Abstract
Background
In retrospective studies, loss of p27Kip1 (p27), a cyclin dependent kinase inhibitor, has been associated with poor prognosis following colorectal cancer treatment. In a prospective study, we validated this relationship in patients enrolled on a trial of adjuvant chemotherapy for Stage III colon cancer.
Methods
Cancer and Leukemia Group B (CALGB) protocol 89803 randomized 1264 stage III colon cancer patients to receive weekly bolus fluorouracil/leucovorin (5FU/LV) or weekly bolus irinotecan, fluorouracil, and leucovorin (IFL). The primary endpoint was overall survival (OS); disease-free survival (DFS) was a secondary endpoint. Expression of p27 and DNA mismatch repair (MMR) proteins were determined by immunohistochemistry (IHC) in primary tumor and normal tissue from paraffin blocks. Data were analyzed using logrank test.
Results
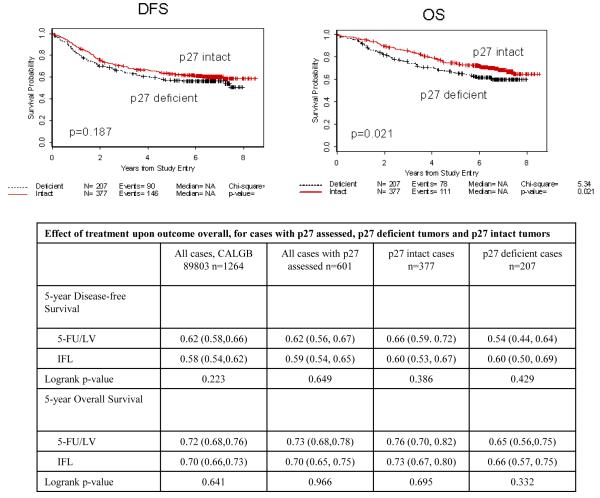
Of 601 tumors analyzed, 207 (34.4%) demonstrated p27 loss, 377 (62.8%) retained p27, and 17 (2.8%) were indeterminate. Patients with p27 negative tumors showed reduced OS (5-year 66%; 95%CI 0.59-0.72 vs. 75%; 95%CI 0.70-0.79, logrank p=0.021). This relationship was not influenced by treatment arm. Combination of p27 status with MMR status, however, identified a small subset of patients that may benefit from IFL (n=36; 5-year DFS 81%; 95%CI 0.64-0.98 vs. 47%; 95%CI 0.21-0.72, logrank p=0.042; 5-year OS 81%; 95%CI 0.64-0.98 vs. 60%; 95%CI 0.35-0.85; logrank p=0.128).
Conclusions
Loss of p27 is associated with reduced survival in stage III colon cancer, but by itself does not indicate a significant difference in outcome between patients treated IFL or 5FU-LV.
Keywords: Colorectal cancer, adjuvant therapy, biomarkers
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States, accounting for approximately 49,000 deaths per year (1). CRC treatment depends upon disease stage. Patients with localized disease receive surgery with or without radiation therapy, whereas local treatment combined with systemic chemotherapy is indicated for patients demonstrating locally advanced tumors or tumors with locoregional metastases. In general, patients with distant metastatic disease are managed by chemotherapy alone, although an increasing body of data suggests that a subset of these patients with isolated hepatic or pulmonary metastases achieve a significant 5-year survival when the metastases are resected (2). Unfortunately, even though treatment decisions depend upon disease stage, conventional histopathological staging methods cannot accurately predict disease behavior. For example, approximately 40% of patients with stage III disease will develop recurrence following surgery and adjuvant chemotherapy. Adjuvant chemotherapy and aggressive surgery carry morbidities and should ideally only be employed for patients who can benefit from these treatments. The variation in clinical behavior found in the current CRC staging system implies that a significant proportion of patients with CRC are currently either over- or under-treated.
Originally identified in cells whose growth was arrested by TGF-α, p27 is a regulatory element of the cell cycle that suppresses the G1-S transition by inhibiting cyclin-dependent kinase activity (3-6). Reduced or absent p27 expression is found in many human malignancies, particularly those of epithelial origin (7-10). Human cancer studies showed that loss of p27 protein is rarely due to inactivation or mutation of the p27 gene (6, 11, 12). Instead, most tumors lacking p27 expression demonstrate elevated expression of ubiquitin ligase and cofactor proteins that target p27 for proteosomal degradation (13, 14).
Retrospective studies showed that p27 expression is associated with a poor prognosis in a variety of epithelial tumors, including those of colon, gastric, breast, prostate, and lung origin (7-9). In a study of 149 surgically resected colon cancers, Loda et al found a significantly improved median overall survival (OS) for patients whose tumors expressed p27 in more than 50% of the cells (241 months), compared to those with expression in less than 50% of cells (149 months) or for which p27 expression was absent (69 months) (7). Multivariate analysis showed that both disease stage and p27 expression were independent indicators of overall survival for this cohort. Other small retrospective studies confirmed this result and also found that low p27 levels are more frequent in right-sided colon cancers (15-19) and predict future development of metastatic disease (20, 21).
