Abstract
Arylhydrocarbon receptor (Ahr) activation by 2,3,7,8-tetrachlordibenzo-p-dioxin (TCDD) interferes with female reproductive functions, but there is little information on the specific targets of TCDD in the hypothalamic-pituitary-gonadal (HPG) axis. In these studies, we found that TCDD upregulated known AhR target genes, cytochrome p450 1a1 (Cyp1a1), Cyp1a2 and Cyp1b1 in the rat pituitary gland. Moreover, 75% of pituitary lactotropes and 45% of gonadotropes contained Ahr mRNA, and most Ahr-containing cells were estrogen receptor 1 (Esr1)-positive. TCDD abrogated estradiol (E2)-induced prolactin (Prl) expression in vivo and in vitro; conversely, E2 blocked TCDD upregulation of luteinizing hormone beta (Lhb) and glycoprotein hormone alpha polypeptide (Cga) expression. TCDD had no effect on levels of Ahr mRNA, but upregulated Esr1 mRNA. E2 independently repressed Ahr and Esr1 expression and blocked TCDD upregulation of Esr1. Thus, complex interactions between Ahr and Esr alter Prl and luteinizing hormone (LH) synthesis by direct actions in lactotropes and gonadotropes. These findings provide important insights into how TCDD disrupts female reproductive functions.
Keywords: Dioxin, Ahr, Antiestrogen, Prl, LH, Estradiol
1. Introduction
During the past 50 years, the incidence of endocrine disorders has dramatically increased, and accumulating evidence suggests that the increase is related, at least in part, to chemical contaminants in the environment (Birnbaum, 1994). Dioxins and dioxin-like molecules of the polyhalogenated aromatic hydrocarbon family are among the most ubiquitous and persistent environmental contaminants linked to endocrine disruption. Of particular concern is the ability of TCDD to interfere with female reproductive functions, thereby decreasing fertility (Myllymaki et al., 2005, Salisbury and Marcinkiewicz, 2002, Wolf et al., 1999).
The mechanisms underlying reduced fertility after TCDD exposure are unclear, but nearly all TCDD effects are mediated by the aryl hydrocarbon receptor (Ahr). Unliganded Ahr (Burbach et al., 1992, Poland et al., 1976) is bound to chaperone proteins (Hsp90/XAP2/p23) in the cytoplasm (Furness et al., 2007, Pollenz et al., 1994, Swanson et al., 1995). When activated by TCDD, Ahr translocates to the nucleus where it dimerizes with Ahr nuclear translocators (Arnts) (Hirose et al., 1996, Kewley et al., 2004, Poland et al., 1976, Reyes et al., 1992). The resulting heterodimeric complex binds to a specific DNA sequence, the dioxin response element (DRE, also called xenobiotic response element, XRE; or aryl hydrocarbon response element, AhrE) (Denison et al., 1989, Hapgood et al., 1989, Nebert et al., 2000), recruits protein coactivators and induces target gene transcription (Whitlock, 1999, Xu et al., 2000).
Importantly, activation of the Ahr pathway also interferes with Esr pathways through a number of mechanisms (Beischlag and Perdew, 2005, DeVito et al., 1992, Kietz et al., 2004, Matthews and Gustafsson, 2006, Matthews et al., 2005, Ohtake et al., 2007, Ohtake et al., 2003, Safe and Wormke, 2003, Tian et al., 1998, Tian et al., 1998). Considering the critical roles of E2 and its receptors in the regulation of the female HPG axis (Green et al., 1986, Greene et al., 1986), the deleterious effects of TCDD on female reproduction may be due to antiestrogenic effects.
The pituitary gland is perhaps the least characterized target of TCDD in the HPG axis. Despite clear evidence that the Ahr pathway can be activated in the pituitary gland (Huang et al., 2000, Huang et al., 2002, Huang et al., 2003), no studies have identified specific pituitary cells or genes directly regulated by both E2 and TCDD. In recent studies, we used targeted microarrays to identify such pituitary-specific genes. We found that TCDD had an antiestrogenic effect on Prl gene expression (Cao and Petersen, unpublished), and also identified the Cga as a possible target of both TCDD and E2. Lhb was not detected as such a target, but other evidence suggests that LH secretion is disrupted by TCDD (Li et al., 1995).
Based on these findings, we tested whether TCDD acts directly in the lactotropes and gonadotropes of the pituitary gland. We next examined whether Ahr and Esr1 mRNAs and proteins are found in the same pituitary cells. Finally, we tested interactions between TCDD and E2 on the regulation of genes encoding Esr1, Ahr, Prl, as well as Lhb and Cga subunits. Our findings suggest that pituitary lactotropes and gonadotropes are direct targets through which Ahr activation can act through E2-dependent and -independent mechanisms to alter LH and Prl synthesis.
2. Materials and Methods
2.1. Animals
Adult female Holtzman Sprague-Dawley rats (Harlan; Indianapolis, IN) were individually housed in a temperature- and light-controlled room (14:10 h light:dark cycle; lights on at 0500 H) with food and water available ad libitum. All animals were maintained according to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and the Institutional Animal Care and Use Committee of the University of Massachusetts approved all treatment protocols.
For studies examining the effects of E2 and TCDD on pituitary gene expression, rats were ovariectomized (OVX), and one week later (Day 0) implanted s.c. with two 3-cm Silastic™ (Dow Corning Corp., Midland, MI) capsules containing either sesame oil (Oil) or E2 (150 μg/ml E2 in sesame oil). Twenty-four hours later (Day 1), rats were weighed and given p.o. TCDD (AccuStandard Inc, New Haven, CT) or dimethylsulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO). The resulting treatment groups were: Oil+DMSO, Oil+TCDD 0.5 μg/kg body weight (bw), Oil+TCDD, 10 μg/kg bw, E2+DMSO, E2+TCDD 0.5 μg/kg bw and E2+TCDD 10 μg/kg bw The higher dose of TCDD was based on results of previous studies demonstrating adverse effects on female reproductive functions (Petroff et al., 2000). TCDD and DMSO were applied to the surface of Keebler Vanilla Wafers™ that were then dried overnight in a hood. On Day 2, all animals were killed between 1530 and 1600 H. Pituitary glands were rapidly removed, frozen on dry ice and stored at −80 °C until used for RNA isolation or cryosections.
2.2. TCDD Effects on Pituitary Cyp1a1, Cyp1a2 and Cyp1b1 Gene Expression
2.2.1. Single-Label In Situ Hybridization (ISH)
Both Prl and Cga genes were found on pituitary microarrays to be regulated by TCDD. To assess whether TCDD acted directly in the pituitary gland, we first used single-label ISH to test whether the Ahr target gene, Cyp1a2, increased after TCDD. ISH also allowed us to visualize pituitary distribution of Cyp1a2 expression. For these studies, the template for in vitro transcription of Cyp1a2 antisense and sense-strand control probes was a 793-bp cDNA fragment (Genbank Accession number NM_012541; nucleotides 805–1598). We used standard methods with 35S-radiolabeled UTP (NEN, Boston, MA) to prepare cRNA probes as described previously (Petersen et al., 2000).
For hybridization, 12-μm cryosections from 3–5 rats/treatment were processed as described previously (Hays et al., 2002, Petersen et al., 1996). We then applied probes (1×106 cpm) in 25 μl of hybridization buffer and covered sections with glass coverslips. Sense-strand controls were included for each probe and sections were hybridized in humid chambers overnight at 55 °C. After post-hybridization washes (Petersen et al., 1996), we dehydrated sections and apposed them to Kodak film for 3 days before developing autoradiograms.
