Abstract
Problem
To better understand the immunoregulatory properties of trophoblasts, we have searched for small immunologically active carbohydrates derived from intact trophoblast-like cells.
Method of study
Using solid phase extraction coupled with HPLC and mass spectrometry methods, we have characterized a low molecular weight carbohydrate-rich fraction associated with JEG-3 cells. We have also tested the bioactivities of selected authentic oligosaccharides found in the oligosaccharide fraction.
Results
The most abundant components of the low molecular weight carbohydrate-rich fraction were maltotriose and maltotetraose, with detectable amounts of maltopentaose. When authentic maltooligosaccharides were tested using lymphocytes, IL-2 inhibition was observed. This activity was dependent upon the number of saccharide subunits, stereochemistry and concentration. To further test maltooligosaccharide properties, maltopentose was attached to glass cover slips. Although spontaneous neutrophil motility was observed on unmodified and control surfaces, it was inhibited on maltooligosaccharide-derivatized surfaces.
Conclusion
Maltooligosaccharides are associated with the trophoblast’s surface where they may exhibit immunoregulatory activities.
Keywords: saccharides, leukocytes, immune regulation
Introduction
Carbohydrates are broadly important molecules in cells and tissues that provide structural assemblies, metabolic energy, and store information as disparate as blood group type, intercellular recognition signals, and trafficking commands within their sequences.1 Cell surface saccharides are well-known regulators of cell-to-cell interactions and downstream signal transduction events in developmental and host defense pathways.2, 3 For example, one mechanism regulating leukocyte trafficking is mediated by selectins, which are lectins expressed by leukocytes, endothelial cells, platelets, and other cell types.4 Selectins recognize the terminal tetrasaccharide sequence: NeuAca2→3Galβ1→4[Fucα1→3]GlcNAcβ1 (sLeX). Selectins mediate initial leukocyte-endothelial cell contact leading to tight integrin-mediated contact and subsequent extravasation.4 Other cell surface receptors such as complement receptor type 3 and dectin-1 bind to polysaccharides and oligosaccharides known as β-1,3-D-glucans, which are found in yeast cell walls.5 Hence, oligosaccharides play central roles in adaptive and innate immune responses with relevance to infectious diseases, autoimmunity, and cancer.6, 7
An important physiological niche exhibiting modified leukocyte function is the maternal-fetal interface. For example, trophoblasts have been shown to protect the conceptus from destruction by activated macrophages.8 Moreover, macrophages activated by multiple mechanisms display cytotoxicity against tumor cell targets, but not trophoblasts.9 Included among the various molecules proposed to influence leukocyte activation in pregnancy are carbohydrates. Previous studies by Arkwright et al.10 demonstrated that neutral oligosaccharides of syncytiotrophoblast membranes suppress allogeneic reactivity using the mixed lymphocyte reaction assay. Muchmore et al.11 have suggested that the disaccharide α-D-Man-(1→6)-α-D-Man, derived from pregnancy urine is capable of inhibiting early events during lymphocyte proliferative responses. We have found that maltooligosaccharides can be released from JEG-3 trophoblast-like cells and that these saccharides reduce IL-2 production by stimulated Jurkat lymphocytes at concentrations expected for interfacial cell-cell interactions. Furthermore, when attached to substrates, maltooligosaccharides reduce the spontaneous motility of neutrophils. We speculate that maltooligosaccharides expressed at the trophoblast’s surface have immunosuppressive characteristics.
Materials and methods
Materials
Maltotriose, maltotetraose, maltopentaose, cellotriose, isomaltotriose and panose were obtained from Sigma-Aldrich (St. Louis, MO). Alexa-488-conjugated PHA (phytohemagglutinin) was obtained from Molecular Probes (Eugene, OR).
Neutrophils
Peripheral blood was collected from healthy human donors in compliance with the guidelines of the University of Michigan Institutional Review Board for Human Subject Research. Neutrophils were isolated using Ficoll-Histopaque (Sigma, St. Louis, MO) density gradient centrifugation, re-suspended and washed in PBS by centrifugation.
Lymphocytes
Jurkat cells (ATCC, Manassas, VA) were maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA) containing 10% FCS and 1% antibiotics.
JEG-3 Trophoblasts
JEG-3 cells, derived from human choriocarcinoma, were obtained from the ATCC (Manassas, VA). JEG-3 cytotrophoblast-like cells were grown in RPMI 1640 containing 10% FCS and 1% penicillin G/streptomycin/amphotericin B (Invitrogen). The cell line was occasionally replaced with the original cell line as a quality control measure.
Cell treatment
As many as 10 to 20 tissue culture flasks were used in each treatment. JEG-3 cells on tissue culture plates were washed with PBS (without Ca2+ and Mg2+; Invitrogen) then incubated with PBS (without Ca2+ and Mg2+) on a shaker at 37°C for 24 hrs. to non-lytically promote the release of glycocalyx materials without cell debris. Alternatively, in some experiments cells were incubated with recombinant PNFase F (BioLabs, Boston, MA) (10 min. at 37°C) to promote the release of these materials. After the supernatant was collected from multiple flasks, the combined supernatants were ultrafiltrated with CentriPlus filter (YM-10, Millipore), which has a molecular weight cut-off of 10K, to isolate a low molecular weight fraction. As negative controls, other cell lines (e.g., Jurkat cells) were exposed to this protocol. The samples were stored at −80°C until used.
