Abstract
Purpose
To establish which embryo parameters, in frozen thawed embryo transfers, have the highest prognosis value in the establishment of pregnancy. The relative importance of different embryo parameters is used to develop an embryo score.
Methods
Retrospective analysis of the implantation rate in 356 frozen/thawed single embryo transfers. A logistic regression model is used to establish an embryo score.
Results
A direct correlation is established between the implantation rate and fresh embryo development (number of blastomeres and their symmetry), survival rate after thawing and mitosis resumption after overnight culture.
Conclusions
An embryo score is developed to determine the implantation potential of frozen/thawed embryos.
Keywords: Cryopreserved embryos, Single embryo transfer, Embryological parameters, Embryo score
Introduction
Since the early days of embryo cryopreservation [1–3], this technique has become an essential part of Assisted Reproduction Techniques (ART) when surplus developing embryos are available, and is now a standard routine procedure with clinical application.
Cryopreservation techniques have evolved considerably and now offer two main advantages: on one hand, the possibility of increasing the cumulative pregnancy rate per cycle [4–6] and on the other, of reducing the number of embryos to be transferred both in the fresh cycle and in successive frozen embryo transfer cycles, thereby decreasing the risk of multiple pregnancy. Other significant advantages include the possibility of delaying embryo transfer, thereby enabling the assessment of infectious diseases, the possibility of performing unsynchronised embryo donations and, in some cases, of preventing ovarian hyperstimulation syndrome (OHSS) [7, 8].
Although the procedure is now standardized, pregnancy rates still remain slightly below the results obtained in fresh cycles [9, 10]. This could be explained by the fact that the best embryos are always selected for transfer in the fresh cycle. The toxic effect of cryoprotectors and the damage caused to embryos by the formation of ice crystals during the freezing-thawing process is also to be taken into account.
An in-depth analysis of the data is needed in order to optimize pregnancy rates and prevent the high incidence of multiple pregnancies. It is important to assess how, and to what extent, different embryo parameters affect the implantation potential of the frozen-thawed embryos. This has already been studied but the relative importance of each parameter has yet to be determined. A number of articles [11–15] have been published showing a direct relationship between the implantation potential of frozen embryos and the following embryo factors: the quality of the fresh embryo, blastomere survival after thawing and the ability to resume mitosis following overnight culture. However, only few studies have analysed these factors in single embryo transfers [16–18].
The main objective of this study is to establish which embryo parameters, in frozen thawed embryo transfers, have the highest prognosis value in the establishment of pregnancy. For this purpose, single frozen embryo transfers (sFET) from our cryopreservation programme have been considered.
Moreover, there is currently a large number of embryo scores to determine the implantation potential of fresh embryos but the implantation potential of frozen-thawed embryos also needs to be assessed. Therefore, an embryo score has been developed for these embryos on the basis of the identified embryo parameters.
Material and methods
All single frozen embryo transfers carried out between 2000 and 2006 at Institut Universitari Dexeus were analysed retrospectively. Three hundred and fifty six transfers performed in this period as part of IVF or ICSI cycles were included, excluding egg donation cycles.
In our cryopreservation programme, there are two situations in which a single embryo transfer is performed: In the first situation, only one embryo is replaced in patients with a good prognosis due to clinical characteristics (49 transfers). These include young maternal age (<35 years old) and/or pregnancy in previous cycle. The second group of patients include those that have only one embryo available for transfer (307 transfers).
Fresh embryo morphology
Embryo morphology was assessed just before cryopreservation under an inverted microscope (×40). The number of blastomeres, their symmetry (equal, slightly unequal or unequal), cytoplasmic fragmentation (≤20%; >20%) and the occurrence of multinucleation were evaluated. Embryos with >35% fragmentation or with multinucleation were considered unsuitable for cryopreservation. In terms of cleavage, preferably embryos of ≥4 blastomeres on D + 2 and of ≥6 blastomeres on D + 3 were frozen although, in some cases, embryos with a slower cleavage rate on D + 3 were frozen.
Cryopreservation procedure
All embryos included in the study were cryopreserved 48 h (±2 h) (D + 2) or 72 h (±4 h) (D + 3) after insemination using the slow freezing protocol [19] and with a programmable biological freezer (Minicool LC 40; Air Liquide, France) and 1.2-propanediol (PROH) and sucrose as cryoprotectants. Most embryos were frozen at D + 3 of development (79.7%). The freezing and thawing procedures were carried out using commercial media (Freeze Kit1 and Thaw Kit1; Vitrolife, Sweden) and 0.25 ml plastic straws (Cryo Bio System, France), following the protocol previously described [3].
