Abstract
The most prevalent cancer diagnosed in the world is sunlight induced skin cancer. In addition to being a complete carcinogen, UV radiation, the causative agent of skin cancer, induces immune suppression. Because UV-induced immune suppression is a well-recognized risk factor for skin cancer induction, it is crucial to understand the mechanisms underlying UV-induced immune suppression. Mast cells, which have recently emerged as immune regulatory cells, are particularly important in UV-induced immune suppression. UV exposure does not induce immune suppression in mast cell-deficient mice. Here we report that UV-irradiation blocks germinal center (GC) formation, antibody secretion and T follicular helper (Tfh) cell function, in part by altering the expression of transcription factors BCL-6 and BLIMP-1. No suppression of GC formation, Tfh cell IL-21 expression, or antibody secretion was observed in UV-irradiated mast cell-deficient (KitW-sh/W-sh) mice. When mast cell-deficient mice were reconstituted with wild type mast cells, immune suppression was restored. Reconstituting the mast cell-deficient mice with bone marrow derived mast cells from IL-10-deficient mice failed to restore the ability of UV radiation to suppress germinal center formation. Our findings demonstrate a novel function for mast cells, suppression of Tfh production, GC formation and antibody production in vivo.
Introduction
T-dependent antibody responses depend on the generation of germinal centers (GC), which are specialized structures present in B cell follicles in secondary lymphoid tissues. The B cells found in these structures have a high rate of proliferation and are identified by peanut agglutinin (PNA) binding and BCL-6 expression (1, 2). Within the GC class switching, recombination, somatic hypermutation, and selection of high affinity B cells occurs (3, 4). For years it was recognized that CD4+ T helper cell function was critical for GC formation by providing help to antigen-specific B cells and promoting the differentiation of plasma and memory cells. More recently, a specialized subset of CD4+ T cells, called T follicular helper (Tfh) cells was identified that provide help for GC and antibody formation (5). Tfh are characterized by expression of co-stimulatory molecules such as ICOS, CD40L, CTLA-4, PD-1 and BTLA, and by the intense and sustained expression of CXCR-5 (6-8). The transcription factor that mediates the development of Tfh is BCL-6, whereas BLIMP-1 antagonizes the activity of BCL-6 and inhibits Tfh development (9-11). Autocrine production of IL-21 is fundamental for Tfh activation and consequently GC formation and antibody production (12, 13).
UV radiation is one of the most common environmental factors affecting human health. The UV wavelengths present in sunlight contribute significantly to the development of skin cancer (14), the most prevalent type of cancer found in the United States (15). Besides its carcinogenic effect, it is well known that exposure to UV radiation is immune suppressive, as demonstrated by the inhibition of cell-mediated immune reactions such as contact and delayed type hypersensitivity (16, 17). A less well-recognized result of total body UV exposure is the suppression of T-dependent, but not T-independent antibody formation (18-21). Although IL-10 producing T cells have been implicated in this process, the exact mechanism(s) leading to UV-induced suppression of antibody formation are not well defined.
Following UV exposure, several cell populations are implicated in the process leading to immune suppression, including keratinocytes (22), macrophages (23), Langerhans cells (24), NKT cells (25), IL-10 secreting CD4+CD25+ T regulatory cells (26) and mast cells (27). In addition to their well-characterized role in type I hypersensitivity, mast cells have the potential to diminish inflammation and suppress immune responses (28). One of the first examples of mast cells playing a role in regulating adaptive immunity was the suppression of contact and delayed type hypersensitivity following UV exposure (27). Moreover, mast cell migration from UV-irradiated skin to the draining lymph node represents a mechanism by which an immune suppressive signal is transmitted from the skin to the immune system (29). In addition, it has been shown that IL-10 produced by mast cells has the ability to limit inflammation in the skin (30) and it has been suggested that mast cell-derived IL-10 is essential for tolerance induction following UV exposure (31).
Here we examined the role of mast cells in the suppression of antibody formation. Exposing mice to UV radiation suppresses GC formation, antibody formation, the production of IL-21 and the expression of BCL-6 by Tfh. Suppression of antibody formation was blocked when UV-irradiated mice were treated with cromolyn, which blocks mast cell degranulation. No suppression of GC formation, IL-21 expression by Tfh, or the activation of BCL-6 was noted in UV-irradiated mast cell-deficient mice. The suppressive effect was restored when mast cell-deficient mice were reconstituted with wild type bone marrow derived mast cells (BMMC), but not when mast cells from IL-10-/- mice were used. These data demonstrate a novel immunoregulatory role for mast cells, suppressing GC formation by suppressing Tfh cell function.
Materials and Methods
Mice
8-10 week old C57BL/6 wild type mice, mast cell deficient mice (KitW-sh/ W-sh), IL-10 deficient mice (B6.129P2-IL10tmiCgn/J) and PGE2-deficient mice (B6.129 (FVB)-Ptgs2 tm2.1 (ptgs1)Fn/J on the C57BL/6 background were obtained from the Jackson Laboratories Bar Harbor, ME). The mice were maintained in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International, in accordance with current regulations and standards of the United States Department of Agriculture, Department of Health and Human Services, and National Institute of Health. All animal procedures were reviewed and approved by the MD Anderson Cancer Center Animal Care and Use Committee.
To analyze the role of mast cells in UV-induced immune suppression, mast cell deficient mice were reconstituted with BMMC as described previously (29). Bone marrow cells were isolated from the femurs and tibias of 6-8 week-old C57BL/6, PGE2-/- or IL-10-/- mice and then cultured at a concentration of 106 cells/ml in complete RPMI 1640 supplemented with murine recombinant IL-3 (10 ng/ml; Peprotech; Rocky Hill, NJ) and SCF (10 ng/ml; Peprotech). Non-adherent cells were transferred to fresh culture medium twice a week for 4 to 6 weeks at which point more than 98% of viable cells were mast cells as verified by flow cytometry (CD117+FcεRIa+). A total of 1 × 106 BMMC were injected into eight sites underlying the dorsal skin of mast cell-deficient mice. Six weeks later, the mice were exposed to UV radiation.
