Abstract
An important research question is whether Attention Deficit Hyperactivity Disorder (ADHD) is related to early or late stage attentional control mechanisms and whether this differentiates a non-hyperactive subtype (“ADD”). This question was addressed in a sample of 145 ADD/ADHD and typically developing comparison adolescents (aged 13-17). Attentional blink and antisaccade tasks were used to assay early and late stage control, respectively. ADD was defined using normative cutoffs to assure low activity level in children who otherwise met full criteria for ADHD. The ADD group had an attenuated attentional blink versus controls and ADHD-combined (ADHD-C). The effect was not produced using DSM-IV definition of ADHD-primarily inattentive type nor DSM symptom counts. ADHD-C showed greater weakness in response inhibition, as manifest in the antisaccade task. Combining tasks yielded an interaction differentiating group performance on the two tasks.
Keywords: ADHD, ADD, ADHD subtypes, response inhibition, attentional blink, antisaccade, adolescence
In considering the nosology of ADHD subtypes, it may be informative to consider the adolescent period of development. Whereas ADHD often persists into adolescence (Barkley, Fischer, Smallish, & Fletcher, 2002; Mannuzza, Klein, & Moulton, 2003), developmental maturation tends to result in normative reductions in hyperactivity (and perhaps also impulsivity) in adolescence (Hart et al., 1994). Therefore, the profile of subtypes (if subtypes exist) would be expected to modify in adolescence. In adolescence, enduring cases of ADHD with marked hyperactivity/impulsivity may be more easily discriminated from cases marked by only transient hyperactivity/impulsivity during childhood. This may assist the effort to identify a refined non-hyperactive ADHD subtype (referred to herein as “ADD” for simplicity to distinguish it from DSM-IV's ADHD primarily inattentive type or ADHD-PI). Note that in DSM-III, “ADD without hyperactivity” was allowed to have impulsivity. In contrast, as operationalized in the present study the “ADD” group will be low on hyperactivity/impulsivity as a unitary dimension.
Adolescence is a particularly important developmental context in which to consider neural networks and related cognitive problems involved in ADHD and/or ADD. Maturation and myelination of brain regions involved in cognitive control, particularly prefrontal cortex and frontal–subcortical networks, continues into late adolescence and beyond (Benes, 2001). This may be reflected in important anatomical differences between children and adolescents with ADHD (Krain & Castellanos, 2006; Shaw et al, 2006). Thus, adolescents may differ from both adults and children with regard to their neuropsychological profile of cognitive control (Casey, Jones, & Hare, 2008) and (Halperin & Schulz, 2006).
This matters for assessing subtypes, because it has been unclear whether the DSM-IV captures a conceptual non-hyperactive ADHD type or “ADD”. Some researchers have argued that ADD is a distinct disorder from ADHD, but that the ADD entity is not adequately described by DSM-IV ADHD-PI (Diamond, 2005; Milich et al., 2001). This line of thought follows on earlier literature prior to DSM-IV, which suggested that an attentional deficit rooted in posterior cortical networks characterized ADD, whereas a cognitive control deficit rooted in frontal cortical networks characterized ADHD (Hynd et al., 1991). Following on that model, Diamond (2005) explicitly hypothesized that the ADD group would have deficits related to functions of frontal-parietal cortical attention networks, whereas ADHD-C would have dysfunction in frontal-subcortical circuits related to cognitive control. Finally, a recent update on thinking about these types was provided by Adams et al (2008). They provided a carefully reasoned review of literature concluding that both ADD and ADHD would exhibit basic inhibitory control deficits, but they would differ in regard to attentional processing. Taking these different hypotheses together, we considered the following predictions.
First, based on other knowledge about the nature of attention and its distributed networks in the brain, then from these theories we would predict that ADD should be related to problems in early stage information control (e.g., attentional gating) which is supported by parietal-frontal and posterior attention networks. On the other hand, ADHD-C should be characterized by problems in late stage control processes that are supported by frontal-subcortical circuits. This hypothesis was supported by neuroimaging and lesion studies that associate left anterior, medial frontal, and parietal regions, which are hypothesized to relate to ADD, with attentional filtering (Persson et al., 2007), but right inferior frontal regions, basal ganglia, and other regions with cognitive control and response inhibition (Aron & Poldrack, 2005). Second, however, we considered the alternative possibility from the more refined outline provided by Adams et al (2008), suggesting that both ADHD and ADD might show difficulties on inhibitory control, but only ADD would show abnormal attentional gating. Third, Diamond (2005), like Milich et al (2001) and Adams et al (2008), argued that the DSM-IV types would not suffice to test this theory. Indeed, family psychiatric data suggest that although DSM-IV subtypes “breed true” in families to a small extent, ADHD-PI (as defined in the DSM-IV) includes many “subthreshold” ADHD-C cases (Stawicki, Nigg, & von Eye, 2006). Therefore it is important to determine whether a more conservatively defined ADD group can be defined and then validated mechanistically along the lines of their hypotheses.
Furthermore, studies of early stage attentional filtering have failed to show consistent differences between the DSM-IV defined ADHD groups, and, despite the hazards of interpreting null findings, several studies of early stage attentional processes have suggested normal functioning in ADHD-C and ADHD-PI (Booth et al., 2007; Carr et al., 2006; Huang-Pollock & Nigg, 2003; van Mourik et al., 2005). These findings would seem to fail to support the proposed hypothesis. Yet these studies did not operationalize ADD apart from ADHD-PI and thus, could have yielded false negative findings.
In turn, studies of late stage cognitive control often show an ordinal pattern of results in which youth with ADHD-PI perform intermediate to youth with ADHD-C and non-ADHD controls, with differences that are statistically reliable in some studies but not in others (Clark et al., 2007; O'Driscoll et al. 2005; Geurts, Verte, Oosterlaan, Roeyers, & Sergeant, 2005; Nigg, Blaskey, Huang-Pollack, & Rappley, 2002). Most of those data might be compatible with the view of ADHD as a spectrum of severity across DSM-IV types, rather than having mechanistically distinct subtypes. Nevertheless, ADD was not operationalized apart from DSM-IV ADHD-PI in these studies.
Defining ADD versus ADHD-PI
If ADD exists and is qualitatively distinct from ADHD, this group should exhibit a qualitatively distinct pattern of attention problems from those of ADHD-C, not merely a milder set of impairments. Two problems must be simultaneously solved to evaluate that hypothesis. The first problem concerns how to operationally define the clinical profile of the putative ADD group since, as noted, DSM-IV ADHD-PI criteria do not meet this conceptual goal. The relevant clinical profile should include no evidence of clinically significant problems with hyperactivity/impulsivity, to prevent identifying children with subthreshold ADHD-C. In children, it recently has been suggested based on latent class analyses that a cutoff might exist at ≤ 2 symptoms of hyperactivity/impulsivity on the DSM-IV symptoms list (Volk et al., 2009). This cut point therefore has at least some empirical support, albeit quite preliminary. However, given the attenuation of activity level with age cited above, it is unclear how many symptoms would be normative in adolescence. Therefore, until such data could be obtained, it seemed prudent for the adolescent sample to consider normative behavior levels rather than, or in addition to, symptom counts in identifying ADD.
The second problem is to demonstrate a mechanistic distinction that is not merely one of severity. A recent study by Derefenko et al. (2008) used cued reaction time (tapping general inhibitory function) and go/no-go (tapping motivational contingencies) tasks to compare inhibition and response style in children with ADHD-C and ADHD-PI. The ADHD-PI group was carefully defined: Conners T score of ≥ 60 for the Conners Cognitive Problems/Inattention, and Conners T score of ≤ 60 for the Conners Hyperactivity. The authors found that only the inattentive group exhibited slow reaction times across both tasks contexts. The authors also note that further studies are needed to examine whether performance can be attributed to slowed information processing and/or motivational elements that could raise arousal and subsequently enhance performance (Diamond, 2005).