CALGB Protocol 89803 was a prospective randomized trial comparing adjuvant chemotherapy using 5-FU and leukovorin (FU/LV) to 5-FU, LV and irinotecan (IFL), administered for 32 and 30 weeks, respectively, following the resection of a stage III colon cancer. This trial showed no difference in either DFS or OS between the 5-FU/LV and IFL treatment arms. This trial also prospectively assessed the effect of tumor p27 status by immunohistochemistry upon DFS and OS, and determined the relationship between tumor p27 status and other markers of tumor behavior.
Materials and Methods
Characteristics of Study Population
All patients enrolled on CALGB protocol 89803, had histologically confirmed stage III colon cancer. All patients underwent definitive surgical resection, and started treatment no earlier than 21 days and no later than 56 days following resection. The primary objective was to compare OS between the two treatment arms; DFS was compared as a secondary objective. Additional secondary aims addressed the relationship between tumor-associated risk factors and treatment outcome, and required submission of paraffin blocks containing tumor and normal tissue to the CALGB Pathology Coordinating Office (PCO) at Ohio State University. This protocol was reviewed by the institutional review board of each participating center and all patients gave written informed consent prior to participation.
Trial Structure and Organization
This trial was written and coordinated by CALGB with participation by the North Central Cancer Treatment Group (NCCTG), National Cancer Institute of Canada Clinical Trials Group (NCIC-CTG), Eastern Cooperative Oncology Group (ECOG), Southwest Oncology Group (SWOG), and the NCI Cancer Trials Support Unit (CTSU). The CALGB data safety monitoring board reviewed safety data semi-annually and efficacy data at protocol specified intervals in accordance with CALGB policies and procedures.
Treatment
Eligible patients were assigned electronically (randomized fixed block) using central registration to receive either IFL or 5FU/LV. The 5FU/LV group received leucovorin 500 mg/m2 intravenously over two hours, with a bolus of 5FU 500 mg/m2 given by intravenous injection at one hour after initiation of leucovorin. Treatments were scheduled to be administered weekly for six consecutive weeks followed by a two week rest, for a total of four cycles or 32 weeks of total therapy. The IFL group received irinotecan 125 mg/m2 over 90 minutes followed immediately by an intravenous bolus injection of leucovorin 20 mg/m2 and then 5FU 500 mg m2 also by intravenous bolus injection. Treatment was scheduled to be given for four consecutive weeks followed by a two week rest for five cycles or a total of 30 weeks. Further details concerning the treatment portion of this trial have been published previously (22).
Characterization of tumor p27 status
Formalin-fixed, paraffin-embedded specimens from 601 patients out of a total of 1264 enrolled on CALGB protocol 89803 were studied. Central pathology review confirmed the histology of each specimen, and blocks were sectioned at 4μm. Laboratory analysis of tissue specimens was performed without knowledge of the patient’s clinical outcome or treatment regimen.
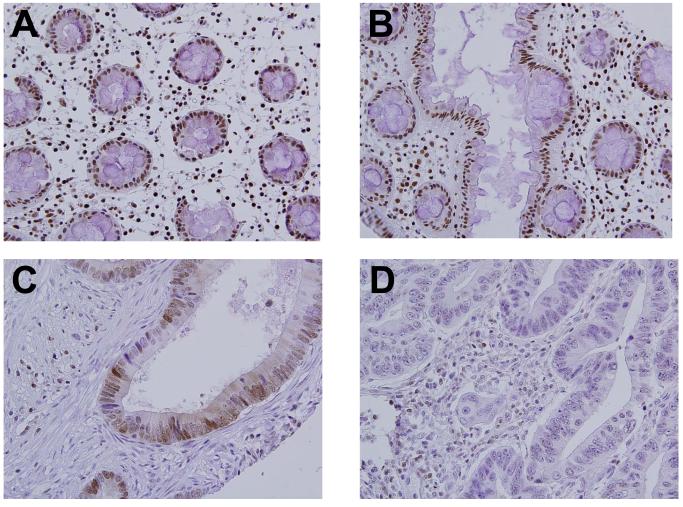
Immunohistochemistry (IHC) was used to detect the presence of p27, MLH1 and MSH2 proteins in primary tumor specimens, using methods described in previous reports (15, 19, 23). Positive controls were provided by examining staining of normal colonic mucosa from each case; tumors known to lack p27, MLH1 or MSH2 were stained concurrently and served as negative controls. A number of different scoring systems have been used for p27 studies (7, 16, 19, 24). In this report, we scored the tumors using a modification of our previous methods that we believe provides best reproducibility, and yields the same outcome result as that using our previous scoring method (data not shown) (7). Nuclear expression of p27 was evaluated in a total of 10 randomly-selected high powered fields per tumor. A tumor cell was counted as p27 positive when its nuclear reaction was equal to or stronger than the reaction in surrounding lymphocytes, which were used as an internal control. All cases were scored as either positive (≥ 10% of tumor cells with strong nuclear staining), negative (<10% of tumor cells with strong nuclear staining), or non-informative (Figure 1). Cases designated as mismatch repair deficient (MMR-D) were those with a negative IHC score for either MLH1 or MSH2, and cases designated mismatch repair intact (MMR-I) retained expression of both proteins. A single study pathologist (E.M.) scored all p27 immunostains. For MLH1 and MSH2, each case was scored by two independent reviewers (CCC, HPH). In cases of disagreement, a third reviewer (MR) examined the case to provide the final score.
