Abstract
IL-2 controls the survival of regulatory T cells (Tregs), but it is unclear whether IL-2 also directly affects Treg suppressive capacity in vivo. We have found that eliminating Bim-dependent apoptosis in IL-2- and CD25-deficient mice restored Treg numbers but failed to cure their lethal autoimmune disease, demonstrating that IL-2-dependent survival and suppressive activity can be uncoupled in Tregs. Treatment with IL-2/anti-IL-2-Ab complexes enhanced the numbers and suppressive capacity of IL-2-deprived Tregs with striking increases in CD25, CTLA-4 and CD39/CD73 expression. Although cytokine treatment induced these suppressive mechanisms in both IL-2−/− and IL-2−/− Bim−/− mice, it only reversed autoimmune disease in the latter. Our results suggest that successful IL-2 therapy of established autoimmune diseases will require a threshold quantity of Tregs present at the start of treatment and show that the suppressive capacity of Tregs critically depends on IL-2 even when Treg survival is independent of this cytokine.
INTRODUCTION
Interleukin-2 plays dual and often opposing roles in immune responses, contributing to both the generation of effector and memory T cells and the maintenance of regulatory T cells (1, 2). The unexpected observation that mice deficient in components of the IL-2/IL-2 receptor pathway develop autoimmune diseases (3, 4) led to the realization that the non-redundant function of IL-2 is to maintain functional regulatory T cells (Tregs) (5). This conclusion has generated considerable interest in therapeutically administering IL-2 to control autoimmune disorders. The feasibility of this therapy was recently enhanced by the finding that IL-2 in complexes with certain antibodies acts with increased potency in vivo, compared to the cytokine itself, and can preferentially stimulate Tregs (6, 7). The goal of our study was to better define the different effects of IL-2 on Tregs and to correct the autoimmune disease of IL-2–knockout mice by administering IL-2/anti-IL-2 Ab complexes. The initial problem we faced was that, in the absence of IL-2, the proportion of Tregs surviving in the periphery is reduced, making it difficult to analyze these cells or enhance their function. To overcome this problem, we chose to promote Treg survival by preventing apoptosis. We show here that genetic ablation of Bim, to block mitochondrial apoptosis pathways, allows Foxp3+ CD4 T cells to survive in mice that lack IL-2 or CD25. However, these surviving Tregs function poorly and fail to prevent the autoimmune disease that develops in mice with a disrupted IL-2 pathway. Treatment with IL-2/anti-IL-2 Ab complexes does cure a significant fraction of the mice, but only if apoptosis of Tregs is first prevented. The ability of IL-2 to promote the function of Foxp3+ cells is associated with striking increases in the expression of CD25, CTLA-4 and CD39/CD73, but only modest effects on IL-10. This study identifies mechanisms by which IL-2 participates in the maintenance and function of Tregs and suggests that IL-2 therapy may cooperate with anti-apoptotic signals to correct states of severe Treg deficiency.
MATERIALS AND METHODS
Mice
Mice were housed in a specific pathogen-free facility in accordance with institutional guidelines. BALB/c mice were purchased from Charles River Laboratories and the Jackson Laboratory. IL-2−/− (3), CD25−/− (4)and Bim−/− (8) mice were back-crossed >12 generations to BALB/c; Foxp3/GFP mice (9) were back-crossed ≥7 generations.
Antibodies
The following clones were used: 145-2C11 (for CD3), GK1.5, RM4-5 (CD4), PC-61 (CD25), UC10-4F10-11 (CTLA-4), FJK-16s (Foxp3), JES6-1A12 (IL-2), 24DMS1 (CD39), eBioTY/11.8 (CD73), JES5-16E3 (IL-10), and B56 (Ki-67).
Anemia and anti-erythrocyte antibodies
Hematocrits, the percentages of blood volume filled with erythrocytes, were measured using a Hemavet 950 instrument (Drew Scientific). Endogenous anti-erythrocyte antibodies were detected using flow cytometry by staining erythrocytes with FITC-conjugated anti-IgM Ab or anti-IgG F(ab′)2 (Jackson ImmunoResearch Laboratories) (10).
Cell culture
Cells were cultured in RPMI 1640 media (Sigma-Aldrich) with 1mM each L-glutamine, non-essential amino acids, sodium pyruvate, HEPES, penicillin, streptomycin (Life Technologies), 50mM 2-ME, and 10% FCS (Omega Scientific). To measure cytokines, cells were activated for 4h with 75 ng/mL Phorbol Myristate Acetate (PMA) plus 750 ng/mL Ionomycin (Sigma-Aldrich) and, for the last 2h, 10mg/mL Brefeldin A (Epicentre Biotechnologies).