Concentration and desalting of samples
A non-porous graphitized carbon column (4 ml, Carbograph SPE, Alltech, Deerfield, IL) was pre-activated with 2 ml of 25% MeOH and 2 ml of water. Thirty ml of sample were passed through the column at a flow rate of 1 ml/min. The column was washed with water to remove salts and then eluted with 2 ml of 25% MeOH in 10µM NH4OAc to obtain the oligosaccharide fraction.12, 13 The eluate was concentrated to 0.5 ml in a Speed-Vac evaporator.
Electrospray mass spectrometry
ESI-MS spectra were recorded on a ThermoElectron Finnigan LTQ linear ion trap mass spectrometer equipped with an electrospray ionization source. Full scan mass range m/z was 100–2000. For positive ion mode MS experiments, the ESI voltage was set at 3.2 kV, the capillary voltage at 25.5V, and the tube lens voltage at 131V. For negative mode MS experiments, the ESI voltage was set at −2.1 kV, the capillary voltage at − 25V, and tube lens voltage at −79V. The capillary temperature, sheath gas and auxiliary gas were 250°C, 20 units and 5 units, respectively, for both modes.
High-Performance Anion-Exchange Chromatography (HPAEC) analysis
The chromatography system was a Dionex BioLC, which included a GP50 gradient pump and an ED50A electrochemical detector. The standard carbohydrate waveform was used for ED50A. Oligosaccharides were separated on a CarboPac PA100 column (9 × 250 mm, Dionex) with 0.1 M NaOH as solvent A and 0.1 M NaOH/0.5 M NaOAc as solvent B. The following linear gradient was used: 0 –12.5% B for 15 min., and then increased to 25% B for 25 min.
IL-2 production
Aliquots of 106 cells/ml/well of Jurkat cells (clone E6-1) were treated with various concentrations of maltooligosaccharides (DP3, DP4, DP5, DP6; DP is the degree of polymerization), isomaltotriose, cellotriose, and panose (Sigma, MO) in the presence or absence of the stimulant PHA (10 µg/ml) for 24 hr. in a 5% CO2 incubator at 37°C. Culture supernatants were collected for IL-2 measurement using a kit from R&D Systems (MN). Briefly, supernatants were incubated in the anti-IL-2 antibody-coated plates at room temperature for 2 hrs.; after washing, plates were incubated with HRP-conjugated secondary antibody at room temperature for another 2 hrs. before color generation. Optical density was measured by a Spectra Max 190 (Molecular Devices, CA).
PHA receptor staining
Jurkat cells were pre-treated with 20 mM DP4 or DP5 at room temperature for 30 min. followed by the addition of 10 µg/ml Alexa-488-PHA (Molecular Probes) for an additional 30 min. Microscopy was performed as described below.
Synthesis of maltooligosaccharide surfaces
As maltooligosaccharides are highly soluble in water and adsorb poorly to glass, we covalently linked DP5 to glass surfaces. The synthetic organic preparation was carried out as outlined in the scheme 1. Briefly, glass cover slips (25 × 25 mm, Corning) were cleaned for 30 min. in 0.1 M NaOH followed by 30 min. in concentrated H2SO4 then rinsed exhaustively with deionized water. After drying under a nitrogen flow, silanization was performed for 2 hr. in 10% v/v 3-aminopropyltrimethoxysilane solution in toluene. The cover slips were rinsed sequentially with toluene, ethanol, and deionized water, respectively. After drying, the cover slips were treated with 10 mM DP5 or cellotriose (β-D-Glc-(1→4)-β-D-Glc-(1→4)-D-Glc) in DMSO/AcOH (17:3) for 2 hr. and followed by 1M NaBH3CN in DMSO for 3 hr. After the synthetic procedure was complete, the modified cover slips were washed with DMSO and deionized water in that order.
Scheme 1.
Immobilization of DP5 on the surface of cover slips
Neutrophil spontaneous motility
The spontaneous motility of neutrophils was assessed using time-lapse optical microscopy. Control or oligosaccharide-modified cover slips were employed. Cells were allowed to acclimate to the surface at 37 °C for one hour. A low magnification field was then chosen to permit visualization of 50–60 cells. A time series of images was taken using a QImaging Retiga 1300 camera and a 40×/0.6 NA Nikon Plan Fluor objective. The series of photographs were taken at a rate of one per minute using MetaMorph software. Cell movement was scored over 30 minutes, with movement being defined as a cell moving at least 10 µm, or the approximate average diameter of a neutrophil. Cells were tagged using the manual count function in MetaMorph, and results were tabulated and plotted using Microsoft Excel.
Microscopy
Cells were observed using a Nikon Eclipse TE2000 Quantum inverted fluorescence microscope (Nikon Instruments, Inc., Melville, NY) with mercury illumination interfaced to a computer using Metamorph (Molecular Devices, Danville, PA) software. Images were taken with an Andor Technologies iXon model DV8 16-bit electron multiplying CCD camera cooled to −90°C (Andor Technologies, South Windsor, CT). A filter module comprised of a D470/40× exciter, 505DCLP dichroic, and an E515LPv2 emitter was used for imaging Alexa-488-conjugated PHA. Time lapse imaging was performed in transmitted bright field imaging using the Metamorph software without the electron-multiplying channel activated.
