Abstract
Non-linguistic auditory processing and working memory update were examined with event-related potentials (ERPs) in 18 children who stutter (CWS) and 18 children who do not stutter (CWNS). Children heard frequent 1kHz tones interspersed with rare 2kHz tones. The two groups did not differ on any measure of the P1 and N1 components, strongly suggesting that early auditory processing of pure tones is unimpaired in CWS. However, as a group, only CWNS exhibited a P3 component to rare tones suggesting that developmental stuttering may be associated with a less efficient attentional allocation and working memory update in response to auditory change.
1. Introduction
Stuttering is characterized by involuntary disruptions in the flow and rhythm of speech. It has been hypothesized to result from interactions between a vulnerable speech motor system and multiple other factors, such as genetic predisposition, emotion regulation, and cognitive and language skills (Smith & Kelly, 1997; Bloodstein & Bernstein-Ratner, 2008). Although traditionally the main focus in stuttering research has been on speech production, a growing literature suggests that stuttering may also be characterized by atypical neural mechanisms underlying at least some aspects of speech perception. For example, individuals who stutter and normally fluent speakers (NFS) have been found to differ both in the efficiency of phonological processing (Blood, Ridenour, Qualls, & Scheffner Hammer, 2003; Corbera, Corral, Escera, & Idiazábal, 2005; Sasisekaran & De Nil, 2006; Weber-Fox, Spruill III, Spencer, & Smith, 2008; but see also Bajaj, Hodson, & Schommer-Aikins, 2004; Weber-Fox, Spencer, Spruill III, & Smith, 2004; Gregg & Yairi, 2007) and in the nature of cognitive mechanisms underlying the perception of syntactic and semantic anomalies (Weber-Fox, 2001; Cuadrado & Weber-Fox, 2003; Bajaj et al., 2004). However, the fact that speech perception differences between individuals who do and do not stutter are typically subtle in nature, do not always manifest themselves in standardized behavioral tests, and involve multiple levels of linguistic structure (at least phonology, semantics, and syntax) raises the question of whether they are indeed language-specific or may be the result of more general differences in cognitive mechanisms involved in sound perception, including non-linguistic auditory processing.
Existing studies of non-linguistic auditory perception in individuals who stutter are few and sometimes report conflicting results. This is possibly due to a continuum of auditory skills in both clinical and typical groups, a diverse nature of experimental approaches used by researchers, and the subtlety of observed cognitive differences associated with stuttering. Additionally, the majority of research in this area is limited to adults who stutter, making it difficult to determine whether reported differences reflect inherent neurophysiological variations in the brain function of individuals who stutter or are the result of many years of compensatory strategies employed by this clinical population.
Auditory perception, however, is not limited to correct encoding of physical properties of sound, but also involves such cognitive processes as working memory and attention. For example, working memory has been proposed to play a role in child language acquisition (Baddeley, 2006), and children with specific language impairment (SLI) show a reliable deficit on working memory tests (Ellis Weismer et al., 2000; Hoffman & Gillam, 2004). Likewise, some researchers suggested that attention facilitates perceptual learning (Bahrick, Lickliter, & Flom, 2004), speech stream segregation, and successful word learning in infants (Hollich, Newman, & Jusczyk, 2005). Further, event-related potential (ERP) studies showed that at least under certain conditions attention may enhance sensory encoding of stimuli (Woldorff et al., 1993). Therefore, a detriment in the efficiency of working memory and attention may negatively affect an individual’s ability to process auditory information even if early neural encoding of sound is normal. A number of researchers have suggested that working memory and/or attention may be impaired in stuttering (e.g., Bajaj, 2007; Bosshardt, 2006; Ratcliff-Baird, 2001); however, direct physiological evidence for this hypothesis is scarce.
We have combined the event-related potentials (ERP) method with an auditory oddball paradigm in order to examine non-linguistic auditory processing in 4 and 5 year-old children who stutter (CWS) and their normally developing peers. Voltage deflections of an ERP recording reflect postsynaptic potentials of large assemblies of similarly oriented neurons in the brain’s cortex. Importantly, the temporal order of deflections within a recording is believed to reflect “the flow of information through the brain” (Luck, 2005, p.11), with earlier components being sensitive to acoustic properties of the auditory signal and later components to cognitive analyses of the perceived sound. Such selective sensitivity of individual ERP components coupled with excellent temporal resolution of the ERP method allowed us to examine in greater detail which processes may contribute to sound perception differences between individuals who do and do not stutter. Below, we first review relevant literature on non-linguistic auditory processing, working memory, and attention in stuttering and then present our study in greater detail.
1.1 Non-Linguistic Auditory Processing in Stuttering
Relatively little is known about the role of non-linguistic auditory processing in stuttering. Early studies focused on the brain stem function in stuttering and compared the strength of acoustic reflex and the ability to cope with low signal-to-noise ratios in adults who stutter (AWS) and normally fluent speakers (NFS). They reported conflicting results, with some studies finding a difference between AWS and NFS (e.g., Hall & Jerger, 1978; Liebetrau & Daly, 1981; Kramer, Green & Guitar, 1987) and some not (e.g., Hannley & Dorman, 1982; Dmitrieva & Gel’man, 2001). In their review of the low-level auditory function in stuttering, Hannley and Dorman (1982) conclude that the lack of replicable differences between AWS and NFS is likely due to the heterogeneity of the stuttering disorder and to typically small group sizes (often less than 10 individuals in each) used for comparisons.
Some studies also probed the contribution of higher neural structures to auditory perception in stuttering. For example, Nudelman, Herbrich, Hess, Hoyt, & Rosenfield (1992) found that AWS are poorer than NFS at tracking linear frequency increases and decreases in frequency-modulated tones by humming. By manipulating the rate of frequency changes, the authors were able to determine that the overall delay in corrective vocal movements of AWS was due to longer times required to detect a change, and not to greater difficulty in initiating a vocal response. The authors attributed this group difference to a disturbance in an “outer cognitive loop that provides the reasoning behind and choice of the words being said” (p. 1883) in AWS.
One recent study (Hampton & Weber-Fox, 2008) recorded ERPs elicited by pure tones in AWS and age-matched NFS in order to evaluate non-linguistic auditory processing in these groups. On average, AWS were slower and less accurate in detecting rare changes in tone frequency, a finding that is in agreement with the results of Nudelman et al. (1992) described above. Although AWS displayed an overall greater variability in the peak amplitude of N1 elicited by both tone types, which is believed to reflect the encoding of physical properties of sound, no group difference in any of the early exogenous ERP measures was found. The authors concluded that at least at the group level, the early neural encoding of sound properties is similar in AWS and NFS.
Overall, studies of non-linguistic auditory processing in stuttering reveal subtle differences between AWS and NFS. Such differences may include a suppressed ability to cope with low signal to noise ratios and a greater difficulty in detecting a change in the auditory environment. Additionally, at least some AWS may have a less efficient neural encoding of sound properties as evidenced by ERP measures. More research with larger groups of participants and measures that are sensitive to subtle differences between individuals who do and do not stutter is clearly needed. Importantly, behavioral measures often disallow conclusions about the source of auditory processing differences, which may include not only a proper encoding of physical features of sound but also such cognitive processes as working memory and attention. Reviewed literature also exposed a significant gap in our knowledge about auditory perception in stuttering – namely, no information is currently available about non-linguistic auditory processing in children who stutter.
1.2 Working Memory and Attention in Stuttering
The strongest evidence for the involvement of working memory and attention in the disorder of stuttering comes from studies that employ dual tasks to examine the effect of cognitive load on speech dysfluency. Such studies require that their participants not only successfully divide attention between two demanding tasks, but also effectively maintain task-relevant information in working memory. For example, in a series of experiments Bosshardt (1999, 2002; see also 2006 for a review) tested the influence of concurrent linguistic and non-linguistic tasks on fluency of word repetitions and sentence production in AWS and NFS. He found that adding mental calculation to the primary task of word repetition significantly increased the stuttering rate only in some AWS (Bosshardt, 1999), while adding another linguistic task, such as silent reading and word memorization, affected stuttering frequency in AWS more profoundly, resulting in a significant group difference between AWS and NFS (Bosshardt, 2002). The author suggested that “the phonological and articulatory systems of persons who stutter are protected less efficiently from interference by attention-demanding processing than those of persons who do not stutter” (Bosshardt, 2006, p.377).
The outcome of dual-task experiments, however, appears to depend at least in part on the difficulty of the primary task and the nature of the secondary task, which together may affect the amount of effort necessary to maintain task-relevant information in working memory. For example, contrary to the findings by Bosshardt, Arends, Povel, and Kolk (1988) reported a decrease in stuttering rates in AWS in three speech tasks when their participants had to simultaneously track a small dot on a computer screen. The effect was most pronounced for severe stutterers. A similar result was also obtained by Vasić and Wijnen (2005) who reported a small but significant decrease in stuttering among their participants when they concurrently either played a video version of a ping-pong game or monitored their speech for a frequently occurring pronoun. Based on these findings, the authors suggested that stuttering is the result of the overly stringent speech production monitoring in individuals who stutter. When such excessive monitoring resources are diverted to other tasks, fluency increases. Thus, contrary to the findings of Bosshardt (1999, 2002), reports by Arends et al. (1988) and by Vasic and Wijnen (2005) claim a beneficial effect of increased cognitive load on fluency in stuttering, at least under certain conditions.
A few studies also examined the difference in attentional functions between individuals who do and do not stutter through more specialized tests. Heitmann, Asbjørnsen, and Helland (2004) employed the Posner Test of Covert Attention Shifts, among other measures, to examine the differences between school-aged CWS, clutterers, and children who do not stutter (CWNS). The task required participants to fixate on the centrally presented cross mark and press a response key once a target appeared in one of the peripheral boxes. Reaction times of CWS were significantly longer than those of CWNS, suggesting an impaired ability to focus attention in this group of participants. In an electroencephalographic (EEG) study of attention-related brain function in stuttering, Ratcliff-Baird (2001) reported a significant increase in theta and decrease in alpha EEG recordings in individuals who stutter compared to NFS in six different tasks. Importantly, the same pattern of EEG activity was previously reported for individuals with attention deficit hyperactivity disorder (ADHD). The author draws parallels between both EEG and behavioral characteristics of individuals who stutter and individuals with ADHD, suggesting the existence of an attentional component to stuttering.