UV exposure and immunization
The shaved backs of the mice were exposed to an immunosuppressive dose of UV radiation (15 kJ/m2 of UVB; 290 to 320 nm) supplied by a 1000W xenon arc solar simulator (Oriel Instruments, Stratford, CT), as described previously (32). The intensity and spectral output of the radiation source was measured with an Optronics model OL-754 scanning spectrophotometer (Optronics Labs, Orlando, FL). 72 hours after irradiation the mice were injected with 100 μg of DNP-KLH (Calbiochem, San Diego, CA) into the dermis on the lower back. Inguinal lymph nodes were removed 7 days post-immunization for Tfh analysis, at day 14 for GC analysis and at day 28 sera were collected to measure antigen-specific IgG1 by ELISA.
To assess the effect of disodium cromoglycate (Sigma Aldrich, St Louis, MO) on antibody production we modified a protocol described previously (33). Briefly, C57BL/6 mice were injected i.p. with 10 mg/kg of disodium cromoglycate for 5 days. 12 hours after last injection, mice were UV-irradiated and immunized as described above.
ELISA
Maxisorp plates (Thermo Fisher Scientific, Waltham, MA) were coated overnight with 1 μg/ml KLH in coating buffer, blocked with blocking buffer and incubated with sera serially diluted in blocking buffer. For detection, horseradish peroxidase-conjugated antibody specific for mouse IgG1 (Serotec, Raleigh, NC) was used. Signals were developed using substrate reagent, ended with stop solution, and recorded at 450 nm using a microplate reader. All solutions were from BD Bioscience (San Jose, CA).
Flow cytometry
Single-cell suspensions of lymph nodes were prepared by gentle mechanical disruption. Cells were suspended in PBS supplemented with 2%FBS and stained with the appropriate antibody. To detect Tfh, cells were stained with anti-CD4 (eBioscience, San Diego, CA), CD44 (eBioscience), BTLA (eBioscience) and biotin-CXCR5 (BD Bioscience) followed by APC-streptavidin (BD Bioscience). GC B cells were labeled with CD19 (BD Bisocience), IgD (BD Bioscience) and PNA (Vector Laboratories, Burlingame, CA). Staining was measured with a FACS Calibur flow cytometer (BD Biosciences) and analyzed with Flowjo software (Tree Star, Ashland, OR).
Quantitative RT-PCR
Total RNA was extracted with Trizol (Invitrogen, Carlsbad, CA) from sorted CD4+ CD44+ cells and further purified by treating with RNeasy RNA cleanup protocol (Qiagen, Germantown, MD). cDNA was reverse transcribed from total RNA using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). 25ng of cDNA was subjected to quantitative RT-PCR using a sequence detector (Model ABI Prism 7500) and target mixes for IL-21, BCL-6, BLIMP-1 and β-actin (Taqman Gene Expression Assay, Applied Biosystems). Cycle threshold values (CT) for IL-21, BCL-6 or BLIMP-1 were normalized to β-actin, as described previously (29), using the following equation: (1.8 (β actin - BCL6)/1.8 (β actin - BLIMP-1) × 1000) where β-actin is the CT of each actin control, BCL6 is the CT of BCL-6, and BLIMP-1 is the CT of BLIMP-1, and 1000 is an arbitrary factor to bring all values above one.
Immunofluorescence analysis
Lymph nodes were collected and immediately frozen in OCT tissue-freezing medium. Sections were cut to 5μm thickness on a cryostat. Sections were stained with the following antibodies: IgD (BD Bioscience), BCL-6 (Santa Cruz Biotechnology, Santa Cruz, CA), mast cell tryptase (Santa Cruz), IL-10 (Abcam, Cambridge, MA), goat IgG Alexa 488 (Invitrogen), rat IgG-Alexa 350 (Invitrogen), rabbit IgG-Alexa 594 (Invitrogen), biotinylated PNA (Vector Labs), and Streptavidin-Alexa 594 (Invitrogen). Slides were mounted with Prolong Gold (Invitrogen). Images were obtained with an Olympus DP70 microscope (Olympus, Melville, NY).
Statistical analysis
Statistical difference between the control group and experimental groups were determined using a one-way ANOVA followed by Bonferroni's multiple comparison test (GraphPad Prism Software V4, San Diego, CA). Representative experiments are shown; each experiment was repeated independently at least three times.
Results
UV radiation blocks GC formation and antibody production
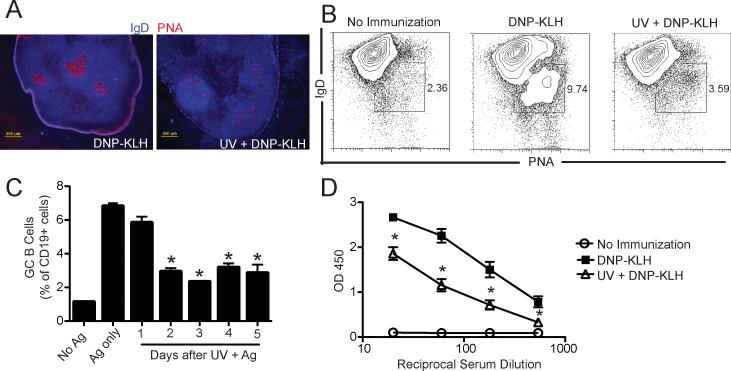
Although it is known that UV exposure can suppress antibody formation (18-21), little is known about the mechanisms involved, so we first determined whether UV exposure was able to suppress GC formation. C57BL/6 mice were exposed to a dose of UV that suppresses delayed type hypersensitivity (15 kJ/m2), immunized with DNP-KLH, and 14 days post immunization, GC formation was examined. We noted a decrease in GC formation as evidenced by a lack of PNA staining in the lymph nodes of mice exposed to UV and immunized with antigen (Fig. 1A). The fraction of PNA+ CD19+ IgD- cells in the lymph nodes of UV-irradiated and immunized mice was reduced when compared to what was found in non-irradiated but immunized controls (Fig. 1B). GC formation was decreased when mice were immunized as early as 2 days post-UV irradiation (Fig. 1C; P < 0.001 UV + Ag vs. Ag only). The suppression of GC formation correlated with a diminished production of antigen-specific IgG1 (Fig. 1D), which was the only IgG subclass detected in the serum of DNP-KLH-immunized mice (Supplemental Fig. 1). These results indicate that GC formation is suppressed by UV exposure.