Results
Structural Studies
The low molecular weight carbohydrate-rich fraction obtained by using the protocol described above was analyzed by MS. In the positive ion mode (Fig. 1a), there are two major ions at m/z 527.4 and 689.5, whereas in the negative ion MS mode, more major ions were observed (503.1, 539.3, 563.1, 665.2, 701.2, 725.1, 677.3, 839.3 and 611.3) (Fig. 1b). When the positive ion mode species at m/z 527.4 and 689.5 were further investigated using MS2, they showed typical oligosacchride characteristics in their spectra (see Fig. 1c and d). In the negative ion mode, we assign m/z 503.1 and 665.2 as a trisaccharide (M1) and tetrasaccharide (M2), respectively. Furthermore, the species at m/z 539.3 and 563.1 are [M1+Cl]− and [M1+CH3CHOO]− and the species at 701.2 and 725.1 are [M2+Cl]− and [M2+CH3CHOO]−, respectively. In the positive mode, the species at m/z 527.2 and 689.2 are sodium adducts of M1 and M2. The component at m/z 611.3 is glutathiol (confirmed by multi-MS and HPLC). The species at m/z 677.3 and 839.3 also exhibit oligosaccharide characteristics; they are likely adducts of M1 and M2 (677.3 = [M1+173]−, 839.3 = [M2+173]−). As the m/z 527.4 peak of Fig. 1a appears as a breakdown product in the MS2 spectrum of the m/z 685.5 component in Fig. 1d, as well as several smaller fragments, M1 is a fragment of M2. Therefore, we focused on the structural analysis of M1.
Fig. 1.
Mass spectrometry of trophoblast oligosaccharides. MS spectra of SPE fraction in the positive ion mode (panel a) and negative ion mode (panel b) are shown. In addition, MS2 spectra of m/z 527.4 (panel c) and m/z 685.5 (panel d) in the positive ion mode are given. The similarities in these fragmentation patterns in panels c and d suggest structural similarities in these two compounds.
Further experiments were performed to better understand the nature of M1. The fragmentation pattern in positive-ion mode MS may not be structure-specific. For example, oligosaccharides may be protonated at several positions, resulting in a potential lack of linkage specificity. Consequently, most carbohydrate linkage determinations have been performed using negative-ion mode MS. However, in our experiments using Na+ adducts, the positive-ion MS mode gave us more structural information. Moreover, the sensitivity was improved dramatically in comparison to the negative-ion mode. In these experiments, three commercially available trisaccharides containing glucose with differences in the anomeric carbon and linkage positions were selected as standard samples for comparison with M1. These saccharides were maltotriose (α-D-Glc-(1→4)-α-D-Glc-(1→4)-D-Glc), cellotriose (β-D-Glc-(1→4)-β-D-Glc-(1→4)-D-Glc), panose (α-D-Glc-(1→6)-α-D-Glc-(1→4)-D-Glc) and isomaltotriose (α-D-Glc-(1→6)-α-D-Glc-(1→6)-D-Glc). Like M1, these trisaccharides only exhibit sodium adducts, because the sodiated ions are more stable than their protonated counterparts in positive ion mode MS (Fig. 2). Fig. 2 shows that trisaccharides with different linkages (1→4 and 1→6) and anomeric configurations (α and β) show different fragmentation profiles. The molecular weight and MS2 spectrum of maltotriose is identical to that of M1, indicating the identity of these two molecules. These findings are summarized in Fig. 3, which shows the molecular fragments corresponding to the peaks given in Figs. 1 and 2 as well as the measured abundances of these various fragments for the listed panel of glucose-containing isomeric trisaccharides. These data further support the identification of M1 as maltotriose.
Fig. 2.
Analysis of a family of structural isomers. MS2 of maltotriose, cellotriose, panose, and isomaltotriose in the positive ion mode are shown in panels a–d, respectively. Note that panel a in this figure is identical to panel c in Fig. 1.
Fig. 3.
Relative abundancies of fragments for M1 and standard trisaccharides using the fragmentation nomenclature of Domon and Costello.28 This analysis shows clearly the correspondence between M1 and maltotriose.