A handful of studies used an oddball paradigm in combination with ERPs to examine working memory and attention in AWS. In such studies, participants are presented with one frequent tone (a standard) and one rare one (a deviant). A rare tone typically elicits a P3 ERP component which is thought to index attentional allocation and a working memory update in response to a detected change as described in detail in section 1.3.2. Ferrand, Gilbert, and Blood (1991) used the peak latency of the P3 ERP component elicited by rare simple tones, among other measures, for determining the locus of vocal response delay in AWS compared to NSF. The authors used the peak latency of P3 as an indicator of the end of stimulus evaluation, independent of response selection and execution. They found no difference between AWS and NFS. However, the interpretation of this result is complicated by the absence of group average ERP waveforms and information on ERP recordings in the report. Additionally, no information on the peak amplitude of the P3 or of any of the earlier ERP components was reported. A study by Morgan, Cranford, and Burk (1997) extended the paradigm used by Ferrand et al. (1991) and recorded ERPs elicited by frequent and rare pure tones from two electrodes positioned over the left and right hemispheres of the brain in AWS and NFS. The results showed a greater P3 peak amplitude over the right-hemisphere site in NFS, while 5 out of 8 AWS produced the opposite pattern. The authors concluded that AWS and NFS differ in the relative involvement of cerebral hemispheres during processing of non-linguistic sounds. However, the very small number of electrodes used in this study significantly limits its interpretive power. Additionally, similar to the study by Ferrand and colleagues (1991), this report does not include group average ERP waveforms and does not present any information on ERP components preceding P3, which makes the evaluation of ERP findings difficult. Lastly, in a recent study by Hampton and Weber-Fox (2008) rare deviants elicited a relatively smaller P3 component in AWS at longer inter-stimulus intervals (1000 ms), indicating a less robust attentional allocation and working memory update in this group. Interestingly, a study by Corbera et al. (2005) found no difference between AWS and NFS in the mismatch negativity (MMN) ERP component elicited by frequency and duration changes of pure tones. Because MMN is believed to index a rather automatic perception mechanism (Näätänen & Picton, 1986; Näätänen, 1995; Näätänen & Alho, 1995), while P3 reflects attentional allocation and working memory update in response to some task relevant change (Donchin, 1981; Donchin and Coles, 1988; Polich, 2003; Linden, 2005), this finding suggests that AWS differ from NFS during a relatively late stage of sound processing.
In general, studies of working memory and attention in stuttering reveal a great need for further research in this area. Very few attempts have been made to examine whether individuals who stutter differ from normally fluent speakers in the neural functioning underlying working memory and attention. Those studies that did address this question used adults as participants, which precludes conclusions about the role of working memory and attention in the development of stuttering. The current study extends the existent research by examining ERP measures associated with these cognitive functions in young CWS.
1.3 Current Study
The goal of the current study was two-fold. First, it was designed to provide a sensitive test of early cortical non-linguistic auditory processing in CWS. Second, it aimed to examine whether CWS and CWNS differ in the efficiency of attentional allocation and working memory update in response to a rare change in tone frequency. By testing pre-school CWS and their normally developing peers, we were able to evaluate both auditory and cognitive functions close to the onset of stuttering, thus addressing an important gap in stuttering research.
We recorded ERP responses while participants listened to two pure tones, one occurring frequently and one rarely (an oddball paradigm). We examined P1 and N1 ERP components elicited by both types of tones in order to evaluate similarities and differences in early neural encoding of sound between CWS and CWNS. Additionally, we evaluated later components N2 and P3 elicited by rare tones in order to compare the robustness of attentional allocation and working memory update in the two groups.
1.3.1 Auditory P1 and N1 ERP Components
The exogenous components P1 and N1 are sensitive to physical properties of sound, such as frequency, duration, inter-stimulus interval, stimulus complexity, and intensity. They may be elicited without any task as long as an individual remains alert. These components are often referred to as “obligatory” because their absence suggests impairment to the primary auditory pathway. Multiple studies showed that the latency of both P1 and N1 becomes shorter as the child matures, and this process may extend well into the teenage years (McGee & Kraus, 1996; Sharma, Kraus, McGee, & Nicole, 1997; Ponton, Eggermont, Jos, Kwong, & Don, 2000; Wunderlich & Cone-Wesson, 2006). Consequently, P1 and N1 latency may be used for evaluating the development of the auditory system, especially in individuals who are unable to provide a reliable behavioral response. For example, it has been reported that P1 may be either absent or its latency significantly lengthened in congenitally deaf children and children who received a cochlear implant more than 7 years after birth (Dorman, Sharma, Gilley, Martin, & Roland, 2007). Further, Hyde (1997) describes in detail the use of the N1 component for the evaluation of the auditory function in populations that are unable to either understand or comply with the requirements of the more traditional behavioral audiometric test as long as passive compliance can be obtained.
Scalp recordings of P1 and N1 are likely to have multiple generators. It is believed that P1 is generated in the temporal lobe, possibly in the auditory association cortex (Scherg, Vajsar, & Picton, 1989; Liégeois-Chauvel, Musolino, Badier, Marquis, & Chauvel, 1994; for a review see also McGee and Kraus, 1996). Näätänen and Picton (1987) present evidence for multiple N1 sub-components, which together may contribute to its parameters in the average waveform. At least one of these sub-components is believed to originate from the supratemporal plane of the brain (Näätänen and Picton, 1987; Scherg et al., 1989), which houses the primary auditory cortex. In young adults, P1 peaks approximately 50 ms post-stimulus onset, and N1 approximately 100 ms post-stimulus onset. Both components are significantly delayed in children. For example, Sharma et al. (1997) report a mean peak latency of 87 ms for the P1 and 221 ms for the N1 components in 6 year old children. P1 and N1 peak latencies reach adult-like measures by late teenage years. Comparison of P1 and N1 between CWNS and CWS allowed us to evaluate whether early sensory encoding of frequency changes is similar across the two groups.
1.3.2 MMN, N2b, and P3 ERP Components
When participants pay attention to presented stimuli, a rare stimulus change typically elicits at least two components of the N2 family (MMN and N2b) and a centro-parietal P3. The MMN is sensitive to any detectible stimulus change and is thought to index a mismatch between a frequently occurring “standard” and a rare deviant (Näätänen & Gaillard, 1983; for a review of the MMN component, see also Näätänen, 1995). Näätänen (1985) suggested that MMN implies the existence of an automatically elicited and rapidly decaying memory trace for recently presented stimuli. When the incoming stimulus does not match such a memory trace, the detection of a mismatch manifests itself as MMN. It has been shown that these memory traces may last up to 4–5 seconds post-stimulus onset in adults. Importantly, even in young children (7–9 years old) neural traces of recent auditory information may be present for well over 1400 ms, which is substantially longer than the longest ISI used in the current study (Čeponienė, Cheour, & Näätänen, 1998). MMN is often described as being independent of attentional allocation to the stimuli; however, this issue is still being debated. A rather large body of literature exists that suggests that MMN may be elicited independently of the direction of attention. For example, in some dichotic listening tasks stimuli indexing an identical change elicit MMN of the same amplitude regardless of whether they are presented to the attended ear or to the unattended one (e.g., Näätänen, Gaillard, & Mantysalo, 1978). Support to this position also comes from the fact that MMN has been found in the ERPs of coma patients 1 to 2 days before they regain consciousness (Kane, Curry, Butler, & Cummins, 1993) and in the ERPs collected during certain stages of sleep (Loewy, Campbell, & Bastien, 1996; but see also Paavilainen et al., 1987). At the same time, a number of researchers have shown that MMN may be modified by attention under certain conditions, such as for example strongly focused selective attention and increased difficulty of a concurrent task (e.g., Muller-Gass, Stelmack, & Campbell, 2006; Sabri, Liebenthal, Waldron, Medler, & Binder, 2006; Yucel, Petty, McCarthy, & Belger, 2005; Woldorff, Hackley, & Hillyard, 1991). Yet other researchers try to reconcile both sides of the debate by pointing out that MMN is highly dependent on the context in which stimuli occur. Such context, they argue, may be shaped by both stimulus-driven and attention dependent factors (Sussman, Winkler, & Wang, 2003; Sussman, 2007).
Unlike MMN, the N2b component is dependent on attentional allocation and task-relevance of deviant stimuli. It typically occurs later than MMN and has a more peak-like appearance (Näätänen & Picton, 1987). This component is also sensitive to perceptual novelty and is believed to reflect a stimulus categorization process (Näätänen, Simpson, & Loveless, 1982; Patel & Azzam, 2005).
The P3 component of the N2-P3 complex is arguably the most studied component in the ERP literature. Multiple theories have been proposed concerning the cognitive processes it encodes (Donchin, 1981; Donchin & Coles, 1988; Verleger, 1988; Picton, 1992; Polich, 2007); however, the most commonly accepted interpretation is that P3 reflects an update in the current schema of the environment (or a working memory update) in response to a detected change (Donchin, 1981; Donchin & Coles, 1988; Polich, 2003, 2007). According to this view, an organism strives to maintain an up-to-date schema of its environment. When a change is detected, attentional resources are allocated to the memory system, which then updates the mental representation of the environment. It is this process of working memory update that is indexed by the P3 component (Polich, 2003, 2007). Such interpretation is supported by a number of observations. For example, the amplitude of P3 is sensitive to the probability of rare changes, with low probability events eliciting larger P3 amplitudes (e.g., Duncan-Johnson & Donchin, 1977). Further, in a more direct test of the relationship between P3 amplitude and memory, some studies showed that during a rote rehearsal task, items that elicited a greater P3 amplitude were associated with better subsequent recall (Fabiani, Karis, & Donchin, 1986, 1990).
Working memory processes reflected in the P3 component are dependent on attentional resources. For example, Polich (2007) states that a comparison between the previous and the current event that precedes the schema update is attention driven. Indeed, rare events typically do not elicit P3 in the absence of attention to the stimuli. Attentional resources are also needed for the working memory update itself. A number of dual task studies showed that when a rare change detection is performed concurrently with some other, primary, task, the P3 amplitude elicited by rare changes decreases with an increase in the primary task demands (Wickens, Kramer, Vanasse, & Donchin, 1983; Kramer, Wickens, & Donchin, 1985).