Flow cytometry
After washing, blocking and staining for surface antigens, cells were fixed with 2% paraformaldehyde. For intracellular staining, fixed cells were permeabilized with0.5% saponin (Sigma-Aldrich) or processed using Cytofix/Cytoperm and Perm/Wash buffers(BD Biosciences). Foxp3 was detected using the Foxp3 Staining Set (eBioscience). Flowcytometry was performed on a FACSCalibur or LSR II instrument using CellQuest or FACSDiva software (BD Bioscience).
Co-culture suppression assay
Lymph node CD4+ GFP+ (Foxp3+) Tregs and CD4+ CD25- GFP- (Foxp3-) responder T cells were purified with matching GFP levels using a MoFlo high-speed cell sorter (DakoCytomation). APCs were prepared from erythrocyte depleted, Mitomycin C (Sigma-Aldrich) treated WT spleens. Cultures were set up in U bottom96 well plates with 4 × 104 APCs, 2 × 104 responder T cells, 3 μg/mL soluble anti-CD3 Ab, and a titrated number of Tregs per well, incubated 48h, pulsed with 1.25 mCi [3H] Thymidine, and harvested at 60h using a Harvester 96 Mach III instrument (Tomtec). Proliferation was measured by scintillation counting using a Trilux 1450 Microbeta instrument (Wallac-Perkin Elmer).
Treatment with IL-2 immune complexes
IL-2-containing immune complexes (6) were made by incubating 1.5 μg mouse rIL-2 with 50 μg functional grade purified anti-IL-2 Ab, clone JES6-1A12, (eBioscience) perdose for 15–30 min at 37° in PBS. Mice were treated by i.p. injection beginning at 9–18 dof age 1/d for 3 d, then 3/wk. Treatment was decreased to 2/wk in 4–5 wk old mice whose hematocrits stabilized, or was stopped if hematocrits fell below 15 and mice became moribund.
Statistical analyses
Data were analyzed using Prism 5.02 for Windows software (GraphPad Software) applying: t test with Welch’s correction (two-tailed Fig. 1B, S1B, S2B, one-tailed Fig. 3D, Fig. 4), Mann-Whitney test (Fig. 1C, 2B, 2C, 3B, 3C, S1C), Log-rank test (Fig. 2A), 1-way ANOVA (Fig. 2D), and Fisher’s exact test (Fig. 3A). Significance is indicated as p ≤ 0.05 (*), p ≤ 0.01 (**), p ≤ 0.001 (***), and p >0.05not significant (ns).
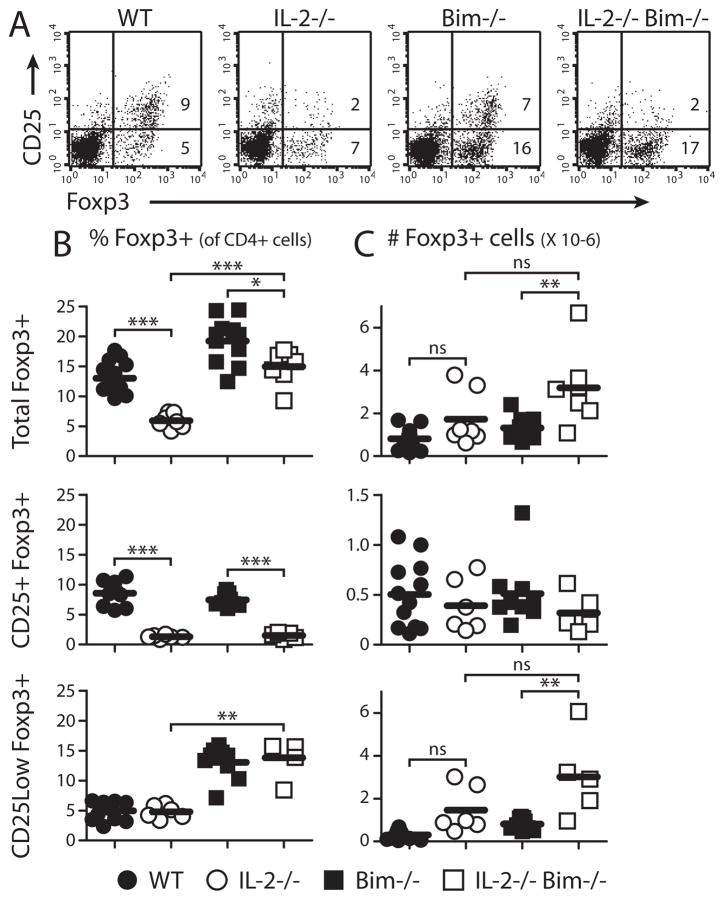
Figure 1. Bim deficiency restores peripheral Foxp3+ cells in IL-2-deficient mice.