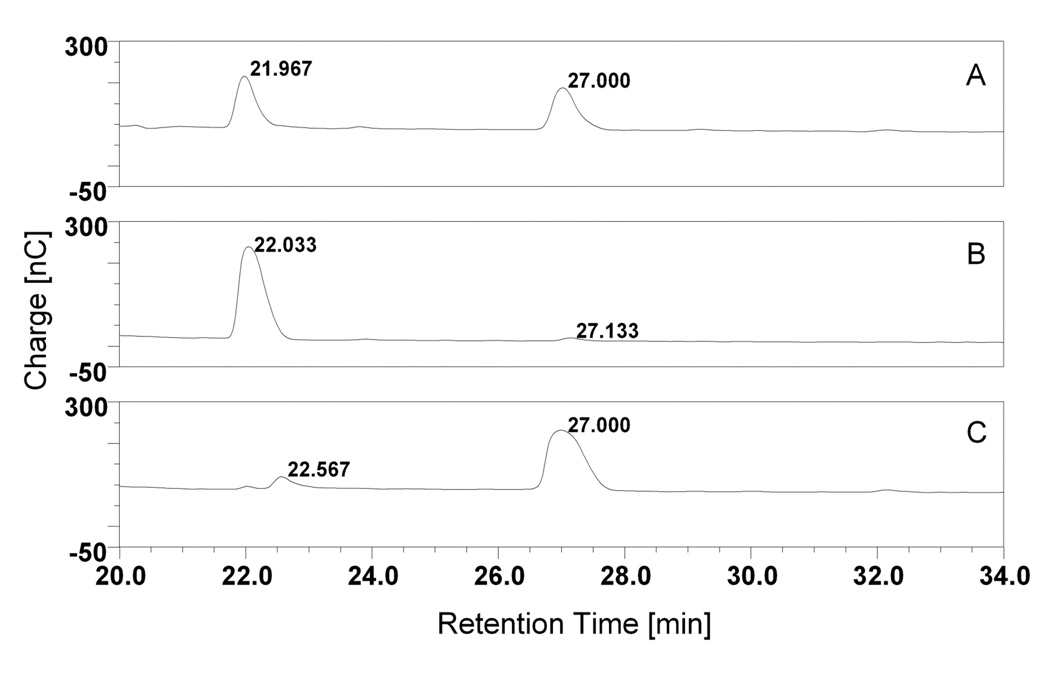
To provide an independent line of evidence to test the correspondence of M1 with maltotriose, we performed HPAEC analyses for M1, M2, maltotriose and maltotetraose under identical conditions. The chromatographs are shown in Fig. 4. Fig. 4a shows the separation of SPE fraction. Two major peaks with retention times of 21.97 and 27.00 min. are observed. These two peaks correspond to the most abundant molecules in the extract, namely M1 and M2. The retention times and the shapes of peaks match well with those of authentic maltotriose and maltotetraose (Fig. 4b and 4c). Hence, the two major compounds released from JEG-3 cells were maltotriose and maltotetraose, which were determined by ES-MS and HPEAC. Although maltooligosaccharides were found to be associated with JEG-3 cells, it is possible that they originated in the culture medium then adsorbed to the cells. When the cell growth medium was examined by HPAEC, maltooligosaccharies could not be detected (data not shown), thus indicating that the maltooligosaccharides originate from the cells, not the culture medium. Furthermore, maltooligosaccharides could not be isolated from Jurkat cells (data not shown), indicating that they are not a general feature of cells in culture.
Fig. 4.
HPAE chromatographs for the SPE fraction (panel a), as well as authentic maltotriose (panel b) and maltotetraose (panel c) are shown. The chromatogram peaks for the trophoblast carbohydrate sample at 22 min. and 27 min. agree with those of maltotriose and maltotetraose.
Functional Studies
Previous studies from other laboratories10 have suggested that trophoblast oligosaccharides exhibit immunoregulatory capacity. To test the potential immunoregulatory capacity of maltooligosaccharides, Jurkat T cells were tested for IL-2 production during treatment with various oligosaccharides. In the first series of experiments a saccharide concentration of 20 mM was used because it approximates the interfacial maltooligosaccharide concentration expected at a site of cell-cell contact. Cells were incubated with or without PHA for 24 hr. at 37°C. As shown in Fig. 5, the isomers cellotriose (β-D-Glc-(1→4)-β-D-Glc-(1→4)-D-Glc) and isomaltotriose (α-D-Glc-(1→6)-α-D-Glc-(1→6)-D-Glc) did not have a statistically significant effect on IL-2 production. However, DP3, DP4, and DP5 increasingly reduced IL-2 production, reaching about 50% inhibition for DP5 at 20 mM. DP6 had somewhat less efficacy than DP5, which is likely due to the fact that as the sequence nears DP7, the conformation becomes more circular in shape.14 These findings strongly suggest that the reported effects are specific, as small changes in oligosaccharide stereochemistry resulted in significant changes in IL-2 production.
Fig. 5.
Effect of DP4 (panel A) and DP5 (panel B) on IL-2 production by Jurkat cells. Cells were treated with various concentrations of DP4 or DP5 in the presence or absence of 10 µg/ml PHA for 24 hr. at 37°C. IL-2 production was assessed using an ELISA kit and plate reader. A dose-dependent reduction in IL-2 release was observed, although DP5 exhibited greater efficacy than DP4. (For DP4 and DP5, PHA vs. 2.5, 5.0, 10, 20, and 40mM, P<0.005).
We also examined the dose-response properties of maltooligosaccharides. In this series of experiments we incubated T cells with various concentrations of DP4 and DP5, as described in the previous paragraph. As shown in Fig. 6a and b, respectively, DP4 and DP5 dose-dependently inhibit IL-2 production by Jurkat T cells. At higher concentrations, DP5 was somewhat more effective than DP4. At 40 mM, DP5 reduced IL-2 production to about 30% of control values. Hence, maltooligosaccharides significantly inhibit the production of IL-2 by T cells.
Fig. 6.