The stimuli that elicit the N2b and P3 are usually either novel, significant, or task-relevant. A recent study by Ferrari, Bradley, Codispoti, and Lang (in press) sheds more light on the role of the N2b and P3 components in encoding different aspects of stimuli. The authors created a visual paradigm in which task relevance and perceptual novelty of rare changes were independently manipulated. The findings showed that the N2b amplitude is sensitive only to perceptual novelty while P3 is modulated by both novelty and task-relevance.
In sum, while MMN reflects a relatively automatic detection of a stimulus change, the N2b-P3 complex indexes both attentional allocation to the stimuli and a working memory update in response to the task relevant change detection. Therefore, a comparison of the MMN, N2b, and P3 components elicited by rare frequency changes in the two groups allowed us to evaluate the robustness of these processes in CWNS and CWS.
1.3.3 Hypotheses
Although Hampton and Weber-Fox (2008) found no group difference between AWS and NFS in any characteristics of early ERP components, a relatively greater variability in the peak amplitude of N100 in AWS reported by the authors suggested that at least some AWS may have exhibited atypical sensory encoding of sound. Therefore, we thought that young CWS might also differ from CWNS in the efficiency of early auditory encoding as indexed by P1 and N1 ERP components.
Based on the reviewed literature and the outcome of an earlier study by Hampton and Weber-Fox (2008) we also hypothesized that similar to AWS, CWS may have an attenuated attentional allocation and working memory update in response to rare tones, which could result either in a reduced peak amplitude of the N2 and P3 components or in their relative delay compared to those in CWNS.
2. Methods
2.1 Participants
Eighteen pre-school CWNS (6 girls) and 18 pre-school CWS (5 girls) participated in the study. Data were collected at two research sites, one at Purdue University and one at the University of Iowa. All precautions were taken to ensure identical experimental setups at both sites. Seven CWNS and 6 CWS participants were tested at the University of Iowa. The rest were tested at Purdue University. Participants’ ages ranged from 4 years 0 months to 5 years 10 months for the CWNS group (mean age 4;10) and from 4 years 0 months to 5 years 11 months for the CWS group (mean age 4;10). Three participants in the CWNS group and 2 in the CWS group were left-handed as reported by children’s parents and confirmed by an abbreviated handedness inventory (5 tasks adapted from Oldfield, 1971). All participants passed a hearing screening at a level of 20 dB HL at 1000, 2000, and 4000 Hz and had normal or corrected-to-normal vision as per parent report. All children were free of neurological disorders, including ADHD, and had no history of taking medications that may affect neural function (for example, medications for depression, seizures, or attention-deficit hyperactivity disorder). They also demonstrated normal non-verbal intelligence as assessed by the Columbia Mental Maturity Scale (Burgemeister, Blum, & Lorge, 1972), showed no symptoms of impaired reciprocal social interaction and restriction of activities (DSM IV criteria of autism and pervasive developmental disorder: American Psychiatric Association, 1994) as assessed by the Childhood Autism Rating Scale (Schopler, Reichler, & Renner, 1988), and had no history of previous treatment for emotional problems, based on parental report.
All children were administered a battery of language evaluation tests. Language tests reported in this study assessed children’s spoken language as measured by the Structured Photographic Expressive Language Test – 3 (SPELT-3, Dawson, Stout, & Eyer, 2003), language comprehension as measured by the Test for Auditory Comprehension of Language -3 (TACL-3, Carrow-Woolfolk, 1999), and phonological abilities as measured by the Bankson-Bernthal Test of Phonology (BBTOP, Bankson & Bernthal, 1990).
Additionally, verbal working memory measurements were obtained through the use of the digit- and word-span subtests of the Test of Auditory-Perceptual Skills (TAPS, Gardner, 1985). In order to obtain non-verbal measures of working memory, a series of color blocks were used to estimate color and order memory span. The experimenter and the child had identical sets of color blocks. During testing, the child was shown a short sequence of blocks (starting with just two) for several seconds and was asked to replicate it with his or her own set of blocks after the sequence was no longer visible. The experimenter would assign separate scores for the use of the correct colors and the correct order of colored blocks in a sequence. Each child was given two different trials with the same number of blocks before the block number could be increased by one. The testing would end after two consecutive errors (Goffman, 2002, unpublished).
Because recent research suggests that socio-economic background may have a significant influence on a number of cognitive abilities (Stevens, Lauinger, & Neville, 2009; Hackman & Farah, 2009), we have also recorded the level of mothers’ education as a measure of children’s socio-economic status (SES). We evaluated the level of maternal education with the help of Hollingshead’s education scale (Hollingstead, 1975, unpublished) and assigned a score of 4 for completed high school, 5 for partial college or specialized training (e.g., technical school), 6 for a completed college degree, and 7 for a graduate degree.
2.1.1 Group Inclusion Criteria
Participants were classified as CWS if all of the following criteria were met (Yairi & Ambrose, 1999): the child was regarded as having a stuttering problem by at least one clinician involved in the project; the child’s stuttering severity was rated as 2 or higher on an eight point severity scale by either his/her parents or a speech-language pathologist; and the child exhibited at least three stuttering-like dysfluencies per 100 syllables of spontaneous speech. CWS who scored below the normal range (a standard score of less than 85) on either the TACL-3 or SPELT -3 tests were excluded from the current study to ensure that all participants had normal language comprehension abilities and showed no signs of specific language impairment (SLI) at the time of testing. However, CWS who performed below the normal range on the BBTOP test were included. Out of 18 CWS, 5 received standard scores that were more than one standard deviation below the standard mean on either one or both sections of the BBTOP test. The normally fluent children were excluded from participation if they failed any of the screening procedures or scored below the normal range on any of the language evaluation tests.
Data presented in this report are part of a longitudinal study currently under way in our laboratory. Each participating child is evaluated on the same battery of tests once a year for 3 to 5 years. The youngest age at which children start their testing is 4 years, although some may start at an older age. The goal of this study was to evaluate non-linguistic auditory processing and cognitive function in very young CWS, close to the onset of stuttering. Therefore, we included data from all qualified 4 and 5 year-olds regardless of whether the data came from the first or second year of testing for 5 year-olds. The availability of useable ERP data determined which year was included in the group average. If useable data were available for multiple years, the earliest available year was included. Altogether, second year ERP data were used for 1 CWNS and 4 CWS. Despite a larger number of children whose second year data was used in the CWS group, groups’ ages were still very well matched, and the two groups did not differ in age (see Section 2.1.2 and Table 1).
Table 1.
Age, linguistic, and socio-economic characteristics of CWNS and CWS groups
| Participant # | Age | SPELT-3 | TACL-3 | BBTOP-CI | BBTOP-PPI | Non-verbal intelligence | Mother’s education (Hollingshead scale) |
|---|---|---|---|---|---|---|---|
| CWNS | |||||||
| 1 | 5;5 | 110 | 113 | 111 | 113 | 105 | 6 |
| 2 | 4;6 | 117 | 121 | 117 | 118 | 119 | 6 |
| 3 | 4;8 | 121 | 141 | 110 | 107 | 124 | 7 |
| 4 | 4;0 | 119 | 139 | 105 | 104 | 119 | 7 |
| 5 | 4;5 | missing | 89 | 96 | 92 | 100 | 4 |
| 6 | 5;10 | 121 | 139 | 95 | 89 | 124 | 7 |
| 7 | 4;0 | 93 | 119 | 101 | 100 | 110 | 7 |
| 8 | 4;1 | 104 | 126 | 98 | 106 | 108 | 7 |
| 9 | 5;10 | 106 | 121 | 100 | 111 | 116 | 6 |
| 10 | 4;11 | 114 | 126 | 93 | 86 | 116 | 7 |
| 11 | 4;2 | 102 | 109 | 99 | 98 | 119 | 6 |
| 12 | 5;9 | 105 | 119 | 105 | 99 | 102 | 7 |
| 13 | 4;9 | 114 | 113 | 106 | 100 | 105 | 7 |
| 14 | 4;2 | 112 | 128 | 105 | 104 | 109 | 6 |
| 15 | 5;7 | 122 | 121 | 101 | 100 | 116 | 7 |
| 16 | 4;11 | 116 | 111 | 115 | 116 | 100 | 5 |
| 17 | 4;4 | 115 | 126 | 104 | 101 | 117 | 6 |
| 18 | 5;4 | 123 | 126 | 111 | 113 | 118 | 6 |
| Mean | 4;10 | 112.6 | 121.5 | 104 | 103.2 | 112.6 | 6.3 |
| SE | 0.16 | 2 | 2.9 | 1.6 | 2.1 | 1.9 | 0.2 |
| CWS | |||||||
| 1 | 4;0 | 93 | 117 | 81* | 80* | 108 | 5 |
| 2 | 5;7 | 92 | 121 | 72* | 65* | 111 | 4 |
| 3 | 4;2 | 95 | 109 | 113 | 109 | 96 | 4 |
| 4 | 5;1 | 93 | 109 | 110 | 115 | 107 | 4 |
| 5 | 4;0 | 105 | 91 | 110 | 104 | 115 | 6 |
| 6 | 5;1 | 121 | 132 | 93 | 86 | 132 | 6 |
| 7 | 5;1 | 92 | 104 | 98 | 98 | 94 | 4 |
| 8 | 5;11 | 103 | 113 | 104 | 108 | 110 | 4 |
| 9 | 4;1 | 107 | 109 | 92 | 100 | 112 | 6 |
| 10 | 4;6 | 100 | 117 | 105 | 107 | 100 | 6 |
| 11 | 4;9 | 102 | 111 | 99 | 103 | 117 | 6 |
| 12 | 4;5 | 103 | 126 | 74* | 71* | 113 | 5 |
| 13 | 4;0 | 102 | 117 | 108 | 107 | 111 | 7 |
| 14 | 4;10 | 110 | 124 | 102 | 107 | 114 | 7 |
| 15 | 4;10 | 122 | 102 | 115 | 106 | 105 | 4 |
| 16 | 5;10 | 111 | 113 | 98 | 95 | 90 | 6 |
| 17 | 5;8 | 114 | 98 | 90 | 84* | 109 | 5 |
| 18 | 4;10 | 86 | 102 | 72* | 66* | 112 | 4 |
| Mean | 4;10 | 102.8 | 111.9 | 96.4 | 95.1 | 108.7 | 5.2 |
| SE | 0.15 | 2.4 | 2.4 | 3.3 | 3.7 | 2.3 | 0.3 |
Asterisks mark standard scores that are one standard deviation or more below the mean.
Bolded means identify measures that differentiated the two groups, with poorer performance and lower SES in CWS.