Figure 1. Germinal center formation is blocked by UV exposure.
C57BL/6 mice were exposed to UV and 3 days later were immunized with DNP-KLH. 14 days after immunization draining lymph nodes were collected and analyzed for GC formation. (a) Representative immunofluorescence image from lymph nodes sections stained with anti-IgD (blue) and PNA (red), Bar = 200 μm. (b) Representative flow cytometric dot plot of IgD- PNA+ germinal center B cells gated on total CD19+. (c) Mice were immunized at different days after UV exposure and GC B cells analyzed at day 14 by flow cytometry. The data are expressed as mean ± SEM, N = 3 mice/group; * P < 0.001 vs. Ag only. (d) Mice were bled 28 d post immunization And KLH-specific IgG1 was measured by ELISA. The data are expressed as means ± SEM, N = 5 mice/group; *P < 0.05 compared to mice immunized with DNP-KLH. Representative experiments are shown; each experiment was repeated independently at least three times.
GC formation in mast cell deficient mice are unaffected by UV radiation
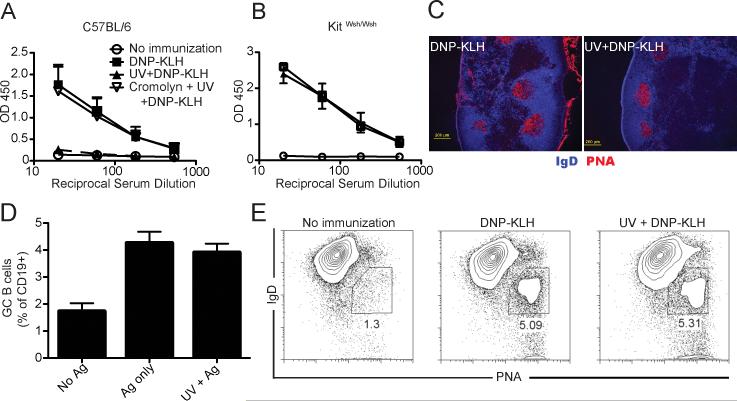
Because of the growing evidence for the role of mast cells in regulating adaptive immune responses, we asked whether mast cells could be involved in UV-induced suppression of GC formation. A group of mice was pretreated with cromolyn, which prevents mast cell degranulation (34) daily for the 5 days prior to UV exposure. UV exposure suppressed antigen-specific IgG1 formation, and cromolyn treatment reversed this effect (Fig. 2A). To confirm this finding, mast cell deficient mice (KitW-sh/W-sh) were exposed to UV radiation and then immunized with DNP-KLH. Immunizing the mast cell-deficient mice resulted in antibody formation, and prior UV exposure did not suppress antibody formation in mast cell-deficient mice (Fig. 2B). Nor did we see any suppression of GC formation (Fig. 2C), or any depression of CD19+PNA+IgD- cell numbers when mast cell-deficient mice were exposed to UV and then immunized with antigen (Fig. 2D). These data indicate that mast cells regulate antibody production and GC formation.
Figure 2. Mast cell deficient mice are resistant to UV-induced suppression of GC formation.
(a) C57BL/6 mice received 5 daily injections of cromolyn (10 mg/kg; ip) and then were exposed to UV radiation. 3 days later they were immunized, and 28 days later serum levels of anti-KLH IgG1 were measured. The data are expressed as means ± SEM, N = 5 mice/group. (b) Mast cell deficient KitW-sh/W-sh mice were UV-irradiated and immunized 3 days later. IgG1 specific for KLH was evaluated 28 days after immunization. The graph shows means ± SEM, N = 5 mice/group. (c) KitW-sh/W-sh mice were exposed to UV and 3 days later were immunized with DNP-KLH. 14 days after immunization draining lymph nodes were collected and analyzed for GC formation. A representative immunofluorescence image from lymph nodes sections stained with anti-IgD (blue) and PNA (red) is shown, Bar = 200 μm. (d) Mice were immunized 3 days after UV exposure and GC B cells analyzed 14 days later by flow cytometry. (e) Flow cytometric dot plot of IgD- PNA+ germinal center B cells, gated on total CD19+ from mast cell deficient mice. Representative experiments are shown; each experiment was repeated independently at least three times.
