Abstract
In this case control study from 2005–2008 of euthyroid first-cycle IVF patients ≥38 years old with singleton baby, miscarriage, biochemical pregnancy, and no pregnancy outcomes, we assayed frozen serum for autoimmune thyroid disease (AITD) and thyroid function at cycle start, trigger, 4 and 5 weeks gestation. AITD prevalence in older infertile women was similar across clinical outcomes, and although AITD was associated with a higher baseline TSH, TSH remains within acceptable range, suggesting that thyroxine supplementation may not affect maternal outcomes in older euthyroid AITD patients through 5 weeks gestation.
Keywords: thyroid autoimmunity, hypothyroidism, subclinical hypothyroidism, infertility, IVF, thyroid peroxidase antibody, thyroglobulin antibody, miscarriage
The prevalence of autoimmune thyroid disease (AITD) has been reported to increase with age (1) and to be higher in infertile populations (2). While no apparent effect of AITD has been observed on pregnancy rates (3,4), many studies (5–7) have concluded that even in the absence of overt thyroid dysfunction, AITD is associated with a three to fivefold increase in the overall miscarriage rate among women with spontaneous pregnancies. An increased rate of miscarriage has also been observed in most (3, 8–10) but not all (11, 12) studies of women with AITD undergoing ART. It has been proposed that the rapid and robust rise in estradiol concentrations with ART may pose a great stress on the hypothalamic-pituitary-thyroid axis and challenge the ability of the thyroid to maintain a pregnancy (13–15), particularly in women with AITD. Women with AITD may have a slightly higher (but still normal) TSH when compared to women without AITD prior to pregnancy (16), which could lead to subclinical or overt hypothyroidism (14) and poor pregnancy outcomes after ART.
Our aims were to estimate the prevalence of AITD in an older, infertile, female IVF population, to determine if an association exists between the presence of AITD and IVF outcomes, and to compare the effect of gonadotropin stimulation and early pregnancy on thyroid reserve in these older women with and without AITD.
We identified the first fresh IVF cycle of all patients ≥ 38 years from January 2005–December 2008 who consented to research on discarded tissue specimens (IRB#6902). Patients were categorized into 4 outcome groups: 1) Baby: singleton pregnancy with singleton live birth; 2) Miscarriage: loss of pregnancy (+sac) <13 weeks; 3) Biochemical pregnancy (+hcg but no sac seen on sono); 4) No pregnancy. We excluded spontaneous reductions and multiple gestations in an effort to limit confounding effects of HCG and E2 on TSH levels. We excluded those with a history of thyroid disease; hyperprolactinemia, and cycles involving PGD. PCOS, and diminished ovarian reserve, broadly defined as a history of poor response to gonadotropin stimulation and/or day 2 FSH>13.5 mIU/mL, were categorized as ovarian etiologies. IVF cycle preparation, stimulation, embryo culture, embryo transfer, and luteal support protocols were performed as previously described (17, 18). The number of embryos transferred was in accordance with current ASRM guidelines (19). Delivery follow-up was confirmed directly with patients.
Each patient’s previously frozen serum samples were tested for thyroid peroxidase antibody (TPOAb) and thyroglobulin antibody (TGAb). Thyroid function tests were performed at four points during the IVF cycle: 1) Start of IVF cycle (day 2); 2) Day of HCG trigger, 3) Day 28 (4 weeks gestation; 14 days after retrieval), and 4) Day 35 (5 weeks gestation; only in patients with a positive day 28 pregnancy test). Thyroid function tests included thyroid stimulating hormone (TSH), free thyroxine (F4), thyroxine binding globulin (TBG), and total thyroxine (TT4). All assays were conducted at our center’s onsite endocrinology lab using an Immulite 2500 machine (Siemens Medical Solutions Diagnostics, Los Angeles, CA). Normal TPOAb levels were defined as < 35 IU/ml and normal TGAb levels were defined as < 40 IU/ml. AITD was defined as having either positive TPOAb or TGAb.
Independent sample t-tests, Fisher exact, and chi square tests were performed as appropriate. Thyroid function data were positively skewed, necessitating the use of statistical procedures robust against non-normality. Generalized linear models were used for repeated measures analyses with fixed effects of AITD status and time, and repeated observations for each patient. Wilcoxon rank sum tests with a Bonferroni correction were used to compare groups with respect to thyroid response each time point, and change in TSH levels within each patient from time point to time point. A p value of <0.05 was considered significant, using two-sided tests. Analyses were conducted using SAS v 9.2.