Lastly, if a child received a score of 100 or higher on standardized tests of language, working memory, and non-verbal intelligence, he/she was not re-tested in consequent years. In those cases when a child was retested during his/her second year, and we used his/her second year ERP data, behavioral scores obtained during the second year are reported.
2.1.2 Group Comparison
Information on the age, language test performance, non-verbal intelligence, and SES for both groups of participants is summarized in Table 1. Children’s performance on working memory tests is shown in Table 2. One-way ANOVAs with a factor of group performed separately on each measurement revealed that the CWNS and CWS groups differed significantly in their performance on SPELT-3, F(1, 34)=9.658, p=0.004; TACL-3, F(1,35)=6.316, p=0.017; BBTOP-CI, F(1,35)=4.266, p=0.047; and in SES, F(1, 35)=12.815, p=0.001, with lower performance scores and lower SES in CWS. Although none of the children included in this study performed below the normal range on either the SPELT-3 or the TACL-3 tests, the difference in group averages was nonetheless significant. The relationship between these linguistic and SES variables and ERP findings are reported in the Results section. No other group comparisons reached significance.
Table 2.
Working memory scores.
| Participant # | Digit Span | Word Span | Non-Verbal Color | Non-Verbal Order |
|---|---|---|---|---|
| Control | ||||
| 1 | 11 | 5 | 1 | 1 |
| 2 | 9 | 2 | 2 | 2 |
| 3 | 12 | 6 | 3 | 2 |
| 4 | 6 | 4 | 0 | 0 |
| 5 | missing | missing | missing | missing |
| 6 | 11 | 5 | 3 | 2 |
| 7 | 10 | 3 | 1 | 1 |
| 8 | 4 | 2 | 0 | 0 |
| 9 | 11 | 6 | 4 | 4 |
| 10 | 7 | 4 | 2 | 2 |
| 11 | 6 | 3 | 2 | 2 |
| 12 | 11 | 6 | 7 | 7 |
| 13 | 4 | 4 | 2 | 1 |
| 14 | 8 | 6 | 3 | 2 |
| 15 | 12 | 5 | 7 | 6 |
| 16 | 8 | 4 | 2 | 0 |
| 17 | 9 | 4 | 2 | 2 |
| 18 | 7 | 4 | 2 | 2 |
| Mean | 8.6 | 4.3 | 2.5 | 2.1 |
| SE | 0.6 | 0.3 | 0.5 | 0.5 |
| CWS | ||||
| 1 | 6 | 4 | 2 | 2 |
| 2 | 7 | 3 | 1 | 1 |
| 3 | 3 | 2 | 0 | 0 |
| 4 | 12 | 3 | 2 | 2 |
| 5 | 4 | 3 | 2 | 2 |
| 6 | 9 | 3 | 5 | 5 |
| 7 | 4 | 2 | 1 | 1 |
| 8 | 10 | 5 | 3 | 3 |
| 9 | 7 | 4 | 2 | 2 |
| 10 | 8 | 4 | 2 | 2 |
| 11 | 9 | 3 | 1 | 1 |
| 12 | 6 | 2 | 3 | 3 |
| 13 | 5 | 4 | 2 | 2 |
| 14 | 9 | 3 | 5 | 2 |
| 15 | 10 | 4 | 3 | 3 |
| 16 | 10 | 5 | 3 | 5 |
| 17 | 9 | 8 | 3 | 0 |
| 18 | 4 | 3 | 2 | 2 |
| Mean | 7.3 | 3.6 | 2.3 | 2.1 |
| SE | 0.6 | 0.3 | 0.3 | 0.3 |
2.2 Stimuli
An auditory odd-ball paradigm was used in this study. Two 50 ms pure tones – 1000 Hz and 2000 Hz – served as stimuli and were presented via earphones (RadioShack). The 1000 Hz tone occurred on 80 percent of the trials (standard) and the 2000 Hz tone on 20 percent of the trials (deviant). Because shorter inter-stimulus intervals (ISI) may pose a greater challenge for the auditory system and thus expose group differences that may not be evident at slower rates of stimulus presentations, each of the two tones was randomly presented at either a short (200 ms) or a long (1000 ms) ISI with the exception that all deviants were followed by 1000 ms before the next stimulus presentation, while half of standards were followed by 200 ms and another half by 1000 ms before the next stimulus presentation. Further, in order to evaluate whether the side of sound presentation influences the speed and efficiency of its neural encoding, 33% of all tones were presented to the right earphone, 33% to the left earphone, and 34% bilaterally. Each child heard 608 tones, with on average 122 of them being deviants. Left and right earphone tones were presented at 72 dB SPL while the bilateral tones were presented at 69 dB SPL in order to equate all tone presentations in perceived loudness. All sounds were presented via earphones (RadioShack).
2.3 Procedure
Children’s parents completed a consent form. The hearing and stuttering screenings as well as language evaluation tests were administered to children in a prior session. At the beginning of the ERP recording session, an electrode cap (Quik-cap) was placed on the child. The child had a choice of watching a cartoon or playing a computer game appropriate for their age during the capping process. The child was then seated in a single-wall sound attenuating booth (Industrial Acoustics Company Inc., NY) 140 cm from a 48-cm monitor. In order to maintain children’s attention, the paradigm was presented as a game. Every 45–105 seconds (82 tonal stimuli on average) the child would either see a picture displayed in place of a fixation point on the monitor (e.g., apple, balloon) or hear a word spoken over the earphones (e.g., “cat”, “party”). When either one of these events occurred, the child would “fish” for a piece of a magnetic puzzle. This game procedure ensured that the children were fixating on the screen and attending to the auditory stimuli presented via the headphones. A total of 8 play breaks took place during the entire session. Children were told that they would hear many “low beeps” and sometimes they would hear “high beeps.” They were encouraged to listen for high beeps but did not provide any responses. A clinician stayed with each child in the recording booth in order to coordinate play breaks with the tonal presentation and direct children’s attention to the stimuli. Although occasionally children would become distracted or bored with the task, this behavior was not noticeably different between the two groups. In such situations, a clinician would direct the child’s attention back to the stimuli and encourage him/her to stand up and stretch during the nearest play break. The total duration of the ERP recording session, including play breaks, was approximately 15–20 minutes. On the completion of the session, each participant was given a choice of one of five toys as a prize.
2.4 Event-Related Brain Potential Recordings
Electrical activity was recorded from the scalp using 32 Ag-Cl electrodes secured in an elastic cap (Quik-cap). Electrodes were positioned over homologous locations across the two hemispheres according to the criteria of the International 10–20 system (American Electroencephalographic Society, 1994). The specific locations were: midline sites FZ, FCZ, CZ, CPZ, PZ, OZ, medial lateral sites: FP1/FP2, F3/F4, FC3/FC4, C3/C4, CP3/CP4, P3/P4, O1/O2, and lateral sites: F7/F8, FT7/FT8, T7/T8, TP7/TP8, P7/P8. Electroencephalographic (EEG) activity was referenced to physically linked mastoids. The electro-oculograms were bipolar recordings via electrodes placed over the right and the left outer canthi (horizontal eye movement) and left inferior and superior orbital ridge (vertical eye movement). All electrode impedances were kept at 5 kOhms or less. The electrical signals were amplified within a bandpass of 0.1 and 100Hz and digitized online (Neuroscan 4.2) at the rate of 500 samples per second.
2.5 Data Analysis
2.5.1 ERP Measures
The EEG recordings were bandpass filtered between 0.1 and 30 Hz. Individual EEG records were then visually inspected to exclude trials containing blinks, excessive eye movement, and muscular artifacts (Neuroscan 4.2). The remaining trials were averaged separately for standards and deviants for the long and short ISIs. The number of useable trials were not adequate to separately analyze the effects of stimulus location (left ear, right ear, both ears) so the ERPs were averaged across presentations. Only analysis of the 1000 ms ISI tones is reported in the current study. Tones presented at 200 ms ISI failed to elicit ERPs probably due to the auditory refractory period substantially exceeding 200 ms (Coch, Skendzel, & Neville, 2005; Wunderlich & Cone-Wesson, 2006), especially in young children (Rojas, Walker, Sheeder, Teale, & Reite, 1998). This data will be kept for future analyses during the follow-up testing of participants in the ongoing longitudinal study in our laboratory. For the stimuli presented at 1000 ms ISI, an average of 101 (range: 21–157; SD 29.3) useable standard and 19 (range: 12–32; SD 5.6) useable deviant trials were collected from each child. The number of trials averaged from each child across three types of presentation (left only, right only, and bilateral) did not differ for the two groups for either type of tone: standards, F(1,35)<1; deviants, F(1, 35)<1. Within each group, the number of left-only, right-only, and bilateral presentations did not differ for either standards (CWNS: F(2,34)=2.191, p=0.127; CWS: F(2,34)=1.452, p=0.248) or deviants (CWNS: F(2,34)=2.55, p=0.093; CWS: F(2,32)<1). Between group comparisons of left-only, right-only, and bilateral tone presentations for standards and deviants revealed no significant differences.
ERPs elicited by both the standard and deviant tones were epoched starting at 100 ms pre-stimulus and ending at 1100 ms post-stimulus onset. The 100 ms prior to the recording onset served as a baseline. Peak amplitudes of components were computed relative to the baseline while peak latencies were computed relative to the onset of a stimulus (0 ms). The temporal windows used for the ERP measures were selected based on the visual inspection of the grand average waveforms. Peak amplitudes and latencies of the following components were measured for ERPs elicited by both the standard and deviant tones: P1 (90–190 ms post-stimulus onset) and N1 (170–360 ms post-stimulus onset). Deviant tones failed to produce a clear N2b peak in most electrode sites. They elicited a sustained negativity (MMN) between approximately 360 and 440 ms post-stimulus onset. Its mean amplitude was measured between 360 and 440 ms post-stimulus onset. Lastly, the P3 component appeared as a broad positivity, without a clear peak. Consequently, its mean amplitude was measured between 700 and 900 ms post-stimulus onset.