Effect of various saccharides on IL-2 production by Jurkat cells. Cells were stimulated with 10 µg/ml PHA in the absence or presence of DP3, DP4, DP5, DP6, iDP3 (isomaltotriose), and cellotriose (all at 20 mM). The saccharide most effective at blocking IL-2 production was DP5. (PHA vs. DP3 and DP4, P<0.005; PHA vs. DP5 and DP6, P<0.001; iDP3 vs. DP4, DP5 and DP6, P<0.001; iDP3 vs. DP3, P<0.005).
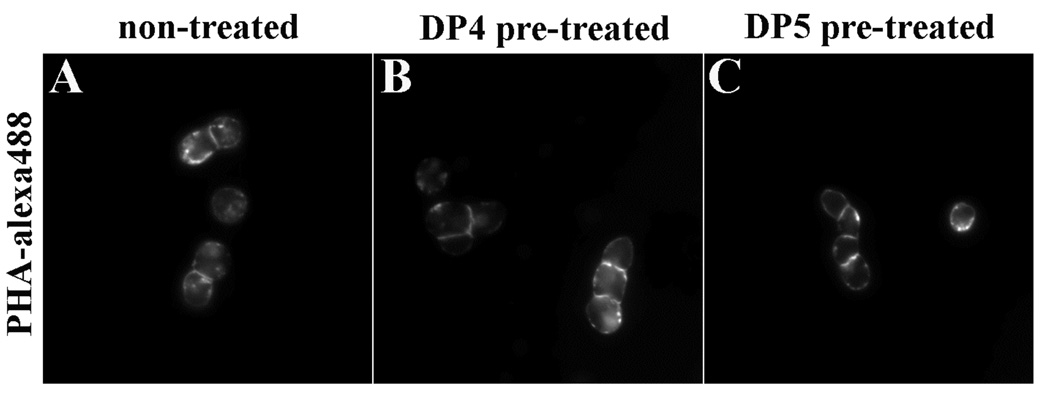
Although maltooligosaccharides do not resemble the complex oligosaccharides recognized by PHA, we nonetheless controlled for the possibility that DP4 and DP5 blocked the binding of PHA to Jurkat cell surfaces. Cells were pre-treated with 20 mM DP4 or DP5 for 30 min. followed by addition of Alexa-488-PHA for 30 min. Figure 7 shows fluorescence micrographs of Jurkat cells labeled with Alexa-488-PHA after incubation in the absence of oligosaccharides (Fig. 7a) or the presence of DP4 or DP5 (Fig. 7b and c, respectively). As these data show, there were no significant differences in the PHA labeling of Jurkat cells during these various conditions. Hence, the differences in IL-2 production cannot be accounted for by competition of the maltooligosaccharides with cell surface oligosaccharides for PHA binding.
Fig. 7.
Effect of maltooligosaccharides on PHA binding to lymphocytes. Cells were incubated with buffer (panel A), 20 mM DP4 (panel B), or 20 mM DP5 (panel C) for 30 min. followed by the addition of 10 µg/ml Alexa-488-PHA for 30 min. at 37°C. Samples were then washed by centrifugation and observed by fluorescence microscopy. As no significant differences in binding intensity or location were observed, these maltooligosaccharides do not inhibit the binding of PHA to cells.
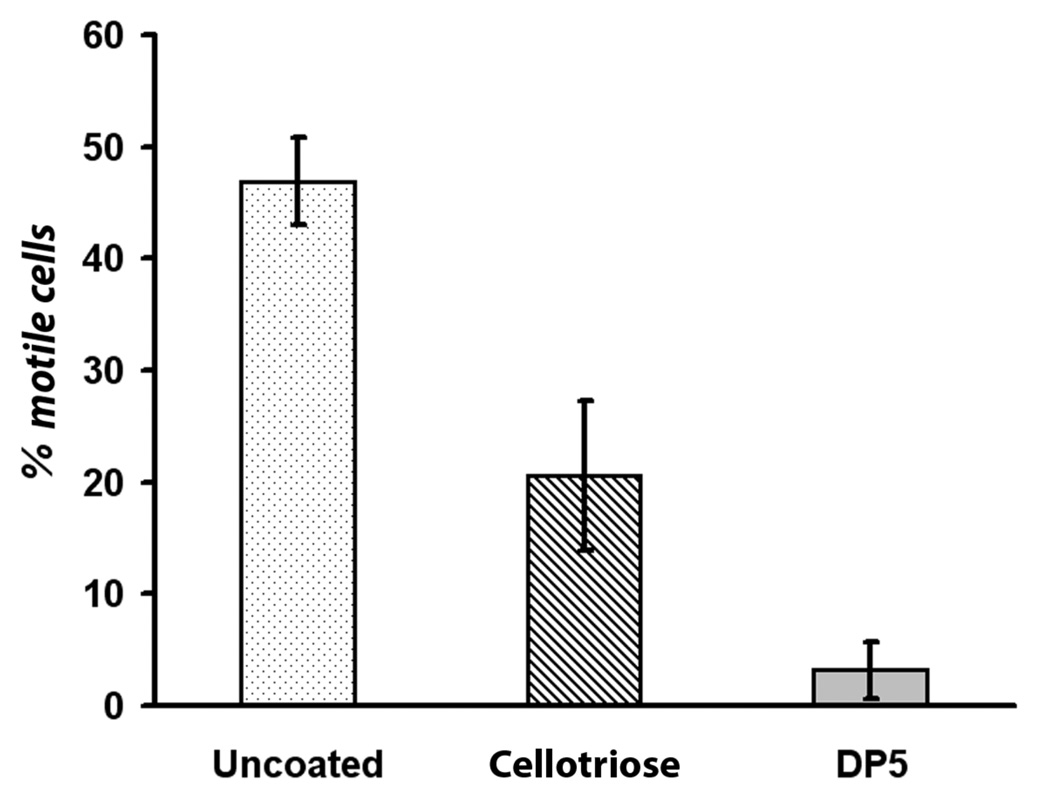
To ascertain the breadth of its potential immunoregulatory capacity, we examined the ability of neutrophils to undergo spontaneous motility on maltooligosaccharide-modified glass cover slips. These covalently modified cover slips were prepared as described in the Materials and Methods. Neutrophils were added to these cover glass-bottom Petri dishes, then allowed to settle and attach to the surface for 1 hr. at 37°C. To avoid confounding maltooligosaccharide effects with those of chemokinetic and chemotaxis effects, we examined spontaneous neutrophil motility. Motile cells were defined as those that moved at least one cell diameter during a timed observation period. Roughly one-half of the neutrophils demonstrated motility on unmodified glass surfaces (Fig. 8). However, this was significantly reduced (P<0.001) on cellotriose-modified surfaces to about 20%. On DP5-modified surfaces, the motile cell percentage was reduced to about 4%, a dramatic reduction in cell movement. It is not surprising that some inhibition was obtained on cellotriose-modified surfaces because it is a glucose-based oligosaccharide whose sequence differs only in the anomeric carbon atom (β vs. α) in comparison to maltooligosaccharides. Hence, maltooligosaccharides have immunoregulatory potential.
Fig. 8.