Task Design for Two Components of Control
In the current study, well-validated behavioral tasks were selected to capture two domains of cognitive control. The attentional blink task was employed to evaluate early stage attentional filtering. Of recent interest in the ADHD literature, it captures temporal rather than spatial selection. This temporal focus was viewed as an advantage in light of evidence that ADHD is associated with difficulties in temporal processing generally (Toplak et al., 2006). In this task, rapid serial visual presentation (RSVP) is used wherein visual stimuli (e.g., letters) are presented one after another in rapid sequence (several per second) (Figure 1). The participant's task is to detect the presence of a probe letter and/or identify a target letter. Early stage processing is evaluated by comparing probe detection across time and across single and dual task conditions. In a single task condition, participants confirm or reject the presence of the probe letter (“Z” in Figure 1). Performance in this condition is quite accurate due to low attentional load. In the dual-task condition, participants must also identify a target stimulus (light gray letter in Figure 1) which can occur in one of several serial positions prior to the probe letter.
FIGURE 1.
A sample trial of events during dual-task condition of the attentional blink task, where a target letter appears in blue (grey in figure) and the probe letter, “Z” occurs at Lag 2.
Detection of the target letter is followed by a brief capacity-limited stage of identification and encoding during which the probe letter can be missed (Chun & Potter, 1995). In typically developing individuals, detection sensitivity during the dual task condition remains high for probe letters that occur immediately after the target (approximately 90-200 ms), termed “Lag 1 sparing” (Chun & Potter, 1995). A drop in probe detection tends to occur 200-500 ms after the target, which is termed, “the attentional blink.” The blink appears to reflect competition for attentional resources as probes pass during target processing, although various interpretations have been offered (Chun & Potter, 1995; Duncan, Ward, & Shapiro, 1994; Hommel et al., 2006; Kawahara et al. 2003). Detection sensitivity begins to rise again for probes occurring more than 500 ms after the target. Functional imaging data implicate early visual areas and frontoparietal circuits in this phenomenon (Hein et al., 2009), supporting its use for our purposes.
With regard to late stage response inhibition, the antisaccade task was chosen and has been used in prior ADHD research. The advantage in this study was that it affords the ability to isolate behaviors that are mainly stimulus driven (i.e., reflexive prosaccades, for which stimulus–response mapping is consistent, and demands on control mechanisms are minimal) from those that are goal driven (voluntary antisaccade, in which stimulus–response mapping is inconsistent, requiring greater top-down control). In the antisaccade condition, reflexive eye movements (saccades) toward a target must be suppressed in order to generate movement in the opposite direction (Miller et al., 2005), assuming eye movements are not compromised by primary problems in the motor system itself (Niedermeyer & Naidu, 1997). Employing the antisaccade task, O'Driscoll et al (2005) found greater impairments in response inhibition and motor planning in adolescent boys with ADHD-C than those with ADHD-PI, with the latter defined as those with fewer than 1.5 symptoms from behavioral checklists for hyperactivity. These findings coupled with a large body of work on the neural underpinnings of saccadic control (Hutton & Ettinger, 2006; Pierrot-Deseilligny et al., 2005), supports the conceptual foundation for selecting this task to evaluate our late stage control component.
Current Study
Based on this background literature, it was hypothesized that the ADHD-C group would show a greater deficit than the ADD group in late-stage response inhibition (O'Driscoll et al., 2005). It was further hypothesized that ADD would be associated with abnormal early-stage filtering as exhibited on the attentional blink task.
Method
Participants
The main results presented here relied on data from 145 participants (aged 13-17), including 74 with ADHD and 71 typically developing non-ADHD comparison youth. A small number of additional cases were available that completed the attentional blink task; for completeness their data are included in the relevant results figures, but those results were almost identical to the main results reported. Potential participants were excluded if they had estimated full scale IQ < 75 on a five subtest (picture completion, information, similarities, block design and vocabulary) short form of the WISC-IV, history of head injury with loss of consciousness greater than 1 min by parent report, sensory–motor handicap, or neurological illness; currently prescribed antipsychotic, antidepressant, or anticonvulsant medications; or if they had current major depressive, manic or hypomanic episode; current substance dependence preventing sober testing; history of psychosis; or prior diagnosis of autistic disorder. Parents of six participants (4, ADHD-C and 2, ADHD-PI) reported a history of learning disability for their child and participants were excluded if the learning disability was considered the primary diagnostic issue.
Procedures
Cases and controls were identified via a multi-gate recruitment strategy, similar to the recruitment procedure used in the Multimodal Treatment Study for ADHD (MTA) study (MTA Cooperative Group, 1999). The process began with widespread public advertisement and outreach to draw volunteer participants. Prospective participants' parents contacted the project, at which point key exclusionary criteria were checked (no sensory–motor handicap or neurological or medical illness that would preclude participation). Eligible participants were then scheduled for a diagnostic visit wherein they completed semi-structured clinical interviews, standardized rating scales, IQ testing (Weschler Intelligence Scale for Children; WISC-IV) and achievement testing. Parents and teachers completed the ADHD Rating Scale (DuPaul et al., 1998), Conners (1997) Rating Scale Revised Short Form, and Child Behavior Checklist (Achenbach et al., 1991).
The Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS-E; Puig-Antich & Ryan, 1986) was administered to the parent by a trained clinician with a masters-degree of relevant education (either M.S.W. or M.A. in psychology or equivalent). The interview assessed all major Axis I disorders of childhood. The child, after completing intellectual and academic screens, was interviewed briefly by the clinician regarding problems and strengths. The clinician and the psychometrists who tested the child recorded observations in the file.
Reliability of KSADS interviews was assessed by review of videotaped interviews. These were calibrated to a reference interviewer who was certified by fidelity to ratings of outside experts. The other interviewers watched videotapes by the lead interviewer and vice versa. Reliability was then computed between the reference rater and the second interviewers. For ADHD, inter-interviewer agreement that there were 6 or more symptoms of inattention was excellent (kappa=.81, n=178); as was the agreement on presence of 6 or more symptoms of hyperactivity/impulsivity (kappa=.76; n=178). Agreement on number of ADHD symptoms was also good (for inattention, intraclass r=.80, for hyperactivity/impulsivity, intraclass r=.88). Agreement for common comorbid diagnoses (ODD, major depression, generalized anxiety disorder, separation anxiety disorder) was acceptable as well (inter-interviewer kappa=.67-.88, n=156).
Best Estimate Diagnosis for ADHD
A diagnostic team (a licensed clinical psychologist and a board-certified child psychiatrist, both with > 20 years clinical experience with clinical assessment of youth) then reviewed the entire file on each child (that is, the written record of the KSADS, as well as the standardized ratings data and interview notes). They had the ability to query the clinician if needed but they did not see the child. They were blind to the experimental measures reported here. The clinicians implemented a best estimate diagnostic procedure as commonly reported in the ADHD literature (Faraone et al., 2000). In that process, each clinician independently reviewed all available clinical information, and arrived at a judgment about whether ADHD was present or absent, ADHD DSM-IV subtype, and comorbid disorders. The team followed Diagnostic and Statistical Manual for Mental Disorders (4th ed.; DSM–IV; American Psychiatric Association, 1994) criteria strictly, requiring evidence of six symptoms in at least one symptom domain, impairment, duration, onset, and evidence that problems were not better accounted for by a coexisting condition. They required evidence of impairing symptoms from both parent and teacher report, but if both reporters indicated significant problems, clinicians used an “or” algorithm to combine parent and teacher data when counting symptoms. Inter-clinician agreement on presence or absence of ADHD (any type), ADHD DSM-IV subtype, and presence of oppositional or conduct disorder was satisfactory (all kappa > .89). Disagreements were readily resolved by conference.