UV exposure suppress T follicular cell generation
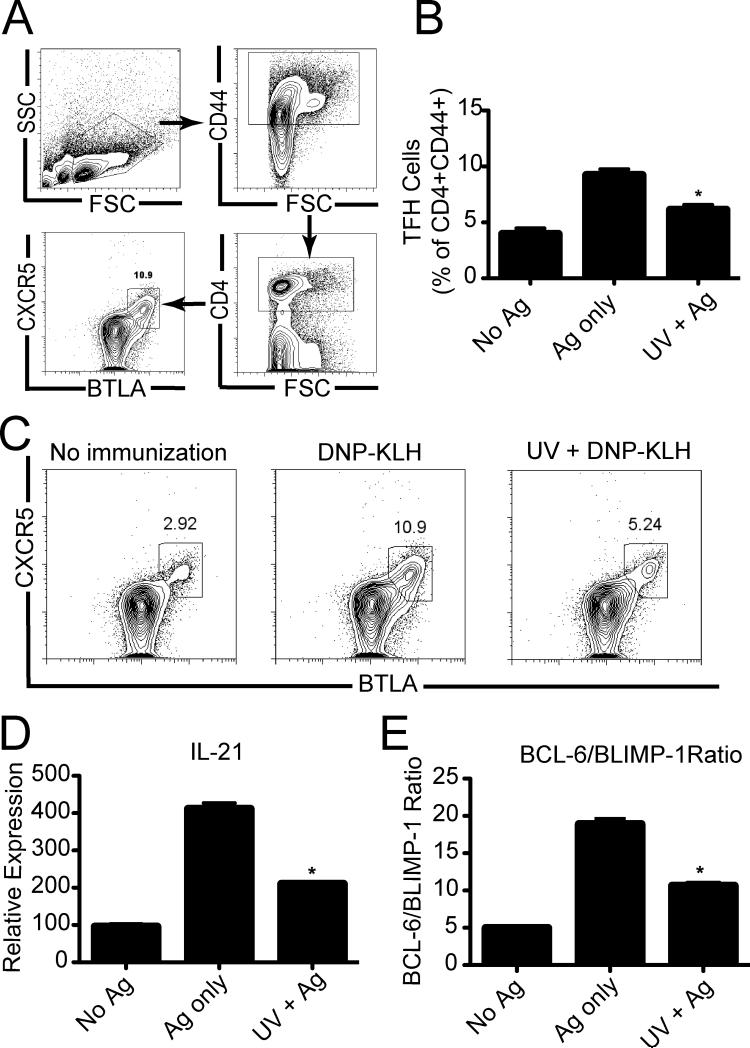
Tfh cells are essential for GC formation (5). Next we evaluated the effect of UV radiation on Tfh cell generation. Mice were exposed to UV and immunized with antigen. Seven days later the inguinal lymph nodes were removed and a single cell suspension was prepared. We gated on the CD4+ CD44+ cells used expression of CXCR5 and BTLA to identify Tfh cells (Fig. 3A). As expected, we saw an up-regulation of Tfh cells in the lymph nodes of immunized mice (Ag only) compared to the non-immunized controls (no Ag). Prior UV exposure suppressed the antigen-induced up-regulation of CXCR5+ and BTLA+ on CD4+ CD44+ cells (Figs. 3B &C).
Figure 3. UV exposure inhibits Tfh generation. Figure 3. UV exposure inhibits Tfh generation.
(a) Gating strategy for detection of Tfh cells. (b) C57BL/6 mice were immunized 3 days after UV exposure and Tfh cells were analyzed 7 days later by flow cytometry, *P<0.05 compared to mice immunized with DNP-KLH (Ag only). (c) Representative flow cytometric dot plot of CXCR5+ BTLA+ Tfh gated on total CD4+, CD44+ cells. (d) mRNA IL-21 expression on sorted CD4+CD44+ positive cells. (e) Ratio of BCL-6/BLIMP-1 by CD4+ CD44+ positive cells. The data are expressed as means ± SEM, N = 5 mice/group, * P < 0.001 compared to Ag only controls. Representative experiments are shown; each experiment was repeated independently at least three times.
IL-21 acts in an autocrine fashion promoting Tfh cell survival and/or activation (12, 13). We next tested whether UV radiation could alter IL-21 expression by the CD4+ CD44+ cells. Lymph nodes were removed from UV-irradiated antigen-immunized mice, or from un-irradiated antigen-immunized controls. The CD4+ CD44+ cells were then isolated by cell sorting. Prior UV exposure resulted in close to 50% reduction in IL-21 expression compared to what was found in the controls (Fig. 3D; P < 0.001, Ag only vs. UV + Ag). The transcription factor BCL-6 is the master regulator for Tfh cell differentiation (9), whereas BLIMP-1 antagonizes Tfh differentiation (10). To determine if UV radiation altered the relation between these transcription factors, CD4+, CD44+ cells were isolated by cell sorting and the expression of BCL-6 and BLIMP-1 was evaluated by quantitative PCR. Immunization resulted in approximately a 4-fold increase in the BCL-6/BLIMP1 ratio compared to the expression found in cells isolated from non-immunized mice. When the BCL-6/BLIMP1 ratio on CD4+, CD44+ cells found in the lymph nodes of UV-irradiated immunized mice was examined, a significant reduction was observed when compared to the control group (Fig. 3E; P < 0.001 Ag only vs. UV + Ag). Thus UV exposure suppresses the activation of Tfh in the lymph node, by affecting relative expression of BCL-6 and BLIMP-1.
It is not clear whether the reduction in Tfh function results from a generalized suppression of CD4 cell function or an antigen-specific event. To address this concern we isolated cells from normal mice KLH-DNP-immunized mice, mice exposed to UV and immunized with DNP-KLH and cells from mice exposed to UV but not immunized. The cells were then stimulated with anti-CD3/anti-CD28 in vitro and their proliferation and IFN-γ production was measured. We observed no difference in the number of proliferating CD4 cells (Supplemental Fig. 2A & B) regardless of in vivo treatment. Nor was any difference in IFN-γ production noted (Supplemental Fig. 2C). When however, the CD4+ cells were stimulated with DNP-KLH in vitro, and IL-21 mRNA levels were measured, we observed suppression of IL-21 expression only in cells isolated from UV-irradiated mice (Supplemental Fig. 2D). Thus these findings, which agree with the majority of reports characterizing UV-induced immune suppression (35), indicate that the UV-induced suppression of Tfh function reflects a antigen-specific event, and not a generalized suppression of CD4 function.