Our de-identified retrospective study was approved for expedited review by the New York University School of Medicine Institutional Review Board (IRB #10-00052), and no investigators declared a conflict of interest.
Among 390 euthyroid patients ≥38 years old (mean ± st dev. 41±2 years, range 38–47), 12% (47/390) were positive for TPOAb, 4.6% (18) were positive for TGAb, and 3.0% (12) were positive for both TPOAb and TGAb. A total of 13.6% (53) met our definition for AITD by testing positive for either TPOAb or TGAb,
In comparing those with and without AITD, there was no difference in mean age (41 years), gravidity, (64% vs. 69%) parity (21% vs. 32%), BMI (23 vs. 24), and day 2 FSH levels (8 vs. 7mIU/ml). There was no difference in the proportions of patients with and without AITD with infertility due to endometriosis (2% vs. 3%), ovarian factors (26% vs. 30%), recurrent miscarriage (8% vs. 5%), tubal factor (6%), uterine factor (8% vs. 5%), multifactorial (11% vs. 18%), or male factor (40% vs. 32%). Mean gonadotropin dosage (3427 vs. 3783 IU) and the number of eggs retrieved (12 vs. 11), fertilized (7 vs. 6), and embryos transferred (3) did not differ between the two groups. There was no difference in the prevalence of AITD in those with a Baby versus a Miscarriage (15% vs. 16%), those with a Baby versus No Pregnancy (15% vs. 15%), and those with a Baby versus a Biochemical Pregnancy (15% vs. 5%).
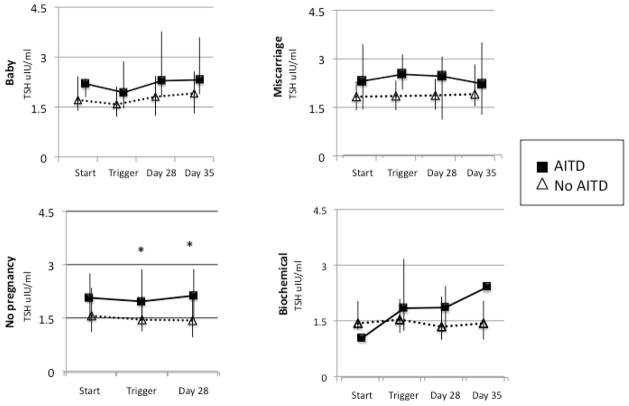
No difference was noted in TBG, TT4 or F4 levels in those with or without AITD as analyzed both overall and at any of the time points. In those with a Baby, patients with AITD had a trend toward higher TSH values than patients without AITD (p=0.06) and values trended higher at each of the four points in the IVF cycle but did not reach significance after adjusting for multiple comparisons: start (p=0.19), trigger (p=0.12), day 28 (p=0.12) and day 35 (p=0.06)(Figure 1). In patient cycles with No Pregnancy, TSH values in women with AITD were higher overall (p=0.01), specifically at trigger (p=0.02) and day 28 (p=0.02) (Figure 1). For the Miscarriage group, TSH values were higher overall (p=0.02), specifically with a trend toward a higher TSH for AITD at Trigger, which did not reach significance after adjusting for multiple comparisons (p=0.12)(Figure 1). There was no difference between TSH values in AITD and no AITD patients in the Biochemical pregnancy group. Across all outcomes, in those with and without AITD, the 75% limit of the interquartile range for TSH remained <4.5mIU/L (Figure 1). We did not need to control for the effect of HCG or E2 on TSH values because there was no difference in HCG or E2 between those with and without AITD at any time point measured throughout the IVF cycle. Thyroid reserve did not appear to be affected, as there was no effect of AITD on the degree of change in Free T4, TBG, TT4, or TSH between the different time points. Though all TSH values remain higher for the AITD patients, there was no difference in the incremental rise in TSH between the two groups.
Figure 1.
Effect of AITD on changes in TSH from the start of the IVF cycle through day 35. Median values with interquartile ranges (IQR) are plotted.
We did not find an increased prevalence of AITD in the miscarriage group, which confirms some studies (11), but is in contrast to other single studies (3, 10, 16, 20, 21), metaanalyses (5), and Endocrine Society Practice Guidelines (22). Similar to other investigators (16), we found that AITD was associated with a higher TSH from baseline through 5 weeks gestation (day 35), but remained <4.5mIU/L at all timepoints. In contrast to other investigators (14), the higher TSH values in women with AITD were not associated with a decrease in F4 or TT4, suggesting that the thyroid gland was able to meet any additional demands placed on it. Unlike previous studies (13–15), we did not find evidence of diminished thyroid reserve as a result of peak estrogen levels of 2000–3000 pg/ml at the time of HCG trigger.