ADD
ADD was then identified post-hoc as follows. Children who met full DSM-IV criteria for ADHD-PI (n=34) were further stratified into those with above and below the mean of parent and teacher Conners ratings of hyperactive/restlessness. On that scale, a T-score was computed that adjusted for national norms for age and sex of the child. Because these T scores have a mean of 50 and a standard deviation of 10, composite parent/teacher T-score ≤50 distinguished the ADD group. An alternative operational definition relying on <= 2 symptoms of hyperactivity/impulsivity on the parent report KSAD was then checked for robustness of any findings, because the item set on the Conners is not the same as the DSM-IV item set.
Medication washout
Participants prescribed psychostimulant medications (amphetamine or extended release amphetamine, n=9; methylphenidate or extended release methylphenidate, n=15) were tested after a minimum washout period of 24 hours. The actual washout period ranged from 31 to >100 hr for extended release preparations (median = 58 hrs), and from 24 to >100 hr for regular release preparations (median = 51 hrs). These washout periods were considered sufficient to minimize medication effects on performance. Participants taking other psychoactive medications (antidepressants) were excluded from participation.
Measures
Attentional blink task
A standard attentional blink paradigm was administered (Raymond et al., 1992). Rapid serial visual presentation was used to present black letters at the central fixation point on a white computer screen. Figure 1 illustrates the procedure graphically. Both single and dual-task blocks contained 80 trials of 22 letters each. Each letter appeared for 90 ms, with no temporal gaps between letters. There were two conditions: the single condition always preceded the dual task condition. Each condition was preceded by six practice trials. For the single task condition, participants were to confirm or reject the presence of a probe (the letter Z) in the letter stream, at the end of each 22 letter trial. The single-task condition contained a blue letter, but participants were asked to ignore this letter and only report on the probe. In the dual-task condition (the main test of attentional filtering), participants were asked at the end of the 22 letter trial to identify the blue letter (target letter) by pressing the corresponding letter on a standard computer keyboard, and to confirm or reject the presence of the Z in the stream by pressing either “1” or “9” on the same keyboard. In both conditions, 50% of the trials contained the probe (the Z). The probe (Z) could appear in one of five temporal positions: 90, 180, 270, 360 or 450 ms after the target (blue) letter. The number of letters occurring before the next target was randomized between 9 and 16 letters. Accuracy was recorded for target identification for each serial position relative to the probe in the dual task condition. Probe detection sensitivity (A′) was calculated for single and dual task conditions (the latter, only for trials on which the target was correctly identified). The presence of the attentional blink is indicated by a task by lag interaction; the interaction is the decrease in probe detection sensitivity in the dual task condition for probe positions greater than 200 ms (2-3 serial positions) after the target. The ADD group was expected to show an attenuated blink effect (detection of more probes) in the dual task condition, which would compromise primary task performance of target identification and encoding. Thus, if the hypothesis was correct. the ADD group would not show the typical task by lag interaction in probe detection characteristic of the attentional blink.
Antisaccade task
A standard computerized antisaccade task with eye movement monitoring was administered as follows. The participant viewed a computer monitor for a total of 60 trials each for the pro- and antisaccade blocks. The pro-saccade condition always preceded the antisaccade condition. Each block was preceded by 6 practice trials. Each trial began with a central fixation point appearing in the center of the screen. A white box (start box) was superimposed over the central fixation to signal the beginning of the trial. Following the offset of the start box, a target box then appeared to the right or left of central fixation. Target box onset was randomly delayed by one of six time intervals ranging from 500 to 1000 ms following the offset of the start box. For the prosaccade condition, participants were instructed to direct their gaze toward the target box, and for the antisaccade condition, they were instructed to direct their gaze in the opposite direction. Eye movements were monitored with an ISCAN (Burlington, MA) ETL-400 pupil and corneal reflection tracking system sampling at 240 Hz. The eye tracker was accurate to within 1° of visual angle both horizontally and vertically. Chin and forehead rests were used to maintain the participant's viewing position and distance. The position of the right eye was tracked, though viewing was binocular. Response latency, directional accuracy, and anticipations were recorded.
Data Analyses
On the attentional blink task, probe (the “z”) detection rates were converted to A′, a nonparametric measure of sensitivity that yields a bias-free measure of signal detection (Stanislaw & Todorov, 1999) by factoring false alarm rate. The primary task of target identification required more than simple detection; thus, for targets accuracy of identification was used rather than a signal detection measure. Dependent variables for the antisaccade task were accuracy (error rate) and percentage of trials with anticipatory eye movements (express saccades). Latency was also evaluated as an index of task integrity. Repeated measures ANCOVA was used to evaluate group differences across conditions. If omnibus comparisons were nonsignificant, further pairwise comparisons were not examined, to minimize family-wise Type I error; otherwise, uncorrected post-hoc Fisher's least significant difference test was conducted (Carmer & Swanson, 1973). To convey effect size, Cohen's partial eta squared (η2) is reported.
Considerable controversy attends to whether IQ should be covaried in cognitive studies of ADHD. Results were therefore first performed with IQ covaried. IQ was never a significant covariate and did not significantly interact with task factors. Results are therefore reported without IQ covaried. The ADHD-C group had a higher ratio of males than the other groups. Thus, all analyses included sex as a covariate.
Results
Sample Description
Table 1 provides a clinical and demographic descriptive overview of the sample and the groups defined; showing variations in sex, so it was always covaried. The table shows that the subtyping was validly reflected in the clinical measures.
Table 1. Demographic and Diagnostic Description of Groups.