Peripheral lymph node cells were stained and analyzed by flow cytometry. (A) Representative CD25 and Foxp3 expression in CD4+ T cells. (B) Percentages and (C) numbers of CD4+ T cells expressing Foxp3, with and without CD25, in 2–4 wk old mice. Individual mice and means are shown.
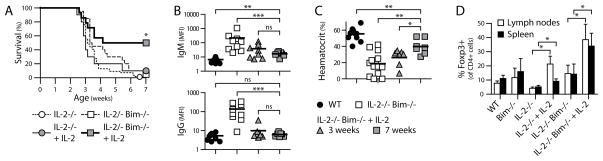
Figure 3. Treatment with IL-2/anti-IL-2-antibody complexes rescues IL-2−/− Bim−/− mice and expands Tregs.
IL-2−/− and IL-2−/− Bim−/− mice were treated with IL-2-Ab complexes beginning at 12–18 d of age, at the onset of detectable anti-erythrocyte Ab. Treatment continued for 7 wk or until mice became moribund from anemia. (A) Survival of treated IL-2−/− or IL-2−/− Bim−/− mice, compared to the untreated controls from Fig. 2A. Treated IL-2−/−, N=10; treated IL-2−/− Bim−/−, N=14. (B) Anti-erythrocyte Ab and (C) hematocrit measurements of surviving treated IL-2−/− Bim−/− mice at 3 or ≥7 wk of age, compared to age-matched controls from Fig. 2B, C. Individual mice and means are shown in (B) and (C). (D) The percentages of Foxp3+ cells were determined by flow cytometry 5–8 d after start of treatment with IL-2/Ab complexes and compared to age-matched controls. Means and SD are shown.
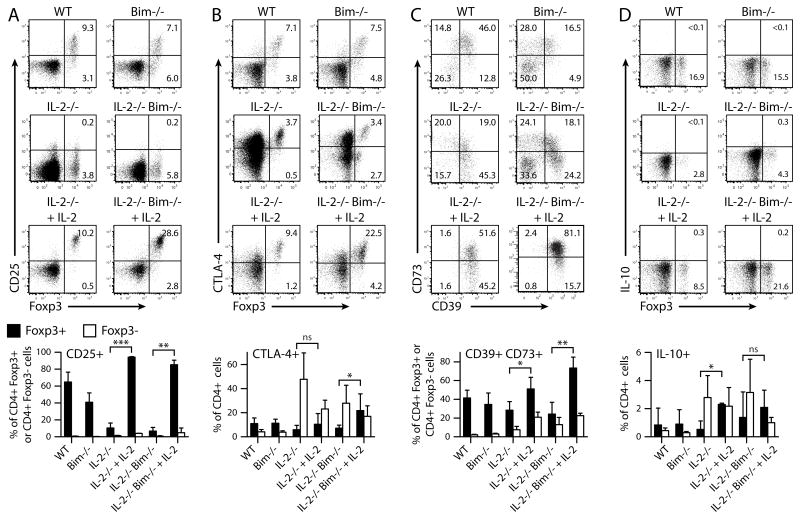
Figure 4. Treatment with IL-2/anti-IL-2-antibody complexes increases the expression of putative suppressive molecules in Tregs.
Splenocytes from Fig. 3D were analyzed by flow cytometry for expression of molecules known to participate in Treg-mediated suppression: (A) CD25, (B) CTLA-4, detected in permeabilized cells, (C) CD39 and CD73, gated on CD4+ Foxp3+ cells, and (D) IL-10, after 4h restimulation with PMA and Ionomycin. Representative dot plots, gated on CD4+ cells except in (C), show the percentages of cells in each quadrant. Bar graphs present means and SD of expression of these molecules in Foxp3+ and Foxp3- cells.