Although current Endocrine Society guidelines recommend increasing the levothyroxine dose in women in whom a TSH value of >2.5 is detected in the first trimester (22), rigorous evidence is currently unavailable. A recent study from our group did not show a difference in clinical outcomes with TSH thresholds above or below 2.5 and 4.5mIU/L (23). Given that a TSH concentration >2.5mU/ml may increase the risk of progression to overt hypothyroidism (24), AITD may identify patients with such predispositions.
Our case control study was designed to minimize the cost of screening for a disease of low prevalence in an older population where pregnancy and delivery are also infrequent, and maximize the detection of AITD, given that previous studies predicted a higher prevalence of AITD in older (5), infertile (8, 25, 26) women and those with miscarriage after ART (3, 9, 10, 20). We note that we lacked information about thrombophilias, pregnancy complications, or child neurodevelopment.
In conclusion, the prevalence of thyroid autoimmunity in our older population was similar to that in previous studies of younger women, and did not differ across varying clinical outcomes. We hypothesize that in older women, other factors play a larger role than thyroid autoimmunity in achieving and maintaining a pregnancy. Although AITD was associated with a higher baseline TSH, free T4 remained the same. Furthermore, TSH remains within a range that has not been unequivocally demonstrated to place either mother or fetus at risk, suggesting that thyroxine supplementation may not affect maternal outcomes in older euthyroid AITD patients within the first 35 days of an IVF cycle.
Acknowledgments
We thank the NYU endocrinology lab of Dr. Joseph Katz, Amee Dharia, and Nandita Ganguly. We thank Lucy Lu for assistance with IRB approval, Dr. Judith Goldberg for statistical planning, and the entire staff of the NYU Fertility Center. This work was supported by NIH/NCRR 1UL1RR029893-01.
Supported in part by grant 1UL1RR029893 from the National Center for Research Resources, National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 2.Poppe K, Velkeniers B, Glinoer D. The role of thyroid autoimmunity in fertility and pregnancy. Nature clinical practice. 2008;4:394–405. doi: 10.1038/ncpendmet0846. [DOI] [PubMed] [Google Scholar]
- 3.Poppe K, Glinoer D, Tournaye H, Devroey P, van Steirteghem A, Kaufman L, et al. Assisted reproduction and thyroid autoimmunity: an unfortunate combination? J Clin Endocrinol Metab. 2003;88:4149–52. doi: 10.1210/jc.2003-030268. [DOI] [PubMed] [Google Scholar]
- 4.Grassi G, Balsamo A, Ansaldi C, Balbo A, Massobrio M, Benedetto C. Thyroid autoimmunity and infertility. Gynecol Endocrinol. 2001;15:389–96. [PubMed] [Google Scholar]
- 5.Prummel MF, Wiersinga WM. Thyroid autoimmunity and miscarriage. European journal of endocrinology/European Federation of Endocrine Societies. 2004;150:751–5. doi: 10.1530/eje.0.1500751. [DOI] [PubMed] [Google Scholar]
- 6.Stagnaro-Green A, Glinoer D. Thyroid autoimmunity and the risk of miscarriage. Best practice & research. 2004;18:167–81. doi: 10.1016/j.beem.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 7.Prummel MF, Strieder T, Wiersinga WM. The environment and autoimmune thyroid diseases. European journal of endocrinology/European Federation of Endocrine Societies. 2004;150:605–18. doi: 10.1530/eje.0.1500605. [DOI] [PubMed] [Google Scholar]
- 8.Negro R, Formoso G, Coppola L, Presicce G, Mangieri T, Pezzarossa A, et al. Euthyroid women with autoimmune disease undergoing assisted reproduction technologies: the role of autoimmunity and thyroid function. J Endocrinol Invest. 2007;30:3–8. doi: 10.1007/BF03347388. [DOI] [PubMed] [Google Scholar]
- 9.Kim CH, Chae HD, Kang BM, Chang YS. Influence of antithyroid antibodies in euthyroid women on in vitro fertilization-embryo transfer outcome. Am J Reprod Immunol. 1998;40:2–8. doi: 10.1111/j.1600-0897.1998.tb00380.x. [DOI] [PubMed] [Google Scholar]