Effect of surface-bound oligosaccharides on spontaneous neutrophil motility. Surfaces were prepared as outlined in Scheme 1. Neutrophils were added to: unmodified glass cover slips (panel A), cellotriose-modified glass cover slips (panel B), or DP5-modified glass cover slips (panel C). Cells moving 10 µm or more during the incubation period were scored as positive. DP5 derivatized surfaces that displayed four glucose subunits (scheme 1) dramatically inhibited spontaneous motility in comparison to both unmodified and cellotriose-modified glass surfaces. (uncoated vs. DP5, P<0.0001; uncoated vs. cellotriose, P=0.0005; cellotriose vs. DP5, P=0.0001).
Discussion
As hemochorial placentation places maternal leukocytes in direct physical contact with villus trophoblasts of fetal origin, mechanisms must exist to modify the mother’s immune response to allow survival of the fetal semiallograft. Although the biochemical machinery responsible for maternal immunoregulation has been incompletely described, emerging evidence suggests that the mitigation of the maternal response is a multi-factorial process affecting both adaptive and innate immune responses. For example, trophoblasts do not express immunostimulatory HLA class I antigens, but rather express HLA-G molecules, which promote tolerance.15, 16 As trophoblasts express B7-H2 and B7-H3 molecules, which interact with members of the CD28 family on lymphocytes, trophoblasts are prone to stimulate Th2-type responses.17 Production of the immunoregulatory enzyme indoleamine-2, 3-dioxygenase may also contribute to immunoregulation during pregnancy.18 Although trophoblasts have been shown to protect the conceptus from destruction by activated macrophages 8 and evade destruction by activated tumoricidal macrophages, 9 the regulation of the innate immune response in pregnancy is also incompletely understood. In the present study we have found that maltooligosaccharides are another cell component that may influence the behavior of neighboring immune cells.
Previous studies have suggested that pregnancy-associated carbohydrates, including those at the trophoblast’s surface, affect the behavior of immune cells.10, 11, 19, 20 Although the structures of some of these compounds are known, the remainder of these potential immunoregulatory molecules is unknown. In the present study, we first identified the major low molecular weight cell surface saccharides of JEG-3 cytotrophoblast-like cells then evaluated their bioactivity. To accomplish the first goal, we used high-performance anion-exchange chromatography with pulsed amperometric detection (HPAE-PAD), which is one of the most powerful techniques for underivated carbohydrate analysis, in conjunction with HPLC-electrospray mass spectroscopy (ES-MS) and MS2. Using these tools, we determined the structures of two components of this fraction to be maltotriose and maltotetraose. Interestingly, the accumulation of maltotriose and maltotetraose in a membrane fraction of a syncytiotrophoblasts has beeen noted21, although they did not determine if maltooligosaccharides were externally expressed nor was their biological role addressed. Our structural analysis of JEG-3 cytotrophoblast-like oligosaccharides demonstrates that maltooligosaccharides are a significant carbohydrate structure of these cells. These maltooligosaccharides must be synthesized by JEG-3 cells, and are presumably non-covalently associated with the cell surface in a fashion similar to hyaluronan and other glycocalyx components.