Embryo morphology following thawing
After thawing, the morphology of the embryos was examined under an inverted microscope (×40) in order to ascertain the number of damaged blastomeres (embryo survival). Four groups were established according to the percentage of intact blastomeres: 100%, ≥80 <100%, >50 <80% and ≤50% embryo survival. Although embryos with a survival rate of <50% were not considered suitable for transfer, in some cases they were in fact transferred at patient’s request, following advice on the expected unfavourable prognosis.
After thawing all embryos were kept in culture overnight. The average time spent in culture was 22.2 ± 2.6 h, allowing enough time to evaluate whether mitosis was resumed. The number of blastomeres that resumed mitosis and/or showed signs of compaction were assessed. The embryos were classified into four groups: non-cleaved, only one cell cleaved, two or more cells cleaved and compacting embryos.
Embryo replacement
The patients underwent an endometrial preparation protocol as described by Coroleu et al. [20].
Uterine transfer of thawed embryos was always performed under ultrasound guidance on the fourth day of progesterone treatment using an Edwards-Wallace embryo replacement catheter (SIMS Portex Ltd, UK) connected to an insulin syringe.
Patients were advised to continue with estrogen and progesterone treatment and were tested for β-HCG serum concentration 14–16 days following embryo transfer. Clinical pregnancy is defined by the presence of high concentration of β-HCG (>75 IU/ml) and the evidence of an intrauterine gestational sac by ultrasonography. Hormonal treatment was maintained until 10 weeks of gestation. Only clinical pregnancies were considered for the evaluation of results. Miscarriage is defined as a spontaneous abortion after the confirmation of a clinical pregnancy.
Statistical analysis
Pearson’s Chi-Square Test or Fisher’s exact test were used to compare qualitative variables and quantitative variables were compared using the T Test or the Wilcoxon Mann-Whitney test.
A multivariable logistic model was adjusted following a model building strategy [21] using variables which were significant in the bivariant analysis for identifying the factors associated with the implantation rate. The embryo score was obtained on the basis of the parameters estimated using the logistic regression model (g = α0 + α1X1+ … + αχXχ) where g is 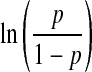 and p is the IR. The scores obtained using the model were translated into a scale of values from 0 to 10, with 0 being the score for embryos with the worst prognosis and 10 for those with the best. The discriminatory capacity of the model was assessed using ROC curves.
and p is the IR. The scores obtained using the model were translated into a scale of values from 0 to 10, with 0 being the score for embryos with the worst prognosis and 10 for those with the best. The discriminatory capacity of the model was assessed using ROC curves.
In order to improve the model management, the uncleaved embryos or those with only a single cleaved blastomere were grouped together as they presented similar IRs.
All the tests were bilateral with an α = 0.05 level of significance and all the analyses were performed using the SPSS v 15.0 statistical package.
Results
A total of 356 single frozen embryo transfers performed between 2000 and 2006 were studied. The average age of the patients at the date of follicular puncture was 35.1 ± 4.4 years of age and 35.6 ± 4.1 at the time of transfer. Seventy-nine point two percent of the embryos were fertilised by ICSI and 20.8% by conventional IVF. Embryos were kept frozen for periods between 2 months and 8 years. The pregnancy rate per transfer, equivalent in this study to the implantation rate, was 19.9%. The miscarriage rate was 33.8%.
The IR achieved in embryos frozen on D + 2 is similar to the one obtained on D + 3.
In those single embryo transfers, in which more frozen embryos were available, the IR was 31.9%, while decreasing to 18.1% in cases where no further embryos were available (p < 0.05).
No statistically significant relationship was found between the patient’s age at the date of embryo cryopreservation or the fertilisation technique used and the implantation and miscarriage rates even though patients over 36 years of age had a lower IR and higher miscarriage rate. However these differences with younger patients were not statistically significant (Table 1).
Table 1.