Repeated-measures ANOVAs were used to evaluate ERP measures. Two ANOVA analyses were performed on each ERP measure – one on medial lateral sites and one on midline sites. Medial lateral ANOVA analyses included one between-group factor (group: CWNS and CWS), and 3 within-group factors: tone type (standard and deviant), hemisphere (left and right), and electrode site. The selection of electrode sites was based on the visual inspection of grand average waveforms and varied slightly depending on the distribution of the measured components. In the case of medial lateral ANOVA analyses, the following electrode sites were used for the P1 and N1 peak amplitude and peak latency measures as well as the P3 mean amplitude measure – F3, FC3, C3, CP3, F4, FC4, C4, CP4. Because the MMN is typically a fronto-central component and was not consistently present across centro-parietal electrodes in our data, its analysis was limited to frontal, fronto-central, and central electrode sites – F3, FC3, C3, F4, FC4, C4. Midline ANOVA analyses included one between-group factor (group: CWNS and CWS), and 2 within-group factors: tone type (standard and deviant) and electrode site. The selection of midline electrodes for analyses of various components paralleled that used for the medial lateral site analyses. More specifically, the same four midline electrode sites were used for the P1 and N1 peak amplitude and peak latency measures: FZ, FCZ, CZ, CPZ; while the analysis of the MMN mean amplitude was based on the FZ, FCZ, and CZ electrode sites only. When medial lateral and midline analyses yielded the same result, only the medial lateral analysis is reported. Significance values were set at p<0.05. In cases where the omnibus analysis produced a significant interaction, it was further evaluated with a step-down ANOVA based on the factors specific to any given interaction. For all repeated measures with greater than one degree of freedom in the numerator, the Huynh-Feldt (H-F) adjusted p-values were used to determine significance (Hays, 1994). Effect sizes, indexed by the partial-eta squared statistic (ηp2), are reported for all significant effects.
2.5.2 Regression Analyses
As mentioned earlier in this report, the CWNS and CWS groups differed significantly on several measures of linguistic aptitude (SPELT-3, TACL-3, BBTOP-CI) and in socio-economic status. In order to examine whether any of these measures might have contributed to the group differences in the mean amplitude of the P3 ERP component, we performed separate linear regression analyses between each measure and the P3 mean amplitude. Additionally, in order to investigate a potential relationship between behavioral measures of working memory (as described in section 2.1) and the P3 mean amplitude, we performed separate regression analyses between (1) the verbal working memory composite (an average of the word span and digit span subtests of the TAPS) and the P3 mean amplitude; and (2) the non-verbal working memory composite (an average of the non-verbal block color and block order tests) and the P3 mean amplitude. The average P3 mean amplitude used for regression analyses was calculated for each participant as a difference between the average voltage over 4 midline electrodes (FZ, FCZ, CZ, and CPZ) elicited by deviant tones and the average voltage over the same 4 electrodes elicited by standard tones. The homogeneity of variance tests performed on the P3 mean amplitude, SPELT-3, TACL-3, BBTOP-CI, SES, and the four working memory tests (digit span, word span, non-verbal block color, and non-verbal block order) showed that the homogeneity of variance assumption for the two groups of participants was violated only for the BBTOP-CI test (Levene statistic=7.959, p=0.008). Consequently, in order to increase power, we pooled data from both groups of participants for regression analyses between the P3 mean amplitude and SPELT-3, TACL-3, and SES. However, regression analyses between the P3 mean amplitude and BBTOP-CI were performed separately on each group.
3. Results
3.1 ERP Results
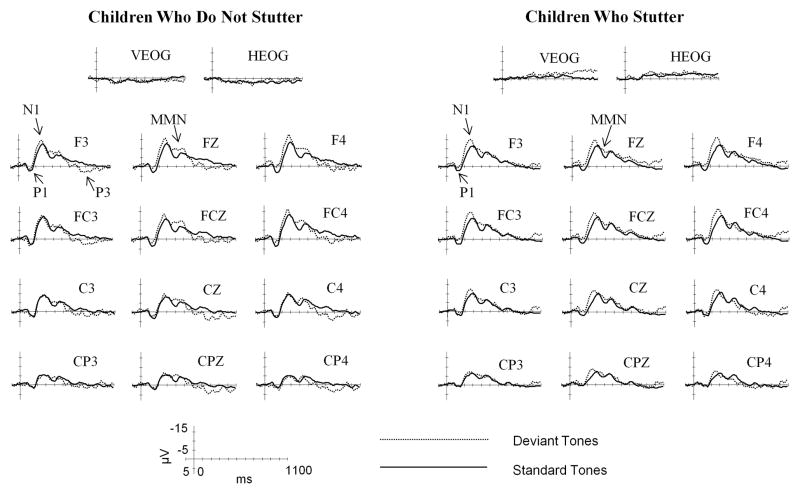
Standard and deviant tones elicited robust ERPs in each group of participants. Figure 1 compares ERPs elicited by standards and deviants in CWNS and CWS groups. Specific similarities and differences between the two groups are summarized below.
Figure 1.
Grand average ERPs elicited by standard and deviant tones in CWNS and CWS. Analyzed ERP components are marked on the F3 and FZ electrodes. Negative potentials are plotted upward. Time 0 ms indicates the onset of the stimulus.
3.2.1 Early Sensory Components P1 and N1
Analysis of the P1 peak amplitude revealed a significant effect of site, F(3,102)=4.1, p=0.027, ηp2=0.108, with relatively greater peak amplitudes over fronto-central and central electrodes compared to frontal and fronto-parietal ones. The effect of site was also significant in the analysis of P1 peak latency, F(3,102)=3.407, p=0.049, ηp2= 0.091 with relatively longer peak latencies over centro-parietal electrodes.
Analysis of the N1 peak amplitude produced a significant effect of tone type, F(1,34)=17.268, p<0.001, ηp2=0.337, with greater peak amplitude in deviant tones. It also yielded a significant effect of site, F(3,102)=83.721, p<0.001, ηp2=0.711, due to larger peak amplitude over frontal and fronto-central electrodes compared to central and centro-parietal ones. Analysis of the N1 peak latency showed a significant effect of tone type, F(1,34)=22.458, p<0.001, ηp2=0.398, with shorter peak latencies elicited by deviant tones.
In sum, the two groups did not differ in either peak amplitude or peak latency measures of the P1 and N1 components. Instead, these analyses showed a group-general effect of tone type on the N1 component, with greater peak amplitude and shorter peak latency elicited by deviant tones.
3.2.2 MMN and P3
Analysis of the MMN mean amplitude revealed a significant effect of tone type, F(1,34)=11.013, p=0.002, ηp2=0.245, with greater negative mean voltage to deviant tones. The effect of electrode site was also significant, F(2,68)=15.598, p<0.001, ηp2=0.314, due to larger mean amplitude over frontal and fronto-central as compared to central electrodes.
Lastly, analysis of the P3 mean amplitude over medial lateral sites showed no significant effect of tone type, F(1,34)=2.655, p=0.112, and no tone type by group interaction, F(1,34)=2.873, p=0.099. However, analysis of the P3 mean amplitude over midline sites yielded a significant group by tone type interaction, F(1,34)=4.49, p=0.041, ηp2=0.117. Step down ANOVAs performed separately on each group showed that deviant tones elicited a significant P3 component only in the CWNS group: CWNS, F(1,17)=5.852, p=0.027, ηp2=0.256; CWS, F(1,17)<1.
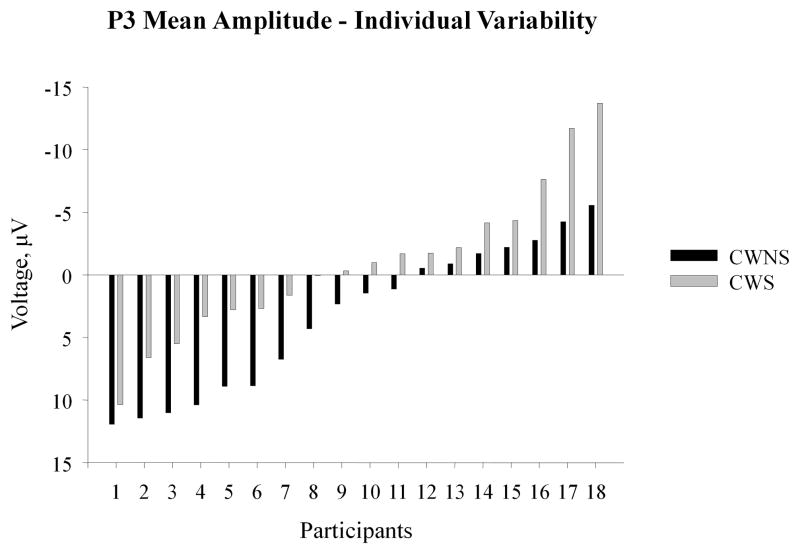
To better visualize group differences in the P3 mean amplitude to deviant tones and to evaluate individual variation in each group, we plotted differences in the P3 mean amplitude between deviant and standard tones averaged over the midline sites (FZ, FCZ, CZ, CPZ) for each participant in Figure 2. This data presentation revealed that, on the one hand, (1) an overall larger proportion of CWS failed to show greater positivity to deviants as compared to standards (CWNS: 7 out of 18, 38.9%; CWS: 11 out of 18, 61.1%), and (2) positive mean amplitudes were on average greater in the CWNS group while negative mean amplitude were greater in the CWS group. On the other hand, P3 mean amplitudes from a number of CWS fell well within the CWNS range.
Figure 2.
Individual variability in the mean amplitude of the P3 component. Each bar represents a difference between the average voltage over FZ, FCZ, CZ, and CPZ electrodes elicited by deviant and standard tones for each participant. In order to maintain consistency with Figure 1, negative voltage is plotted upward.
Overall, while deviant tones elicited MMN in both groups, they led to a significant P3 component over the midline electrode sites only in CWNS. Yet, examination of individual data suggested that in at least some CWS, deviant tones were associated with a pattern of brain responses similar to that of CWNS.
3.3 Regression Analyses
As described in the Methods section of this report, the two groups of participants differed in their performance on the SPELT-3, TACL-3, and BBTOP-CI tests and in SES. In order to determine whether the aspects of linguistic aptitude evaluated by these tests and the socioeconomic background of participants were correlated with the P3 mean amplitude (which differentiated the two groups), we performed a series of linear regression analyses between each of the above measures and the P3 mean amplitude elicited by deviant tones (see Methods). We found that none of the measures correlated significantly with the P3 mean amplitude: SPELT-3: R=0.062, F(1,34)<1; TACL-3: R=0.21, F(1,35)=1.561, p=0.22; SES: R=0.323, F(1,35)=3.948, p=0.055; BBTOP-CI: CWNS, R=0.171, F(1,17)<1 and CWS, R=0.006, F(1,17)<1.