Figure 1.
Variable p27 expression in stage III colon cancer.
A,B: Normal controls, human colon;
C: Adenocarcinoma, colon p27 positive;
D: Adenocarcinoma, colon, p27 negative
Statistical methods
The goal of this correlative study was to determine whether tumor p27 status predicted treatment outcome for patients with stage III colon cancer. The primary endpoint of this study was OS measured from entry onto the clinical trial until death from any cause. DFS was also considered as a study endpoint. A secondary goal was to determine the relationship between tumor p27 status and MMR status, as determined by IHC. The logrank test was used to make survival comparisons among categories defined by MMR status and within treatment and MMR subgroups. The proportional hazards model was used to make survival comparisons controlling for treatment and other clinico-pathologic factors. The Kaplan-Meier method was used to estimate the DFS and OS curves and 5-year survival probabilities. Data were analyzed with continued follow-up for DFS and OS as of March 10, 2008. All statistical analyses were performed by CALGB statisticians.
Results
Clinical and pathological differences between p27 intact and p27 deficient tumors
We examined the relationship between p27 status and other variables associated with tumor behavior (Table 1). Tumors were available for analysis for 601 of a total of 1283 patients randomized to CALGB 89803 . There was no difference between treatment outcome in either experimental arm when patients with available tumors were compared to the entire study cohort (data not shown). Of 601 tumors analyzed, 207 (34.4%) demonstrated loss of p27 expression, 377 (62.8%) retained p27 expression, and 17 (2.8%) were indeterminate. Compared to those with intact p27 by IHC, p27 negative tumors were more likely to be proximal (68.6% vs. 50.9%, p<0.0001). Tumors lacking p27 were also more likely to be poorly differentiated (grade III/IV in 33.3% vs. 18.5%, p<0.0001). The nodal ratio, i.e. the number of positive nodes divided by the total number of nodes examined, was higher for p27 deficient cases (0.33 vs. 0.28, p=0.017) (Table 1). There were no differences between p27 deficient and p27 intact tumors in the proportion of male patients, patient age, or the number of positive nodes per case.
Table 1.
Characteristics of the study cohort by p27 status
| CALGB 89803 overall |
Cases with p27 data |
p27 intact cases | p27 deficient cases |
P-value (intact vs deficient) |
|
|---|---|---|---|---|---|
| Age- mean (range) | 59.8 (21-85) | 59.8 (24-85) | 59.4 (24-83) | 60.6 (26-85) | 0.1833 |
| N= | 1264 | 601 | 377 | 207 | |
|
| |||||
| Male gender – no. (%) | 692 (55.5) | 326 (55.9) | 206 (54.6) | 120 (58.0) | 0.4384 |
| N= | 1264 | 601 | 377 | 207 | |
|
| |||||
| Number of positive nodes – mean (SD) |
3.6 (3.4) | 3.6 (3.4) | 3.4 (3.2) | 3.9 (3.7) | 0.1255 |
| N= | 580 | 374 | 206 | ||
|
| |||||
| Node ratio – mean (SD) |
0.30 (0.25) | 0.30 (0.25) | 0.28 (0.23) | 0.33 (0.28) | 0.0170 |
| N= | 1235 | 579 | 373 | 206 | |
|
| |||||
| Tumor location – no. (%) |
<0.0001 | ||||
| Proximal | 713(56.4) | 346 (57.6) | 192 (50.9) | 142 (68.6) | |
| Distal | 521(41.2) | 249 (41.4) | 180 (47.8) | 64 (31.0) | |
| Unknown | 30(2.4) | 6 (1.0) | 5 (1.3) | 1 (0.5) | |
|
| |||||
| Tumor differentiation – no. (%) |
<0.0001 | ||||
| Well/moderate | 931 (73.66) | 455 (75.71) | 305 (80.90) | 137 (66.18) | |
| Poor | 304 (24.05) | 142 (23.63) | 69 (18.30) | 69 (33.33) | |
| Unknown | 29 (2.29) | 4 (0.67) | 3 (0.80) | 1 (0.48) | |
Loss of p27 is associated with reduced OS and DFS in stage III colon cancer
Comparisons of 5-year DFS and OS for p27 deficient and p27 intact cases are shown in Table 2 and Figure 2. The median duration of follow-up was 6.58 years. Patients enrolled on CALGB 89803 whose tumors were deficient in p27 had a 5-year OS that was 12.0% less in relative terms than those with intact p27 (logrank p=0.021). This same trend was observed for DFS, although the difference was not significant when assessed by the logrank test (9.7% relative decrease at 5 years, p=0.187).
