the pseudanthium partly agrees with our previous findings in Anthosteminae: four prophylls are present in Anthostema (two outer lateral prophylls and two inner median prophylls) but only three prophylls in Dichostemma (a single lateral prophyll and two median prophylls) (Figs 5, 6). These observations indicate a bauplan shift in the Calycopeplus pseudanthium, in which the two median prophylls switched orientation to a transversal position. The accessory buds also switched from a median position in Anthosteminae to a transversal position in Neoguillauminiinae and its sister group, Euphorbiinae (Prenner and Rudall 2007).
Orientation and morphology of the female flower
Linked with this apparent positional shift of the inner prophylls plus accessory buds is an apparent shift in orientation of the female flower. In Euphorbia s.l., Neoguillauminia and Anthostema, an unpaired carpel lies in the same (abaxial) sector as the pseudanthial bract (Prenner and Rudall 2007). In contrast, in Calycopeplus the unpaired carpel lies in the opposite (i.e. adaxial) sector, towards the main axis (Figs 5, 6), indicating that the entire pseudanthium of Calycopeplus was shifted through 90° during evolution. An alternative hypothesis results from comparison of Calycopeplus with the tetramerous female flower of Dichostemma (Prenner and Rudall 2007). The aberrant orientation of the female flower in Calycopeplus could be explained as the result of different reduction processes; in Calycopeplus one abaxial carpel is reduced and it is possible that in all other taxa it is the adaxial carpel that is reduced.
There is currently no clear evidence for either of these two hypotheses. Evidence for some flexibility of carpel number in Euphorbieae lies in the fact that both tetramerous and trimerous carpels are present in early divergent Euphorbieae (Prenner and Rudall 2007), and there have been occasional observations of dimerous gynoecia in Calycopeplus (Fig. 4d–e) and both dimerous and tetramerous gynoecia in Euphorbia (G. Prenner, pers. comm.). Webster (1994a) noted that three carpels are present in the majority of Euphorbiaceae, but he also highlighted that there is reduction to one and increase to six or more carpels in some genera, and he commented that it is ‘not even certain that 3-carpellate gynoecia represent the plesiomorphic condition in Euphorbiaceae’ (see also Radcliffe-Smith 2001). According to Eichler (1878), female flowers of Mercurialis annua show lability in both carpel number (two or three carpels per flower) and carpel orientation. The unpaired carpel of a trimerous gynoecium lies either in a median abaxial or a median adaxial position (see also Durand 1956). Flexibility of carpel orientation also occurs in other families. For example, Eichler (1875) noted that in Polemoniaceae the unpaired carpel lies in a median adaxial position only if bracteoles are present; if the bracteoles are missing, the carpel lies in a median abaxial position and the entire flower is differently oriented (see also Wydler 1860).
Further lability in the female flower of Euphorbieae occurs in the perianth. In Anthosteminae the number of perianth lobes equals the number of carpels (four in Dichostemma, three in Anthostema) and each perianth lobe lies in front of a carpel. Subtribe Neoguillauminiinae is more variable in this respect. In Neoguillauminia the female perianth is somewhat irregular, with two larger lateral lobes and several smaller lobes in abaxial and adaxial positions. In contrast, in Calycopeplus the female perianth consists of six lobes, of which the three that alternate with the carpels are formed slightly earlier than the three lobes in the radii of the carpels (Fig. 4). In cases with only two carpels, the perianth appears to possess five lobes (Fig. 4d–f). Both subtribes Anthosteminae and Neoguillauminiinae share early formation of the female perianth (i.e. before the gynoecium differentiates), which contrasts strikingly with Euphorbiinae in which we found very late formation of lobes at the base of each carpel, when the gynoecium is already differentiated (Prenner and Rudall 2007).