As previous studies have indicated that trophoblast membranes 22, 23 and, specifically, their membrane-associated saccharides,10 affect lymphocyte function, we assessed cytokine production by PHA-activated Jurkat lymphocytes. We found that maltooligosaccharides reduce cytokine release in a dose-dependent fashion, with an optimal chain length of DP5. This optimal chain length is likely due to the formation of secondary structures, such as rings, at longer chain lengths.14 However, the use of millimolar concentrations of maltooligosaccharides in these experiments might seem too high. Although this may appear to be high, it is roughly what one would expect for a region of intercellular contact. Using HPAE chromatography of SPE isolates and authentic DP3, we estimate that 4×10−9 mol of DP3 can be isolated from 107 cells. Given the assumptions that the cell surface area equals 800 µm2 and that maltooligosaccharides are uniformly distributed on their surfaces, we estimate ~3×105 molecules of DP3/µm2. With approximately equal abundances of DP3 and DP4, a maltooligosaccharide value of ~6×105 molecules of per µm2 is suggested, which is roughly a quarter of the density of phospholipids in a membrane (2.5×106/µm2). We estimate that maltooligosaccharides may be distributed in a zone with a thickness of 0.04 µm. This does not seem unreasonable because the trophoectoderm and apical epithelial membranes at the time of implantation approach as close as 0.0075–0.015 µm.24 It is also consistent with the fact that the leukocyte glycocalyx is ~0.012 nm in thickness.25 Using a thickness of 0.04 µm, we conservatively estimate a local concentration of ~24 mM. Hence, for the biological conditions of intercellular contact, the concentrations used in this study do not appear to be unreasonable.
Early studies have also suggested that trophoblasts affect the behavior of adherent mononuclear phagocytes.8, 9 To test this possibility in our maltooligosaccharide system, we synthesized DP5-modified glass cover slips (Scheme 1). In addition, the surface-associated DP5 is a better model of the trophoblast surface (both are essentially two-dimensional), in contrast to the bulk addition of DP5 in the lymphocyte experiments. As phagocytic cells are adherent, these surfaces allowed us to test the hypothetical bioactivity of DP5 in a chemically robust system while retaining a physiologically-appropriate surface context for the assessment of bioactivity. Our experimental findings showed that spontaneous neutrophil motility was reduced on DP5-modified surfaces. This is consistent with other work suggesting that small oligosaccharides, including maltooligosaccharides, affect phagocyte functions such as oxidant production.14 Thus, the biological activities of both neutrophils and lymphocytes are affected by maltooligosaccharides.
Although our findings have suggested a previously unrecognized biological regulatory pathway based upon maltooligosaccharides, there may be practical applications of these findings. For example, biomaterials are often compromised by immunological activation at their surfaces.26 By using maltooligosaccharides as a coating component, in analogy with trophoblasts, it may be possible to improve the biocompatibility of implants. This proposal is not unreasonable because carbohydrates, such as hyaluronic acid, have already proven to be useful in mitigating host responses to implant materials. Indeed, maltooligosaccharide modification may be a generally useful tool in reducing immunologic/inflammatory responses to implants, polymeric injectables, and other substances.
In summary, we have demonstrated that maltooligosaccharides are present at the surface of JEG-3 trophoblasts. Further studies on the biochemical and physiological properties of maltooligosaccharides are underway. From a biological perspective, maltooligosaccharides would be expected to be an excellent immunological evasion tactic. As a component of glycogen, maltooligosaccharides are an ancient self molecule. More importantly, as glycogen is a component of normal human serum (5 mg/100 ml27), mothers producing an anti-maltooligosaccharide antibody would succumb to serum sickness. Moreover, we have also shown that these molecules are capable of influencing the behavior of leukocytes. We suggest that maltooligosaccharides contribute to the carbohydrate surface coat of JEG-3 cells. To examine maltooligosaccharide expression in human placental tissue, we are developing novel fluorescent tools. We speculate that maltooligosaccharides may contribute to the mitigation of adaptive and innate immunologic responses at the maternal-fetal interface.
Acknowledgements
We thank Hongpeng Lui for technical assistance. This work was supported, in part, by the Intramural Program of the National Instutute of Child Health and Human Development, NIH, DHHS.