| Participants | DSM IV Groups | Control v | Control v | ADHD-C v | |||
|---|---|---|---|---|---|---|---|
| Control | ADHD-C | ADHD-PI (DSM-IV) | ADD | ADHD-C (p) | ADD (p) | ADD (p) | |
| n | 74 | 37 | 34 | 15 | -- | -- | -- |
| % male | 54.1 | 75.7 | 62.2 | 46.7 | .029 | .592 | .053 |
| Age | 15.5 (1.0) | 15.0 (1.1) | 15.3 (1.2) | 15.1(1.1) | .031 | .159 | .902 |
| Full-scale IQ | 112.8 (14.8) | 103.1 (11.0) | 105.5 (12.1) | 104.2 (8.5) | <.001 | .024 | .792 |
| Family income† | 80.0 | 55.0 | 60.0 | 67.5 | .315 | .064 | .019 |
| Global Assessment of Functioning | 83.3(9.1) | 70.9(8.6) | 73.9 (8.9) | 76.1 (8.6) | <.001 | .005 | .064 |
| Conners ADHD Index T Score* | 47.9 (5.3) | 73.6 (8.0) | 64.8 (8.7) | 59.7 (8.8) | <.001 | <.001 | <.001 |
| Conners Cognitive Problems T Score* | 47.1 (5.0) | 66.2 (8.2) | 63.9 (10.3) | 58.9 (9.9) | <.001 | <.001 | .001 |
| Conners Hyperactivity T score* | 49.1 (5.1) | 76.9(9.1) | 54.0 (7.9) | 46.6 (2.1) | <.001 | .172 | <.001 |
| Conners Oppositional problems T score* | 47.1 (4.6) | 63.9 (9.4) | 51.5 (7.4) | 47.1 (4.5) | <.001 | .992 | <.001 |
| KSAD Inattentive Symptoms | .77 (.92) | 6.8 (1.5) | 6.2 (1.7) | 5.4 (1.9) | <.001 | <.001 | <.001 |
| KSAD Hyperactive/Impulsive Symptoms | .46 (.67) | 5.6 (1.9) | 1.4 (1.4) | .7 (.87) | <.001 | .472 | <.001 |
| Current ODD N | 1 | 13 | 3 | 0 | -- | -- | -- |
| Current CD N | 0 | 2 | 0 | 0 | -- | -- | -- |
| Current MDD N | 0 | 0 | 0 | 0 | -- | -- | -- |
| Current GAD N | 0 | 1 | 0 | 0 | -- | -- | -- |
| BASC externalizing problems T Score | 45.9 (7.2) | 71.3 (13.2) | 51.6 (8.3) | 47.1 (8.6) | <.001 | .883 | <.001 |
| BASC internalizing problems T Score | 45.6 (7.8) | 57.7 (11.8) | 50.3 (10.8) | 43.4 (6.8) | <.001 | .651 | <.001 |
| YSR externalizing problems T Score | 49.2 (10.1) | 53.2 (9.2) | 54.6 (11.9) | 51.8 (12.4) | .014 | .604 | .307 |
| YSR internalizing problems T Score | 48.3 (8.8) | 60.3 (8.5) | 54.9 (9.7) | 53.4 (6.8) | <.001 | .113 | .041 |
| YSR total T Score | 47.5 (9.3) | 59.8 (7.8) | 56.5 (11.2) | 55.3 (10.9) | <.001 | .024 | .086 |
| WIAT/WRAT Reading Standard Score | 104.6 (8.8) | 100.8 (10.2) | 99.7 (10.8) | 100.14 (10.8) | .038 | .097 | .836 |
| WIAT/WRAT Spelling Standard Score | 104.7 (11.5) | 95.6 (16.3) | 95.3 (11.7) | 94.9 (13.0) | .001 | .029 | .890 |
| WIAT/WRAT Math Standard Score | 107.3 (14.9) | 96.5 (14.8) | 97.8 (16.7) | 98.5 (15.6) | <.001 | .067 | .458 |
Note. Data include the subset of participants who completed both saccade and attentional blink tasks. Values in parentheses indicate standard deviations. ADD = ADHD-PI (with below average mean activity scores (mean of Parent & Teacher Conners Hyperactivity, T<50); ADHD-C = ADHD combined subtype; ODD = oppositional defiant disorder; CD = conduct disorder; MDD = major depressive disorder; GAD = generalized anxiety disorder (includes GAD-Not Otherwise Specified (NOS)); BASC = Behavioral Assessment Scale for Children; YSR = Youth Self-Report.
Conners T scores reflect the mean of mother & teacher T-scores; KSAD inattentive and hyperactivity/impulsivity scores reflect the mean of parent and teacher symptom counts.
Median group income reported in the thousands, based on 66 reports for Control group, 31 for ADHD-PI group and 33 for ADHD-C group.
Attentional Blink Task Results
For the attentional blink task, we focused on two main variables that serve in tandem as indicators of attentional control. The first is accuracy of the primary task of target identification (target letters appeared in blue) under dual task conditions. The accuracy with which participants identify the blue target letters is an indication of the attentional resources apportioned to letter identification, given there is a competing pull for those resources from the presence of a probe letter (“Z”). The second measure is the sensitivity with which participants are able to detect the probe letter itself. As mentioned above, the drop in sensitivity for probes that occur in close temporal proximity to the target letter indicates the presence of the blink effect and serves to preserve the ability to consolidate the target identification.
Probe detection in single and dual task conditions are compared across temporal positions, to evaluate the presence of the attentional blink. A difference between groups with respect to the presence or magnitude of the attentional blink would be indicated by a 3 way interaction of group by task by lag. However, due to limited power to detect the 3 way interaction we also planned to examine the two way interaction (lag × task) within each group to evaluate robustness of the blink effect for each group. If the hypothesis is correct, and the ADD group is associated with impairments in early attentional control, this group should show (1) reductions in target identification, due to a lack of attentional resources; (2) increased probe detection, due to an inability to filter out competing information, or (3) both.
Preliminary task analysis
To evaluate performance on the primary task of target identification, a repeated measures ANCOVA was performed for target accuracy (dual task condition only). It revealed a main effect of group, F(2,122) =6.08, p=.003, η2=.091. Pairwise comparisons indicated that the ADHD-C group was less accurate than controls (57% vs. 67%, p=.001), but did not differ from the ADD group (57% vs 58%, p=.71). The ADD group was marginally worse than the control group (58% vs. 67%, p=.054). Despite the fact that control performance was better, both subtype groups performed comparably on the primary task.
Primary hypothesis tests in attentional blink
We then examined probe detection sensitivity (A′) in single versus dual task conditions. The main effect for task, F(1,122) =27.07, p<.001, η2=.182, showed that probe sensitivity was worse (fewer probes detected) under the dual task than single task condition. This finding suggested that the task operated as intended. We had predicted that the ADD group, due to inefficient attentional filtering, would be uniquely sensitive to probes. Consistent with this hypothesis, a main effect of group was found, F(2,122) =4.63, p=.011, η 2=.071, which was qualified by an interaction between task and group, F(2,122) =3.29, p=.041, η 2=.051. Follow-up of the interaction showed that groups differed in probe sensitivity in the dual task condition, F(2,122) =4.27, p=.016, η2=.065, but not in the single task condition (all p>.05). The ADD group did in fact, detect more probes (67%) than either controls (58%, p=.037) or ADHD-C (.53%, p=.004).
The above results were sustained when we included additional participants who had completed only the attentional blink task; results for that full sample are illustrated in Figure 2 (panel A). The pattern was the same as reported above. In the single task condition, all groups performed comparably across probe lags, but in the dual task condition they differed substantially. However rather than appearing as a group by lag interaction (non-significant), this emerged as a main effect of group on probe sensitivity across all lags. Figure 2, panels B, C, and D show dual task target accuracy relative to probe detection for the three groups. In particular, the pattern of performance suggests that the ADD group may have performed the task differently to both control and ADHD-C groups. It was therefore important to examine task performance and the blink effect within each group, even though in the omnibus analysis the 3-way interaction was not significant.
FIGURE 2.
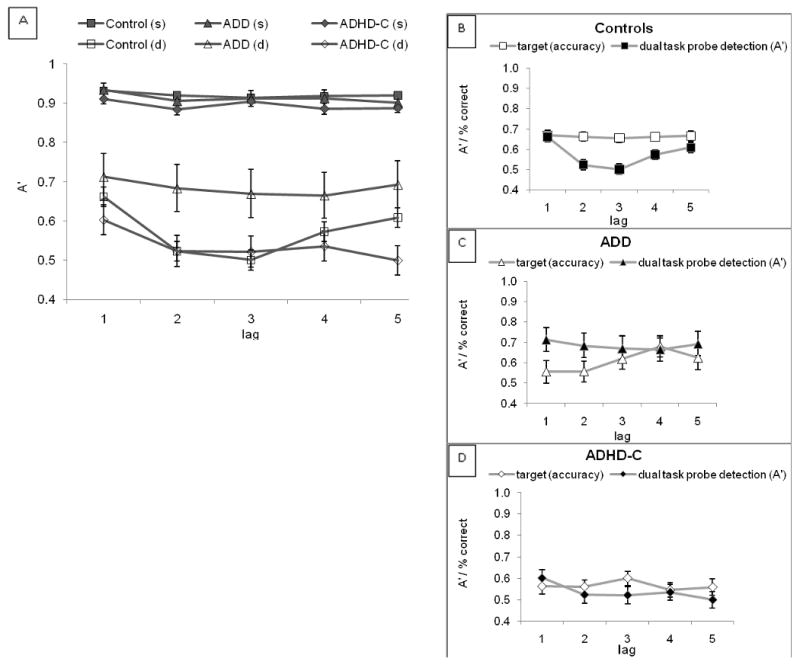
The four panels in this figure illustrate the results for all participants completing the attentional blink task. The panel directly above (Panel A) shows probe detection accuracy for all three groups in both single and dual task conditions. s=single task, d=dual task. As expected, accuracy in the single task condition is equally high for all groups, whereas probe detection suffers at lags 2 and 3 for the two groups showing an attentional blink. The three panels to the right (B-D) show target versus probe accuracy in the dual task condition for each of the three groups (error bars for the control group too small to always exceed the box size).