The male partial inflorescences and male flowers
The male partial inflorescence of Calycopeplus is a dichasial cyme. Its basic bauplan does not differ appreciably either from its sister Neoguillauminia or from Anthosteminae (Figs 3, 6H); however, within Calycopeplus there is a strong tendency towards reduction of male partial inflorescences, ranging from 5–14 male flowers in C. collinus to 3–7in C. paucifolius (present study), or even two or three in C. oligandrus (Forster 1995). Therefore, we conclude that the bauplan of male partial inflorescences in subtribes Neoguillauminiinae and Anthosteminae is a relatively conservative element of the Euphorbieae pseudanthium. Further reductions of the cymes from dichasial to monochasial, as found in Euphorbia and its allies (Prenner and Rudall 2007), must have taken place later (Fig. 6). It will be interesting to study male partial inflorescences of the earliest-divergent species of Euphorbia s.l. (subtribe Euphorbiinae), with emphasis on the evolution of these structures. So far, such studies have been hampered by the lack of a comprehensive and well resolved phylogeny of this huge genus (>2000 species). A promising attempt to tackle this challenge is currently underway in an NSF-funded ‘EuphORBia: global inventory of the spurges’ (principal investigator Paul Berry).
In addition to two lateral bracts, which are distinctly enlarged and probably protect the developing stamens, we occasionally found a third enlarged bract in an adaxial position (Fig. 3i). The presence of enlarged outer male bracts (i.e. the bracts subtending the second- and third-formed male flower of each partial inflorescence) has been repeatedly highlighted for Calycopeplus (e.g. by Pax 1924; Mansfeld 1928). However, since Neoguillauminia, Dichostemma and Anthostema are fairly similar in this character (Prenner and Rudall 2007), it appears that it has less systematic value than previously thought.
Since we had only fairly young material at hand, we only documented male flowers with well developed anthers sitting on a solid stalk (i.e. lacking a constriction zone). Therefore, we conclude that in analogy with the other studied taxa of Euphorbieae the constriction zone is formed late. This constriction is regarded as dividing the stalk into an upper stamen filament and a lower flower pedicel. We could find neither a male perianth nor perianthlike lobes (as previously found in Neoguillauminia; Prenner and Rudall 2007). However, the perianth-like lobes form late in ontogeny and therefore it is necessary to check this character on plant material that is older than that currently at hand.
General characters (size, showiness etc.) of pseudanthia in Euphorbieae and hypotheses on the rapid and highly successful evolution of Euphorbia s.l.
There are two main possibilities for the ancestral condition of the Euphorbia cyathium: it evolved from either a thyrse (e.g. Gilbert 1994) or a single cyme (e.g. Croizat 1937). Gilbert (1994) favoured the scenario of a contracted synflorescence of axillary bisexual cymes leading to the cyathium. The present study supports earlier hypothesis (e.g. Warming 1870; Venkata Rao 1971; Gilbert 1994; Prenner and Rudall 2007) that the cyathium evolved from a thyrse terminated by a single female flower and surrounded by cymose male partial inflorescences.
It is noteworthy that both early divergent Anthosteminae and Calycopeplus (Neoguillauminiinae) possess rather small (<<1-cm-diameter) inconspicuous pseudanthia with no petaloid parts. In contrast, the second taxon of Neoguillauminiinae investigated, Neoguillauminia cleopatra, possesses significantly larger pseudanthia (>>1-cm-diameter) in which the bracts of the male partial inflorescences are enlarged and petaloid (white). In Euphorbiinae, pseudanthial sizes range from just a few millimetres (e.g. in Chamaesyce Gray) to a length of more than 2 cm in the monosymmetrical and bird-pollinated cyathia of some species of Pedilanthus Neck ex Poit. Furthermore, a broad range of structures can become petaloid in Euphorbiinae, including leaves in the ‘Christmas flower’ (E. pulcherrima Willd. ex Klotsch), cyathial bracts in some species (e.g. E. milii Desmoul.) and a broad variety of outgrowths of the cyathial nectaries in others (e.g. E. leucocephala Lotsy, E. tridentata Lam.; Hoppe 1985). In some bird-pollinated species of Pedilanthus, the entire cyathium plus its bract are bright red. Interestingly, to our knowledge, the bracts that subtend the male partial inflorescences never become petaloid (as they are in Neoguillauminia; see above). Overall, probably the majority of species of Euphorbia s.l. could be classified as ‘inconspicuous’, since their cyathia lack particular colouring (i.e. they are greenish) and/or are rather small (i.e. <<1-cm-diameter). Admittedly, little is known about the exact mode of pollination in these inconspicuous taxa (e.g. Meeuse et al. 1989). Ehrenfeld (1976, 1979) found more than 200 insect species visiting cyathia of Euphorbia capitellata, E. albomarginata (both with conspicuous cyathia) and E. hyssopifolia (with inconspicuous cyathia), though the latter received the fewest visits and was found to be self-pollinating (Ehrenfeld 1976). We speculate that cyathia in other taxa with small and/or inconspicuous cyathia are visited by a broad range of small insects; however, we emphasise the scanty knowledge of both the behaviour of these small insects and morphological details of many small and inconspicuous cyathia.