References
- 1.Sharon N, Lis H. Carbohydrates in cell recognition. Sci Am. 1993;268:82–89. doi: 10.1038/scientificamerican0193-82. [DOI] [PubMed] [Google Scholar]
- 2.Haltiwanger RS, Lowe JB. Role of glycosylation in development. Annu Rev Biochem. 2004;73:491–537. doi: 10.1146/annurev.biochem.73.011303.074043. [DOI] [PubMed] [Google Scholar]
- 3.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lasky LA. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science. 1992;258:964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- 5.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 6.Cobb BA, Kasper DL. Coming of age: carbohydrates and immunity. Eur J Immunol. 2005;35:352–356. doi: 10.1002/eji.200425889. [DOI] [PubMed] [Google Scholar]
- 7.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 8.Sionov RV, Yagel S, Har-Nir R, Gallily R. Trophoblasts protect the inner cell mass from macrophage destruction. Biol Reprod. 1993;49:588–595. doi: 10.1095/biolreprod49.3.588. [DOI] [PubMed] [Google Scholar]
- 9.Lu CY, Redline RW, Shea CM, Dustin LB, McKay DB. Pregnancy as a natural model of allograft tolerance. Interactions between adherent macrophages and trophoblast populations. Transplantation. 1989;48:848–855. doi: 10.1097/00007890-198911000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Arkwright PD, Rademacher TW, Boutignon F, Dwek RA, Redman CW. Suppression of allogeneic reactivity in vitro by the syncytiotrophoblast membrane glycocalyx of the human term placenta is carbohydrate dependent. Glycobiology. 1994;4:39–47. doi: 10.1093/glycob/4.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Muchmore AV, Decker JM, Blaese RM, Nilsson B. Purification and characterization of a mannose-containing disaccharide obtained from human pregnancy urine. A new immunoregulatory saccharide. J Exp Med. 1984;160:1672–1685. doi: 10.1084/jem.160.6.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Packer NH, Lawson MA, Jardine DR, Redmond JW. A general approach to desalting oligosaccharides released from glycoproteins. Glycoconj J. 1998;15:737–747. doi: 10.1023/a:1006983125913. [DOI] [PubMed] [Google Scholar]
- 13.Wuttke M, Muller S, Nitsche DP, Paulsson M, Hanisch FG, Maurer P. Structural characterization of human recombinant and bone-derived bone sialoprotein. Functional implications for cell attachment and hydroxyapatite binding. J Biol Chem. 2001;276:36839–36848. doi: 10.1074/jbc.M105689200. [DOI] [PubMed] [Google Scholar]
- 14.Bland EJ, Keshavarz T, Bucke C. The influence of small oligosaccharides on the immune system. Carbohydr Res. 2004;339:1673–1678. doi: 10.1016/j.carres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Carosella ED, Rouas-Freiss N, Paul P, Dausset J. HLA-G: a tolerance molecule from the major histocompatibility complex. Immunol Today. 1999;20:60–62. doi: 10.1016/s0167-5699(98)01387-5. [DOI] [PubMed] [Google Scholar]
- 16.Hunt JS, Petroff MG, Morales P, Sedlmayr P, Geraghty DE, Ober C. HLA-G in reproduction: studies on the maternal-fetal interface. Hum Immunol. 2000;61:1113–1117. doi: 10.1016/s0198-8859(00)00195-6. [DOI] [PubMed] [Google Scholar]
- 17.Petroff MG, Kharatyan E, Torry DS, Holets L. The immunomodulatory proteins B7-DC, B7-H2, and B7-H3 are differentially expressed across gestation in the human placenta. Am J Pathol. 2005;167:465–473. doi: 10.1016/S0002-9440(10)62990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patankar MS, Ozgur K, Oehninger S, Dell A, Morris H, Seppala M, Clark GF. Expression of glycans linked to natural killer cell inhibition on the human zona pellucida. Mol Hum Reprod. 1997;3:501–505. doi: 10.1093/molehr/3.6.501. [DOI] [PubMed] [Google Scholar]
- 20.Muchmore AV, Shifrin S, Decker JM. In vitro evidence that carbohydrate moieties derived from uromodulin, an 85,000 dalton immunosuppressive glycoprotein isolated from human pregnancy urine, are immunosuppressive in the absence of intact protein. J Immunol. 1987;138:2547–2553. [PubMed] [Google Scholar]
- 21.Fischer SJ, Laine RA. Accumulation of malto-oligosaccharides in the syncytiotrophoblastic cells of first-trimester human placentas. Biochem J. 1981;200:93–98. doi: 10.1042/bj2000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thibault G, Degenne D, Lacord M, Guillaumin JM, Girard AC, Bardos P. Inhibitory effect of human syncytiotrophoblast plasma membrane vesicles on Jurkat cells activated by phorbol ester and calcium ionophore. Cell Immunol. 1992;139:259–267. doi: 10.1016/0008-8749(92)90118-9. [DOI] [PubMed] [Google Scholar]
- 23.Thibault G, Degenne D, Girard AC, Guillaumin JM, Lacord M, Bardos P. The inhibitory effect of human syncytiotrophoblast plasma membrane vesicles on in vitro lymphocyte proliferation is associated with reduced interleukin 2 receptor expression. Cell Immunol. 1991;138:165–174. doi: 10.1016/0008-8749(91)90141-w. [DOI] [PubMed] [Google Scholar]
- 24.Lopata A. Implantation of the human embryo. Hum Reprod. 1996;11 Suppl 1:175–184. doi: 10.1093/humrep/11.suppl_5.175. discussion 193-175. [DOI] [PubMed] [Google Scholar]
- 25.Richards KL, Douglas SD. Alterations of the glycocalyx of Fc receptor-bearing cell lines during Fc receptor-ligand interactions. J Reticuloendothel Soc. 1983;33:305–314. [PubMed] [Google Scholar]
- 26.Tang L, Liu L, Elwing HB. Complement activation and inflammation triggered by model biomaterial surfaces. J Biomed Mater Res. 1998;41:333–340. doi: 10.1002/(sici)1097-4636(199808)41:2<333::aid-jbm19>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 27.White A, Handler P, Smith EL. Principles of biochemistry. 5th edn. New York: McGraw-Hill; 1973. [Google Scholar]
- 28.Domon B, Costello CE. Structure elucidation of glycosphingolipids and gangliosides using high-performance tandem mass spectrometry. Biochemistry. 1988;27:1534–1543. doi: 10.1021/bi00405a021. [DOI] [PubMed] [Google Scholar]