In the control group, a normal blink response occurred as seen in Figure 2. In the critical dual task blink region (between lag 1 and lag 3), controls demonstrated typical lag 1 sparing followed by a precipitous drop in probe sensitivity from lag 1 to lag 2. This decrease in probe detection at lags 2 and 3 is the attentional blink (interaction of task and lag within control group, F(4,352)=8.60, p<.001, η2=.09). As expected, performance of target identification remained stable across all positions relative to the probe (p=.98) (Figure 2B), suggesting the “blink” supported the primary task of target identification across temporal positions of the probe.
In the ADHD-C group, there was significant effect of task (p<.001, η2=.82), but the simple effect for lag was not significant (p=.17). The interaction of task and lag was also nonsignificant (p=.54), suggesting the blink was attenuated or variable. However, as in the control group, target accuracy for this group (Figure 2C) also remained relatively consistent across probe positions (p=.39), suggesting sufficient gating of the probe that target detection across temporal positions was not compromised.
The ADD group, on the other hand, showed a very different pattern as illustrated in panel 2C. As the figure suggests, there was no simple effect of lag on probe detection (p=.93) and no interaction of task and lag (p=.93). The pattern of performance in this figure suggests impaired attentional gating failed to insulate target processing from competing probes occurring in the first three lag positions. Probe detection was relatively high for lags 1 and 2, and remained stable across temporal positions. However, target accuracy showed qualitatively inconsistent performance which tended to be worst at the early lags and improving with greater temporal distance from the probe (later lags). This pattern suggests that the ADD group was unable to temporally allocate sufficient attention to consistently gate competing probes. This compromised performance of the primary task of target identification and consolidation.
Comparison of diagnostic models
Table 2 summarizes the results of the statistical analyses of the task for each diagnostic model (that is, ADD defined by normative cut points, ADD defined by ≤ 2 symptoms of hyperactivity/impulsivity, and ADHD-PI as defined in DSM-IV). It shows the results already discussed for the primary model (“Model 1”) in which ADD was defined by the Conners normative cutoff as having less than average activity. Model 2, using KSADS symptom counts (parent interview) at ≤ 2 symptoms of hyperactivity, also showed a task by group interaction, though it did not distinguish between the subtypes with regard to probe detection. Nonetheless, it suggests results rather similar to Model 1. Model 3 relies on the DSM-IV subtype definitions and showed no group interaction or subtype simple effects, indicating that the effect we observed was not detectable when more symptoms of hyperactivity/impulsivity were present as in the DSM-IV definition of ADHD-PI. This suggests a configural pattern in which ADD is detected only when identified with fewer total symptoms.
TABLE 2. Attentional Blink Summary of Group Interactions and Effect Sizes for Diagnostic Models Examined.
| (All participants who completed both tasks) | (All participants who completed blink task) | ||||||
|---|---|---|---|---|---|---|---|
| Blink Task | Blink Task | ||||||
| Model | Diagnostic Criteria | n ADD† | Pairwise Comparisons | Task × Group p (h2) | Pairwise p | Task × Group p (h2) | Pairwise p |
| 1 | Conners (mean [P,T]) Hyper Tscores<50 | 15, 16 | ADD v ADHD-C | .041 (.051) | .004 | .027 (.050) | .003 |
| Controls v ADD | .107 | .037 | |||||
| Controls v ADHD-C | .034 | .083 | |||||
| 2 | KSAD (P) Hyper Symptoms <2 | 23, 27 | ADD v ADHD-C | .041 (.048) | .079 | .022 (.049) | .050 |
| Controls v ADD | .836 | .452 | |||||
| Controls v ADHD-C | .040 | .089 | |||||
| 3 | DSM-IV PI | 34, 39 | ADHD-PI v ADHD-C | n.s. | ‡ | ‡ | n.s. |
| Controls v ADHD-PI | |||||||
| Controls v ADHD-C | |||||||
P=Parent, T=Teacher, Hyper= Hyperactivity
N for ADD participants in both tasks, N for all completing blink task
Main effect of group was not significant
Motor Suppression: Antisaccade Task Results
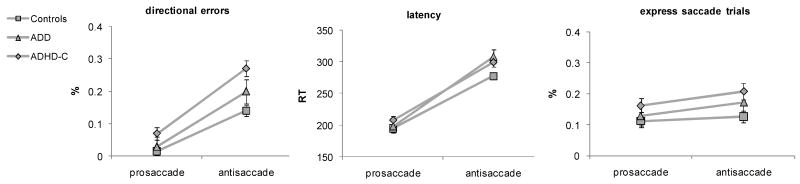
Several variables can be obtained from the antisaccade task, which indicate various aspects of cognitive and motoric processes. We focused on latency as a measure of task engagement and on error rate and anticipatory saccades as primary outcome variables measuring cognitive control. Prosaccade performance involves consistent stimulus-response mapping and manifests as fast reaction time of reflect reflexive, automatic movement, few directional errors and a reduced number of anticipatory movements from fixation. The antisaccade condition represents inconsistent stimulus-response mapping and thus requires top-down control to suppress reflexive movements toward the target, and subsequent generation of movement in the direction opposite that of the target. Compared to the prosaccade condition, this process is time consuming and effortful, and manifests as longer reaction times (when the task is performed according to instructions), increased directional errors and a greater proportion of anticipatory movements from starting fixation (Munoz & Everling, 2004). As has been demonstrated in previous studies, we predicted that antisaccade errors would be worse in the ADHD-C group.
Preliminary task analysis
Analysis of saccade latency showed main effects of task, F(1,122) =52.29, p<.001, η2=.300, group, F(2,122) =3.73, p=.027, η2=.158, and an interaction between task and group, F(2,122)=3.08, p=.049, η2=.048. Follow-up analyses showed that controls were faster in the antisaccade condition than both the ADHD-C group, F(1,86) =5.26, p=.024, η2=.058, and the ADD group, F(1,108) =6.91, p=.010, η2=.060. There were no group differences in the prosaccade condition (p>.1). ADHD and ADD groups did not differ from each other in either condition (both, p=>.4). These effects suggested the task was more effortful for the ADD and ADHD groups than the controls. Further, there is no evidence of speed-accuracy trade-off because both groups showed slowed performance and yet had higher error rates compared to control children. We proceeded to analyze the error data and interpret it in relation to the primary hypotheses.
Primary hypothesis tests in antisaccade
The results of the antisaccade task are illustrated in Figure 3, with the aforementioned latency data in the leftmost panel. Results of the various statistical models are summarized in Table 3. Results showed that both ADHD subtypes tended to have weakness in response inhibition, but did not differ from one another. For directional errors, there were main effects of task, F(1,122) =16.76, p<.001, η2=.121 and group, F(1,122) =9.49, p<.001, η2=.135 which were qualified by an interaction of group and task, F(2,122)=3.12, p=.048, η2=.049. Follow-up multivariate ANCOVAs including pro- and antisaccade error rates showed that the ADHD-C group committed more errors than controls in both pro- and antisaccade conditions (respectively, F(1,108)=4.61, p=.034, η2=.041 and F(1,108) =18.13, p<.001, η2=.144). ADHD and ADD did not differ in either condition (both p>.3). The ADD group committed more errors than controls in the antisaccade condition only, F(1,86) =4.09, p=.046, η2=.045.