- 10.Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, et al. Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: a prospective study. Human Reprod (Oxford, England) 2005;20:1529–33. doi: 10.1093/humrep/deh843. [DOI] [PubMed] [Google Scholar]
- 11.Muller AF, Verhoeff A, Mantel MJ, Berghout A. Thyroid autoimmunity and abortion: a prospective study in women undergoing in vitro fertilization. Fertil Steril. 1999;71:30–4. doi: 10.1016/s0015-0282(98)00394-x. [DOI] [PubMed] [Google Scholar]
- 12.Kutteh WH, Yetman DL, Carr AC, Beck LA, Scott RT., Jr Increased prevalence of antithyroid antibodies identified in women with recurrent pregnancy loss but not in women undergoing assisted reproduction. Fertil Steril. 1999;71:843–8. doi: 10.1016/s0015-0282(99)00091-6. [DOI] [PubMed] [Google Scholar]
- 13.Muller AF, Verhoeff A, Mantel MJ, De Jong FH, Berghout A. Decrease of free thyroxine levels after controlled ovarian hyperstimulation. J Clin Endocrinol Metab. 2000;85:545–8. doi: 10.1210/jcem.85.2.6374. [DOI] [PubMed] [Google Scholar]
- 14.Poppe K, Glinoer D, Tournaye H, Schiettecatte J, Devroey P, van Steirteghem A, et al. Impact of ovarian hyperstimulation on thyroid function in women with and without thyroid autoimmunity. J Clin Endocrinol Metab. 2004;89:3808–12. doi: 10.1210/jc.2004-0105. [DOI] [PubMed] [Google Scholar]
- 15.Poppe K, Glinoer D, Tournaye H, Schiettecatte J, Haentjens P, Velkeniers B. Thyroid function after assisted reproductive technology in women free of thyroid disease. Fertil Steril. 2005;83:1753–7. doi: 10.1016/j.fertnstert.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Bagis T, Gokcel A, Saygili ES. Autoimmune thyroid disease in pregnancy and the postpartum period: relationship to spontaneous abortion. Thyroid. 2001;11:1049–53. doi: 10.1089/105072501753271743. [DOI] [PubMed] [Google Scholar]
- 17.Reh A, Krey L, Noyes N. Are gonadotropin-releasing hormone agonists losing popularity? Current trends at a large fertility center. Fertil Steril. 2010;93:101–8. doi: 10.1016/j.fertnstert.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 18.Grifo JA, Flisser E, Adler A, McCaffrey C, Krey LC, Licciardi F, et al. Programmatic implementation of blastocyst transfer in a university-based in vitro fertilization clinic: maximizing pregnancy rates and minimizing triplet rates. Fertil Steril. 2007;88:294–300. doi: 10.1016/j.fertnstert.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 19.ASRM Practice Committee. Guidelines on number of embryos transferred. Fertil Steril. 2006;86:S51–2. doi: 10.1016/j.fertnstert.2006.07.1473. [DOI] [PubMed] [Google Scholar]
- 20.Bussen S, Steck T, Dietl J. Increased prevalence of thyroid antibodies in euthyroid women with a history of recurrent in-vitro fertilization failure. Human Reprod (Oxford, England) 2000;15:545–8. doi: 10.1093/humrep/15.3.545. [DOI] [PubMed] [Google Scholar]
- 21.Iijima T, Tada H, Hidaka Y, Mitsuda N, Murata Y, Amino N. Effects of autoantibodies on the course of pregnancy and fetal growth. Obstet Gynecol. 1997;90:364–9. doi: 10.1016/s0029-7844(97)00283-4. [DOI] [PubMed] [Google Scholar]
- 22.Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007;92:S1–47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 23.Reh A, Grifo J, Danoff A. What is a normal thyroid-stimulating hormone (TSH) level? Effects of stricter TSH thresholds on pregnancy outcomes after in vitro fertilization. Fertil Steril. 2010 doi: 10.1016/j.fertnstert.2010.06.041. In Press. [DOI] [PubMed] [Google Scholar]
- 24.Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clinical Endocrinol. 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 25.Bellver J, Soares SR, Alvarez C, Munoz E, Ramirez A, Rubio C, et al. The role of thrombophilia and thyroid autoimmunity in unexplained infertility, implantation failure and recurrent spontaneous abortion. Human Reprod (Oxford, England) 2008;23:278–84. doi: 10.1093/humrep/dem383. [DOI] [PubMed] [Google Scholar]
- 26.Kaider AS, Kaider BD, Janowicz PB, Roussev RG. Immunodiagnostic evaluation in women with reproductive failure. Am J Reprod Immunol. 1999;42:335–46. doi: 10.1111/j.1600-0897.1999.tb00110.x. [DOI] [PubMed] [Google Scholar]