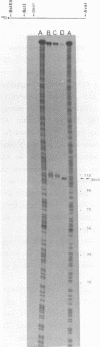
Abstract
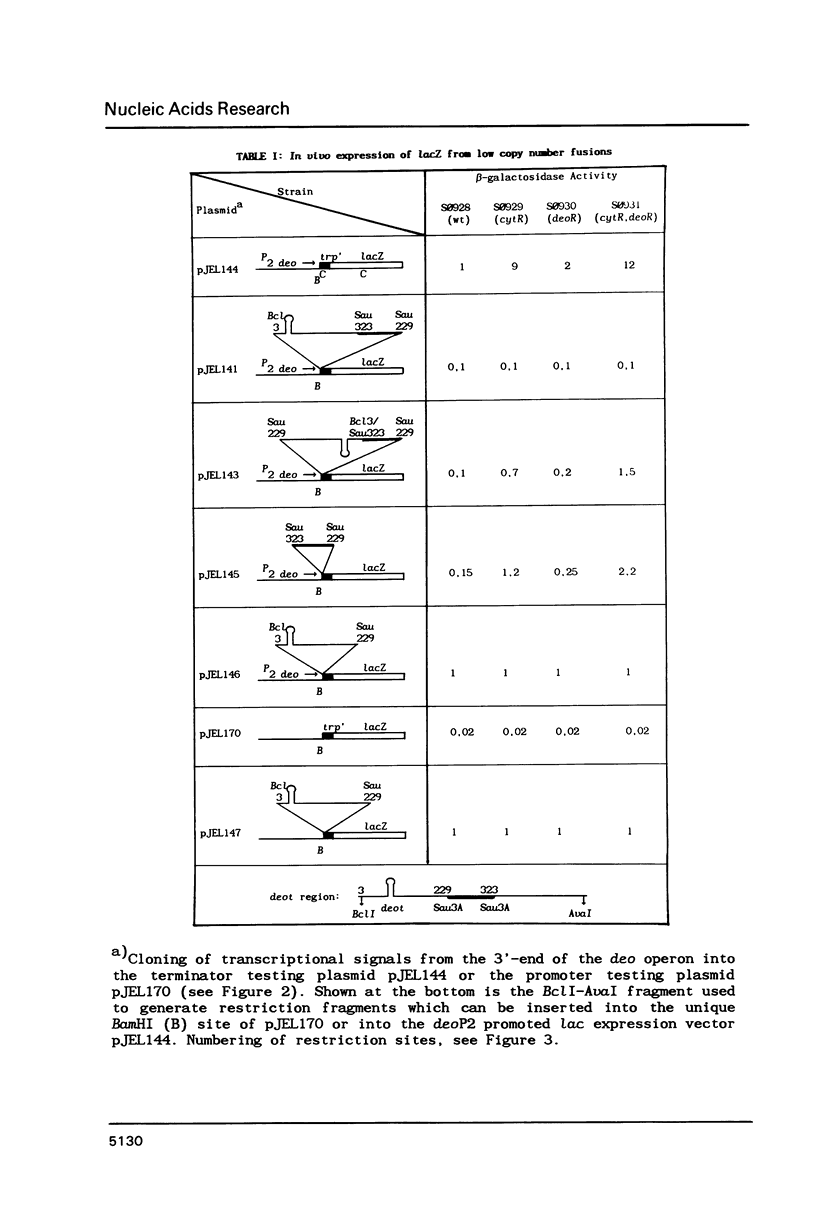
We describe the construction of low copy number operon-fusion vectors, and use one of these vectors for the cloning and transcriptional analysis of the terminator region after the deo operon of Escherichia coli K-12. The new vectors are miniderivatives of plasmid R1 containing the parB stability locus of this plasmid and the lac genes as a selectable marker. Since the copy number of the vectors is only one per genome-equivalent at temperatures below 37 degrees C this system is ideally suited for isolation and characterization of transcriptional and translational signals from E. coli. Our results show that a very strong terminator (deot), which resembles Rho-independent terminators, is located 60 bp downstream from the fourth structural gene of the deo operon. This confirms that deoD is the last gene in the operon. In addition, we have identified a new promoter just after the deot terminator and a short DNA sequence that is able to reduce lacZ expression by 85% when inserted between the deoP2 promoter and the lac genes.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Casadaban M. J., Chou J., Cohen S. N. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. doi: 10.1128/jb.143.2.971-980.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Dandanell G., Valentin-Hansen P., Larsen J. E., Hammer K. Long-range cooperativity between gene regulatory sequences in a prokaryote. 1987 Feb 26-Mar 4Nature. 325(6107):823–826. doi: 10.1038/325823a0. [DOI] [PubMed] [Google Scholar]
- Gerdes K., Rasmussen P. B., Molin S. Unique type of plasmid maintenance function: postsegregational killing of plasmid-free cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3116–3120. doi: 10.1073/pnas.83.10.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes W. M., Platt T., Rosenberg M. Termination of transcription in E. coli. Cell. 1983 Apr;32(4):1029–1032. doi: 10.1016/0092-8674(83)90287-8. [DOI] [PubMed] [Google Scholar]