2.2.2. QPCR
To test whether effects of TCDD were direct, we used QPCR to examine whether TCDD increased expression of the Ahr target genes, Cyp1a1 and Cyp1b1. Using Ultraspec™-II RNA isolation reagents (Biotecx Laboratories Inc., Houston, TX), RNA was extracted from 5–7 pituitaries/treatment group from animals prepared as described above. RNA pellets were resuspended in nuclease-free water, and used in reverse transcription (RT) reactions to synthesize cDNA used for QPCR. We used the Mx3005P™ real time PCR system (Stratagene, La Jolla, CA) and validated primer sets (Sigma-Genosys, St. Louis, MO) for Cyp1a1(Martignoni et al., 2004) and Cyp1b1(Gauger et al., 2007) mRNAs and for the internal standard, beta actin (Actb) (Augustine et al., 2003). QPCR was performed in a 25-μl reaction volume containing 1X iQ SuperMix (Bio-Rad, Hercules, CA), 400 nM forward primer, 400 nM reverse primer and 2 μl cDNA. Samples containing no RT reaction were run as negative controls. All threshold cycle (Ct) values were normalized to values of the internal standard. QPCR reactions for each sample were performed in duplicate or triplicate, and data were analyzed with the −ΔΔCt method (Livak and Schmittgen, 2001). To facilitate comparisons among groups, data were converted to percent control by dividing each −ΔΔCt value by the average −ΔΔCt of the vehicle controls. When the value of the vehicle control group was 0, we expressed data as 2−ΔCT multiplied by 100.
2.3 Colocalization of Ahr with Prl or Lhb
2.3.1 Dual-label ISH
For these studies, we used pituitaries from OVX rats treated as described above (n=3–5 pituitaries/group). We prepared digoxigenin-labeled cRNA probes for Prl mRNA using a full-length, 820-bp cDNA (Genbank Accession number V01249) and methods described previously (Ottem et al., 2004). Digoxigenin-labeled probes to Lhb mRNA were transcribed from a 350-bp PstI-PstI cDNA fragment provided by W.W. Chin (Chin et al., 1983). Two separate 35S-labeled cRNA probes for Ahr were transcribed from 1.2 kbp- and 517-bp subcloned fragments of the full length cDNA, kindly provided by C. Bradfield (University of Wisconsin Madison) (Carver et al., 1994) and used previously in our laboratory (Petersen et al., 2000).
For hybridization, cryosections were processed as described above for single-label ISH. A mixture of the two radiolabeled Ahr cRNA probes (1.0×106 cpm of each probe) and 1 μl digoxigenin-labeled Prl or Lhb probes was applied to each tissue section in 25 μl of hybridization buffer. Sense-strand controls were included for each probe and all sections were hybridized in humid chambers overnight at 55 °C.
After post-hybridization washes, the digoxigenin-labeled probes for Prl or Lhb were detected immunocytochemically as described previously (Hays et al., 2002). Slides were then dipped in NTB emulsion (Kodak, Rochester NY) to detect radiolabeled probes for Ahr mRNA. Slides were exposed for 1–2 weeks at 4 °C and then processed in Dektol developer and Kodak fixer. After development, we counted the number of cells containing Ahr, Prl or Lhb mRNA, as well as cells with both Ahr and Prl or Ahr and Lhb mRNA.
2.3.2 Dual-label ICC
For studies colocalizing Ahr and Prl or Lhb proteins, three adult female rats received wafers containing 10 μg/kg bw TCDD. Pituitaries were removed the following day and fixed with 4% paraformaldehyde in 0.05 M sodium phosphate buffer (pH 7.4) for 24 h. They were immersed in 20% sucrose in paraformaldehyde for another 24 h, then in 0.02 M potassium phosphate-buffered saline (KPBS) containing 20% sucrose overnight at 4 °C. Finally, pituitaries were frozen in powdered dry ice and stored at −80 °C until they were sectioned at 30 μm and stored at −20 °C, free-floating in cryoprotectant solution (20% glycerol, 30% ethylene glycol in KPBS).
For dual-label ICC, 3–5 pituitary sections from each animal were removed and rinsed in six 5-min washes of KPBS with mild agitation, then pre-incubated in 2% normal donkey serum (Colorado Serum Co., Denver, CO) with 0.3% Triton X-100 in KPBS (LKPBS) at 4 °C overnight to eliminate nonspecific signal. Sections were next incubated for 72 h with mild agitation at 4 °C in goat polyclonal anti-Ahr (M-20, 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti-rPrl (anti-rPRL-IC-5, 1:600,000; NIDDK, Bethesda, MD) or rabbit polyclonal anti-rLhb (anti-betaLH-IC-3, 1:100,000; NIDDK). The specificity of these antisera has been described previously (Oesch-Bartlomowicz et al., 2005, Sorenson et al., 2007, Watanabe et al., 2004). Next, sections were rinsed in KPBS and then incubated with Alexa-Fluor donkey anti-goat 488 and Alexa-Fluor donkey anti-rabbit 555 (Invitrogen, Carlsbad, CA) at 1:200 dilutions in LKPBS. After incubation, sections were washed and counterstained with Hoechst 33258 dye (Invitrogen). After KPBS rinses, sections were mounted onto slides and coverslipped with glycerol mounting medium.
For cell counting, one medial and two lateral anterior pituitary sections were chosen randomly from each animal and analyzed using a Leica CTR 5000 microscope fitted with filters for Cy3, FITC and DAPI. We first examined Ahr immunoreactivity (Ahr-ir) with a Cy3 filter and photographed sections at 100X with a Retiga 1300i digital camera. Prl-ir or Lhb-ir was detected in the same region with a FITC filter and photographed. Finally, nuclear DNA of cells in the same region stained with Hoechst 33258 was visualized with a DAPI filter and photographed. The MCID Image Analysis (Interfocus Imaging Ltd., Cambridge, England) software package was used to merge images. The total number of cells (nuclear staining), cells with Ahr-ir, cells with Prl-ir, and cells with both Ahr-ir and Prl-ir, or Ahr-ir and Lhb-ir were counted in each pituitary section.
2.4 TCDD Effects on E2 Regulation of Prl mRNA in GH3 Cells
As another test of whether TCDD directly inhibited E2 regulation of Prl mRNA, we used GH3 cells (American Type Tissue Collection, Rockville, MD), a rat pituitary-derived Prl-producing cell line. Cells were maintained in Hams F-12K media supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine (Mediatech, Herndon, VA) and 10% fetal bovine serum (FBS; Hyclone, South Logan, UT) at 37 °C with 5% CO2. Once cells reached a confluence of 80–90%, they were split into 6-well plates. When cells reached approximately 50% confluence, medium was replaced with 10% dextran/charcoal-stripped FBS (Hyclone, South Logan, UT) in phenol red-free DMEM/F12 medium (Mediatech, Herndon, VA) for two days. GH3 cells were then treated with E2 or ethanol vehicle and with TCDD or DMSO vehicle.
We first performed a logarithmic dose-response study in which GH3 cells were treated for 24 h with vehicle or E2 at doses ranging from 10−7 through 10−11 M. We then performed a time-course study using 10−8 M E2 (concentration found to be saturating) or vehicle and treating cells for 6, 12 or 24 h. Finally, we examined the effect of E2 in the presence of various doses of TCDD (10−10, 10−9, 10−8, 10−7 M) or DMSO vehicle in cells treated for 24 h. After treatment, total RNA was isolated from GH3 cells, and QPCR was used to quantify Prl mRNA levels as described above.
2.5 TCDD and E2 Effects on Prl, Lhb and Cga mRNA Levels
We used QPCR to verify microarray findings that TCDD abrogated E2 induction of Prl gene expression using the animal model described above. Using RNA from the same animals, we also tested effects of TCDD or E2, alone or in combination, on Lhb and Cga mRNA levels.
For each of 5–7 samples/treatment, total RNA was isolated and used in RT reactions to prepare cDNA and perform QPCR as described above. The primer sets and probes used were as described previously for Prl (Langouche et al., 2004) and for the internal standard, 18S RNA (Zhu and Altmann, 2005). The Prl probe was labeled with FAM at the 5′ end and BHQ-1 at the 3′ end, and 18S RNA was labeled with HEX at the 5′ end. QPCR was performed in a 25-μl reaction volume containing 1X iQ SuperMix (Bio-Rad, Hercules, CA), 400 nM forward primer, 400 nM reverse primer and 2 μl cDNA.