Additionally, neither verbal nor non-verbal behavioral measures of working memory were a significant predictor of the P3 mean amplitude: verbal working memory composite, R=0.278, F(1,34)=2.754, p=0.106; non-verbal working memory composite, R=0.061, F(1,34)<1.
4. Discussion
We combined an auditory oddball paradigm with ERP recordings in order to examine non-linguistic auditory processing as well as attentional allocation and working memory update in preschool CWS and their normally developing peers. The component composition of ERP responses elicited by both standard and deviant tones looked remarkably similar across the two groups suggesting that, overall, pure tones were processed similarly by CWS and CWNS.
4.1 Non-Linguistic Auditory Processing in Stuttering
Contrary to our hypothesis, CWNS and CWS did not differ on any measure of the P1 and N1 components, strongly suggesting that early auditory encoding of pure tones is unimpaired in CWS. Our results are in agreement with a magnetoencephalographic (MEG) study by Biermann-Ruben, Salmelin, and Schnitzler (2005) who found no differences in early sensory components between AWS and NFS in response to pure tones. More specifically, the lack of group difference in the peak latency of these components in our study suggests that at least in respect to those sound properties that are present in pure tones, the degree of maturation of the auditory pathway in 4 and 5 year-old CWS is comparable to that in CWNS.
In both groups, the N1 associated with standard tones had both later peak latency and smaller peak amplitude compared to those elicited by deviants. This finding is in agreement with previous reports (Butler, 1968; Picton, Campbell, Baribeau-Braun & Proulx, 1978). Näätänen and Picton (1987) suggested that such amplitude difference may be due to “selective refractoriness” of neuronal populations. According to this view, at least somewhat different populations of neurons respond to standard and deviant tones. Multiple successive repetitions of standards increase the refractoriness of standard-responding neurons and lead to the relative attenuation of the N1 peak amplitude. The authors conclude that “the amplitude of the N1 is therefore jointly determined by the immediate change in the stimulus level and by the refractory state of the generator mechanism” (p.389). Therefore, although 1000 ms ISI was sufficient in order for the deviant-responding neurons to fully recover, repeated presentations of standard tones must have increased the refractoriness of the standard-responding neurons to the extent that would not allow full recovery within a 1000 ms window, thus leading to the attenuation of the N1 amplitude to standards. The fact that both groups exhibited a similar attenuation of the N1 peak amplitude elicited by standard tones suggests that the refractory state of neuronal populations responding to standard and deviant tones were similar in CWNS and CWS.
In sum, early sensory encoding of pure tones appears to be indistinguishable in CWNS and CWS. However, it remains to be investigated whether group differences emerge in response to more complex sounds.
4.2 Working Memory and Attention in Stuttering
Analysis of ERPs elicited by deviant as compared to standard tones revealed that deviant tones were associated with an MMN component in both groups of participants and with a P3 component in CWNS only. No clear N2b component was present in most sites. Because N2b is thought to be sensitive to the task relevance of stimulus change, the lack of a specific task in our paradigm (i.e. children were not required to respond to deviant tones) might have led to a significant reduction of this component. Additionally, MMN appears to overlap N2b at least in some sites, possibly further contributing to the lack of a clear N2b peak in our data.
The MMN component elicited by deviant tones manifested itself as a sustained negativity closely following N1. It was greatest over frontal and fronto-central sites. As described in the Introduction, the MMN is sensitive to any detectible stimulus change and is believed to be elicited rather automatically (Näätänen and Picton, 1987). It has been proposed that MMN reflects auditory sensory memory for physical properties of recently presented stimuli (Näätänen, 1985). When a new stimulus does not match a memory trace for the previously presented sound, a neuronal mismatch response as indexed by MMN is elicited. The lack of a group difference in MMN to a change in pure tones suggests that the neural processes that are involved in the automatic creation and maintenance of a memory trace for recently presented stimuli and in the detection of a stimulus change are similar in CWNS and CWS. This finding is in agreement with an earlier study by Corbera et al. (2005).
Lastly, we found that deviant tones failed to elicit P3 in children who stutter. This finding indicates that the processes contributing to the attentional allocation and working memory update in response to an auditory change are less robust in CWS. This conclusion is in agreement with an earlier report of reduced P3 mean amplitude in adults who stutter compared to NFS (Hampton and Weber-Fox, 2008). The presence of attenuated P3 to deviants in both young children and adults who stutter suggests that it likely reflects a true physiological characteristic of the brain function in stuttering rather than acquired changes due to many years of coping with the disorder.
Defining a specific mechanism that underlies the attenuated neural response to the auditory change detection in CWS awaits further research. One contributing factor may be the vulnerability of the CWS’s auditory system to changes in cognitive load. Even though the appearance of pictures and the presentation of auditory words signaling game breaks never overlapped with tone presentation, the fact that children were expecting these game cues while also monitoring the tones may have increased a cognitive load of our task enough to differentiate the two groups. Previous studies show a decrease in the P3 amplitude with increase in the cognitive load of a concurrent task (Wickens et al., 1983; Kramer et al., 1985). Therefore, one might suggest that a decrease in the P3 mean amplitude in the CWS group may be due to their greater difficulty in distributing attention between the two tasks or their greater distractibility by the game break expectations. This possibility, however, will require future studies that systematically modulate the cognitive load of a given task.
Interestingly, the CWNS and CWS groups did not differ in behavioral measures of working memory, and further a regression analysis between either verbal or non-verbal working memory tests and the P3 mean amplitude failed to find a significant relation between behavioral and electrophysiological measures. Several factors may have contributed to this pattern of results. First, the oddball task used to elicit the ERPs is markedly different from the working memory tests administered to participants. The former only required that children monitored a very simple auditory signal and observed its changes. The latter, on the other hand, were more complex and required an active encoding and retrieval of different types of information. Therefore, behavioral and electrophysiological measures might have tapped very different cognitive processes. Second, our ERP findings may reflect a subclinical attenuation of function in the CWS group, which does not appear in more complex behavioral tasks because participants are able to compensate for it, possibly by allocating extra effort to the task. This would agree with a number of previous studies that report ERP differences between clinical (or atypical) and typical populations in the absence of behavioral differences between the two (e.g., Stevens, Lauinger, & Neville, 2009; Stevens, Sanders, & Neville, 2006; Weber-Fox, 2001).
Inspection of individual data revealed that while an overall larger percentage of CWS did not display a greater positivity to deviant tones – 61.1% of CWS vs. 38.9% of CWNS – a substantial number of CWS in fact showed a pattern of brain responses very similar to that of CWNS. This finding underlines significant individual variability among individuals who stutter and suggests that relatively less efficient attentional allocation and working memory update may contribute to auditory processing challenges of some individuals who stutter while clinical profiles of others may be shaped by other factors. CWS tested in this study were within 1 or 2 years from the onset of stuttering. Previous research shows that approximately 50% of CWS will spontaneously recover from this disorder (Yairi & Ambrose, 1999). One might suggest that an overlap in the P3 values between the two groups may in part reflect the fact that some of our CWS will recover from stuttering. As we continue to follow these children’s development of cognitive and motor skills through the current longitudinal project in our laboratory, we hope to determine whether atypical ERP responses of pre-school CWS can predict their likelihood of developing persistent stuttering or recovering from the disorder.
Although every effort was made to equate CWS and CWNS groups on a number of cognitive, speech-language, and physiological parameters, the two groups nonetheless differed slightly in their performance on SPELT-3, TACL-3, BBTOP-CI, and in SES. In order to evaluate a possible influence of these measures on the P3 mean amplitude, we performed regression analyses between each measure and the P3 mean amplitude difference between standards and deviants (see Methods). None of the regressions were significant suggesting that at least for this set of participants, the above factors did not significantly contribute to group differences in electrophysiological measures. The SPELT-3 test of expressive language and the word-span working memory test both required overt speech production. Because speaking is overall more effortful for CWS as compared to CWNS, their lower scores on these tests may reflect difficulty with speech production rather than a true weakness of language or other cognitive skills. Lastly, all CWS included in this study showed normal performance on SPELT-3 and TACL-3 tests, suggesting that the group difference in these tests was likely due to relatively high scores for CWNS rather than relatively low scores for CWS.
4.3 Study Limitations
Due to the young age of participants, we were unable to collect behavioral responses to deviant tones, which prevented us from examining a relationship between electrophysiological and behavioral measures of auditory change detection. We believe this caveat was outweighed by the need to examine non-linguistic auditory processing as close to the onset of stuttering as possible. In the future, we hope to gain further insight into the development of auditory and executive functions in CWS by using a similar paradigm with school-age children who are able to provide reliable behavioral responses. This study is currently under way in our laboratory.
A traditional oddball paradigm usually employs only one type of auditory change – namely a change in the frequency of sound. However, the natural auditory environment is rich with changes in multiple auditory characteristics, such as intensity, complexity, and timbre. It is therefore difficult to generalize oddball task findings to more natural complex sounds. One might hypothesize that as the complexity of the auditory environment increases and places a greater challenge on the auditory system, differences in the sensory encoding and later cognitive analysis of sound between individuals who do and do not stutter may also increase. This possibility requires further research.
Lastly, although insightful, the finding of attenuated working memory update as indexed by the P3 ERP component in CWS does not immediately distinguish this population from a number of other clinical groups for whom a similar finding had been reported. For example, a reduced P3 peak amplitude has previously been found in schizophrenia, autism, severe depression, dementia, attention deficit disorder, dyslexia, and alcoholism (Picton, 1992). Given the diversity of the above disorders, it is unlikely that an impairment in the same neural mechanism contributed to the reduced amplitude of the P3 ERP measure in all reported cases. Identifying an atypical pattern of brain response to a sensory change is a necessary and important step in understanding any given disorder. However, more complex stimuli and experimental conditions may be necessary in order to understand the unique profile of cognitive function in each clinical population.
4.4 Conclusion
This study extends previous research in stuttering by showing that important differences exist between young CWS and their normally developing peers in the processing of simple non-linguistic auditory information that requires no speech or motor response. The use of pre-school children as participants addresses an important gap in stuttering research and provides an insight into possible neurophysiological characteristics of brain function in CWS before they are reshaped by compensatory strategies. Lastly, evaluation of individual data reveals significant variability in ERP responses to auditory change, with at least some CWS displaying response patterns similar to those of CWNS. This observation advocates for multifactorial framework in stuttering research which assumes that multiple physiological, psychological, and cognitive factors (including attention and working memory) may be weighted differently in the clinical profile of each individual who stutters, allowing for the significant diversity in the onset, progression, and recovery patterns of this disorder (e.g., Smith and Kelly, 1997).