- Küpper H., Sekiya T., Rosenberg M., Egan J., Landy A. A rho-dependent termination site in the gene coding for tyrosine tRNA su3 of Escherichia coli. Nature. 1978 Mar 30;272(5652):423–428. doi: 10.1038/272423a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J. E., Gerdes K., Light J., Molin S. Low-copy-number plasmid-cloning vectors amplifiable by derepression of an inserted foreign promoter. Gene. 1984 Apr;28(1):45–54. doi: 10.1016/0378-1119(84)90086-6. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Roberts J. W., Wu R. Transcription terminates at lambda tR1 in three clusters. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6171–6175. doi: 10.1073/pnas.79.20.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McKenney K., Shimatake H., Court D., Schmeissner U., Brady C., Rosenberg M. A system to study promoter and terminator signals recognized by Escherichia coli RNA polymerase. Gene Amplif Anal. 1981;2:383–415. [PubMed] [Google Scholar]
- Morgan W. D., Bear D. G., Litchman B. L., von Hippel P. H. RNA sequence and secondary structure requirements for rho-dependent transcription termination. Nucleic Acids Res. 1985 May 24;13(10):3739–3754. doi: 10.1093/nar/13.10.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott J. E., Galloway J. L., Platt T. Maturation of Escherichia coli tryptophan operon mRNA: evidence for 3' exonucleolytic processing after rho-dependent termination. EMBO J. 1985 Jul;4(7):1887–1891. doi: 10.1002/j.1460-2075.1985.tb03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Schrenk W. J., Weisberg R. A. A simple method for making new transducing lines of coliphage lambda. Mol Gen Genet. 1975;137(2):101–107. doi: 10.1007/BF00341676. [DOI] [PubMed] [Google Scholar]
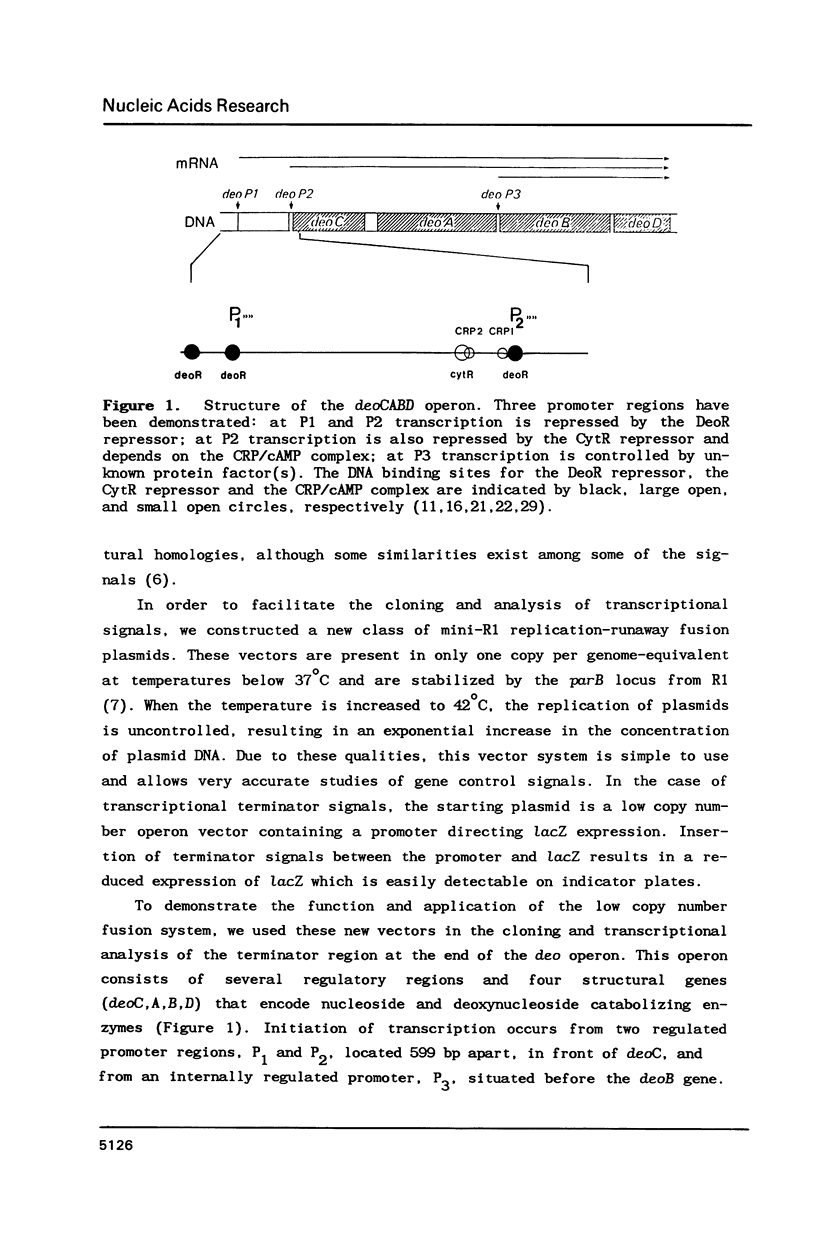
- Valentin-Hansen P., Aiba H., Schümperli D. The structure of tandem regulatory regions in the deo operon of Escherichia coli K12. EMBO J. 1982;1(3):317–322. doi: 10.1002/j.1460-2075.1982.tb01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Albrechtsen B., Løve Larsen J. E. DNA-protein recognition: demonstration