Primers for rat Cga were designed with Light Cycler QPCR primer design software (Roche Applied Scientific, Mannheim, Germany), and forward and reverse primers were 5′CCAAGATCCAGAGTTTGC3′ and 5′GGACCTTGCGGGAGTC3′ respectively. The primer set for rat Lhb were designed similarly and were: 5′TCACCTTCACCACCAGCATC3′ forward and 5′GGTAGGTGCACACTGGCTGA3′ reverse. The primers for the internal control were Actb as described previously (Augustine et al., 2003) and we used 1X iQ MasterMix for SYBR Green (Bio-Rad, Hercules, CA). QPCR reactions for each sample were performed in duplicate or triplicate and data were analyzed with the ΔΔCt method (Livak and Schmittgen, 2001).
2.6 Effects of E2 and TCDD on Ahr and Esr1 mRNA Levels
We used previously described primer sets and probes for rat Ahr (Lovekamp-Swan et al., 2003), and Esr1 mRNA (Spreafico et al., 1992) to examine E2 and TCDD regulation of Ahr and Esr1 expression. Probes for Esr1 were labeled with FAM at the 5′ end and BHQ-1 at the 3′ end and probes for Actb (Augustine et al., 2003) were labeled with HEX at the 5′ end. Relative mRNA levels were determined by QPCR as described above.
2.7 Colocalization of Ahr and Esr1 in Pituitary Cells
To test whether TCDD and E2 could act in the same cells, we performed dual-label ICC for Ahr and Esr1. Sections from the same animals used to colocalize Ahr and Prl (see above) were incubated in goat polyclonal anti-Ahr (M-20, 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit polyclonal anti-Esr1 (C1355, 1:20,000; Upstate Biotechnology, Waltham, MA) for 72 h at 4 °C. Specificity of the latter was verified previously (Wu et al., 2009). After rinsing, sections were incubated with Alexa-Fluor secondary antibodies as described above at a 1:200 dilution in LKPBS, and nuclei were stained with Hoechst 33258. Finally, sections were mounted, coverslipped and photographed. Cells containing Ahr, Esr1 or both were counted as described above.
2.8 Statistical Analyses
Effects of 0.5 and 10 μg TCDD on Cyp1a1 mRNA levels were compared using Student t-tests. Results of all other studies were analyzed using either one-way analysis of variance (ANOVA) or two-way ANOVA with time, dosages or treatments as the main effects. Post-hoc tests, where appropriate, were performed using Newman-Keuls for one-way, or Bonferroni’s t test for two-way ANOVA. Differences between treatments were considered to be significant when p<0.05.
3. Results
3.1. TCDD Induced Cyp1a1, Cyp1a2 and Cyp1b1 Gene Expression in Female Rat Pituitary
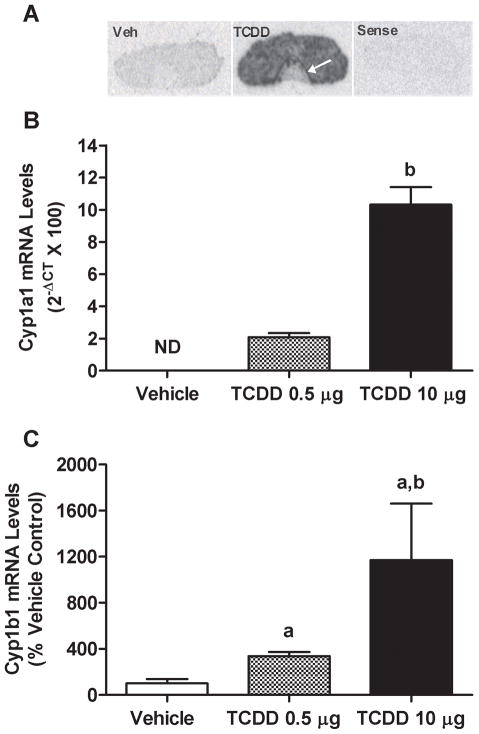
Results of ISH studies showed that TCDD increased Cyp1a2 mRNA levels in the pituitary gland. Interestingly, the intermediate lobe of the pituitary showed the highest level of induced expression (Fig. 1A). Results of QPCR studies showed that TCDD induced expression of both Cyp1a1 and Cyp1b1 genes in a dose-dependent way (Fig. 1B and C), and that Cyp1a1 expression was undetectable without TCDD treatment.
FIG. 1.
Effects of TCDD on Cyp1a2, 1a1 and 1b1 gene expression in the pituitary gland in vivo. (A) Film autoradiogram of ISH using 35S-labeled cRNA probes for Cyp1a2 mRNA in pituitaries from OVX rats treated p.o. with TCDD 10 μg/kg bw or DMSO vehicle. White arrow indicates particularly intense signal in the intermediate lobe. QPCR measurements of Cyp1a1 (B) or Cyp1b1 (C) mRNA levels in OVX rats treated with either 0.5 or 10 μg/kg bw TCDD p.o. Bars represent mean ± SEM of 4–6 samples analyzed in duplicate. ND indicates no detectable signal in vehicle treatment group. aSignificantly different from vehicle-treated group; bsignificantly different from 0.5 μg group. Differences were considered significant if p<0.05.
3.2. Ahr Expression Was Found in Both Lactotropes and Gonadotropes
3.2.1. ISH
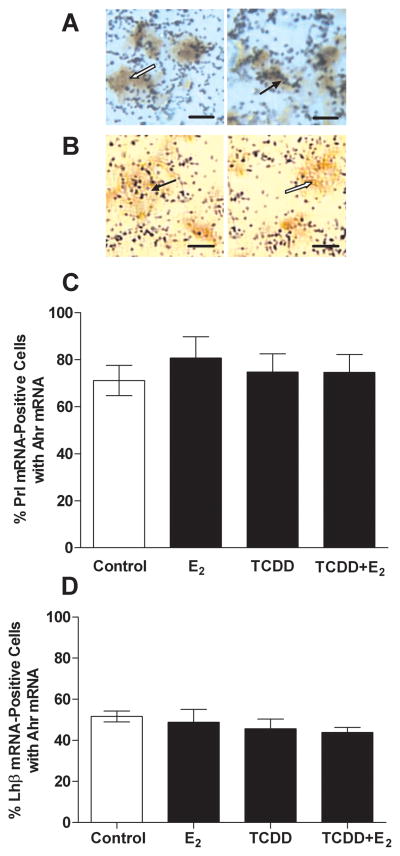
Results of our dual-label ISH studies showed colocalization of Ahr and Prl or Lhb mRNA (Fig. 2A, B) in pituitary cells. Of all the Ahr-positive cells, 48.5 ± 0.5% (mean ± SEM) were lactotropes. Neither E2 nor TCDD treatment altered the percentage of lactotropes expressing Ahr mRNA. Regardless of treatment, between 70 and 80% of Prl mRNA-containing cells also expressed the Ahr gene (Fig. 2C). Lhb mRNA-containing cells accounted for only 6.1 ± 0.5% of all Ahr mRNA-positive cells in the anterior pituitary, but nearly half of Lhb-containing cells also contained Ahr mRNA, regardless of TCDD or E2 treatment (Fig. 2D).
FIG. 2.