Acknowledgments
We thank Barbara Brown for her invaluable help in recruiting and behavioral testing of participants. We also thank John Spruill III and Hayley Arnold for help with collection of electrophysiological data and Amy Mosier for help with organizing the data. This study was presented at the annual conference of the American Speech and Hearing Association (November 2008). This project was supported by NIH-NIDCD R01 grant DC00559-18 awarded to Christine Weber-Fox.
References
- American Electroencephalographic Society. Guideline thirteen: Guidelines for standard electrode placement nomenclature. Journal of Clinical Neurophysiology. 1994;11:111–113. [PubMed] [Google Scholar]
- Arends N, Povel DJ, Kolk H. Stuttering as an attentional phenomenon. Journal of Fluency Disorders. 1988;13:141–151. [Google Scholar]
- Baddeley A. Working memory: An overview. In: Pickering SJ, editor. Working Memory and Education. New York, NY: Academic Press; 2006. pp. 1–31. [Google Scholar]
- Bahrick LE, Lickliter R, Flom R. Intersensory redundancy guides the development of selective attention, perception, and cognition in infancy. Current Directions in Psychological Science. 2004;13(3):99–102. [Google Scholar]
- Bajaj A. Working memory involvement in stuttering: Exploring the evidence and research implications. Journal of Fluency Disorders. 2007;32:218–238. doi: 10.1016/j.jfludis.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Bajaj A, Hodson B, Schommer-Aikins M. Performance on phonological and grammatical awareness metalinguistic tasks by children who stutter and their fluent peers. Journal of Fluency Disorders. 2004;29:63–77. doi: 10.1016/j.jfludis.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Bankson NW, Bernthal JE. Bankson-Bernthal Test of Phonology. Riverside Publishing Company; 1990. [Google Scholar]
- Biermann-Ruben K, Salmelin R, Schnitzler A. Right rolandic activation during speech perception in stutterers: a MEG study. NeuroImage. 2005;25:793–801. doi: 10.1016/j.neuroimage.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Blood GW, Ridenour VJ, Jr, Qualls CD, Scheffner Hammer C. Co-occurring disorders in children who stutter. Journal of Communication Disorders. 2003;36:427–448. doi: 10.1016/s0021-9924(03)00023-6. [DOI] [PubMed] [Google Scholar]
- Bloodstein O, Bernstein Ratner N. A Handbook on Stuttering. 6. New York: Thomson Delmar Learning; 2008. [Google Scholar]
- Bosshardt HG. Effects of concurrent mental calculation on stuttering, inhalation and speech timing. Journal of Fluency Disorders. 1999;24:43–72. [Google Scholar]
- Bosshardt HG. Effects of concurrent cognitive processing on the fluency of word repetition: Comparison between persons who do and do not stutter. Journal of Fluency Disorders. 2002;27:93–114. doi: 10.1016/s0094-730x(02)00113-4. [DOI] [PubMed] [Google Scholar]
- Bosshardt HG. Cognitive processing load as a determinant of stuttering: Summary of a research programme. Clinical Linguistics and Phonetics. 2006;20(5):371–385. doi: 10.1080/02699200500074321. [DOI] [PubMed] [Google Scholar]
- Burgemeister B, Blum L, Lorge I. Columbia Mental Maturity Scale. 3. New York: Harcourt Brace Jovanovich; 1972. [Google Scholar]
- Butler RA. Effect of changes in stimulus frequency and intensity on habituation of the human vertex potential. Journal of the Acoustical Society of America. 1968;44:945–950. doi: 10.1121/1.1911233. [DOI] [PubMed] [Google Scholar]
- Carrow-Woolfolk E. Test for Auditory Comprehension of Language – Revised (TACL-R) Allen, TX: DLM Teaching Resources; 1985. [Google Scholar]
- Čeponienė R, Cheour M, Näätänen R. Interstimulus interval and auditory event-related potentials in children: Evidence for multiple generators. Electroencephalography and Clinical Neurophysiology. 1998;108:345–354. doi: 10.1016/s0168-5597(97)00081-6. [DOI] [PubMed] [Google Scholar]
- Coch D, Skendzel W, Neville HJ. Auditory and visual refractory period effects in children and adults: An ERP study. Clinical Neurophysiology. 2005;116:2184–2203. doi: 10.1016/j.clinph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Corbera S, Corral MJ, Escera C, Idiazábal MaA. Abnormal speech sound representation in persistent developmental stuttering. Neurology. 2005;65:1246–1252. doi: 10.1212/01.wnl.0000180969.03719.81. [DOI] [PubMed] [Google Scholar]
- Cuadrado EM, Weber-Fox CM. Atypical syntactic processing in individuals who stutter: Evidence from event-related potentials and behavioral measures. Journal of Speech, Language, and Hearing Research. 2003;46:960–976. doi: 10.1044/1092-4388(2003/075). [DOI] [PubMed] [Google Scholar]
- Dawson JI, Stout CE, Eyer JA. Structured Photographic Expressive Language Test. 3. DeKalb, IL: Janelle Publications; 2003. [Google Scholar]
- Dmitrieva ES, Gel’man VYa. Perception of the emotional speech component by stuttering children associated with noise: Communication II. Analysis of the temporal characteristics of the recognition of different emotions. Human Physiology. 2001;27(1):36–41. [PubMed] [Google Scholar]
- Donchin E. Surprise!... Surprise? Psychophysiology. 1981;18(5):493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Precommentary: Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Dorman MF, Sharma A, Gilley P, Martin K, Roland P. Central auditory development: Evidence from CAEP measurements in children fit with cochlear implants. Journal of Communication Disorders. 2007;40:284–294. doi: 10.1016/j.jcomdis.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Donchin E. Effects of a priori and sequential probability of stimuli on event–related potential. Psychophysiology. 1977;14:95. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Ellis Weismer S, Tomblin JB, Zhang X, Buckwalter P, Chynoweth J, Jones M. Nonword repetition performance in school-age children with and without language impairment. Journal of Speech, Language, and Hearing Research. 2000;43:865–878. doi: 10.1044/jslhr.4304.865. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Karis D, Donchin E. P300 and recall in an incidental memory paradigm. Psychophysiology. 1986;23:298–308. doi: 10.1111/j.1469-8986.1986.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Karis D, Donchin E. Effects of mnemonic strategy manipulation in a Von Restorff paradigm. Electroencephalography and Clinical Neurophysiology. 1990;75:2–35. doi: 10.1016/0013-4694(90)90149-e. [DOI] [PubMed] [Google Scholar]
- Ferrand CT, Gilbert HR, Blood GW. Selected aspects of central processing and vocal motor function in stutterers and nonstutterers: P300, laryngeal shift, and vibratory onset. Journal of Fluency Disorders. 1991;16:101–115. [Google Scholar]
- Ferrari V, Bradley MM, Codispoti M, Lang PJ. Detecting novelty and significance. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2009.21244. in press. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MF. TAPS Test of Auditory–Perceptual Skill. San Francisco, CA: Children’s Hospital of San Francisco; 1985. [Google Scholar]
- Goffman L. Color Block Non-Verbal Memory Task. Purdue University; West Lafayette, Indiana: 2002. [Google Scholar]
- Gregg BA, Yairi E. Phonological skills and disfluency levels in preschool children who stutter. Journal of Communication Disorders. 2007;40:97–115. doi: 10.1016/j.jcomdis.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in Cognitive Sciences. 2009;13 (2):65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JW, Jerger J. Central auditory function in stutterers. Journal of Speech and Hearing Research. 1978;21:324–337. doi: 10.1044/jshr.2102.324. [DOI] [PubMed] [Google Scholar]
- Hampton A, Weber-Fox C. Journal of Fluency Disorders. 2008;33(4):253–273. doi: 10.1016/j.jfludis.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannley M, Dorman MF. Some observations on auditory function and stuttering. Journal of Fluency Disorders. 1982;7:93–108. [Google Scholar]
- Hays M. Statistics. 5. Fort Worth, TX: Harcourt Brace College Publishers; 1994. [Google Scholar]
- Heitmann RR, Asbjørnsen A, Helland T. Attentional functions in speech fluency disorders. Logopedics Phoniatrics Vocology. 2004;29:119–127. doi: 10.1080/14015430410017379. [DOI] [PubMed] [Google Scholar]
- Hoffman L, Gillam R. Verbal and spatial information processing constraints in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2004;47:114–125. doi: 10.1044/1092-4388(2004/011). [DOI] [PubMed] [Google Scholar]
- Hollich G, Newman R, Jusczyk P. Infants use of synchronized visual information to separate streams of speech. Child Development. 2005;76:598–613. doi: 10.1111/j.1467-8624.2005.00866.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Department of Sociology, Yale University; New Haven, Connecticut: 1975. Unpublished manuscript. [Google Scholar]
- Hyde M. The N1 response and its applications. Audiology and Neurootology. 1997;2:281–307. doi: 10.1159/000259253. [DOI] [PubMed] [Google Scholar]
- Kane NM, Curry SH, Butler SR, Cummins BH. Electrophysiological indicator of awakening from coma. The Lancet. 1993;341:688. doi: 10.1016/0140-6736(93)90453-n. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Wickens CD, Donchin E. Processing of stimulus properties: evidence for dual-task integrality. Journal of Experimental Psychology: Human Perception and Performance. 1985;11:393–408. doi: 10.1037//0096-1523.11.4.393. [DOI] [PubMed] [Google Scholar]
- Kramer MB, Green D, Guitar B. A comparison of stutterers and nonstutterers on masking level differences and synthetic sentence identification tasks. Journal of Communication Disorders. 1987;20:379–390. doi: 10.1016/0021-9924(87)90026-8. [DOI] [PubMed] [Google Scholar]
- Liebetrau RM, Daly DA. Auditory processing and perceptual abilities of “organic” and “functional” stutterers. Journal of Fluency Disorders. 1981;6:219–231. [Google Scholar]