of three genetically separated operator elements that are required for repression of the Escherichia coli deoCABD promoters by the DeoR repressor. EMBO J. 1986 Aug;5(8):2015–2021. doi: 10.1002/j.1460-2075.1986.tb04458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Boëtius F., Hammer-Jespersen K., Svendsen I. The primary structure of Escherichia coli K12 2-deoxyribose 5-phosphate aldolase. Nucleotide sequence of the deoC gene and the amino acid sequence of the enzyme. Eur J Biochem. 1982 Jul;125(3):561–566. doi: 10.1111/j.1432-1033.1982.tb06719.x. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P., Hammer-Jespersen K., Boetius F., Svendsen I. Structure and function of the intercistronic regulatory deoC-deoA element of Escherichia coli K-12. EMBO J. 1984 Jan;3(1):179–183. doi: 10.1002/j.1460-2075.1984.tb01781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Hammer K., Løve Larsen J. E., Svendsen I. The internal regulated promoter of the deo operon of Escherichia coli K-12. Nucleic Acids Res. 1984 Jul 11;12(13):5211–5224. doi: 10.1093/nar/12.13.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P., Svenningsen B. A., Munch-Petersen A., Hammer-Jespersen K. Regulation of the deo operon in Escherichia coli: the double negative control of the deo operon by the cytR and deoR repressors in a DNA directed in vitro system. Mol Gen Genet. 1978 Feb 16;159(2):191–202. doi: 10.1007/BF00270893. [DOI] [PubMed] [Google Scholar]
- Valentin-Hansen P. Tandem CRP binding sites in the deo operon of Escherichia coli K-12. EMBO J. 1982;1(9):1049–1054. doi: 10.1002/j.1460-2075.1982.tb01295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and properties of S1 nuclease from Aspergillus. Methods Enzymol. 1980;65(1):248–255. doi: 10.1016/s0076-6879(80)65034-4. [DOI] [PubMed] [Google Scholar]
- Wu A. M., Christie G. E., Platt T. Tandem termination sites in the tryptophan operon of Escherichia coli. Proc Natl Acad Sci U S A. 1981 May;78(5):2913–2917. doi: 10.1073/pnas.78.5.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]