Results of ISH studies colocalizing Ahr and Prl or Lhb mRNAs in pituitary glands of rats treated with E2, TCDD or both. Photomicrographs in the top panel show results of dual-label ISH with digoxigenin-labeled cRNA probes for (A) Prl mRNA (brown signal) or (B) Lhb mRNA (brown signal) and 35S-labeled cRNA probes for Ahr mRNA (black silver grains) hybridized to pituitary cryosections. White arrows denote cells containing either Prl mRNA (A) or Lhb mRNA (B) only. Black arrows indicate cells with both Ahr and Prl mRNAs (A) or Ahr and Lhb mRNAs (B). Scale bars = 5 μm. Graphs in the bottom panels show effects of TCDD and E2 on the percentages of lactotropes (C) and gonadotropes (D) that contained Ahr mRNA. Bars represent mean ± SEM.
3.2.2. ICC
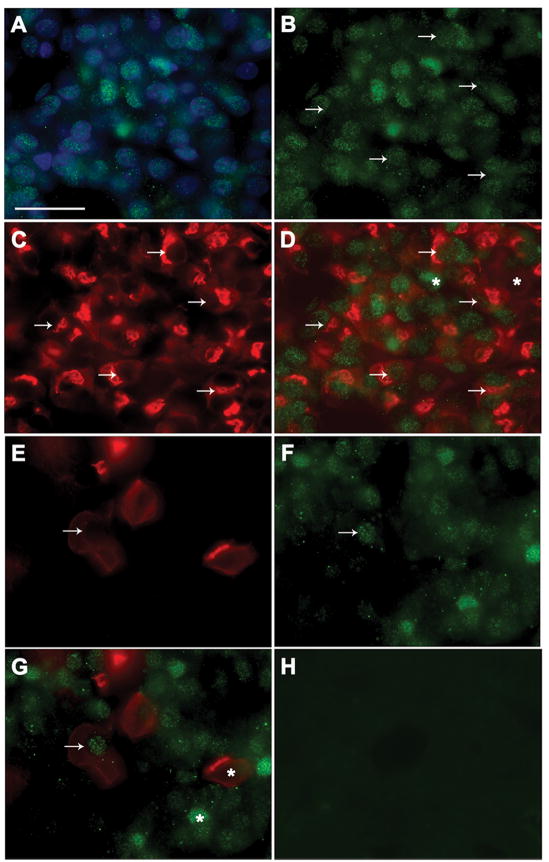
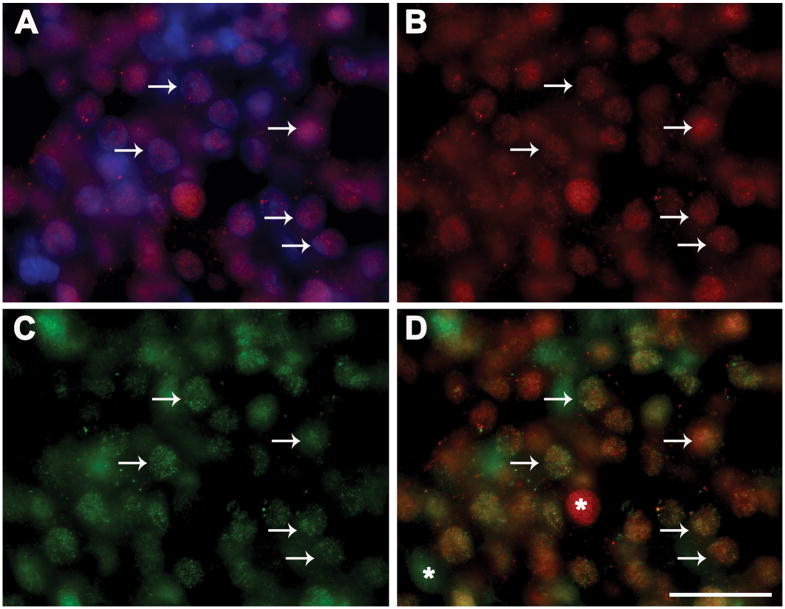
Fig. 3 shows results of dual-label ICC colocalizing Ahr-ir and Prl-ir (A-D) or Lhb-ir (E-H). All Ahr-ir in pituitaries from TCDD-treated animal was localized in cell nuclei and 72.3 ± 2.9% of stained nuclei were Ahr-ir (Fig. 3A and B). Prl-ir cells (Fig. 3C) accounted for 43.6 ± 8.3% of pituitary cells. Consistent with results of our ISH studies, 74.6 ± 13.7% of Prl-ir cells were also Ahr-ir and 50.8 ± 8.9% of the Ahr-ir cells were Prl-ir cells (Fig. 3D).
Fig. 3.
Results of ICC studies colocalizing Ahr and Prl or Lhb proteins in rat anterior pituitary gland. (A-D) Photomicrographs of Ahr-ir in Hoeschst 33258-stained nuclei (A); cells with Ahr-ir (B, green), Prl-ir (C, red) or both Prl-ir and Ahr-ir (D). (E-H) Photomicrographs of cells with Lhb-ir (E, red), Ahr-ir (F, green), or both Lhb-ir with Ahr-ir (G); (H) example of negative control in which primary antibody was omitted. Arrows indicate dual-labeled cells and asterisks denote cells that express only Ahr-ir (D and G, green) or only Prl-ir (D, red around unstained nucleus) or Lhb-ir (G, red). Scale bar, 25 μm
Of the total cells counted, 8.7 ± 0.6% were Lhb-ir (Fig. 3E). Similar to our ISH findings, Lhb-ir cells accounted for only 11.3 ± 4.1 % Ahr-ir cells (Fig. 3F), but 53.7 ± 1.6% of Lhb-ir cells were also Ahr-ir (Fig. 3G). Specificity of the antibodies was verified by absence of signal when primary antibodies were omitted (see example in Fig. 3H).
3.3. TCDD Interfered with E2 Activation of Prl Gene Expression In Vivo and In Vitro
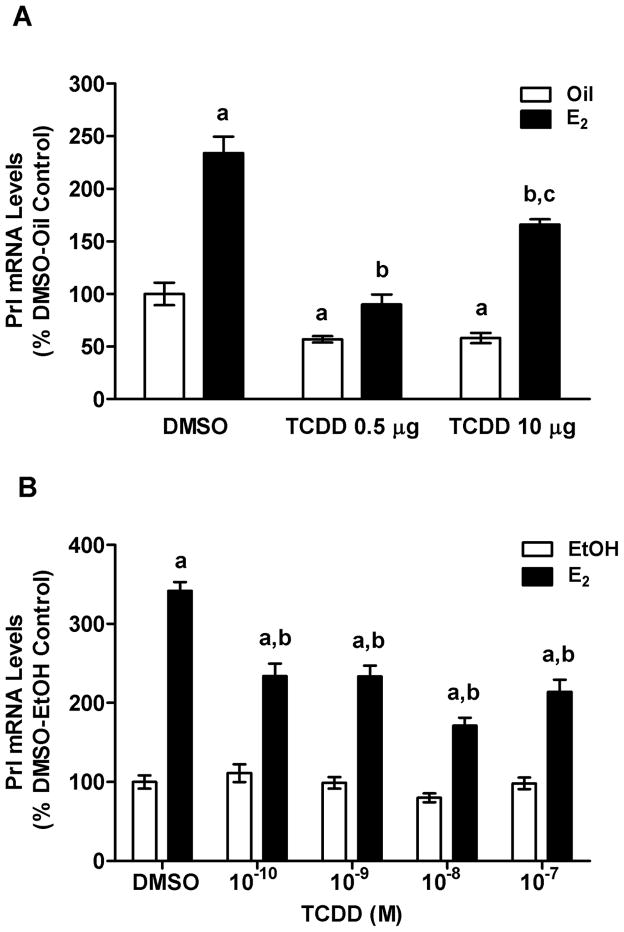
QPCR analysis of mRNA from our in vivo studies showed that E2 increased Prl mRNA levels and TCDD blunted the stimulatory effects of E2 (Fig. 4A). Interestingly, TCDD 0.5 μg/kg bw was more effective than the 10 μg/kg bw dose. In fact, the lower dose completely abolished E2-induced Prl gene expression. Moreover, both doses of TCDD alone decreased Prl mRNA levels significantly.