- Liégeois-Chauvel C, Musolino A, Badier JM, Marquis P, Chauvel P. Evoked potentials recorded from the auditory cortex in man: Evaluation and topography of the middle latency components. Electroencephalography and Clinical Neurophysiology. 1994;92:204–214. doi: 10.1016/0168-5597(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Linden DE. The P300: Where in the brain is it produced and what does it tell us? The Neuroscientist. 2005;11(6):563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- Loewy DH, Campbell KB, Bastien C. The mismatch negativity to frequency deviant stimuli during natural sleep. Electroencephalography and Clinical Neurophysiology. 1996;98:493–501. doi: 10.1016/0013-4694(96)95553-4. [DOI] [PubMed] [Google Scholar]
- Luck S. An Introduction to the Event-Related Potential Technique. Cambridge, MA: The MIT Press; 2005. [Google Scholar]
- McGee T, Kraus N. Auditory development reflected by middle latency response. Ear and Hearing. 1996;17(5):419–429. doi: 10.1097/00003446-199610000-00008. [DOI] [PubMed] [Google Scholar]
- Morgan MD, Cranford JL, Burk K. P300 event-related potentials in stutterers and nonstutterers. Journal of Speech, Language, and Hearing Research. 1997;40:1334–1340. doi: 10.1044/jslhr.4006.1334. [DOI] [PubMed] [Google Scholar]
- Muller-Gass A, Stelmack RM, Campbell KB. The effect of visual task difficulty and attentional direction on the detection of acoustic change as indexed by the mismatch negativity. Brain Research. 2006;1078:112–130. doi: 10.1016/j.brainres.2005.12.125. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Selective attention and stimulus processing: Reflections in event-related potentials, magnetoencephalogram and regional cerebral blood flow. In: Posner MI, Marin OS, editors. Attention and Performance XI. Hillsdale, NJ: Erlbaum; 1985. pp. 355–373. [Google Scholar]
- Näätänen R. The mismatch negativity: A powerful tool for cognitive neuroscience. Ear and Hearing. 1995;16(1):6–18. [PubMed] [Google Scholar]
- Näätänen R, Alho K. Mismatch negativity - a unique measure of sensory processing in audition. International Journal of Neuroscience. 1995;80:317–337. doi: 10.3109/00207459508986107. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Gaillard AWK. The orienting relfex and the N2 deflection of the event-related potential (ERP) In: Gaillard AWK, Ritter W, editors. Tutorials in ERP Research: Endogenous Components. Amsterdam: North-Holland; 1983. pp. 119–141. [Google Scholar]
- Näätänen R, Gaillard AWK, Mantysalo S. The N1 effect of selective attention reinterpreted. Acta Psychologica. 1978;42:313–329. doi: 10.1016/0001-6918(78)90006-9. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton TW. N2 and automatic versus controlled processes. Cerebral Psychophysiology: Studies in Event-Related Potentials (EEG Supplement 38) 1986:169–186. [PubMed] [Google Scholar]
- Näätänen R, Picton T. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Simpson M, Loveless NE. Stimulus deviance and evoked potentials. Biological Psychology. 1982;14:53–98. doi: 10.1016/0301-0511(82)90017-5. [DOI] [PubMed] [Google Scholar]
- Nudelman HB, Herbrich KE, Hess KR, Hoyt BD, Rosenfield DB. A model of the phonatory response time of stutterers and fluent speakers to frequency-modulated tones. Journal of the Acoustical Society of America. 1992;92(4 Pt 1):1882–1888. doi: 10.1121/1.405263. [DOI] [PubMed] [Google Scholar]
- Oldfield R. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychology. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paavilainen P, Alho K, Reinikainen K, Sams M, Näätänen R. Event-related potentials to pitch change in an auditory stimulus sequence during sleep. Current trends in event-related brain potetial research. In: Johnson R, Rohrbaugh JW, Parasuraman R, editors. Electroencephalography and Clinical Neurophysiology Supplement. Vol. 40. 1987. pp. 246–255. [PubMed] [Google Scholar]
- Patel SH, Azzam PN. Characterization of N200 and P300: Selected studies of the event-related potential. International Journal of Medical Sciences. 2005;2(4):147–154. doi: 10.7150/ijms.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW. The P300 wave of the human event-related potential. Journal of Clinical Neurophysiology. 1992;9(4):456–479. doi: 10.1097/00004691-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Picton TW, Campbell KB, Baribeau-Braun J, Proulx GB. The neurophysiology of human attention: A tutorial review. In: Requin J, editor. Attention and Performance VII. Hillsdale, NJ: Lawrence Erlbaum; 1978. pp. 429–467. [Google Scholar]
- Polich J. Theoretical overview of P3a and P3b. In: Polich J, editor. Detection of Change: Event-Related Potential and fMRI Findings. NewYork: Kluwer Academic Publishers; 2003. [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clincial Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponton CW, Eggermont, Jos J, Kwong B, Don M. Maturation of human central auditory system activity: Evidence from multi-channel evoked potentials. Clinical Neurophysiology. 2000;111:220–236. doi: 10.1016/s1388-2457(99)00236-9. [DOI] [PubMed] [Google Scholar]
- Ratcliff-Baird B. ADHD and stuttering: Similar EEG profiles suggest neurotherapy as an adjunct to traditional speech therapies. Journal of Neurotherapy. 2001;5(4):5–22. [Google Scholar]
- Rojas DC, Walker JR, Sheeder JL, Teale PD, Reite ML. Developmental chagnes in refractoriness of the neuromagnetic M100 in children. NeuroReport. 1998;9:1543–1547. doi: 10.1097/00001756-199805110-00055. [DOI] [PubMed] [Google Scholar]
- Sabri M, Liebenthal E, Waldron EJ, Medler DA, Binder JR. Attentional modulation in the detection of irrelevant deviants: a simultaneous ERP/fMRI study. Journal of Cognitive Neuroscience. 2006;18(5):689–700. doi: 10.1162/jocn.2006.18.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasisekaran J, De Nil LF. Phoneme monitoring in silent naming and perception in adults who stutter. Journal of Fluency Disorders. 2006;31:284–302. doi: 10.1016/j.jfludis.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Scherg M, Vajsar J, Picton TW. A source analysis of the late human auditory evoked potentials. Journal of Cognitive Neuroscience. 1989;1(4):336–355. doi: 10.1162/jocn.1989.1.4.336. [DOI] [PubMed] [Google Scholar]
- Schopler E, Reichler RJ, Renner BR. The Childhood Autism Rating Scale (CARS) for Diagnostic Screening and Classification of Autism. Los Angeles: Western Psychological Services; 1988. [Google Scholar]
- Sharma A, Kraus N, McGee TJ, Nicol TG. Developmental changes in P1 and N1 central auditory responses elicited by consonant-vowel syllables. Electroencephalography and Clinical Neurophysiology. 1997;104:540–545. doi: 10.1016/s0168-5597(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Smith A, Kelly E. Stuttering: a dynamic, multifactorial model. In: Curlee RF, Siegel GM, editors. Nature and Treatment of Stuttering. Boston: Allyn and Bacon; 1997. pp. 204–217. [Google Scholar]
- Stevens C, Lauinger B, Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related potential study. Developmental Science. 2009;12(4):634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C, Sanders L, Neville H. Neurophysiological evidence for selective auditory attention deficits in children with specific language impairment. Brain Research. 2006;1111:143–152. doi: 10.1016/j.brainres.2006.06.114. [DOI] [PubMed] [Google Scholar]
- Sussman ES. A new view on the MMN and attention debate: the role of context in processing auditory events. Journal of Psychophysiology. 2007;21(3–4):164–175. [Google Scholar]
- Sussman E, Winkler I, Wang W. MMN and attention: competition for deviance detection. Psychophysiology. 2003;40:430–435. doi: 10.1111/1469-8986.00045. [DOI] [PubMed] [Google Scholar]
- Vasic N, Wijnen F. Stuttering as a monitoring deficit. In: Hartsuiker RJ, Rostma BR, Wijnen F, editors. Phonological Encoding and Monitoring in Normal and Pathological Speech. Hove (East Sussex): Psychology Press; 2005. [Google Scholar]
- Verleger R. Event-related potentials and cognition: A critique of the context updating hypothesis and an alternative interpretation of P3. Behavioral and Brain Sciences. 1988;11:343–427. [Google Scholar]
- Weber-Fox C. Neural systems for sentence processing in stuttering. Journal of Speech, Language, and Hearing Research. 2001;44:814–825. doi: 10.1044/1092-4388(2001/064). [DOI] [PubMed] [Google Scholar]
- Weber-Fox C, Spencer RMC, Spruill JE, III, Smith A. Phonological processing in adults who stutter: Electrophysiological and behavioral evidence. Journal of Speech, Language, and Hearing Research. 2004;47:1244–1258. doi: 10.1044/1092-4388(2004/094). [DOI] [PubMed] [Google Scholar]
- Weber-Fox C, Spruill JE, III, Spencer R, Smith A. Atypical neural functions underlying phonological processing and silent rehearsal in children who stutter. Developmental Science. 2008;11(2):321–337. doi: 10.1111/j.1467-7687.2008.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens C, Kramer A, Vanasse L, Donchin E. Performance of concurrent tasks: A psychophysiological analysis of the reciprocity of information-processing resources. Science. 1983;221:1080–1082. doi: 10.1126/science.6879207. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Gallen CC, Hampson SA, Hillyard SA, Pantev C, Sobel D, Bloom FE. Modulation of early sensory processing in human auditory cortex during auditory selective attention. Proceedings of the National Academy of Science. 1993;90:8722–8726. doi: 10.1073/pnas.90.18.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldorff MG, Hackley SA, Hillyard SA. The effects of channel-selective attention on the mismatch negativity wave elicited by deviant tones. Psychophysiology. 1991;28(4):30–42. doi: 10.1111/j.1469-8986.1991.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK. Maturation of CAEP in infants and children: A review. Hearing Research. 2006;212:212–223. doi: 10.1016/j.heares.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Yairi E, Ambrose N. Early childhood stuttering I: Persistency and recovery rates. Journal of Speech, Language, and Hearing Research. 1999;42:1097–1112. doi: 10.1044/jslhr.4205.1097. [DOI] [PubMed] [Google Scholar]
- Yucel G, Petty C, McCarthy G, Belger A. Graded visual attention modulates brain responses evoked by task-irrelevant auditory pitch changes. Journal of Cognitive Neuroscience. 2005;17(12):1819–1828. doi: 10.1162/089892905775008698. [DOI] [PubMed] [Google Scholar]