FIGURE 3.
Saccade task results for directional errors, latency and percentage of anticipator saccades for pro- and antisaccade conditions. Controls (n=74), ADHD-PI (-H) (n=15), and ADHD-C (n=37). Error bars show one standard error above and below the mean (two standard error total span).
TABLE 3. Antisaccade Task Summary of Group Interactions and Effect Sizes for Diagnostic Models Examined.
| Saccade Task | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Latency | Errors | Express Saccades | |||||||
| Model | Diagnostic Criteria | n ADD Group | Pairwise Comparisons | Task × Group p (h2) | Pairwise p | Task × Group p (h2) | Pairwise p | Task × Group p (h2) | Pairwise p |
| 1 | Conners (mean [P,T]) Hyper Tscores<50 | 15 | ADD v ADHD-C | .049 (.048) | .947 | .048 (.049) | .086 | .050 (.048) | .395 |
| Controls v ADD | .090 | .212 | .400 | ||||||
| Controls v ADHD-C | .015 | <.001 | .015 | ||||||
| 2 | KSAD (P) Hyper Symptoms <2 | 23 | ADD v ADHD-C | n.s. | .858 | .024 (.056) | .044 | n.s. | ‡ |
| Controls v ADD | .048 | .124 | |||||||
| Controls v ADHD-C | .012 | <.001 | |||||||
| 3 | DSM-IV PI | 34 | ADHD-PI v ADHD-C | .023 (.052) | .609 | n.s. | .075 | n.s. | .612 |
| Controls v ADHD-PI | .053 | .022 | .093 | ||||||
| Controls v ADHD-C | .011 | <.001 | .023 | ||||||
P=Parent, T=Teacher, Hyper= Hyperactivity
Main effect of group was not significant.
The same set of analyses was performed for the percentage of trials on which participants made express saccades (anticipations) during the delay before target onset. There were main effects of task, F(2,122) =4.76, p=.031, η2=.038, and group, F(2,122) =3.06, p=.050, η2=.048, but no interaction. Pairwise comparisons showed that the ADHD-C group had greater difficulty maintaining fixation during the delay period compared to the control group, (p=.015). The ADD group differed neither from controls (p=.40), nor from the ADHD-C group (p=.36). Qualitatively, there was an ordinal pattern to express saccade rate from the control to ADD to ADHD-C group (12%, 15% and 18%, respectively).
Comparison of diagnostic models
Once again, two additional diagnostic models were tested. Table 3 summarizes the results of the above analyses (“Model 1”) as well as analyses using a cutoff of ≤ 2 symptoms of hyperactivity on the KSADS to define ADD (“Model 2”), and by using DSM-IV subtyping (“Model 3”). Unlike Model 1, Model 2 did differentiate the subtypes: there was a significantly higher error rate in the antisaccade condition for ADHD-C than both controls (p<.001) and ADD (p=.044). In Model 3, the DSM-IV subtypes did not differ from one another.
Summary Analysis Across Both Antisaccade and Attentional Blink
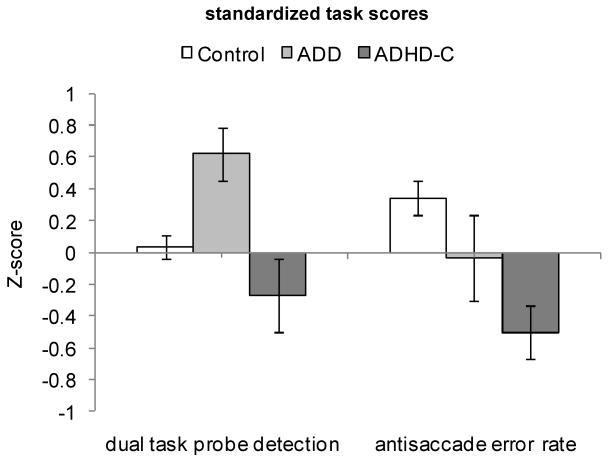
The clearest evidence of a distinction between the types would be a dissociation in which the differences in effect size between the tasks are different between the groups. To attempt to directly compare how the groups differed with respect to the two domains of attentional filtering and cognitive control, standardized scores were computed for (a) antisaccade directional errors and (b) attentional blink dual task probe detection. Figure 4 depicts the standardized scores for each task in each group. Scores were submitted to MANCOVA with task (blink, antisaccade) as the within-subjects factor and diagnostic group (control, ADD and ADHD-C) as the between-subjects factor. Consistent with the dissociation hypothesis, the task × group interaction was significant, F(2,122)=3.94, p=.022, η2=.061. This indicates that the pattern of group differences was significantly different across the two types of tasks and supports the supposition that the tasks are picking up distinct problems in the two ADHD subgroups. The interaction was due to differential effects on the two tasks: ADD had greater difficulty than ADHD-C on blink, and ADHD-C showed greater deficits than ADD on antisaccade (although as noted above, that particular difference was not significant under model 1 though it was significant under model 2). This result helps to confirm that the lack of group differences on the antisaccade task on Model 1 was not reflecting merely a subtler version of the same effect as on the blink task but shows that the tasks did perform differentially for the two affected groups, consistent with a configural interpretation.
FIGURE 4.
Standardized scores for antisaccade error rate and overall dual task probe detection performance for controls (n=74), ADD (n=15) and ADHD-C (n=37) groups. Attentional blink task z-scores were based on overall probe detection sensitivity in the dual-task condition. Error bars are mean standard errors.
Discussion
This study examined two cognitive mechanisms to attempt to validate a distinction between ADD (here, serving as a shorthand for a refined inattentive subtype with no evidence of problems in hyperactivity/impulsivity) and ADHD. It was hypothesized that ADD would be associated with particular difficulty in early-stage attentional control, whereas ADHD-C would be associated with difficulty in late-stage response inhibition. With some caveats, the findings supported this hypothesis: the ADD group tended to have different (worse) early stage attention filtering than the ADHD-C or control group, whereas both the ADD and ADHD-C groups tended to have weakness in response inhibition, though qualitatively greater for ADHD-C. A composite analysis showed significantly different results across the tasks (task by group interaction). The findings therefore are closely in line with the formulation provided by Adams et al (2008) and Diamond (2005) in that some type of inhibitory problem emerged in both ADD and ADHD, but ADD had unique problems in attentional control.
It has long been suggested that ADD should have specific weakness in the domain of early-stage attentional processing that distinguish it from ADHD-C (Hynd et al., 1991). Tests of this hypothesis have been stymied by lack of research criteria for ADD (no formal criteria were provided in DSM-III), and reliance on DSM-IV subtypes, which evidently do not capture the ADD entity if it exists. Therefore this study relied on a simple conceptual definition: children who otherwise meet criteria for ADHD (inattentive type) but are clearly within the normal range on the activity scale of a nationally normed rating scale (defined as T<50). We also tested a second idea, ≤2 symptoms of hyperactivity, as proposed by Volk et al (2009).
Consistent with many prior studies, there were no clear differences between ADHD-C and ADHD-PI as defined by DSM-IV on these tasks. However, differences began to emerge once ADD was operationalized as a subgroup of the ADHD-PI group. On the attentional blink task, the pattern of findings was consistent with the proposal of Diamond (2005), Hynd et al (1991) and Milich et al (2001), suggesting that ADD was not merely a mild version of ADHD but rather had distinct cognitive abnormalities in early stage attentional processing. This is consistent with suggestions by Diamond (2005) and Hynd (1991) that ADD is related to abnormalities in frontal-posterior cortical networks. The ADD group had the most atypical performance, with abnormal early-stage attentional control that differentiated it from ADHD-C and typically developing youth. However, this finding only emerged using our normative cut-off definition of ADD. It did not fully reproduce using a definition of ≤2 symptoms of DSM-IV hyperactivity/impulsivity. The pattern of dual task performance in the ADHD-C group, though qualitatively somewhat abnormal as well, did not statistically differ from controls.