FIG. 4.
Effects of E2 and TCDD on Prl mRNA levels in pituitaries of OVX rats in vivo and in GH3 cells in vitro. (A) QPCR measurements of Prl mRNA from rats OVX rats treated with Oil or E2 and DMSO or TCDD as described in Materials and Methods. Bars represent mean ± SEM of values from 5–6 animals run in duplicate. aSignificantly different from Oil-DMSO vehicle control; bsignificantly different from E2-DMSO group; csignificantly different from E2-TCDD 0.5 μg/kg bw group. (B) GH3 cells treated with 10−8 M E2 or ethanol (EtOH) and various concentrations of TCDD. Bars represent mean ± SEM of 10–11 samples each run in duplicate. aSignificantly different from corresponding EtOH control; bsignificantly different from E2-DMSO group. Differences were considered significant if p<0.05.
Our in vitro time-course study in GH3 cells showed maximal stimulatory effects of E2 on Prl mRNA at 24 h (data not shown), so this time point was used in subsequent studies. Our log dose-response of Prl mRNA to E2 was linear with saturation at 10−8 M E2 (not shown). We replicated our in vivo findings by showing that TCDD blocked E2 (10−8 M)-induced increases in Prl mRNA levels (Fig. 4B).
3.4 TCDD Increased and E2 Decreased Levels of Cga and Lhb in Female Rat Pituitary
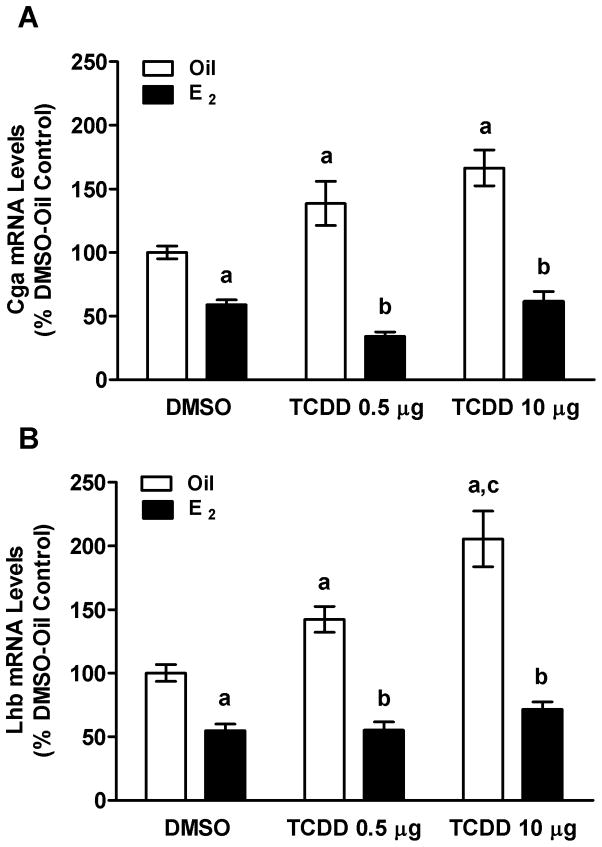
Results of QPCR analyses showed that E2 decreased Cga (Fig. 5A) and Lhb mRNA levels (Fig. 5B). TCDD did not abrogate these effects of E2 Interestingly, TCDD alone significantly increased levels of both Cga (Fig. 5A) and Lhb (Fig. 5B), and E2 blocked these increases.
FIG. 5.
Effects of E2 and TCDD (0.5 or 10 μg/kg bw) or Oil or DMSO vehicle on Cga (A) and Lhb (B) mRNA levels in pituitary glands of OVX rats (See Materials and Methods for details of treatments). Bars represent mean ± SEM of values from 5–7 animals run in duplicate. aSignificantly different from Oil-DMSO vehicle control; bsignificantly different from corresponding Oil control; csignificantly different from Oil-TCDD 0.5 μg group. Differences were considered significant if p<0.05.
3.5 TCDD and E2 Exerted Different Effects on Esr1 and Ahr mRNA Levels
QPCR analyses showed that TCDD increased Esr1 mRNA at both 0.5 μg and 10 μg/kg bw TCDD (Fig. 6A). However, TCDD had no effect on Ahr mRNA levels, regardless of the dose (Fig. 6B). In contrast, E2 decreased pituitary levels of both AhR and Esr1 mRNAs and TCDD did not abrogate the E2 effects.
FIG. 6.
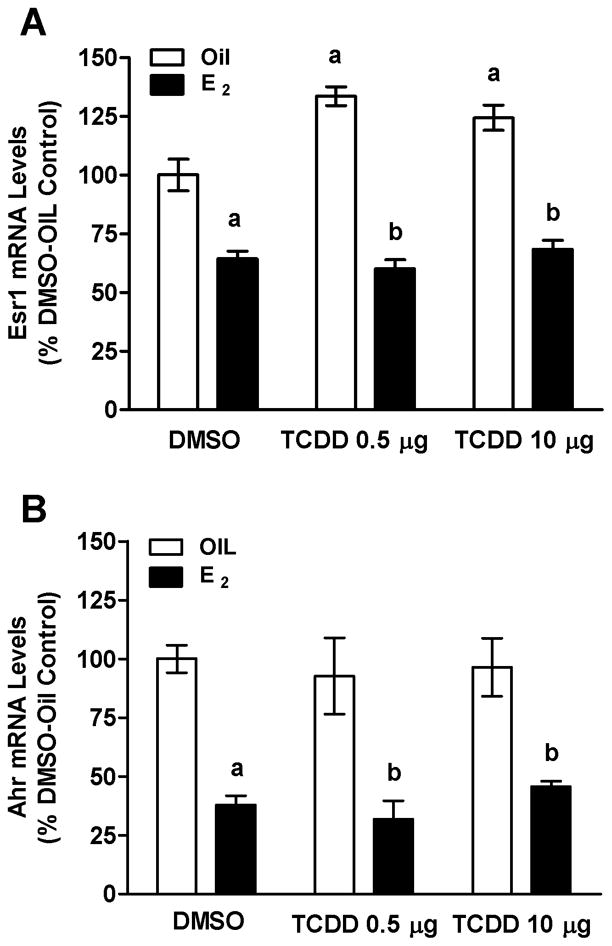
Effects of E2 and TCDD on Esr1 (A) or Ahr (B) mRNA levels in the pituitary glands of OVX rats measured using QPCR. Bars represent mean ± SEM of values from 5–7 animals run in duplicate. aSignificantly different from Oil-DMSO vehicle control; bsignificantly different from corresponding Oil control. Differences were considered significant if p<0.05.
3.6 Esr1-ir Was Detected in Most Ahr-ir Cells
We found that 67.8 ± 8.9% of all pituitary cells were Esr1-ir (Fig. 7A). Of the Esr1-ir cells (Fig. 7B, 7D), 83.1 ± 2.3 % were also Ahr-ir (Fig. 7C, 7D). Finally, 76.9 ± 8.4 % of Ahr-ir cells were also Esr1-ir (Fig. 7D).
FIG. 7.
Colocalization of Ahr and Esr1 proteins in rat anterior pituitary gland using ICC and Hoeschst 33258 to counterstain cell nuclei (blue, Panel A). Photomicrographs show Esr1-ir and Hoeschst 33258 stain (A), Esr1-ir (red; Panel B); Ahr-ir (green; Panel C) and colocalization of Esr1-ir with Ahr-ir (D). Arrows denote cells that contain both Esr1-ir and Ahr-ir; asterisks in (D) denote cells that express only Ahr-ir (green) or Esr1 (red). Scale bar, 25μm.