Implantation and miscarriage rate according to age, fertilization method and day of cryopreservation
| Parameter | N (%) | Pregnancies | Implantation rate | Miscarriage rate | |
|---|---|---|---|---|---|
| Patient age | ≤32 | 91 (25.6) | 24 | 26.4% | 20.8% |
| 33–35 | 98 (27.5) | 20 | 20.4% | 30.0% | |
| 36–38 | 91 (25.6) | 18 | 18.8% | 50.0% | |
| ≥39 | 76 (21.3) | 9 | 11.8% | 44.4% | |
| ns | ns | ||||
| Fertilization method | IVF | 74 (20.8) | 13 | 17.6% | 46.2% |
| ICSI | 282 (79.2) | 58 | 20.6% | 31.0% | |
| ns | ns | ||||
| Day of cryopreservation | D + 2 | 72 (20.2) | 14 | 19.4% | 35.7% |
| D + 3 | 284 (79.8) | 57 | 20.1% | 33.3% | |
| ns | ns | ||||
| Total | 356 | 71 | 19.9% | 33.8% |
ns not significant
Embryo parameters
The classical parameters for embryo quality were assessed before freezing and their relationship with the implantation rate was analysed. Embryos frozen at 6 to 9 cells on D + 3 presented a significantly higher IR (23.8%) than those with abnormal cleavage rates, including both slow and fast cleaving embryos (11.0%). When studying the relationship between the symmetry of the blastomeres and the IR of the embryos, statistically significant differences were observed between the groups studied (p < 0.05), with higher IR in embryos with equal or slightly unequal blastomeres. With respect to the influence of cytoplasmatic fragmentation on the IR, there is a tendency towards a lower IR in embryos with fragmentation rates over 20% (11.4%) when compared to 21.2% in embryos with fragmentation of ≤20%, although the differences were not statistically significant.
In the evaluation of embryo characteristics after thawing, the survival rate and the ability of the embryo to resume mitosis following overnight culture were assessed. The implantation rate of embryos with a survival rate of ≥80% of the blastomeres is significantly higher than the one of embryos with <80% (25.3% vs 10.4%) (p < 0.05). There is a direct correlation between the degree of development of the embryos after overnight culture and their implantation potential. The embryos in which at least two blastomeres cleaved and those that displayed signs of compaction presented a statistically significant increase in the IR (28% and 25%, respectively) when compared to the uncleaved group of embryos and those in which a single blastomere resumed mitosis (12.6% and 15.4% respectively) (p < 0.05) (Table 2).
Table 2.
Implantation rate according to embryo parameters
| Parameter | Number of embryos (%) | Number of pregnancies | Implantation rate | |
|---|---|---|---|---|
| Fresh embryo quality | ||||
| Number of blastomeres | D + 2 | |||
| 4–6 cells | 72 (20.2) | 14 | 19.4% | |
| D + 3 | ||||
| 6–9 cells | 202 (56.7) | 48 | 23.8% | |
| <6> 9 cells | 82 (23.0) | 9 | 11.0% | |
| p < 0.05 | ||||
| Blastomere symmetry | Equal | 58 (16.3) | 20 | 34.5% |
| Slightly unequal | 93 (26.1) | 25 | 26.9% | |
| Unequal | 205 (57.6) | 26 | 12.7% | |
| p < 0.05 | ||||
| Cytoplasmic fragmentation | ≤20% | 312 (87.6) | 66 | 21.2% |
| >20% | 44 (12.4) | 5 | 11.4% | |
| ns | ||||
| After thawing | ||||
| Blastomere survival rate | 100% | 170 (47.8) | 44 | 25.9% |
| 80−< 100% | 51 (14.3) | 12 | 23.5% | |
| >50−< 80% | 80 (22.5) | 13 | 16.3% | |
| ≤ 50% | 55 (15.4) | 2 | 3.6% | |
| p < 0.05 | ||||
| Resumption of mitosis | Non-cleaved | 119 (33.4) | 15 | 12.6% |
| 1 Cell cleaved | 65 (18.3) | 10 | 15.4% | |
| ≥2 cells cleaved | 100 (28.1) | 28 | 28.0% | |
| Compacting | 72 (20.2) | 18 | 25.0% | |
| p < 0.05 | ||||
ns not significant
Logistic regression model: embryo score
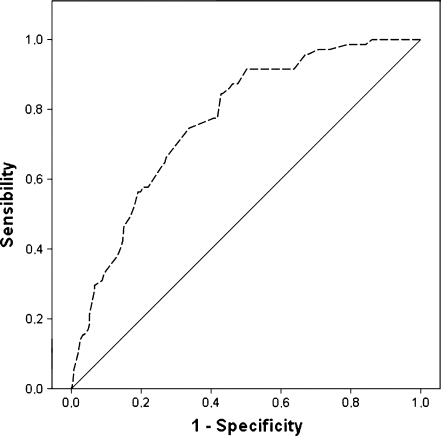
Our results (Fig. 1) show that age does not have an influence on the model’s discriminatory capacity and therefore this variable has not been taken into account in the development of the score.