Table 2.
Prognostic value of tumor p27 status
| All cases, CALGB 89803 n=1264 |
All cases with p27 assessed n=601 |
p27 intact cases n=377 |
p27 deficient cases n=207 |
logrank p | |
|---|---|---|---|---|---|
| 5-year Disease-free Survival - % (95% CI) | |||||
| 0.60 (0.58,0.63) |
0.61 (0.57,0.64) |
0.63 (0.58,0.68) |
0.57 (0.50,0.64) |
0.187 | |
|
| |||||
| 5-year Overall Survival - % (95% CI) | |||||
| 0.72 (0.68,0.74) |
0.71 (0.68,0.75) |
0.75 (0.70,0.79) |
0.66 (0.59,0.72) |
0.021 | |
|
| |||||
| Prognostic value of tumor MMR status | |||||
|
| |||||
| All cases with MMR assessed n=783 |
MMR-I cases n=677 |
MMR-D cases n=106 |
logrank p | ||
| 5-year Disease-free Survival - % (95% CI) | |||||
| 0.61 (0.58,0.65) |
0.60 (0.56,0.64) |
0.67 (0.58,0.76) |
0.180 | ||
|
| |||||
| 5-year Overall Survival - % (95% CI) | |||||
| 0.72 (0.68,0.75) |
0.72 (0.68,0.75) |
0.73 (0.64,0.81) |
0.470 | ||
Figure 2.
Kaplan-Meier plots for DFS and OS comparing patients with p27 intact tumors to those with p27 deficient tumors.
Addition of irinotecan to 5-FU + LV does not improve treatment outcome for patients with p27 deficient tumors
For the 1264 patients treated on CALGB protocol 89803, addition of irinotecan to 5-FU/LV did not improve 5-year DFS or OS (22). We examined the impact of tumor p27 status upon treatment outcome for either 5FU/LV or IFL. The results are presented in Table 3. This analysis showed that p27 intact and p27 deficient tumors responded similarly to adjuvant treatment with 5-FU/LV and IFL.
Table 3A.
Relationship between tumor p27 and MMR status
| All cases with p27 and MMR assessed |
p27 intact cases | p27 deficient cases, |
p= | |
|---|---|---|---|---|
| MMR-D – n (%) | 74 (12.6) | 34 (9.1) | 36 (18.1) | 0.0017 |
| MMR-I – n (%) | 515 (87.4) | 341 (90.9) | 163 (81.9) | |
| Total | 589 | 375 | 199 |
p27 status may help predict OS
In a multivariate analysis of p27 status (intact; deficient), treatment arm (IFL; 5FU/LV), age (years, continuous), gender (male; female), tumor location (distal; proximal), tumor differentiation (grades I/II; grades III/IV), tumor invasion (T1/T2; T3/T4), extracellular mucin (≤ 50%; > 50%), and nodal ratio (number of positive nodes versus number of nodes sampled), only nodal ratio (logrank p<0.0001; HR=3.91) significantly predicted DFS. When predicting OS, nodal ratio (logrank p<0.0001; HR=4.38) and p27 status (logrank p=0.0448; HR=0.742) remained significant at α=0.05.
Relationship between tumor p27 status and microsatellite instability
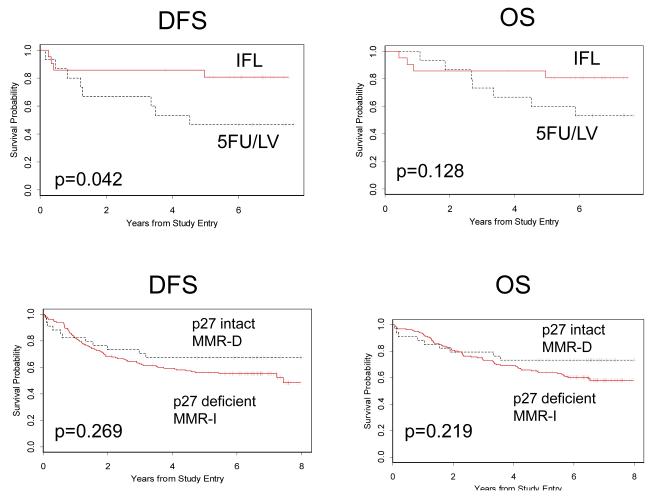
We determined tumor MMR status using IHC to identify loss of expression of either of two mismatch repair proteins, MLH1 or MSH2. We found no difference in outcome for MMR-D compared to MMR-I tumors (Table 2). In additional studies of this same cohort, we compared tumor IHC and genotyping using Bethesda criteria microsatellite loci (25). This analysis showed that these two methods of identifying mismatch repair deficiency were equivalent for predicting treatment outcome. In this study, we chose MLH1 and MSH2 IHC for study in combination with p27 IHC because results obtained with this common method could be readily applied to clinical practice. Like the p27 deficient tumors examined here, colon cancers that exhibited MMR-D were more likely to be proximally located (91.5% vs 51.3%, p<0.0001) and poorly differentiated (49.0% vs 21.0%, p<0.0001) than those that were MMR-I. Also like p27 deficient tumors, MMR-D cases had a lower nodal ratio than MMR-I cases (0.18 vs. 0.23, p=0.005). MMR status was determined by IHC on 589 tumors for which p27 status was known. The results are shown in Table 3. Tumors deficient in p27 were more likely to be MMR-D than tumors with intact p27 (p=0.0017). Tumors that were MMR-D and p27 intact had the best outcome, with a 19.6% increase in DFS at 5 years (67%; 95% CI 0.52, 0.83 vs 56%; 95% CI 0.48, 0.64, logrank p=0.268), and a 14.1% increase in OS at 5 years (73%; 95% CI 0.58, 0.88 vs 64%; 95% CI 0.56,0.71, logrank p=0.219). This is in comparison to the category with the worst outcome, which included patients with p27 deficient, MMR-I tumors (Table 3 and Figure 3).