4. Discussion
These studies are the first to show that TCDD interferes with E2 induction of pituitary Prl gene expression and upregulates expression of Lhb and Cga independently of E2. It is likely that the observed antiestrogenic effects of TCDD on Prl mRNA levels were exerted directly on the pituitary gland because we replicated our findings in vitro. In addition, we showed that the majority of lactotropes expressed the Ahr, and that most Ahr-expressing cells also contain Esr1, the Esr isoform important for Prl gene regulation (Glidewell-Kenney et al., 2008, Wersinger et al., 1999). The antiestrogenic effects of TCDD on Prl expression may be attributable to localized metabolism of E2 through induction of genes encoding estrogen hydroxylases, Cyp1a1, Cyp1a2 and Cyp1b1 in the pituitary. However, TCDD was unable to block the inhibitory effects of E2 on Esr1, Ahr, Cga or Lhb mRNA levels and TCDD upregulated Esr1, Cga and Lhb mRNA levels independent of E2. Thus, generalized antiestrogenicity cannot explain all the effects of TCDD in the pituitary. These findings indicate that the pituitary gland is an important target through which TCDD interferes with Prl and LH synthesis through both E2-dependent and -independent mechanisms.
The idea that TCDD acts directly in the pituitary is supported by our in vivo and in vitro studies. Using the OVX animal model, we showed that the antiestrogenic effects of TCDD on Prl regulation were not secondary to effects in the ovary. Moreover, our in vitro studies in Prl-expressing GH3 cells replicated our in vivo results. These data complement our findings that approximately 75% of lactotropes contained Ahr, and that most Ahr-containing cells were Esr1-positive. Importantly, previous work documented pituitary expression of Arnt (Huang et al., 2000, Huang et al., 2002), a protein required for Ahr function. Together this body of evidence suggests that TCDD-activated Ahr exerts antiestrogenic activity directly in lactotropes to inhibit E2 upregulation of Prl synthesis.
Interestingly, TCDD alone repressed Prl gene expression in vivo, but not in vitro. One interpretation of these findings is that TCDD exerts E2-independent effects on Prl regulation through a hypothalamic mechanism. No studies have evaluated this idea directly, but others report that acute TCDD exposure suppresses circulating Prl levels (Moore et al., 1989, Russell et al., 1988), and that suppression is reversed by blocking the action of dopamine (DA), a potent inhibitor of Prl release (Russell et al., 1988). In addition, TCDD dramatically increases levels of DA in the median eminence of males, but has no direct effects on pituitary Prl release in vitro (Russell et al., 1988). These data provide convincing evidence for DA mediation of TCDD suppression of Prl release in males. It may be that a similar mechanism explains our findings that TCDD increases Prl gene expression in females independently of E2 in vivo, but not in vitro.
Somewhat surprisingly, TCDD alone also increased levels of both Lhb and Cga mRNAs, but in this case E2 abolished the effect. It is unlikely for several reasons that these effects are mediated by E2 suppression and TCDD activation of gonadotropin-releasing hormone (GnRH) neurons. First, E2 enhances, rather than represses, TCDD induction of LH release (Petroff et al., 2003). In addition, neither native GnRH neurons (Cao and Petersen, unpublished) nor immortalized GnRH neurons (GT1-7 cells) (Petroff et al., 2003) contain AhR. Similarly, TCDD affects neither GnRH transcription nor release from GT1-7 cells. Thus, GnRH neurons are probably not responsible for opposing effects of TCDD and E2 on Lhb and Cga expression.
In contrast, there is substantial evidence that TCDD and E2 regulate Lhb and Cga through direct actions in the pituitary. First, at least 45% of gonadotropes contained Ahr mRNA and protein, and most Ahr-expressing cells also contained Esr1. In addition, previous work shows that the repressive effects of E2 on Lhb mRNA levels require Esr1 binding to EREs (Glidewell-Kenney et al., 2008). Thus, the ability of E2 to block TCDD effects on Lhb expression is also likely to occur at the transcriptional level. This interpretation is supported by evidence that liganded Ahr interferes with Esr1 transactivation of genes (Safe and Wormke, 2003). These findings combined with evidence that Esr1 can act as a coactivator in Ahr transactivation of gene expression (Matthews et al., 2005), suggest the intriguing possibility that Esr1 might serve as a corepressor of Ahr activation of the Lhb gene.
Regardless of the mechanisms through which Esr and Ahr interact to regulate Lhb and Cga mRNA levels, our data may explain previous findings of aberrant LH release after acute TCDD exposure. Immature rats treated with TCDD and then with equine chorionic gonadotropin (to increase E2 levels) show a dramatic rise in serum LH levels well before E2 levels begin to rise (Gao et al., 1999, Li et al., 1995). Then, as E2 levels peak, serum LH levels begin to fall. Consistent with these findings, we found that TCDD substantially increased both Lhb and Cga mRNA levels in the absence of E2, and that E2 blocked the increases. Taken together, these findings indicate that inappropriate stimulation of LH synthesis in the absence of E2 may play a role in the premature surge in LH release triggered by TCDD. In turn, this surge in LH before follicles and oocytes mature likely contributes to the reduced ovulatory rate observed in TCDD-exposed rats (Li et al., 1995).
Similar to its effects on Lhb mRNA levels, TCDD alone stimulated Cga mRNA levels and E2 repressed them. LH was the only glycoprotein we examined in this study, but Cga is also a component of follicle-stimulating hormone and thyroid-stimulating hormone (TSH). In view of our findings that lactotropes and gonadotropes together make up only 60% of the Ahr-containing population, thyrotropes may also be targets. This idea is consistent with previous work showing that TCDD elevates serum TSH levels (Kohn, 2000). Finally, we found that the intermediate lobe of the pituitary contained Ahr mRNA and that TCDD increased expression of Cyp1a2 in that region. The intermediate lobe contains proopiomelanocortin (POMC) and TCDD increases POMC gene expression in vivo and in vitro (Huang et al., 2002), and also increases secretion of a POMC product, ACTH (Bestervelt et al., 1998). Thus, it seems possible that TCDD exerts direct effects on the regulation of multiple pituitary hormones.
5. Conclusions
We conclude that pituitary lactotropes and gonadotropes are direct targets of TCDD. In addition, TCDD and E2 act antagonistically to regulate Prl, as well as Lhb and Cga gene expression. Considering that Prl and LH impact nearly all aspects of female reproductive functions, our findings may explain how TCDD interferes with such diverse functions as ovulation (Li et al., 1995), blastocyst implantation (Kitajima et al., 2004) and pup survival (Vorderstrasse et al., 2004).
Acknowledgments
This work was supported by NIH grant RO1ES08774 to SLP. We thank Jason Luszcz and Dr. Clifford Carpenter for technical assistance in preparing cDNA transcriptional templates used in these studies and Dr. Paula Moura for preparing brain tissue for ICC. The authors declare that they have no competing financial interests that would influence the interpretation of the studies reported herein.
Non-Standard Abbreviations Used
- Ahr
aryl hydrocarbon receptor
- TCDD
2,3,7,8-tetrachlorodibenzo-p-dioxin
- PCR
polymerase chain reaction
- OVX
ovariectomized
- Prl
preprolactin
- ISH
in situ hybridization
- ICC
immunocytochemistry
- Cga
glycoprotein hormones, alpha subunit
- LH
luteinizing hormone
- Lhb
luteinizing hormone beta
- Arnt
Ahr nuclear translocator
- DRE
dioxin response element
- XRE
xenobiotic response element
- AhrE
Ahr response element
- Esr1
oestrogen receptor 1; also called ER alpha
- Esr2
oestrogen receptor 2; also called ER beta
- HPG
hypothalamic-pituitary-gonadal
- RT
reverse transcription
- FBS
fetal bovine serum
- KPBS
potassium phosphate buffered saline
- Tx-KPBS
Triton X-100 in KPBS
- ir
immunoreactivity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
JinYan Cao, Email: jcao4@ncsu.edu.
Heather B. Patisaul, Email: Heather_Patisaul@ncsu.edu.