Fig. 1.
Discriminatory capacity of the model without age (ROC curve). AUC: 0.779 [0.723–0.835] without age
On a bivariant analysis (Table 2), the only variables associated separately with the IR were the number of blastomeres of the fresh embryo, either at D + 2 or D + 3 of development, their symmetry, the blastomere survival rate after thawing and the resumption of mitosis after overnight culture. The logistic regression model was constructed on the basis of these variables (Table 3). The area under the ROC curve to verify the discriminatory capacity of the score resulted in a value of AUC = 0.769 [0.712–0.826] (p < 0.05).
Table 3.
Logistic regression model
| Parameter | B | E.T | Wald | df | Sig. | OR | CI 95% OR | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Number of blastomeres in fresh embryo | D + 3; 6–9 | 0 | – | – | – | – | 1 | – | – |
| D + 2; 4–5 | −0.817 | 0.425 | 3.694 | 1 | 0.055 | 0.442 | 0.192 | 1.016 | |
| D + 3; <6– >9 | −1.235 | 0.422 | 8.575 | 1 | 0.003 | 0.291 | 0.127 | 0.665 | |
| Symmetry of blastomeres | Equal | 0 | – | – | – | – | 1 | – | – |
| Slightly unequal | −0.713 | 0.411 | 3.011 | 1 | 0.083 | 0.490 | 0.219 | 1.097 | |
| Unequal | −1.707 | 0.396 | 18.604 | 1 | 0 | 0.181 | 0.084 | 0.394 | |
| Survival rate | 100% | 0 | – | – | – | – | 1 | – | – |
| 80− <100% | −0.356 | 0.423 | 0.711 | 1 | 0.399 | 0.700 | 0.306 | 1.603 | |
| >50– <80% | −0.921 | 0.392 | 5.531 | 1 | 0.019 | 0.398 | 0.185 | 0.858 | |
| ≤ 50% | −2.497 | 0.769 | 10.551 | 1 | 0.001 | 0.082 | 0.018 | 0.372 | |
| Resumption of mitosis | ≥ 2 blastomeres | 0 | – | – | – | – | 1 | – | – |
| ≤1 blastomere | –0.901 | 0.353 | 6.52 | 1 | 0.011 | 0.406 | 0.203 | 0.811 | |
| Compacting | −0.675 | 0.411 | 2.69 | 1 | 0.101 | 0.509 | 0.227 | 1.141 | |
| Constant | 1.139 | 0.493 | 5.335 | 1 | 0.021 | 3.124 | |||
The model to be applied is as follows:
1.139—[0 (when 6-9 blastomeres at D + 3) or 0.817 (when 4-5 blastomeres at D + 2) or 1.235 (when <6> 9 blastomeres at D + 3)]—[0 (when equal blastomeres) or 0.713 (when slightly unequal blastomeres) or 1.707 (when unequal blastomeres)]—[0 (when 100% survival) or 0.356 (when ≥80 <100% survival) or 0.921 (when >50 <80% survival) or 2.497 (when ≤50% survival)]—[0 (when division of ≥2 blastomeres) or 0.901 (when division of 0-1 blastomere) or 0.675 (when signs of compaction)]. The score obtained was transformed by multiplying by 1.5773 and adding 8.20. The score is thus transformed into values within a range of 0 to 10. For example, for an embryo frozen on D + 3 with eight blastomeres (a value of 0), that are slightly unequal (value of 0.713), where seven out of eight blastomeres survive after thawing (87.5%) (value of 0.356), with only one blastomere cleaved after overnight culture (value of 0.901), the score would be:
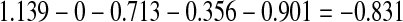 |
to be transformed into−0.831 (×1.5773) + 8.20 = 6.89.