Our results (Prenner and Rudall 2007, and the present paper) highlight the reduction of male partial inflorescences from a dichasial type in the early divergent tribe Anthosteminae to a monochasial type in Euphorbiinae (Fig. 6). Both male and female perianths are highly reduced in Euphorbiinae, leaving late-developing perianth-like vestigial structures in only a few species. Furthermore, male floral bracts, which are well developed in the other subtribes, including Calycopeplus, are highly reduced in Euphorbiinae. Taken together, these processes of strong floral reductions and bauplan shifts resulted in a very flowerlike structure subtended by a single abaxial bract and laterally preceded by two cyathial bracteoles, which themselves bear accessory buds in their axils in a dichasial manner (Fig. 6). Steinmann and Porter (2002) hypothesised that the nectar-producing involucral glands situated on the rim of the involucre (rather than partially enclosed as in typical flowers) enhanced successful insect attraction and pollination. However, there is no obvious difference in nectar accessibility among the three subtribes of Euphorbieae. Steinmann and Porter (2002) also speculated that the considerable diversification within Euphorbia resulted from one or more key innovations – including the cyanthium – that promoted rapid evolution and diversification within this lineage, but provided no obvious mechanism for this. Unfortunately, to our knowledge there are no records of floral visitors in Anthosteminae. Clearly, more detailed pollination studies are required to understand what role the evolution of a secondary flower-like structure could have played in the diversification of Euphorbiinae.
Acknowledgements
Gerhard Prenner acknowledges funding from the Austrian Science Fund (Erwin–Schrödinger Fellowship, FWF-J2504). We thank three anonymous referees for valuable comments on the manuscript.
References
- Baillon H. Species Euphorbiacearum Euphorbiacées Australiennes. Adansonia. 1866;6:282–345. [Google Scholar]
- Bentham G. Flora australiensis: a description of the plants of the Australian territory, Vol. 6, Thymeleae to Dioscorideae. Reeve & Co.; London: 1873. [Google Scholar]
- Bentham G. Notes on Euphorbiaceae. Journal of the Linnean Society, London. 1880;17:185–267. [Google Scholar]
- Boissier PE. Subordo Euphorbieae. In: de Candolle A, editor. Prodromus systematis naturalis regni vegetabilis. 2. Vol. 15. V Masson; Paris: 1862. pp. 3–188. [Google Scholar]
- Croizat L. Notes on Euphorbiaceae, with a new genus and a new subtribe of the Euphorbieae. Philippine Journal of Science. 1937;64:397–412. [Google Scholar]
- Durand B. L'organisation morphologique de la fleur des Mercuriales annuelles. Naturalia Monspeliensia. Serie Botanique. 1956;8:105–124. [Google Scholar]
- Ehrenfeld JG. Reproductive biology of three species of Euphorbia subgenus Chamaesyce (Euphorbiaceae) American Journal of Botany. 1976;63:406–413. doi: 10.2307/2441907. [Google Scholar]
- Ehrenfeld JG. Pollination of three species of Euphorbia subgenus Chamaesyce, with special reference to bees. American Midland Naturalist. 1979;101:87–98. doi: 10.2307/2424904. [Google Scholar]