Mast cell deficient mice are resistant to UV-induced suppression of Tfh cell generation
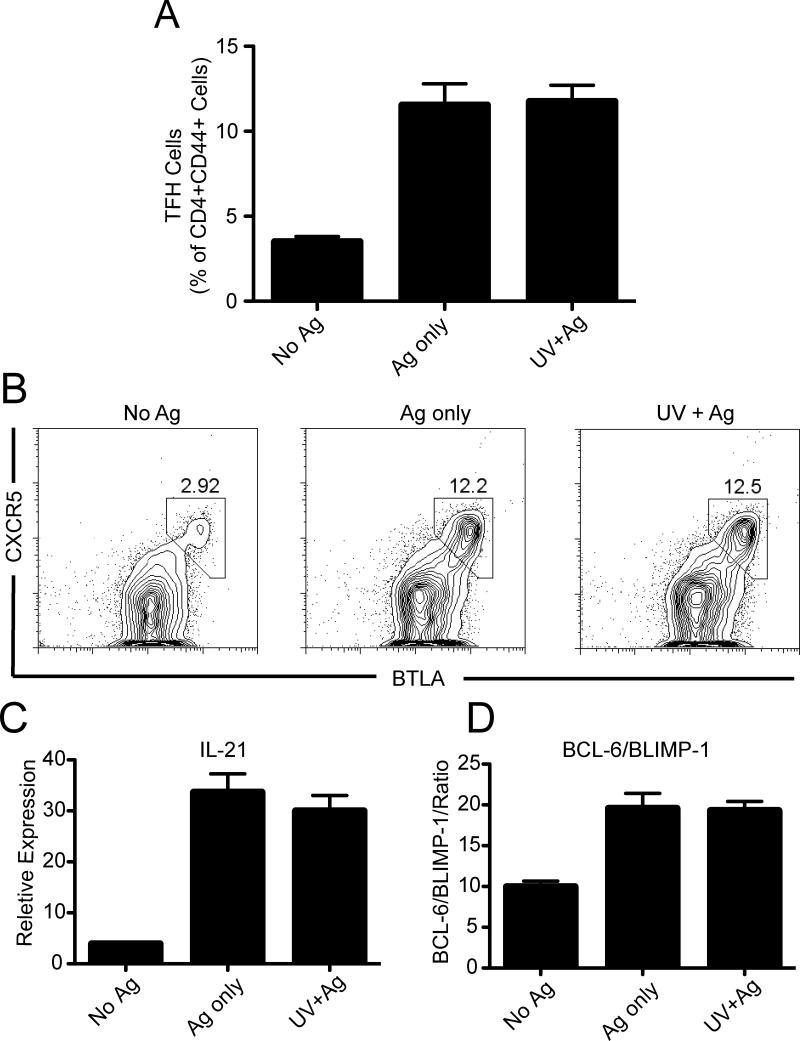
Next we asked if the generation of Tfh cells is altered in mast cell deficient mice. First, we observed that the expression of CXCR5+ and BTLA+ on CD4+ CD44+ cells was similar in UV-irradiated and non-irradiated mast cell-deficient mice (Figs. 4 A & B). Similarly, sorted CD4+ CD44+ cells from both groups of mice expressed almost identical levels of IL-21 mRNA (P > 0.05, Ag only vs. UV + Ag) (Fig. 4C). Finally, CD4+ CD44+ sorted cells from both groups showed similar BCL-6/BLIMP-1 ratios (Fig. 4D; P > 0.05, Ag only vs. UV + Ag). These results indicate that Tfh are generated in mast cell deficient mice, and are unaffected by prior UV exposure.
Figure 4. UV exposure does not suppress Tfh function in Mast cell deficient mice.
(a) Mast cell deficient mice were exposed to UV, and 3 days later were immunized with antigen. Tfh cells were analyzed 7 days later by flow cytometry. (b) Representative flow cytometric dot plot of CXCR5+ BTLA+ Tfh gated on total CD4+, CD44+ cells. (c) mRNA IL-21 expression by sorted CD4+ CD44+ positive cells. (d) Ratio of BCL-6/BLIMP-1 expression by sorted CD4+ CD44+ positive cells. The data are expressed as means ± SEM, N = 5 mice/group. Representative experiments are shown; each experiment was repeated independently at least three times.