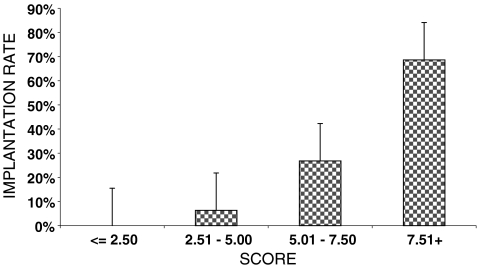
Four score categories were established: ≤2.5; 2.51–5; 5.01–7.50; >7.50. When the score was applied retrospectively to the sample under study, no pregnancies were recorded in the embryos with a score of ≤2.5 (0/32), only 6.1% of embryos with a score between 2.51–5 implanted (6/98), the embryos with a score between 5.01–7.5 implanted in 23.9% of the cases and the implantation rate reached 47.8% (43/180) for the group of embryos with the highest score (>7.5) (22/46). Following the example described before and based on our model, an embryo with a score of 6.89 would have an implantation potential of 23.9% (Fig. 2).
Fig. 2.
Implantation rate plus CI 95% according to the embryo score
Discussion
The implantation and miscarriage rate observed in our results (19.9% and 33.8% respectively) were comparable to the rates obtained in other retrospective studies in which single frozen/thawed embryo transfers were analysed [16–18, 22, 23], ranging from 8.9% to 26.7% and from 18.4% to 29.7% respectively.
In 49 cases (13.8%), a single embryo was transferred due to patient’s clinical characteristics and this group constitutes a good prognosis group, as shown by the results obtained. The implantation rate observed was higher than in the group where no further frozen embryos were available (31.9% and 18.1% respectively). In the study performed by Hyden-Granskog et al. [22], such “elective” transfers were analysed separately (18% of the total) from the “non-elective” (compulsory) ones with pregnancy rates of 40.7% and 23.6%, respectively, comparable to our findings. These results can be explained by the higher potential of implantation of embryos from young women or derived from cohorts of embryos that already implanted in fresh cycles which mainly constitute the “elective” transfer group.
No correlation was found between the age of the patients on the date of embryo cryopreservation and the IR and miscarriage rate. However, our results show a tendency towards lower IR and higher miscarriage rate with increasing maternal age. There is a degree of controversy in this respect and recent studies show a correlation between age and pregnancy rate [17, 18] whereas in others, the authors find no such correlation [16]. Moreover, in a recent publication [23] the authors reported significant differences in the live birth rate but no differences in IR with increasing age.
As shown in numerous studies, no correlation has been established between implantation rate and the fertilisation technique employed [17, 23, 24]. In the latter study, significant differences were however established in terms of the rate of children born favouring the conventional IVF method.
There is some controversy as to the day of development on which embryo cryopreservation should be carried out. Certain authors propose D + 2 of development as the optimum day for embryo cryopreservation and obtain a significantly higher pregnancy rates in this group of embryos compared to the ones that are cryopreserved on D + 3 [13, 24]. Other authors have described significantly higher pregnancy rates in embryos cryopreserved on D + 3 compared to D + 2 [25, 26]. Our findings do not show significant differences in the implantation rate according to the day on which cryopreservation takes place. Embryo quality and embryo selection are key points to obtain high implantation rates in cryopreservation programmes; since freezing on D + 3 enables to perform a better morphological selection of the embryos and our implantation rate reaches 20.1%, it does not seem advisable to change the protocol currently used.
The embryo parameters usually correlated to the implantation capacity of an embryo in a fresh cycle are the number of blastomeres, their symmetry and the cytoplasmic fragmentation percentage. In the case of cryopreserved embryos, the percentage of blastomeres that survive the freezing-thawing process and their development capacity after overnight culture have to be added to these factors.
In our data, embryos cryopreserved on D + 3 at the adequate developmental stage (6–9 blastomeres) have significantly higher implantation rates than the embryos cleaving at a slower or faster rate. The poor prognosis for slow cleaving embryos in fresh cycles [27], as well as in frozen embryo transfer cycles [12] is well known and, therefore, the lower implantation rate observed in this group (<6 blastomeres on D + 3; 9.7%) is not surprising. Gabrielsen et al. [15] analysed embryo cleaveage timming in embryos cryopreserved on D + 3 and found similar results with respect to its correlation with the implantation rate.
According to our results, a significantly higher implantation rate is observed in frozen-thawed embryos with a symmetrical cleavage pattern before freezing as compared to those with an asymmetrical pattern. These results are in agreement with the reports on fresh embryo transfer cycles [27, 28]. To our knowledge, there are no published data on the relationship between the symmetry of blastomeres in the fresh embryos and the results of frozen embryo transfer cycles.