Figure 3.
Top: Kaplan-Meier plots for DFS and OS for patients with p27 deficient, MMR-D tumors comparing those treated with IFL to those treated with 5FU/LV.
Bottom: Kaplan-Meier plots for DFS and OS comparing patients with p27 intact, MMR-D tumors to those with p27 deficient, MMR-I tumors.
In a previous analysis of this cohort, we showed that patients with MMR-D tumors experienced similar treatment outcome when compared to those whose tumors were MMR-I (25). However, this same prospective study found that patients with MMR-D tumors may be more likely to benefit from IFL. To explore the relationship between the predictive value of tumor MMR determination and the prognostic value of tumor p27 status, we examined treatment outcome for cases from CALGB 89803 for which both p27 and MMR status were known (Table 3). This analysis identified the p27 deficient, MMR-D subset as one that may benefit from the irinotecan-containing regimen, with 72% better 5-year DFS (logrank p=0.042), and 35% better 5-year OS (logrank p=0.128) for the IFL arm (Figure 3).
Discussion
Our results indicate that tumor p27 status is a prognostic factor for patients receiving adjuvant chemotherapy following surgery for stage III colon cancer, as patients with p27 deficient tumors treated with either FU/LV or IFL had a 5-year OS that was 12% less than those with p27 intact tumors. When the interaction between p27 status and treatment was examined, we found that loss of p27 did not predict improved response to the irinotecan-containing regimen used in this study. As a result, tumor p27 status, taken alone, is not currently useful in guiding choice of adjuvant chemotherapy. However, low levels of p27 in colorectal cancers result from enhanced ubiquitin-dependent degradation of this tumor suppressor, and recent studies identified the ubiquitin ligase, Pirh2, as a major effector of p27 degradation. These studies confirmed an inverse relationship between p27 and Pirh2 expression in colorectal cancer (26), suggesting that selective inhibition of Pirh2 would be beneficial in treating patients with p27 deficient tumors.
In an analysis from the Nurses Health Study and the Health Professional Follow-up Study, Ogino, et. al. examined the relationship between tumor MSI and p27 status for 634 cases of colorectal cancer that developed during prospective follow-up (27). Using a definition of p27 status similar to that employed here, they found that 36.4% of cancers were p27 deficient, and 16.6% exhibited MSI-H (27). MMR status in this study was determined by tumor genotyping to identify high levels of microsatellite instability (MSI-H), rather than IHC. These investigators found that p27 deficient tumors were more likely to be MSI-H (26.0% p27 deficient/MSI-H, 11.2% p27 deficient/MSS or MSI-L, p<0.001). Our results are consistent with this, as 19.2% of the p27 deficient cases were MMR-D, compared to 9.1% of those with intact p27.
A study of the relationship between treatment outcome and tumor MMR status for this same cohort suggested that MMR-D status predicted improved IFL response, although MMR status was not a significant prognostic factor overall (25). We therefore performed an exploratory analysis combining a prognostic factor (tumor p27 status) with a predictive factor (tumor MMR status). The p27 deficient tumors in this series showed some similarities to those that were MMR-D, as these categories were both associated with proximal location, poorly differentiated histology, and higher nodal ratio. Despite these similar clinicopathologic characteristics, p27 deficient and MMR-D tumors show distinct clinical behavior. While patients with p27 deficient tumors have a worse outcome, those with MMR-D tumors generally have a better prognosis, or at least do not demonstrate the aggressive behavior typical of poorly differentiated cancers (28, 29). In this trial, both treatment arms contained 5FU+LV, and we found no difference in outcome for the MMR-D and MMR-I cases, although there was a trend toward improved 5-year DFS in those with MMR-D tumors (0.67 vs 0.60, p=0.18). In addition, we found that only 18% of tumors that were p27 deficient were also MMR-D. Given these results, we predicted that the best outcome would be observed for patients whose tumors were p27 intact and MMR-D, and the worst outcome for those whose tumors were p27 deficient and MMR-I. This was what we observed for the DFS analysis, although the 19.6% difference in DFS between the categories with the best outcome (p27 intact, MMR-D) and worst outcome (p27 deficient, MMR-I) was not statistically significant (logrank p-value for this comparison = 0.219).