References
- Augustine RA, Kokay IC, Andrews ZB, Ladyman SR, Grattan DR. Quantitation of prolactin receptor mRNA in the maternal rat brain during pregnancy and lactation. J Mol Endocrinol. 2003;31:221–232. doi: 10.1677/jme.0.0310221. [DOI] [PubMed] [Google Scholar]
- Beischlag TV, Perdew GH. ER alpha-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J Biol Chem. 2005;280:21607–21611. doi: 10.1074/jbc.C500090200. [DOI] [PubMed] [Google Scholar]
- Bestervelt LL, Pitt JA, Piper WN. Evidence for Ah receptor mediation of increased ACTH concentrations in primary cultures of rat anterior pituitary cells exposed to TCDD. Toxicol Sci. 1998;46:294–299. doi: 10.1006/toxs.1998.2548. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS. Endocrine effects of prenatal exposure to PCBs, dioxins, and other xenobiotics: implications for policy and future research. Environ Health Perspect. 1994;102:676–679. doi: 10.1289/ehp.94102676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci USA. 1992;89:8185–8189. doi: 10.1073/pnas.89.17.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver LA, Hogenesch JB, Bradfield CA. Tissue specific expression of the rat Ah-receptor and ARNT mRNAs. Nucleic Acids Res. 1994;22:3038–3044. doi: 10.1093/nar/22.15.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin WW, Godine JE, Klein DR, Chang AS, Tan LK, Habener JF. Nucleotide sequence of the cDNA encoding the precursor of the beta subunit of rat lutropin. Proc Natl Acad Sci USA. 1983;80:4649–4653. doi: 10.1073/pnas.80.15.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison MS, Fisher JM, Whitlock JP., Jr Protein-DNA interactions at recognition sites for the dioxin-Ah receptor complex. J Biol Chem. 1989;264:16478–16482. [PubMed] [Google Scholar]
- DeVito MJ, Thomas T, Martin E, Umbreit TH, Gallo MA. Antiestrogenic action of 2,3,7,8-tetrachlorodibenzo-p-dioxin: tissue-specific regulation of estrogen receptor in CD1 mice. Toxicol Appl Pharmacol. 1992;113:284–292. doi: 10.1016/0041-008x(92)90126-d. [DOI] [PubMed] [Google Scholar]
- Furness SG, Lees MJ, Whitelaw ML. The dioxin (aryl hydrocarbon) receptor as a model for adaptive responses of bHLH/PAS transcription factors. FEBS Lett. 2007;581:3616–3625. doi: 10.1016/j.febslet.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Gao X, Son DS, Terranova PF, Rozman KK. Toxic equivalency factors of polychlorinated dibenzo-p-dioxins in an ovulation model: validation of the toxic equivalency concept for one aspect of endocrine disruption. Toxicol Appl Pharmacol. 1999;157:107–116. doi: 10.1006/taap.1999.8649. [DOI] [PubMed] [Google Scholar]
- Gauger KJ, Giera S, Sharlin DS, Bansal R, Iannacone E, Zoeller RT. Polychlorinated biphenyls 105 and 118 form thyroid hormone receptor agonists after cytochrome P4501A1 activation in rat pituitary GH3 cells. Environ Health Perspect. 2007;115:1623–1630. doi: 10.1289/ehp.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Weiss J, Hurley LA, Levine JE, Jameson JL. Estrogen receptor alpha signaling pathways differentially regulate gonadotropin subunit gene expression and serum follicle-stimulating hormone in the female mouse. Endocrinology. 2008;149:4168–4176. doi: 10.1210/en.2007-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Hapgood J, Cuthill S, Denis M, Poellinger L, Gustafsson JA. Specific protein-DNA interactions at a xenobiotic-responsive element: copurification of dioxin receptor and DNA-binding activity. Proc Natl Acad Sci USA. 1989;86:60–64. doi: 10.1073/pnas.86.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays LE, Carpenter CD, Petersen SL. Evidence that GABAergic neurons in the preoptic area of the rat brain are targets of 2,3,7,8-tetrachlorodibenzo-p-dioxin during development. Environ Health Perspect. 2002;110(Suppl 3):369–376. doi: 10.1289/ehp.02110s3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose K, Morita M, Ema M, Mimura J, Hamada H, Fujii H, Saijo Y, Gotoh O, Sogawa K, Fujii-Kuriyama Y. cDNA cloning and tissue-specific expression of a novel basic helix-loop-helix/PAS factor (Arnt2) with close sequence similarity to the aryl hydrocarbon receptor nuclear translocator (Arnt) Mol Cell Biol. 1996;16:1706–1713. doi: 10.1128/mcb.16.4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Rannug A, Ahlbom E, Hakansson H, Ceccatelli S. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on the expression of cytochrome P450 1A1, the aryl hydrocarbon receptor, and the aryl hydrocarbon receptor nuclear translocator in rat brain and pituitary. Toxicol Appl Pharmacol. 2000;169:159–167. doi: 10.1006/taap.2000.9064. [DOI] [PubMed] [Google Scholar]
- Huang P, Ceccatelli S, Hakansson H, Grandison L, Rannug A. Constitutive and TCDD-induced expression of Ah receptor-responsive genes in the pituitary. Neurotoxicology. 2002;23:783–793. doi: 10.1016/S0161-813X(02)00040-2. [DOI] [PubMed] [Google Scholar]
- Huang P, Ceccatelli S, Hoegberg P, Sten Shi TJ, Hakansson H, Rannug A. TCDD-induced expression of Ah receptor responsive genes in the pituitary and brain of cellular retinol-binding protein (CRBP-I) knockout mice. Toxicol Appl Pharmacol. 2003;192:262–274. doi: 10.1016/s0041-008x(03)00296-5. [DOI] [PubMed] [Google Scholar]
- Kewley RJ, Whitelaw ML, Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int J Biochem Cell Biol. 2004;36:189–204. doi: 10.1016/s1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- Kietz S, Thomsen JS, Matthews J, Pettersson K, Strom A, Gustafsson JA. The Ah receptor inhibits estrogen-induced estrogen receptor beta in breast cancer cells. Biochem Biophys Res Commun. 2004;320:76–82. doi: 10.1016/j.bbrc.2004.05.132. [DOI] [PubMed] [Google Scholar]
- Kitajima M, Khan KN, Fujishita A, Masuzaki H, Koji T, Ishimaru T. Expression of the arylhydrocarbon receptor in the peri-implantation period of the mouse uterus and the impact of dioxin on mouse implantation. Arch Histol Cytol. 2004;67:465–474. doi: 10.1679/aohc.67.465. [DOI] [PubMed] [Google Scholar]
- Kohn MC. Effects of TCDD on thyroid hormone homeostasis in the rat. Drug Chem Toxicol. 2000;23:259–277. doi: 10.1081/dct-100100114. [DOI] [PubMed] [Google Scholar]
- Langouche L, Hersmus N, Papageorgiou A, Vankelecom H, Denef C. Melanocortin peptides stimulate prolactin gene expression and prolactin accumulation in rat pituitary aggregate cell cultures. J Neuroendocrinol. 2004;16:695–703. doi: 10.1111/j.1365-2826.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Li X, Johnson DC, Rozman KK. Reproductive effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in female rats: ovulation, hormonal regulation, and possible mechanism(s) Toxicol Appl Pharmacol. 1995;133:321–327. doi: 10.1006/taap.1995.1157. [DOI] [PubMed] [Google Scholar]
- Li X, Johnson DC, Rozman KK. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on estrous cyclicity and ovulation in female Sprague-Dawley rats. Toxicol Lett. 1995;78:219–222. doi: 10.1016/0378-4274(95)03252-g. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Jetten AM, Davis BJ. Dual activation of PPARalpha and PPARgamma by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol Cell Endocrinol. 2003;201:133–141. doi: 10.1016/s0303-7207(02)00423-9. [DOI] [PubMed] [Google Scholar]