Although embryos with a high degree (>35%) of fragmentation are not cryopreserved in our programme, we have analyzed if implantation rate is affected by embryo fragmentation before freezing. The cut-off point was established at 20% fragmentation, which is the percentage most broadly used [29]. No significant differences were detected between the two groups (≤20% and >20%), although few embryos are included in the latter group (44/356). In a previous study [4] the correlation between embryo fragmentation rate and implantation rate could not be established among the three groups established (<10%, 10–20% and >20–50%).
The parameters studied following thawing were the survival rate and mitosis resumption.
With respect to the percentage of blastomeres that survived the freezing and thawing process, our data show that there are no statistically significant differences in the implantation rate between the group of intact embryos and the group in which at least 80% of the blastomeres survived. Under this percentage, implantation rates fell significantly. Numerous studies have shown that the implantation potential of embryos on D + 2 of development is affected by the partial lysis of the embryo [9, 14, 30]. However, recent studies could not establish the relationship between embryo survival following thawing of embryos on D + 2 and implantation rate [15, 18]. In all these studies, performed on D + 2 embryos, partial lysis involves at least 25% of the embryo, whereas our data, mainly from embryos frozen on D + 3, show that implantation potential does not seem to be affected by partial lysis of the embryo, involving 20% or less of the embryo. Similarly, Tang et al. [16], with embryos cryopreserved on D + 3, show that lysis of ≤25% of the blastomeres does not affect implantation potential.
With regard to mitosis resumption following overnight culture, our data show that embryo cleavage has a considerable predictive value. However, the differences in the implantation rate only become significant when at least two blastomeres have cleaved or the embryo shows signs of compaction. Numerous studies have previously reported differences in implantation rates when at least one blastomere has cleaved [17, 18, 30]. It must be remembered that these studies were carried out on embryos cryopreserved on D + 2 of development and, therefore, the percentage of blastomeres that resume mitosis is the same as in this study. The parameter to be considered is probably the percentage, not the absolute number of blastomeres that resume mitosis. There is some controversy concerning this percentage since certain authors studying cryopreserved embryos on D + 2 [15] established in 50% the percentage of cleaved blastomeres required to observe differences in the implantation rate. Similarly to our data, other authors have reported differences in the implantation rate of embryos cryopreserved on D + 3 [16] when at least 25% of the blastomeres cleave. It would seem that a minimum percentage of the embryo should have the capacity of resuming mitosis in order to ensure full embryo viability.
A score for frozen/thawed embryos has been developed which includes the different embryo parameters that significantly influence the implantation rate: cleavage rate, symmetry of the blastomeres, embryo survival rate and resumption of mitosis.
The proposed embryo score for thawed embryos is a useful tool for determining the implantation potential of these embryos. Thus, in cases in which the embryos have a score denoting a low implantation potential (≤5), it can be suggested to the patient to thaw more embryos and postpone the transfer for 1 day, provided that more embryos are available. The fact that the score takes into account mitosis resumption following overnight culture means that it cannot be used to prevent multiple pregnancy since its calculation requires a timing that is incompatible with the synchronisation of the patient’s cycle.
In a similar study [23], a number of clinical variables to determine the number of embryos to be thawed and transferred was analyzed. In this multivariate analysis, four parameters were defined which were to be taken into account when deciding whether to transfer one or two thawed embryos. Blastomere survival rate, number of previous fresh cycles and IVF as the fertilisation method, compared with ICSI, were found to be positive predictors of live birth. The number of embryos thawed to achieve one transfer was negatively associated with pregnancy rate. However the predictive value of this parameter was found to be low.
In conclusion, our study shows that the embryos with the best implantation prognosis are those that are frozen on D + 3, with 6-9 blastomeres of equal size, a survival rate following thawing of 80–100% and with two or more cleaved blastomeres or signs of compaction after overnight culture. There is a need to validate the proposed score in a prospective way to assess its clinical value both in single and multiple embryo transfers.
Acknowledgements
The authors wish to thank the members of the Servei de Medicina de la Reproducció of Institut Universitari Dexeus in Barcelona for providing clinical and embryological data essential for the development of this study. This work was done under the auspices of the Catedra d’Investigacio en Obstetricia i Ginecologia.
Footnotes
Capsule
In a retrospective study, the relative importance of diferent embryo parameters in a cryopreservation programe is used to develop an embryo score for determining the implantation potential.