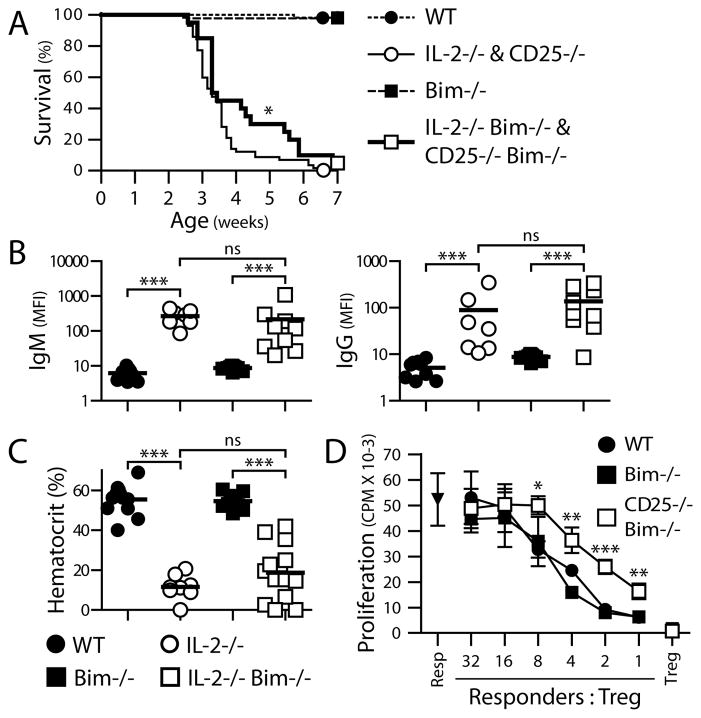
Figure 2. Rescued Foxp3+ T cells fail to protect IL-2−/− Bim−/− and CD25−/− Bim−/− mice from autoimmune disease and are less suppressive in culture.
(A) Survival of the indicated strains was monitored from weaning to 7 wk of age. WT, N=53; IL-2−/− and CD25−/−, N=57; Bim−/−, N=42; IL-2−/− Bim−/− and CD25−/− Bim−/−, N=20. (B) Anti-erythrocyte Ab titers in mice > 3 wk of age. Erythrocytes were stained with anti-IgM or anti-IgG secondary Ab, to detect bound endogenous Ab, and analyzed by flow cytometry. Anti-erythrocyte Ab levels were determined by the mean fluorescence intensity (MFI) of secondary Ab staining. (C) Anemia was tested with hematocrit readings of blood from mice > 4 wk of age. Individual mice and means are shown in (B) and (C). (D) CD25−/− Bim−/− and control mice were bred to express a Foxp3/GFP fusion reporter. Peripheral CD4+ GFP+ (Foxp3+) T cells of each genotype were purified with matching GFP levels by high speed cell sorting. In vitro suppression was compared by titrating the number of Foxp3+ T cells per well in a co-culture assay and measuring the proliferation of naïve CD4+ Foxp3- responder T cells in response to anti-CD3 Ab.
Resp: Foxp3- cells cultured without Tregs. Treg: Foxp3+ cells cultured without responder T cells. Means and SD from 1 of 3 experiments with similar results are shown.
RESULTS AND DISCUSSION
Deletion of Bim rescues Foxp3+ cells in IL-2−/− and CD25−/− mice
Mice lacking IL-2 or CD25 have a >2-fold reduction in the % of Foxp3+ CD4 T cellsin peripheral lymphoid organs (Fig. 1) (11, 12). The total number of CD4+ Foxp3+ T cells in IL-2−/− and CD25−/− mice did not decline, or even increased, but this measurement was confounded by the markedly expanded size of the lymphoid organs even in young animals. One reason for this Treg deficiency may be that IL-2 acts as a survival factor and its absence leads to apoptotic death of IL-2–dependent cells. The principal sensor of growth factor deprivation in most cell types is Bim, a “BH3-only” protein that triggers apoptosis by the mitochondrial (or intrinsic) pathway (13). To ask if ablating this death pathway would rescue Foxp3+ cells, we crossed IL-2−/− and CD25−/− mice with Bim−/− mice. In double-knockout mice lacking IL-2 or CD25 and Bim there was a significant increase in the proportion of Foxp3+ cells (Fig. 1). Interestingly, many of these cells expressed low levels of CD25, supporting the idea that IL-2 powerfully stimulates expression of its own receptor (2). It is also interesting that Bim deletion itself increased the percentage of peripheral and thymic Foxp3+ cells (Fig. S1). IL-2 participates in but is not required for thymic Treg development (11, 12, 14), while Bim deficiency may permit more self-reactive thymocytes to escape negative selection (8, 13) and expand the pool of T cells from which Tregs can develop.