- Martignoni M, de Kanter R, Grossi P, Mahnke A, Saturno G, Monshouwer M. An in vivo and in vitro comparison of CYP induction in rat liver and intestine using slices and quantitative RT-PCR. Chem Biol Interact. 2004;151:1–11. doi: 10.1016/j.cbi.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl Recept Signal. 2006;4:e016. doi: 10.1621/nrs.04016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J, Wihlen B, Thomsen J, Gustafsson JA. Aryl hydrocarbon receptor-mediated transcription: ligand-dependent recruitment of estrogen receptor alpha to 2,3,7,8-tetrachlorodibenzo-p-dioxin-responsive promoters. Mol Cell Bio. 2005;25:5317–5328. doi: 10.1128/MCB.25.13.5317-5328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RW, Parsons JA, Bookstaff RC, Peterson RE. Plasma concentrations of pituitary hormones in 2,3,7,8-tetrachlorodibenzo-p-dioxin-treated male rats. J Biochem Toxicol. 1989;4:165–172. doi: 10.1002/jbt.2570040305. [DOI] [PubMed] [Google Scholar]
- Myllymaki SA, Haavisto TE, Brokken LJ, Viluksela M, Toppari J, Paranko J. In utero and lactational exposure to TCDD; steroidogenic outcomes differ in male and female rat pups. Toxicol Sci. 2005;88:534–544. doi: 10.1093/toxsci/kfi308. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59:65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- Oesch-Bartlomowicz B, Huelster A, Wiss O, Antoniou-Lipfert P, Dietrich C, Arand M, Weiss C, Bockamp E, Oesch F. Aryl hydrocarbon receptor activation by cAMP vs. dioxin: divergent signaling pathways. Proc Natl Acad Sci USA. 2005;102:9218–9223. doi: 10.1073/pnas.0503488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T, Fujii-Kuriyama Y, Kato S. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562–566. doi: 10.1038/nature05683. [DOI] [PubMed] [Google Scholar]
- Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, Yanagisawa J, Fujii-Kuriyama Y, Kato S. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL. Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci. 2004;24:8097–8105. doi: 10.1523/JNEUROSCI.2267-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Gardner E, Adelman J, McCrone S. Examination of steroid-induced changes in LHRH gene transcription using 33P-and 35S-labeled probes specific for intron 2. Endocrinology. 1996;137:234–239. doi: 10.1210/endo.137.1.8536618. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Curran MA, Marconi SA, Carpenter CD, Lubbers LS, McAbee MD. Distribution of mRNAs encoding the arylhydrocarbon receptor, arylhydrocarbon receptor nuclear translocator, and arylhydrocarbon receptor nuclear translocator-2 in the rat brain and brainstem. J Comp Neurol. 2000;427:428–439. doi: 10.1002/1096-9861(20001120)427:3<428::aid-cne9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Petroff BK, Gao X, Rozman KK, Terranova PF. Interaction of estradiol and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in an ovulation model: evidence for systemic potentiation and local ovarian effects. Reprod Toxicol. 2000;14:247–255. doi: 10.1016/s0890-6238(00)00075-7. [DOI] [PubMed] [Google Scholar]
- Petroff BK, Croutch CR, Hunter DM, Wierman ME, Gao X. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) stimulates gonadotropin secretion in the immature female Sprague-Dawley rat through a pentobarbital- and estradiol-sensitive mechanism but does not alter gonadotropin-releasing hormone (GnRH) secretion by immortalized GnRH neurons in vitro. Biol Reprod. 2003;68:2100–2106. doi: 10.1095/biolreprod.102.010439. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976;251:4936–4946. [PubMed] [Google Scholar]
- Pollenz RS, Sattler CA, Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Mol Pharmacol. 1994;45:428–438. [PubMed] [Google Scholar]
- Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Russell DH, Buckley AR, Shah GN, Sipes IG, Blask DE, Benson B. Hypothalamic site of action of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Appl Pharmacol. 1988;94:496–502. doi: 10.1016/0041-008x(88)90290-6. [DOI] [PubMed] [Google Scholar]
- Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha crosstalk and mechanisms of action. Chem Res Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- Salisbury TB, Marcinkiewicz JL. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin and 2,3,4,7,8-pentachlorodibenzofuran reduces growth and disrupts reproductive parameters in female rats. Biol Reprod. 2002;66:1621–1626. doi: 10.1095/biolreprod66.6.1621. [DOI] [PubMed] [Google Scholar]
- Sorenson RL, Stout LE, Brelje TC, Jetton TL, Matschinsky FM. Immunohistochemical evidence for the presence of glucokinase in the gonadotropes and thyrotropes of the anterior pituitary gland of rat and monkey. J Histochem Cytochem. 2007;55:555–566. doi: 10.1369/jhc.6A7117.2007. [DOI] [PubMed] [Google Scholar]
- Spreafico E, Bettini E, Pollio G, Maggi A. Nucleotide sequence of estrogen receptor cDNA from Sprague-Dawley rat. Eur J Pharmacol. 1992;227:353–356. doi: 10.1016/0922-4106(92)90016-o. [DOI] [PubMed] [Google Scholar]
- Swanson HI, Chan WK, Bradfield CA. DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins. J Biol Chem. 1995;270:26292–26302. doi: 10.1074/jbc.270.44.26292. [DOI] [PubMed] [Google Scholar]
- Tian Y, Ke S, Thomas T, Meeker RJ, Gallo MA. Transcriptional suppression of estrogen receptor gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) J Steroid Biochem Mol Biol. 1998;67:17–24. doi: 10.1016/s0960-0760(98)00067-3. [DOI] [PubMed] [Google Scholar]
- Tian Y, Ke S, Thomas T, Meeker RJ, Gallo MA. Regulation of estrogen receptor mRNA by 2,3,7,8-tetrachlorodibenzo-p-dioxin as measured by competitive RT-PCR. J Biochem Mol Toxicol. 1998;12:71–77. doi: 10.1002/(sici)1099-0461(1998)12:2<71::aid-jbt1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Vorderstrasse BA, Fenton SE, Bohn AA, Cundiff JA, Lawrence BP. A novel effect of dioxin: exposure during pregnancy severely impairs mammary gland differentiation. Toxicol Sci. 2004;78:248–257. doi: 10.1093/toxsci/kfh062. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Suzuki A, Goto M, Ohsako S, Tohyama C, Handa H, Iguchi T. Comparative uterine gene expression analysis after dioxin and estradiol administration. J Mol Endocrinol. 2004;33:763–771. doi: 10.1677/jme.1.01529. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Haisenleder DJ, Lubahn DB, Rissman EF. Steroid feedback on gonadotropin release and pituitary gonadotropin subunit mRNA in mice lacking a functional estrogen receptor alpha. Endocrine. 1999;11:137–143. doi: 10.1385/ENDO:11:2:137. [DOI] [PubMed] [Google Scholar]
- Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- Wolf CJ, Ostby JS, Gray LE., Jr Gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) severely alters reproductive function of female hamster offspring. Toxicol Sci. 1999;51:259–264. doi: 10.1093/toxsci/51.2.259. [DOI] [PubMed] [Google Scholar]
- Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor alpha in male rats. J Comp Neurol. 2009;512:688–701. doi: 10.1002/cne.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Li AP, Kaminski DL, Ruh MF. 2,3,7,8 Tetrachlorodibenzo-p-dioxin induction of cytochrome P4501A in cultured rat and human hepatocytes. Chem Biol Interact. 2000;124:173–189. doi: 10.1016/s0009-2797(99)00149-0. [DOI] [PubMed] [Google Scholar]
- Zhu LJ, Altmann SW. mRNA and 18S-RNA coapplication-reverse transcription for quantitative gene expression analysis. Anal Biochem. 2005;345:102–109. doi: 10.1016/j.ab.2005.07.028. [DOI] [PubMed] [Google Scholar]