References
- 1.Trounson A, Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305:707–709. doi: 10.1038/305707a0. [DOI] [PubMed] [Google Scholar]
- 2.Zeilmaker GH, Alberda AT, Gent I, Rijkmans CM, Drogendijk AC. Two pregnancies following transfer of intact frozen-thawed embryos. Fertil Steril. 1984;42:293–296. doi: 10.1016/s0015-0282(16)48029-5. [DOI] [PubMed] [Google Scholar]
- 3.Veiga A, Calderon G, Barri PN, Coroleu B. Pregnancy after the replacement of a frozen-thawed embryo with less than 50% intact blastomeres. Hum Reprod. 1987;2:321–323. doi: 10.1093/oxfordjournals.humrep.a136542. [DOI] [PubMed] [Google Scholar]
- 4.Tiitinen A, Halttunen M, Harkki P, Vuoristo P, Hyden-Granskog C. Elective single embryo transfer: the value of cryopreservation. Hum Reprod. 2001;16:1140–1144. doi: 10.1093/humrep/16.6.1140. [DOI] [PubMed] [Google Scholar]
- 5.Lannou D, Griveau JF, Laurent MC, Gueho A, Veron E, Morcel K. Contribution of embryo cryopreservation to elective single embryo transfer in IVF-ICSI. Reprod Biomed Online. 2006;13:368–375. doi: 10.1016/S1472-6483(10)61441-1. [DOI] [PubMed] [Google Scholar]
- 6.Lundin K, Bergh C. Cumulative impact of adding frozen-thawed cycles to single versus double fresh embryo transfers. Reprod Biomed Online. 2007;15:76–82. doi: 10.1016/S1472-6483(10)60695-5. [DOI] [PubMed] [Google Scholar]
- 7.Sills ES, McLoughlin LJ, Genton MG, Walsh DJ, Coull GD, Walsh AP. Ovarian hyperstimulation syndrome and prophylactic human embryo cryopreservation: analysis of reproductive outcome following thawed embryo transfer. J Ovarian Res. 2008;1:7. doi: 10.1186/1757-2215-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verwoerd GR, Mathews T, Brinsden PR. Optimal follicle and oocyte numbers for cryopreservation of all embryos in IVF cycles at risk of OHSS. Reprod Biomed Online. 2008;17:312–317. doi: 10.1016/S1472-6483(10)60213-1. [DOI] [PubMed] [Google Scholar]
- 9.Edgar DH, Bourne H, Jericho H, McBain JC. The developmental potential of cryopreserved human embryos. Mol Cell Endocrinol. 2000;169:69–72. doi: 10.1016/S0303-7207(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 10.Andersen AN, Gianaroli L, Felberbaum R, de Mouzon J, Nygren KG, The European IVF-monitoring programme (EIM), European Society of Human Reproduction and Embryology (ESHRE). Assisted reproductive technology in Europe, 2001. Results generated from European registers by ESHRE. Hum Reprod 2005;20:1158–76. [DOI] [PubMed]
- 11.Schalkoff ME, Oskowitz SP, Powers RD. A multifactorial analysis of the pregnancy outcome in a successful embryo cryopreservation program. Fertil Steril. 1993;59:1070–1074. [PubMed] [Google Scholar]
- 12.Edgar DH, Jericho H, Bourne H, McBain JC. The influence of prefreeze growth rate and blastomere number on cryosurvival and subsequent implantation of human embryos. J Assist Reprod Genet. 2001;18:135–138. doi: 10.1023/A:1009416205265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salumets A, Tuuri T, Makinen S, Vilska S, Husu L, Tainio R, et al. Effect of developmental stage of embryo at freezing on pregnancy outcome of frozen-thawed embryo transfer. Hum Reprod. 2003;18:1890–1895. doi: 10.1093/humrep/deg339. [DOI] [PubMed] [Google Scholar]
- 14.El-Toukhy T, Khalaf Y, Al-Darazi K, Andritsos V, Taylor A, Braude P. Effect of blastomere loss on the outcome of frozen embryo replacement cycles. Fertil Steril. 2003;79:1106–1111. doi: 10.1016/S0015-0282(03)00072-4. [DOI] [PubMed] [Google Scholar]
- 15.Gabrielsen A, Fedder J, Agerholm I. Parameters predicting the implantation rate of thawed IVF/ICSI embryos: a retrospective study. Reprod Biomed Online. 2006;12:70–76. doi: 10.1016/S1472-6483(10)60983-2. [DOI] [PubMed] [Google Scholar]