IL-10 produced by mast cells is critical for UV-induced suppression of germinal center formation
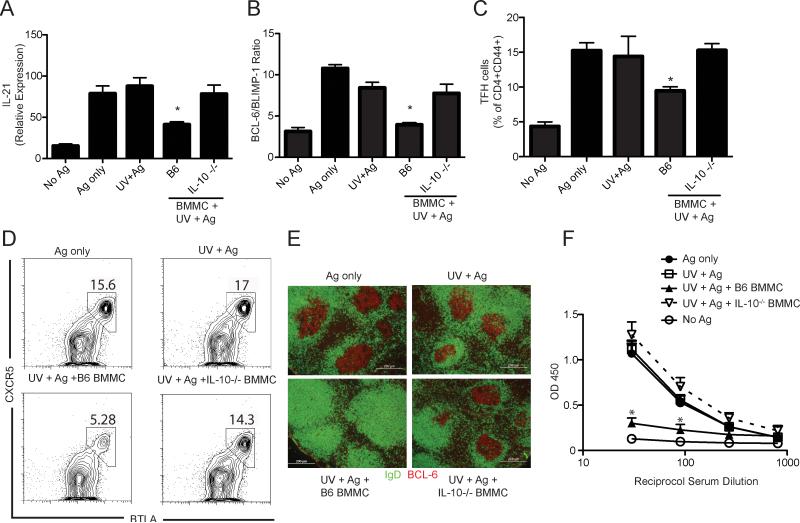
The immunoregulatory cytokine IL-10 plays a critical role in UV-induced immune suppression (22). We detected IL-10 producing mast cells 24 h after UV exposure in the inguinal lymph nodes that drained the UV-irradiated dorsal skin, but not in non-skin draining popliteal lymph nodes (Supplemental Fig. 3). We then asked whether mast cell-derived IL-10 is mediating UV-induced suppression of humoral immunity. Mast cell deficient mice that were reconstituted by injecting BMMC from either wild type or IL-10 deficient animals into the skin showed a similar mast cell density in the reconstituted area when compared to the density of mast cells found in wild type mice (Supplemental Fig. 4A). In the absence of UV exposure, reconstituting KitW-sh/W-sh mice with normal or IL-10-deficient BMMC did not affect antibody production or Tfh generation (Supplemental Fig. 4B & C). When mast cell deficient mice were reconstituted with normal BMMC, and then UV-irradiated, a significant reduction in IL-21 mRNA expression by CD4+ CD44+ cells was observed (Fig. 5A; P < 0.05, UV+BMMC B6 vs. Ag only). These cells also expressed a diminished BCL-6/BLIMP-1 ratio compared to cells isolated from the non-irradiated immunized controls (Fig. 5B; P < 0.001, UV+BMMC B6 vs. Ag only) and exhibited a decreased expression of CXCR5 and BTLA on CD4+ CD44+ cells (Fig. 5C). In contrast, IL-21 expression (Fig. 5A), the BCL-6/BLIMP-1 ratio (Fig. 5B), and the numbers of Tfh (Fig. 5C) were not suppressed in UV-irradiated mast cell-deficient mice reconstituted with IL-10-/- BMMC (p > 0.05 vs. Ag only control). Similarly, the numbers of Tfh was unaltered in mice reconstituted with IL-10-/- BMMC, compared to the positive control (Ag only; Fig. 5D). We also found no suppression of GC formation when IL-10-deficient BMMC reconstituted mice were exposed to UV radiation (Fig. 5E). Similarly, no suppression of antibody production was observed when IL-10-/- BMMC were used to reconstitute UV-irradiated mast cell deficient mice (Fig. 5E). As a control, another group of mast cell-deficient mice was reconstituted with BMMC isolated from prostaglandin endoperoxidase synthase 2-deficient (PGE2-/-) animals. GC formation and Tfh activation in these mice was suppressed by prior UV exposure, indicating the mast cell-derived prostaglandin-E2 is not involved in suppressing GC formation (Supplemental Fig. 5). Taken as a whole, these results indicate that mast cells can suppress humoral immunity. Furthermore, they indicate that mast cell-derived IL-10 is responsible for suppressing GC formation, Tfh activation, and antibody production in vivo.
Figure 5. Mast cell-derived IL-10 is crucial for UV-induced humoral immune suppression.
Mast cell deficient mice, or mast cell deficient mice reconstituted with wild type (B6) or IL-10-/- BMMC were exposed to UV and immunized with antigen. Lymph node CD4+CD44+ positive cells were isolated by cell sorting 7 days post immunization (a) IL-21 mRNA expression by CD4+ CD44+ cells. * P < 0.05 vs. mast cell deficient mice immunized with DNP-KLH (Ag only). (b) Ratio of BCL-6/BLIMP-1 expression by CD4+ CD44+ positive cells. *P < 0.001 vs. mast cell deficient mice immunized with DNP-KLH (Ag only). (c) Mast cell deficient mice and BMMC reconstituted mice were exposed to UV, and 3 days later were immunized with antigen. Tfh cells were analyzed 7 days later by flow cytometry. * P < 0.05 vs. mast cell deficient mice immunized with DNP-KLH (Ag only). (d) Representative flow cytometric dot plot of CXCR5+ BTLA+ Tfh, gated on total CD4+ CD44+. (e) Representative immunofluorescence image from lymph nodes sections stained with anti-IgD (green) and BCL-6 (red), Bar = 200 μm. (f) Mice were bled 28 days post immunization and KLH-specific IgG1 was measured by ELISA. The data are expressed as means ± SEM, N = 5 mice/group. *P < 0.05 compared to non-irradiated immunized (Ag only) mice. Representative experiments are shown; each experiment was repeated independently at least three times.
Discussion
In these experiments we used the environmentally relevant carcinogen, UV radiation to activate immune suppression. The immune suppressive properties of UV radiation first became evident in experiments studying the immunobiology of UV-induced skin cancers (36). Since that time, the immunosuppressive properties of UV radiation have been confirmed many times, in both mice and humans (35). Most of the studies employing UV as an immunosuppressive agent have examined its effects on cell-mediated immunity (35). Although it is also known that UV exposure suppresses humoral immunity (18-20), little is understood about the mechanisms involved. Recent studies have shown that Tfh are the crucial cells that provide co-stimulatory signals and cytokines necessary for B cell activation, GC formation and antibody production (37). Here we provide evidence that UV radiation suppress antibody production by inhibiting GC development as early as 48 hours post irradiation. Furthermore, we observed a decreased fraction of CXCR5+ CD4+ cells and decreased production of IL-21 by activated T cells isolated from UV-exposed mice. We hypothesize that the reduction in Tfh cell number is due to decreased levels of IL-21, which works in an autocrine manner to promote Tfh activation and the help necessary for GC formation and antibody production (13). However, because many other cells types in addition to Tfh produce IL-21 (37), we could not rely solely on IL-21 as a indicator of Tfh activation. The recent identification of BCL-6 as the master transcription factor (9), and BLIMP-1 as the repressor of Tfh differentiation (10) allowed us to more precisely identify the effect of UV exposure on Tfh activation. Our results show that CD4+, CD44+ cells from UV-irradiated antigen-immunized mice had a depressed BCL-6/BLIMP-1 ratio compared to what was observed with cells from non-irradiated immunized controls, indicating that UV radiation targets Tfh cell generation.