- Eichler AW. Blüthendiagramme construiert und erläutert. Vol. 1. W. Engelmann; Leipzig, Germany: 1875. [Google Scholar]
- Eichler AW. Blüthendiagramme construiert und erläutert. Vol. 2. W. Engelmann; Leipzig, Germany: 1878. [Google Scholar]
- Forster PI. A taxonomic revision of Calycopeplus Planch. (Euphorbiaceae) Austrobaileya. 1995;4:417–428. [Google Scholar]
- Gilbert MG. The relationships of the Euphorbieae (Euphorbiaceae) Annals of the Missouri Botanical Garden. 1994;81:283–288. doi: 10.2307/2992098. [Google Scholar]
- Hoppe J. Die Morphogenese der Cyathiendrüse und ihrer Anhänge, ihre blattypologische Deutung und Bedeutung. Botanische Jahrbücher für Systematik. 1985;105:497–581. [Google Scholar]
- Mansfeld R. Beitrag zur Morphologie des Euphorbia–Cyathiums. Berichte der Deutschen Botanischen Gesellschaft. 1928;46:674–677. [Google Scholar]
- Meeuse AD, Vinkenoog S, Vroege PW. Anthecology of Euphorbia–preliminary studies. Acta Botanica Neerlandica. 1989;38:493–502. [Google Scholar]
- Pax F. Die Phylogenie der Euphorbiaceae. Botanische Jahrbücher. 1924;59:129–182. [Google Scholar]
- Planchon MJ-E. La vraie nature de la fleur des euphorbes expliquée par un nouveau genre d'euphorbiacées. Bulletin de la Société Botanique de France. 1861;8:29–33. [Google Scholar]
- Prenner G, Rudall PJ. Comparative ontogeny of the cyathium in Euphorbia and its allies: exploring the organ–flower–inflorescence boundary. American Journal of Botany. 2007;94:1612–1629. doi: 10.3732/ajb.94.10.1612. doi: 10.3732/ajb.94.10.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenner G, Box MS, Cunniff J, Rudall PJ. The branching stamens of Ricinus and the homologies of the angiosperm stamen fascicle. International Journal of Plant Sciences. 2008 in press. [Google Scholar]
- Radcliffe-Smith A. Genera Euphorbiacearum. Royal Botanic Gardens, Kew; London: 2001. [Google Scholar]
- Steinmann VW, Porter JM. Phylogenetic relationships in Euphorbieae (Euphorbiaceae) based on ITS and ndhF sequence data. Annals of the Missouri Botanical Garden. 2002;89:453–490. doi: 10.2307/3298591. [Google Scholar]
- Venkata Rao C. Anatomy of the inflorescence of some Euphorbiaceae with a discussion on the phylogeny and evolution of the inflorescence including the cyathium. Botanical Notiser. 1971;124:39–64. [Google Scholar]
- Warming E. Über die Entwicklung des Blüthenstandes von Euphorbia. Flora. 1870;53:385–397. [Google Scholar]
- Webster GL. Conspectus of a new classification of the Euphorbiaceae. Taxon. 1975;24:593–601. doi: 10.2307/1220725. [Google Scholar]
- Webster GL. Classification of the Euphorbiaceae. Annals of the Missouri Botanical Garden. 1994a;81:3–32. doi: 10.2307/2399908. [Google Scholar]
- Webster GL. Synopsis of the genera and suprageneric taxa of Euphorbiaceae. Annals of the Missouri Botanical Garden. 1994b;81:33–144. doi: 10.2307/2399909. [Google Scholar]
- Wurdack KJ, Hoffmann P, Chase MW. Molecular phylogenetic analysis of uniovulate Euphorbiaceae (Euphorbiaceae sensu stricto) using plastid rbcL and trnL-F DNA sequences. American Journal of Botany. 2005;92:1397–1420. doi: 10.3732/ajb.92.8.1397. doi: 10.3732/ajb.92.8.1397. [DOI] [PubMed] [Google Scholar]
- Wydler H. Kleinere Beiträge zur Kenntniss einheimischer Gewächse. Flora. 1860;42:657–662. [Google Scholar]