- 16.Tang R, Catt J, Howlett D. Towards defining parameters for a successful single embryo transfer in frozen cycles. Hum Reprod. 2006;21:1179–1183. doi: 10.1093/humrep/dei490. [DOI] [PubMed] [Google Scholar]
- 17.Salumets A, Suikkari AM, Makinen S, Karro H, Roos A, Tuuri T. Frozen embryo transfers: implications of clinical and embryological factors on the pregnancy outcome. Hum Reprod. 2006;21:2368–2374. doi: 10.1093/humrep/del151. [DOI] [PubMed] [Google Scholar]
- 18.Edgar DH, Archer J, McBain J, Bourne H. Embryonic factors affecting outcome from single cryopreserved embryo transfer. Reprod Biomed Online. 2007;14:718–723. doi: 10.1016/S1472-6483(10)60674-8. [DOI] [PubMed] [Google Scholar]
- 19.Lassalle B, Testart J, Renard JP. Human embryo features that influence the success of cryopreservation with the use of 1, 2 propanediol. Fertil Steril. 1985;44:645–651. doi: 10.1016/s0015-0282(16)48981-8. [DOI] [PubMed] [Google Scholar]
- 20.Coroleu B, Barri PN, Carreras O, Martinez F, Veiga A, Balasch J. The usefulness of ultrasound guidance in frozen-thawed embryo transfer: a prospective randomized clinical trial. Hum Reprod. 2002;17:2885–2890. doi: 10.1093/humrep/17.11.2885. [DOI] [PubMed] [Google Scholar]
- 21.Hosmer DW, Lemeshow S. Applied logistic regression. 2. New York: Willey; 2000. [Google Scholar]
- 22.Hyden-Granskog C, Unkila-Kallio L, Halttunen M, Tiitinen A. Single embryo transfer is an option in frozen embryo transfer. Hum Reprod. 2005;20:2935–2938. doi: 10.1093/humrep/dei133. [DOI] [PubMed] [Google Scholar]
- 23.Olivius C, Lundin K, Bergh C. Predictive factors for live birth in cryopreservation single embryo transfer cycles. Reprod Biomed Online. 2008;17:676–683. doi: 10.1016/S1472-6483(10)60315-X. [DOI] [PubMed] [Google Scholar]
- 24.Mandelbaum J, Belaisch-Allart J, Junca AM, Antoine JM, Plachot M, Alvarez S, et al. Cryopreservation in human assisted reproduction is now routine for embryos but remains a research procedure for oocytes. Hum Reprod. 1998;Suppl 3:161–174. doi: 10.1093/humrep/13.suppl_3.161. [DOI] [PubMed] [Google Scholar]
- 25.Lahav-Baratz S, Koifman M, Shiloh H, Ishai D, Wiener-Megnazi Z, Dirnfeld M. Analyzing factors affecting the success rate of frozen-thawed embryos. J Assist Reprod Genet. 2003;20:444–448. doi: 10.1023/B:JARG.0000006705.46147.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sifer C, Sellami A, Poncelet C, Martin-Pont B, Porcher R, Hugues JN, et al. Day 3 compared with day 2 cryopreservation does not affect embryo survival but improves the outcome of frozen-thawed embryo transfers. Fertil Steril. 2006;86:1537–1540. doi: 10.1016/j.fertnstert.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 27.Hardarson T, Hanson C, Sjogren A, Lundin K. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Hum Reprod. 2001;16:313–318. doi: 10.1093/humrep/16.2.313. [DOI] [PubMed] [Google Scholar]
- 28.Holte J, Berglund L, Milton K, Garello C, Gennarelli G, Revelli A, et al. Construction of an evidence-based integrated morphology cleavage embryo score for implantation potential of embryos scored and transferred on day 2 after oocyte retrieval. Hum Reprod. 2007;22:548–557. doi: 10.1093/humrep/del403. [DOI] [PubMed] [Google Scholar]
- 29.Royen E, Mangelschots K, Neubourg D, Valkenburg M, Meerssche M, Ryckaert G, et al. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum Reprod. 1999;14:2345–2349. doi: 10.1093/humrep/14.9.2345. [DOI] [PubMed] [Google Scholar]
- 30.Guerif F, Bidault R, Cadoret V, Couet ML, Lansac J, Royere D. Parameters guiding selection of best embryos for transfer after cryopreservation: a reappraisal. Hum Reprod. 2002;17:1321–1326. doi: 10.1093/humrep/17.5.1321. [DOI] [PubMed] [Google Scholar]