Deletion of Bim fails to prevent autoimmune disease in IL-2−/− and CD25−/− mice
The critical question we were interested in is whether or not Foxp3+ cells that survived in the absence of IL-2 signals were functional. The most stringent test for the functional competence of Tregs is their ability to prevent autoimmunity. BALB/c IL-2−/− and CD25−/− mice develop severe autoimmune hemolytic anemia and >80% die by 4 wk after birth (10, 15). IL-2−/− Bim−/− and CD25−/− Bim−/− mice showed the same disease onset and ultimate lethality as IL-2 and CD25 single-knockout mice (Fig. 2A). The percentage of Foxp3+ cells did not decline with age and only weakly correlated with anemia (data not shown), consistent with the idea that simply restoring Treg numbers is not adequate for preventing autoimmunity. Moreover, IL-2−/− and IL-2−/− Bim−/− mice developed the same titers of anti-erythrocyte Abs and fall in hematocrits (Fig. 2B, 2C). Bim likely alters the number and TCR repertoire, and perhaps activation (16), of non-Tregs but, critically, such effects did not change the course of this disease.
Besides survival, IL-2 has been proposed to maintain Tregs by promoting the expression of genes involved in metabolism and cell cycle gene control (12). However, we observed the same fraction of Foxp3+ cells dividing in WT and IL-2−/− mice (Fig. S2). Since restoring the quantity of Foxp3+ cells fails to prevent the autoimmune disease caused by the complete absence of IL-2 signaling, we examined Treg function. We crossed CD25−/− mice with Foxp3/GFP reporter mice (9), purified Foxp3/GFP+ cells, and assessed their suppressive activity by co-culture with Foxp3- responder CD4 T cells. We chose to use CD25−/− Foxp3+ cells rather than IL-2−/− cells because any defects of the latter might be corrected by IL-2 secreted by responder cells in the co-cultures. The Foxp3+ cells from the CD25−/− mice were 2 to 4-fold less suppressive than wild-type Tregs (Fig. 2D). It is interesting that this modest defect of suppressive function in vitro corresponds to a complete inability to prevent autoimmunity in vivo. Similar discrepancies between the results of suppression assays using cell cultures versus in vivo outcomes have been reported in other experimental systems (17, 18).
Treatment with IL-2/anti-IL-2 antibody complexes ameliorates autoimmune disease in IL-2−/− Bim−/− mice
To further examine the effect of IL-2 on Tregs, we asked if the autoimmune disease of IL-2−/− mice could be prevented by treatment with IL-2/anti-IL-2 Ab complexes beginning as early as 12 d after birth. We selected clone JES6-1A12 as the anti-IL-2 Ab, since it induces strong Treg proliferation with little effect on CD4 and CD8 effector/memory T cells (6). This treatment failed to prevent lethality of IL-2−/− mice (Fig. 3A) despite being sufficient to induce CD25 on ~90% of Tregs (Fig. 4A). Other investigators have made similar observations (O. Boyman, personal communication). In contrast, treating IL-2−/− Bim−/− mice rescued 50% of the animals, with reversal of autoantibody production and hemolytic anemia and an increase in the proportion of Foxp3+ T cells (Fig. 3). From 7 successfully treated mice, 3 were anemic at the start of treatment, demonstrating that IL-2/Ab complexes not only prevent, but also reverse, a progressing autoimmune disease. These results indicate that the surviving Tregs in IL-2−/− Bim−/− double-knockout mice can be made functionally competent by exposure to IL-2.
Effects of IL-2 on Foxp3+ cells
The ability to rescue Foxp3+ cells from apoptosis in the absence of IL-2 allows us to examine how IL-2 affects Tregs independently of their survival. To address this question, we analyzed the Foxp3+ and Foxp3- populations in the lymphoid organs of 2–3 wk old IL-2−/− and IL2−/−Bim−/− mice treated with IL-2–Ab complexes. Treatment markedly increased the proportion and numbers of Foxp3+ cells, with little effect on the level of Foxp3 expression, in both the single- and double-knockout mice (Fig. 3, 4). The increase in Foxp3+ cells was greater when apoptosis was also prevented, i.e. in IL-2−/− Bim−/− double-knockout, compared to the IL-2 single-knockout, mice.