This trial allowed us to explore whether a combination of tumor p27 and MMR status predicted better response to IFL. Although the sample size is small, patients whose tumors were both p27 deficient and MMR-D had a DFS that was improved by 72% (logrank p=0.042) and OS that was improved by 35% (logrank p=0.128) when they received the regimen containing irinotecan. This relationship was not observed in tumors that were p27 intact/MMR-D, p27 intact/MMR-I, or p27 deficient/MMR-I. This subset analysis of a small number of samples in the p27 deficient, MMR-D category (N=36) must be interpreted with caution, however several factors make these data intriguing. First, this study was a pre-specified prospective analysis of both MMR and p27 as markers of disease response and treatment outcome, and is therefore free of the bias inherent in retrospective tumor bank studies. Similarities in marker frequency and tumor location between p27 deficient and MMR-D tumors suggest a biological relationship between these factors. It is possible that this yet unidentified relationship is responsible for enhanced tumor sensitivity to irinotecan.
These results provide important insight into the ways in which prognostic and predictive factors can interact, potentially leading to inaccuracy when single factors are used to predict disease behavior. Tumors that are p27 deficient are less likely to respond to adjuvant chemotherapy, an effect that is largely independent of treatment. However, tumors that are p27 deficient are also somewhat more likely to exhibit MMR-D, a condition that appears to convey a differential response to chemotherapy in this colon cancer adjuvant treatment trial. This study, therefore, illustrates the importance of testing prognostic and predictive factors in large randomized trials where the relationships between treatment and multiple tumor-specific variables can be assessed.
Statement of Translational Relevance.
The current AJCC staging system for colon cancer is insufficient, as evidenced by a wide variation in adjuvant treatment outcome among patients with stage III disease. In this prospective study of adjuvant chemotherapy following surgery for stage III colon cancer, we showed that loss of tumor p27 expression is associated with post-treatment disease recurrence. This prognostic result allows clinicians to subdivide stage III colon cancer into high- and low-risk groups, and identifies p27 signaling pathway as a potential target for new agent development.
Table 3B.
Prognostic and predictive values of combined p27; MMR variable
| n= | 5-year Disease-free Survival |
5-year Overall Survival | |
|---|---|---|---|
| Prognostic | |||
| p27intact/MMR-D | 34 | 0.67 (0.52, 0.83) | 0.73 (0.58, 0.88) |
| p27 intact/MMR-I | 341 | 0.62 (0.57, 0.68) | 0.75 (0.70, 0.80) |
| p27 deficient/MMR-D | 36 | 0.66 (0.51, 0.82) | 0.72 (0.57, 0.87) |
| p27 deficient/MMR-I | 163 | 0.56 (0.48, 0.64) | 0.64 (0.56, 0.71) |
| Logrank p | 0.347 | 0.079 | |
|
| |||
| Predictive | |||
| p27intact/MMR-D | 34 | ||
| 5-FU/LV | 18 | 0.61 (0.38,0.83) | 0.72 (0.51,0.93) |
| IFL | 16 | 0.75 (0.54, 0.96) | 0.75 (0.54, 0.96) |
| Logrank p | 0.358 | 0.856 | |
|
| |||
| p27 intact/MMR-I | 341 | ||
| 5-FU/LV | 174 | 0.66 (0.59, 0.73) | 0.77 (0.70, 0.83) |
| IFL | 167 | 0.59 (0.51, 0.66) | 0.73 (0.66, 0.80) |
| Logrank p | 0.233 | 0.661 | |
|
| |||
| p27 deficient/MMR-D | 36 | ||
| 5-FU/LV | 15 | 0.47 (0.21, 0.72) | 0.60 (0.35, 0.85) |
| IFL | 21 | 0.81 (0.64, 0.98) | 0.81 (0.64, 0.98) |
| Logrank p | 0.042 | 0.128 | |
|
| |||
| p27 deficient/MMR-I | 163 | ||
| 5-FU/LV | 76 | 0.56 (0.45, 0.67) | 0.66 (0.56, 0.77) |
| IFL | 87 | 0.56 (0.46, 0.67) | 0.61 (0.51, 0.72) |
| Logrank p | 0.957 | 0.845 | |
Acknowledgments
Funding The research for Cancer and Leukemia Group B protocol 89803 was supported, in part, by Public Health Service grant CA31946 from the National Cancer Institute (NCI), National Institutes of Health, Department of Health and Human Services, to the CALGB (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NCI.