We also demonstrated that mast cells, and mast cell derived IL-10, plays a role in suppressing Tfh cell generation, IL-21 expression, GC formation and antibody production. These findings add to the appreciation of the important role IL-10 plays in UV-induced immune suppression and UV-induced skin cancer formation (35). How mast cells alter T activation in the lymph node is not completely understood. Others have suggested that activated mast cells can act as antigen-presenting cells, form an immunological synapse with T cells, and directly affect their activation (38). Evidence also exists indicating that cytokines produced by activated mast cells (IL-4) can induce Th2 differentiation (39). Our studies further support the important role that mast cell-derived cytokines play in immune regulation by demonstrating that mast cell derived IL-10 is the critical immunosuppressive factor that blocks Tfh generation, GC formation and antibody production.
Although it is well known that UV radiation only is absorbed in the top layers of the skin, it can clearly effect GC formation in skin-draining lymph nodes. The data presented here support the concept that dermal mast cells transmit the immune suppressive signal from the skin to the lymph nodes, although the exact mechanism is not entirely clear. Two potential mechanisms may be involved. Previously we reported that UV triggers mast cells migration from the skin to the lymph node, and agents to block mast cell migration (i.e., CXCR4 antagonists) block the induction of immune suppression (29). We suggest that dermal mast cells are triggered by UV exposure to leave the skin and migrate to the draining lymph nodes, where they release IL-10 and suppress GC formation. Another mechanism that activated mast cells can use to deliver immune regulatory signals from the skin to distant lymph nodes is via the release of small spherical cytokine containing heparin-based particles. Kunder and colleagues recently presented data demonstrating that these micro-particles can enter lymphatic vessels, migrate to draining lymph nodes, and release bioactive TNF (40). It is possible that UV-activated dermal mast cells release IL-10-containing heparin-granules, which enter the lymphatics, migrate to the draining lymph nodes, and suppress Tfh function.
Although the results presented here indicate that mast cells suppress antibody formation, it should be pointed out that a report by McLachlan and colleagues indicates that mast cells can also serve as adjuvants for antibody formation. Their data indicates that administration of mast cell activators with antigen induces mast cells to secrete TNF, which promotes the migration of dendritic cells to the lymph nodes and enhances antibody production (41). The critical difference between their findings and the data presented here appears to be the agents that are used to activate the mast cell. McLachlan et al, used compound 48/80 a classic mast cell activator to induce TNF release and promote the immune response. When we reconstituted mast cell-deficient mice with normal BMMC and simply immunized the animals, we saw no effect, positive or negative on antibody formation (Supplemental Fig. 4). When however, we exposed mice to a classic immunosuppressive agent, UV radiation, the mast cells were activated to release IL-10 and suppress Tfh function. These observations support the idea that mast cells are “tunable” regulatory cells, that depending on the environment and the signals they receive, can either promote or suppress the immune response (42).
In conclusion, our results show a new function for mast cells. We find that mast cells suppress Tfh cell generation, the formation of GC and antibody production via the production of IL-10. These findings support the growing appreciation of mast cells as immunoregulatory elements. We suggest that mast cells, in addition to blocking classic cell mediated immune reactions (i.e. DTH) (27, 29, 30), also regulate humoral immune reactions by negatively affecting Tfh function.
Supplementary Material
Acknowledgments
We thank Roza Nurieva for valuable suggestions regarding BCL-6 and BLIMP-1 measurements, Nasser Kazimi for technical help with animal experiments, Pamela Grant for help with tissue sections and Karen Ramirez for assistance in cell sorting.
This work was supported by grants from the National Cancer Institute (CA131207 & CA112660). The animal, histology, and flow cytometry facilities at the MDACC are supporting in part by a core grant from the NCI (CA16672).