In the ADD group, performance was consistent with the hypothesis that early-stage attentional processing may be a specific cognitive weakness for this group. The suggestion by Adams et al (2008) that ADD might evidence slow processing speed and would tend to support our prediction that ADD would feature impairment in maximizing attentional resources when needed, and filtering temporally competing information in a temporally demanding context. The pattern of dual task performance suggests that the ADD group could not adequately recruit capacity-limited attentional resources needed to encode and identify the target letter, but was capable of the less demanding process of probe detection. Thus, in the ADD group, probes that occurred at typical blink lag positions interfered with or prevented consolidation of primary task targets. This finding would be consistent with disturbances in frontoparietal networks as suggested by Diamond (2005). The pattern of response is also striking in its similarity to data from patients with unilateral visual neglect from stroke in right parietal, frontal and basal ganglia regions (Husain et al, 1997). In addition, a recent visuospatial choice reaction time study by Querne & Berquin (2009) showed markedly slow reaction times in the predominantly inattentive subtype group (defined by DSM-IV) and markedly fast response times in the hyperactive/impulsive group, suggesting different mechanisms of processing between the subtypes.
It is important to recognize that the results here are not simply a case of results getting clearer with “more extreme” group definition. The ADD group did not have more inattention symptoms than the ADHD-PI group as a whole. They only had fewer hyperactive impulsive symptoms. Thus, the ADD group is less extreme in clinical severity when seen in the context of the entire ADHD-PI group from which it was selected. Instead it represents a different configuration of symptoms in which inattention is present but hyperactivity-impulsivity is not.
Although we cannot rule out a strategy effect (or failure to do the task as intended), this appears unlikely based on the task parameters. Results relied on main effects of accuracy. While these findings are interpretable, they are somewhat weaker evidence for inferring a formal attentional deficit than a 3-way interaction would have been. Prior findings with the attentional blink task in ADHD have been mixed. However, earlier studies had not delineated an inattentive group with few if any hyperactive/impulsive symptoms and are thus difficult to directly comparable to ours. However, Carr et al. (2006) showed an essentially normal attentional blink response in both ADHD subtypes in adults. In children with ADHD, Li et al. (2004) found a protracted and larger magnitude of blink which differs from the results obtained here. In another study, Li et al. (2005) examined a nonclinical group of adolescents with high and low impulsivity and found that participants high on impulsivity showed a temporally displaced blink effect; but that study did not specifically examine ADHD subtypes.
With regard to response inhibition, the ADHD-C group committed more directional errors in both pro- and antisaccade conditions compared to controls, as predicted; the ADD group differed from controls but only in the antisaccade condition. The ADHD-C finding here is not new, and the ADD finding tends to confirm other reports (e.g., Derefinko et al., 2008) that ADD also tends to show some problems in response inhibition. However, when the diagnostic model relied on the DSM-IV symptoms (≤2) and not on the normative scores, the ADHD-C group showed reliably worse performance than the ADD group.
The ADD group also showed the qualitatively slowest responses (longer response latency) on the anti-saccade task. Human and nonhuman primate studies of pro- and antisaccade behavior has shown that activity in frontal regions is increased to a greater extent on antisaccade than on prosaccade trials. Further, activity is decreased for antisaccade trials on which a direction error is made (Brown et al., 2007; Munoz & Everling, 2004). Thus, the increased latency on antisaccade trials for the ADD group could be the result of a slower progression of neural activity needed to suppress reflexive saccades. In any event, the response inhibition findings confirm in a large adolescent sample, an apparently developmentally stable weakness in top-down control of saccadic movements in ADHD similar to what is seen in children and adults (Carr et al, 2006; Karatekin, 2006; Klein et al, 2003; Munoz et al, 2003).
Limitations
There are several limitations to note. First, the sample size of the ADD group obviously was small. The present findings may be viewed as suggestive that pursuit of this approach may be fruitful in evaluating an ADD entity. Further, the ADHD-C group did not have a completely normal attentional blink, suggesting that even though we identified group differences, overlap (dimensionality) in the observed problems may still exist. Third, the present study did not examine temporal positions beyond approximately 500 ms on the attentional blink task. Studies that have included later-occurring probes have sometimes found that ADHD groups tend to recover from the blink more slowly than controls (Hollingsworth et al., 2001). It remains possible that additional ADHD effects might emerge in the ability to recover from the blink with extended probe positions. This is particularly notable for the ADD group, which appeared to adapt to the dual task at a later temporal stage.
Implications for Research, Policy, and Practice
For some cognitive tasks, both subtype groups may demonstrate poor performance compared to controls. However, the etiology of that performance may be very different between the subtype groups. Diagnostic descriptions of behavior need to be quantifiable and testable, taking into consideration both cognitive and neurobiological profiles of symptomatology. Tasks like the antisaccade and attentional blink, for which parameters have been empirically validated as measuring some of the putative component mechanisms of cognitive control, can help to further delineate the cognitive and potentially neurobiological differences between subtypes. As noted earlier, further studies are needed to specifically discern motivational aspects of task performance and level of arousal in ADD from those of processing speed and their potential interaction. Clinically, the data suggest that it remains important for clinicians to take note of children with high inattention but very low levels of hyperactivity. When defined in this way, our results suggest that an ADD entity may exist which is mechanistically distinguishable not only in severity but in cognitive profile from ADHD-C.
TABLE 4. Analysis Summary of Standardized Scores for Antisaccade Error Rate and Attentional Blink Dual Task Probe Detection.
| Analysis of Standardized Antisaccade + Blink Scores* | Dual Task Probe Detection | Antisaccade Error Rate | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Diagnostic Criteria | n ADD Group | Pairwise Comparisons | Task × Group p (η2) | Pairwise p | Group p | Pairwise p | Group p | Pairwise p |
| 1 | Conners (mean [P,T]) Hyper Tscores<50 | 15 | ADD v ADHD-C | .022 (.061) | .001 | 0.016 | .004 | <.001 | .104 |
| Controls v ADD | .573 | .037 | .148 | ||||||
| Controls v ADHD-C | <.001 | .201 | <.001 | ||||||
| 2 | KSAD (P) Hyper Symptoms <2 | 23 | ADD v ADHD-C | .024 (.055) | .007 | n.s. | -- | <.001 | .117 |
| Controls v ADD | .573 | .034 | |||||||
| Controls v ADHD-C | <.001 | <.001 | |||||||
| 3 | DSM-IV PI | 34 | ADHD-PI v ADHD-C | .029 (.049) | .019 | n.s. | -- | <.001 | .162 |
| Controls v ADHD-PI | .193 | .007 | |||||||
| Controls v ADHD-C | <.001 | <.001 | |||||||
P=Parent, T=Teacher, Hyper= Hyperactivity
Antisaccade condition error rate and attentoinal blink dual task probe detection (A′) scores were transformed to z-scores; z-scores served as the dependent variable in a repeated measures ANCOVA with task as the within group factor.
Acknowledgments
This work was supported by NIH R01 MH-59105 and MH- MH63146.
Contributor Information
Laurie Carr, Michigan State University.
John Henderson, University of Edinburgh.