Footnotes
The following institutions participated in this study:
Baptist Cancer Institute CCOP, Memphis, TN–Lee S. Schwartzberg, M.D. [CA71323], Christiana Care Health Services, Inc. CCOP, Wilmington, DE–Stephen Grubbs, M.D. [CA45418], Dana-Farber Cancer Institute, Boston, MA–Eric P. Winer, M.D. [CA32291], Dartmouth Medical School -Norris Cotton Cancer Center, Lebanon, NH–Marc S. Ernstoff, M.D. [CA04326], Duke University Medical Center, Durham, NC–Jeffrey Crawford, M.D. [CA47577], Georgetown University Medical Center, Washington, DC–Minetta C. Liu, M.D. [CA77597], Cancer Centers of the Carolinas, Greenville, SC–Jeffrey K. Giguere, M.D [CA29165], Long Island Jewish Medical Center, Lake Success, NY–Kanti R. Rai, M.D. [CA11028], Massachusetts General Hospital, Boston, MA–Jeffrey W. Clark, M.D. [CA12449], Memorial Sloan-Kettering Cancer Center, New York, NY–Clifford A. Hudis, M.D. [CA77651], Missouri Baptist Medical Center, St. Louis, MO–Alan P. Lyss, M.D. [CA114558], Mount Sinai Medical Center, Miami, FL–Rogerio C. Lilenbaum, M.D. [CA45564], Mount Sinai School of Medicine, New York, NY–Lewis R. Silverman, M.D. [CA04457], Nevada Cancer Research Foundation CCOP, Las Vegas, NV– John A. Ellerton, M.D. [CA35421], North Shore-Long Island Jewish Medical Center, Manhasset, NY–Daniel R Budman, M.D. [CA35279], Rhode Island Hospital, Providence, RI–William Sikov, M.D. [CA08025], Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, M.D. [CA02599], Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC–James N. Atkins, M.D. [CA45808], State University of New York Upstate Medical University, Syracuse, NY–Stephen L. Graziano, M.D. [CA21060], The Ohio State University Medical Center, Columbus, OH–Clara D Bloomfield, M.D. [CA77658], University of California at San Diego, San Diego, CA–Barbara A. Parker, M.D. [CA11789], University of California at San Francisco, San Francisco, CA–Alan P. Venook, M.D. [CA60138], University of Chicago, Chicago, IL –Gini Fleming, M.D. [CA41287], University of Illinois MBCCOP, Chicago, IL–Lawrence E. Feldman, M.D. [CA74811], University of Iowa, Iowa City, IA–Daniel A. Vaena, M.D. [CA47642], University of Maryland Greenebaum Cancer Center, Baltimore, MD–Martin Edelman, M.D. [CA31983], University of Massachusetts Medical School, Worcester, MA–William V. Walsh, M.D. [CA37135], University of Minnesota, Minneapolis, MN–Bruce A Peterson, M.D. [CA16450], University of Missouri/Ellis Fischel Cancer Center, Columbia, MO–Michael C Perry, M.D. [CA12046], University of Nebraska Medical Center, Omaha, NE–Anne Kessinger, M.D. [CA77298], University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, M.D. [CA47559], University of Tennessee Memphis, Memphis, TN–Harvey B. Niell, M.D. [CA47555], University of Vermont, Burlington, VT–Hyman B. Muss, M.D. [CA77406], Wake Forest University School of Medicine, Winston-Salem, NC–David D Hurd, M.D. [CA03927], Walter Reed Army Medical Center, Washington, DC–Thomas Reid, M.D. [CA26806], Washington University School of Medicine, St. Louis, MO–Nancy Bartlett, M.D. [CA77440], Weill Medical College of Cornell University, New York, NY–John Leonard, M.D. [CA07968]
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Miller G, Biernacki P, Kemeny NE, et al. Outcomes after resection of synchronous or metachronous hepatic and pulmonary colorectal metastases. J Am Coll Surg. 2007;205:231–8. doi: 10.1016/j.jamcollsurg.2007.04.039. [DOI] [PubMed] [Google Scholar]
- 3.Hershko DD, Shapira M. Prognostic role of p27Kip1 deregulation in colorectal cancer. Cancer. 2006;107:668–75. doi: 10.1002/cncr.22073. [DOI] [PubMed] [Google Scholar]
- 4.Ponce-Castandea MV, Lee MH, Latres E, et al. p27kip1: chromosomal mapping to 12p12-12p13.1 and absence of mutations in human tumors. Cancer Res. 1998;58:542–8. [PubMed] [Google Scholar]
- 5.Slingerland JM, Hengst L, Pan CH, Alexander D, Stampfer MR, Reed SI. A novel inhibitor of cyclin-Cdk activity detected in transforming growth factor beta-arrested epithelial cells. Mol Cell Biol. 1994;14:3683–94. doi: 10.1128/mcb.14.6.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polyak K, Lee MH, Erdjument-Bromage H, et al. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59–66. doi: 10.1016/0092-8674(94)90572-x. [DOI] [PubMed] [Google Scholar]
- 7.Loda M, Cukor B, Tam SW, et al. Increased proteosome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231–4. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 8.Porter PL, Malone KE, Heagerty PJ, et al. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;3:222–5. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 9.Esposito V, Baldi A, De Luca A, et al. Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res. 1997;57:3381–5. [PubMed] [Google Scholar]