Abbreviations
- BMMC
bone marrow-derived mast cells
- GC
germinal center
- PGE2
prostaglandin E-2
- PNA
peanut agglutinin
- Tfh
T follicular helper
Footnotes
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Butcher EC, Rouse RV, Coffman RL, Nottenburg CN, Hardy RR, Weissman IL. Surface phenotype of Peyer's patch germinal center cells: implications for the role of germinal centers in B cell differentiation. J Immunol. 1982;129:2698–2707. [PubMed] [Google Scholar]
- 2.Cattoretti G, Chang CC, Cechova K, Zhang J, Ye BH, Falini B, Louie DC, Offit K, Chaganti RS, Dalla-Favera R. BCL-6 protein is expressed in germinal-center B cells. Blood. 1995;86:45–53. [PubMed] [Google Scholar]
- 3.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 4.Liu YJ, Malisan F, de Bouteiller O, Guret C, Lebecque S, Banchereau J, Mills FC, Max EE, Martinez-Valdez H. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 1996;4:241–250. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 5.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 6.Walker LS, Gulbranson-Judge A, Flynn S, Brocker T, Raykundalia C, Goodall M, Forster R, Lipp M, Lane P. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5-positive CD4 cells and germinal centers. J Exp Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, Forster R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD, Wang YH, Dong C. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL, Srivastava M, Linterman M, Zheng L, Simpson N, Ellyard JI, Parish IA, Ma CS, Li QJ, Parish CR, Mackay CR, Vinuesa CG. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity. 2009;31:457–468. doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Urbach F. Ultraviolet radiation and skin cancer of humans. J. Photochem. Photobiol. B: Biol. 1997;40:3–7. doi: 10.1016/s1011-1344(97)00029-8. [DOI] [PubMed] [Google Scholar]
- 15.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993-2002. BMC Cancer. 2006;6:264–272. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kripke ML. Immunosuppressive effects of ultraviolet (280-320 nm) radiation and psoralen plus ultraviolet (320-400 nm) radiation in mice. J Natl Cancer Inst. 1982;69:171–173. [PubMed] [Google Scholar]
- 17.Norval M, McLoone P, Lesiak A, Narbutt J. The effect of chronic ultraviolet radiation on the human immune system. Photochem Photobiol. 2008;84:19–28. doi: 10.1111/j.1751-1097.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 18.Alderson MR, Keast D. The effect of ultraviolet radiation on humoral immune responses to T-dependent antigens. Environ Res. 1986;40:321–331. doi: 10.1016/s0013-9351(86)80107-4. [DOI] [PubMed] [Google Scholar]
- 19.Ullrich SE. The effect of ultraviolet radiation-induced suppressor cells on T cell activity. Immunology. 1987;60:353–360. [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Toda M, Saito K, Hori T, Horii T, Shiku H, Kuribayashi K, Kato T. Post-immune UV irradiation induces Tr1-like regulatory T cells that suppress humoral immune responses. Int Immunol. 2008;20:57–70. doi: 10.1093/intimm/dxm124. [DOI] [PubMed] [Google Scholar]
- 21.Brown EL, Rivas JM, Ullrich SE, Young CR, Norris SJ, Kripke ML. Modulation of immunity to Borrelia burgdorferi by ultraviolet irradiation: differential effect on Th1 and Th2 immune responses. Eur J Immunol. 1995;25:3017–3022. doi: 10.1002/eji.1830251105. [DOI] [PubMed] [Google Scholar]
- 22.Rivas JM, Ullrich SE. Systemic suppression of delayed-type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- 23.Kang K, Hammerberg C, Meunier L, Cooper KD. CD11b+ macrophages that infiltrate human epidermis after in vivo Ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10. J. Immunol. 1994;153:5256–5264. [PubMed] [Google Scholar]
- 24.Stingl GL, Gazze-Stingl LA, Aberer W, Wolff K. Antigen presentation by murine epidermal Langerhans cells and its alteration by ultraviolet light. J. Immunol. 1981;127:1701–1713. [PubMed] [Google Scholar]
- 25.Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521–525. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 26.Schwarz T. 25 years of UV-induced immunosuppression mediated by T cells-from disregarded T suppressor cells to highly respected regulatory T cells. Photochem Photobiol. 2008;84:10–18. doi: 10.1111/j.1751-1097.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- 27.Hart PH, Grimbaldeston MA, Swift GJ, Jaksic A, Noonan FP, Finlay-Jones JJ. Dermal mast cells determine susceptibility to Ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J. Exp. Med. 1998;187:2045–2053. doi: 10.1084/jem.187.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrne SN, Limon-Flores AY, Ullrich SE. Mast cell migration from the skin to the draining lymph nodes upon ultraviolet irradiation represents a key step in the induction of immune suppression. J Immunol. 2008;180:4648–4655. doi: 10.4049/jimmunol.180.7.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–1104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 31.Alard P, Kurimoto I, Niizeki H, Doherty JM, Streilein JW. Hapten-specific tolerance induced by acute, low-dose ultraviolet B radiation of skin requires mast cell degranulation. Eur J Immunol. 2001;31:1736–1746. doi: 10.1002/1521-4141(200106)31:6<1736::aid-immu1736>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 32.Nghiem DX, Kazimi N, Clydesdale G, Ananthaswamy HN, Kripke ML, Ullrich SE. Ultraviolet a radiation suppresses an established immune response: implications for sunscreen design. J Invest Dermatol. 2001;117:1193–1199. doi: 10.1046/j.0022-202x.2001.01503.x. [DOI] [PubMed] [Google Scholar]
- 33.Nautiyal KM, Ribeiro AC, Pfaff DW, Silver R. Brain mast cells link the immune system to anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:18053–18057. doi: 10.1073/pnas.0809479105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orr TS, Hall DE, Gwilliam JM, Cox OS. The effect of disodium cromoglycate on the release of histamine and degranulation of rat mast cells induced by compound 48-80. Life Sci I. 1971;10:805–812. doi: 10.1016/0024-3205(71)90035-x. [DOI] [PubMed] [Google Scholar]
- 35.Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005;571:185–205. doi: 10.1016/j.mrfmmm.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 36.Kripke ML. Antigenicity of murine skin tumors induced by ultraviolet light. J Natl Cancer Inst. 1974;53:1333–1336. doi: 10.1093/jnci/53.5.1333. [DOI] [PubMed] [Google Scholar]
- 37.King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol. 2009;9:757–766. doi: 10.1038/nri2644. [DOI] [PubMed] [Google Scholar]
- 38.Gaudenzio N, Espagnolle N, Mars LT, Liblau R, Valitutti S, Espinosa E. Cell-cell cooperation at the T helper cell/mast cell immunological synapse. Blood. 2009;114:4979–4988. doi: 10.1182/blood-2009-02-202648. [DOI] [PubMed] [Google Scholar]
- 39.Huels C, Germann T, Goedert S, Hoehn P, Koelsch S, Hultner L, Palm N, Rude E, Schmitt E. Co-activation of naive CD4+ T cells and bone marrow-derived mast cells results in the development of Th2 cells. Int Immunol. 1995;7:525–532. doi: 10.1093/intimm/7.4.525. [DOI] [PubMed] [Google Scholar]
- 40.Kunder CA, St John AL, Li G, Leong KW, Berwin B, Staats HF, Abraham SN. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J Exp Med. 2009;206:2455–2467. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, Staats HF, Abraham SN. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14:536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 42.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.