We were also interested in which suppressive mechanisms of Tregs might be enhanced by IL-2-Ab treatment. The suppressive activity of Foxp3+ Tregs has been attributed to many, potentially cooperative, mechanisms (17, 19), including the B7-binding receptor CTLA-4 (20), the adenosine-producing enzyme combination of CD39 and CD73 (21, 22), and the immune-modulating cytokine IL-10 (23). Consumption of IL-2 by Tregs with high CD25 expression may be a mechanism of suppression (24), but it obviously cannot account for suppressing an autoimmune response that develops in the absence of IL-2. IL-2-Ab treatment strikingly increased the proportion of CTLA-4-expressing Foxp3+ cells, especially in the IL-2−/− Bim−/− mice (Fig. 4B). It has been suggested that CTLA-4 antagonizes B7 on antigen-presenting cells and thus inhibits immune responses. CD39/CD73 co-expression also increased after treatment, especially on Foxp3+ cells in IL-2−/− Bim−/− mice (Fig. 4C). By catabolizing extracellular ATP to adenosine, CD39 and CD73 can convert a pro-inflammatory trigger to a signal inhibiting T cell responses (21, 22). In contrast to a prior study with IL-2-Ab therapy (7), we observed little effect on IL-10 production by either Foxp3+ or Foxp3- cells (Fig. 4D).
Qualitatively, the effects of IL-2-Ab treatment are similar on Foxp3+ cells from IL-2−/− and IL-2−/− Bim−/− mice. The greater efficacy of this treatment in the double-knockout mice is likely because more Foxp3+ cells are available at the start of treatment to respond to IL-2. This suggests that quantitative thresholds of Foxp3+ cells may determine the effectiveness of IL-2 therapy in inflammatory diseases.
The study described here has, for the first time, uncoupled IL-2-dependent survival and suppressive activity in Foxp3+ Tregs. Our experiments formally demonstrate that IL-2 serves multiple roles in the maintenance of functional Tregs. The absence of IL-2 signals reduces the proportion of Foxp3+ cells and this population can be restored, at least partially, by blocking apoptosis. In addition, IL-2 is critical for the normal function of Tregs. It maintains the functional competence of these cells by optimizing expression of CTLA-4, a key mediator of Treg suppression. It also enhances CD39 and CD73 levels, which may concurrently reduce inflammation and inhibit effector T cells. Since multiple pathways may be essential but not sufficient for fully functional Tregs (17, 19, 20, 23), the seemingly pleiotropic effects of IL-2 could boost immune regulation in a range of situations. Therapeutic strategies relying on the administration of IL-2, especially in the form of immune complexes, should consider the numbers of available Tregs as well as the actions of the cytokine on the activities of these cells.
Acknowledgments
The authors are grateful to Shuwei Jiang for expert cell sorting and Carlos Benitez for mouse husbandry.
GRANT SUPPORT
Supported by NIH grants RO1 AI073656 and PO1 AI35297 (AKA)
ABBREVIATIONS
- Tregs
regulatory T cells
References
- 1.Hoyer KK, Dooms H, Barron L, Abbas AK. Interleukin-2 in the development and control of inflammatory disease. Immunol Rev. 2008;226:19–28. doi: 10.1111/j.1600-065X.2008.00697.x. [DOI] [PubMed] [Google Scholar]
- 2.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–479. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 3.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 4.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 5.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 6.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 7.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Hoyer KK, Wolslegel K, Dooms H, Abbas AK. Targeting T cell-specific costimulators and growth factors in a model of autoimmune hemolytic anemia. J Immunol. 2007;179:2844–2850. doi: 10.4049/jimmunol.179.5.2844. [DOI] [PubMed] [Google Scholar]
- 11.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 13.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 14.Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadlack B, Lohler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle RJ, Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 16.Ludwinski MW, Sun J, Hilliard B, Gong S, Xue F, Carmody RJ, DeVirgiliis J, Chen YH. Critical roles of Bim in T cell activation and T cell-mediated autoimmune inflammation in mice. J Clin Invest. 2009;119:1706–1713. doi: 10.1172/JCI37619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 21.Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5′ adenosine monophosphate to adenosine. J Immunol. 2006;177:6780–6786. doi: 10.4049/jimmunol.177.10.6780. [DOI] [PubMed] [Google Scholar]
- 22.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]