- 10.Tsihlias J, Kapusta LR, DeBoer G, et al. Loss of cyclin-dependent kinase inhibitor p27Kip1 is a novel prognostic factor in localized human prostate adenocarcinoma. Cancer Res. 1998;58:542–8. [PubMed] [Google Scholar]
- 11.Kawamata N, Morosetti R, Miller CW, et al. Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res. 1995;55:2266–9. [PubMed] [Google Scholar]
- 12.Fernando AA, Balbin M, Pendas AM, Vizoso F, Velasco G, Lopez-Otin C. Mutational analysis of the human cyclin-dependent kinase inhibitor p27Kip1 in primary breast carcinomas. Hum Genet. 1996;97:91–4. doi: 10.1007/BF00218840. [DOI] [PubMed] [Google Scholar]
- 13.Hershko D, Bornstein G, Ben-Izhak O, et al. Inverse relation between levels of p27(Kip1) and of its ubiquitin ligase subunit Skp2 in colorectal carcinomas. Cancer. 2001;91:1745–51. doi: 10.1002/1097-0142(20010501)91:9<1745::aid-cncr1193>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Shapira M, Ben-Izhak O, Bishara B, et al. Alterations in the expression of the cell cycle regulatory protein cyclin kinase subunit 1 in colorectal carcinoma. Cancer. 2004;100:1615–21. doi: 10.1002/cncr.20172. [DOI] [PubMed] [Google Scholar]
- 15.Palmquist R, Stenling R, Oberg A, Landberg G. Prognostic significance of p27(Kip1) expression in colorectal cancer: a clinico-pathological characterization. J Pathol. 1999;188:18–23. doi: 10.1002/(SICI)1096-9896(199905)188:1<18::AID-PATH311>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Sun XF. Loss of p27 expression predicts poor prognosis in patients with Dukes’ B or proximal colorectal cancer. Int J Oncol. 2001;19:49–52. [PubMed] [Google Scholar]
- 17.Shapira M, Ben-Izhak O, Linn S, Futerman B, Minkov I, Hershko DD. The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer. 2005:7. doi: 10.1002/cncr.20917. [DOI] [PubMed] [Google Scholar]
- 18.Nalepa G, Harper JW. Therapeutic anti-cancer targets upstream of the proteosome. Cancer Treat Rev. 2003;29:49–57. doi: 10.1016/s0305-7372(03)00083-5. [DOI] [PubMed] [Google Scholar]
- 19.Palmqvist R, Stenling R, Oberg A, Landberg G. Prognostic significance of p27(Kip1) expression in colorectal cancer: a clinico-pathological characterization. J Pathol. 1999;188:18–23. doi: 10.1002/(SICI)1096-9896(199905)188:1<18::AID-PATH311>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Thomas GV, Szigeti K, Murphy M, Draetta GF, Pagano M, Loda M. Down-regulation of p27 is associated with development of colorectal adenocarcinoma metastases. Am J Pathol. 1998;153:681–7. doi: 10.1016/S0002-9440(10)65610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirano K, Minamoto T. Altered expression of p52 and p27 proteins, alone or combined, as a predictor of metastatic potential in early invasive carcinoma of the colon and rectum - a comparative clinicopathologic and molecular analysis. Cancer Detect Prevent. 2000;24:343–55. [PubMed] [Google Scholar]
- 22.Saltz L, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: results of CALGB 89803. J Clin Oncol. 2007;25:3456–61. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 23.Thibodeau SN, French AJ, Roche PC, et al. Altered expression of hMLH1 and hMSH2 in tumours with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res. 1996;56:4836–40. [PubMed] [Google Scholar]
- 24.Walsh S, Murphy M, Silverman M, et al. p27 expression in inflammatory bowel disease-associated neoplasia. J Pathol. 1999;155:1511–8. doi: 10.1016/S0002-9440(10)65466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertagnolli MM, Niedzwiecki D, Compton CC, et al. Microsatellite instability predicts improved response to adjuvant therapy with irinotecan, 5-fluorouracil and leukovorin (IFL) in stage III colon cancer: CALGB Protocol 89803. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.18.2071. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hattori T, Isobe T, Abe K, et al. Pirh2 promotes ubiquitin-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. Cancer Res. 2007;67:10789–95. doi: 10.1158/0008-5472.CAN-07-2033. [DOI] [PubMed] [Google Scholar]
- 27.Ogino S, Kawasaki T, Kirkner GJ, Yamaji T, Loda M, Fuchs CS. Loss of nuclear p27 (CDKN1B/KIP1) in colorectal cancer is correlated with microsatellite instability and CIMP. Mod Pathol. 2007;20:15–22. doi: 10.1038/modpathol.3800709. [DOI] [PubMed] [Google Scholar]
- 28.Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342:69–77. doi: 10.1056/NEJM200001133420201. [DOI] [PubMed] [Google Scholar]
- 29.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–18. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]