Joel T. Nigg, Oregon Health & Science University
References
- Achenbach T. Manual for the Young Adult Self-Report and Young Adult Behavior Checklist. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Adams ZW, Derefinko KJ, Milich R, Fillmore MT. Inhibitory functioning across ADHD subtypes: recent findings, clinical implications, and future directions. Developmental Disabilities Research Reviews. 2008;14:268–275. doi: 10.1002/ddrr.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1285–92. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. Journal of Abnormal Psychology. 2002;111:279–289. [PubMed] [Google Scholar]
- Benes FM. The development of prefrontal cortex: The maturation of neurotransmitter systems and their interactions. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2001. pp. 79–92. [Google Scholar]
- Booth JE, Carlson CL, Tucker DM. Performance on a neurocognitive measure of alerting differentiates ADHD combined and inattentive subtypes: a preliminary report. Archives of Clinical Neuropsychology. 2007;22:423–32. doi: 10.1016/j.acn.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Brown MR, Vilis T, Everling S. Frontoparietal activation with preparation for antisaccades. Journal of Neurophysiology. 2007;98:1751–62. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Carmer SG, Swanson MR. An evaluation of ten pairwise multiple comparison procedures by Monte Carlo methods. Journal of the American Statistical Association. 1973;68:66–74. [Google Scholar]
- Carr LA, Nigg JT, Henderson JM. Attentional versus motor inhibition in adults with attention-deficit/hyperactivity disorder. Neuropsychology. 2006;20:430–441. doi: 10.1037/0894-4105.20.4.430. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones R, Hare T. The adolescent brain. In: Kingstone A, Miller MB, editors. Annals of the New York Academy of Sciences: The Year in Cognitive Neuroscience. Vol. 1124. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Potter MC. A two-stage model for multiple target detection in rapid serial visual presentation. Journal of Experimental Psychology: Human Perception and Performance. 1995;21:109–27. doi: 10.1037//0096-1523.21.1.109. [DOI] [PubMed] [Google Scholar]
- Clarke SD, Kohn MR, Hermens DF, Rabbinge M, Clark CR, Gordon E, Williams LM. Distinguishing symptom profiles in adolescent ADHD using an objective cognitive test battery. International Journal of Adolescent Medicine and Health. 2007;19:355–67. doi: 10.1515/ijamh.2007.19.3.355. [DOI] [PubMed] [Google Scholar]
- Conners CK, Erhardt D, Sparrow E. Adult ADHD Rating Scales: Technical Manual. Toronto, Ontario, Canada: Multi-Health Systems; 1999. [Google Scholar]
- Derefinko KJ, Adams ZW, Milich R, Fillmore MT, Lorch EP, Lynam DR. Response style differences in the inattentive and combined subtypes of attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2008;36:745–758. doi: 10.1007/s10802-007-9207-3. [DOI] [PubMed] [Google Scholar]
- Diamond A. Attention-deficit disorder (attention-deficit/hyperactivity disorder without hyperactivity): A neurobiologically and behaviorally distinct disorder from attention-deficit/hyperactivity disorder (with hyperactivity) Developmental Psychopathology. 2005;17:807–825. doi: 10.1017/S0954579405050388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Ward R, Shapiro K. Direct measurement of attentional dwell time in human vision. Nature. 1994;369(6478):313–5. doi: 10.1038/369313a0. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale–IV: Checklists, Norms, and Clinical Interpretations. New York: Guilford Press; 1998. [Google Scholar]
- Faraone SV, Biederman J, Spencer T, Wilens T, Seidman LJ, Mick E, Doyle AE. Attention-deficit/hyperactivity disorder in adults: An overview. Biological Psychiatry. 2000;48:9–20. doi: 10.1016/s0006-3223(00)00889-1. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Verte S, Oosterlaan J, Roeyers H, Sergeant JA. ADHD subtypes: Do they differ in their executive functioning profile? Archives of Clinical Neuropsychology. 2005;20:457–477. doi: 10.1016/j.acn.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132:560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Hart EL, Lahey BB, Loeber R, Hanson KS. Criterion validity of informants in the diagnosis of disruptive behavior disorders in children: A preliminary study. Journal of Consulting and Clinical Psychology. 1994;62:410–4. doi: 10.1037/0022-006X.62.2.410. [DOI] [PubMed] [Google Scholar]
- Hein G, Alink A, Kleinschmidt A, Müller NG. The attentional blink modulates activity in the early visual cortex. Journal of Cognitive Neuroscience. 2009;21:197–206. doi: 10.1162/jocn.2008.21026. [DOI] [PubMed] [Google Scholar]
- Hollingsworth DE, McAuliffe SP, Knowlton BJ. Temporal allocation of visual attention in adult attention deficit hyperactivity disorder. Journal of Cognitive Neuroscience. 2001;13:298–305. doi: 10.1162/08989290151137359. [DOI] [PubMed] [Google Scholar]
- Hommel B, Kessler K, Schmitz F, Gross J, Akyurek E, Shapiro K, Schnitzler A. How the brain blinks: Towards a neurocognitive model of the attentional blink. Psychological Research. 2006;70:425–435. doi: 10.1007/s00426-005-0009-3. [DOI] [PubMed] [Google Scholar]
- Huang-Pollock CL, Nigg JT. Searching for the attention deficit in attention deficit hyperactivity disorder: The case of visuospatial orienting. Clinical Psychology Review. 2003;23:801–830. doi: 10.1016/s0272-7358(03)00073-4. [DOI] [PubMed] [Google Scholar]
- Husain M, Shapiro K, Martin J, Kennard C. Abnormal temporal dynamics of visual attention in spatial neglect patients. Nature. 1997;385:154–156. doi: 10.1038/385154a0. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Ettinger U. The antisaccade task as a research tool in psychopathology: A critical review. Psychophysiology. 2006;43:302–313. doi: 10.1111/j.1469-8986.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- Hynd GW, Lorys AR, Semrud-Clikeman M, Nieves N, Huettner MI, Lahey BB. Attention deficit disorder without hyperactivity: A distinct behavioral and neurocognitive syndrome. Journal of Child Neurology. 1991;6:S37–S43. doi: 10.1177/0883073891006001s05. [DOI] [PubMed] [Google Scholar]
- Karatekin C. Improving antisaccade performance in adolescents with attention-deficit/hyperactivity disorder (ADHD) Experimental Brain Research. 2006;174:324–341. doi: 10.1007/s00221-006-0467-x. [DOI] [PubMed] [Google Scholar]
- Kawahara J, Zuvic SM, Enns JT, Di Lollo V. Task switching mediates the attentional blink even without backward masking. Perception & Psychophysics. 2003;65:339–51. doi: 10.3758/bf03194565. [DOI] [PubMed] [Google Scholar]
- Klein CH, Raschke A, Brandenbusch A. Development of pro- and antisaccades in children with attention-deficit hyperactivity disorder (ADHD) and healthy controls. Psychophysiology. 2003;40:17–28. doi: 10.1111/1469-8986.00003. [DOI] [PubMed] [Google Scholar]
- Krain AL, Castellanos FX. Brain development and ADHD. Clinical Psychology Review. 2006;26(4):433–44. doi: 10.1016/j.cpr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Li CS, Lin WH, Chang HL, Hung YW. A psychophysical measure of attention deficit in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Psychology. 2004;113(2):228–36. doi: 10.1037/0021-843X.113.2.228. [DOI] [PubMed] [Google Scholar]
- Li CS, Chen SH, Lin WH, Yang YY. Attentional blink in adolescents with varying levels of impulsivity. Journal of Psychiatry Research. 2005;39(2):197–205. doi: 10.1016/j.jpsychires.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Luciana M. Cognitive neuroscience and the prefrontal cortex: Normative development and vulnerability to psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Volume 2: Developmental neuroscience. Hoboken, NJ: John Wiley & Sons; 2006. pp. 292–330. [Google Scholar]
- Mannuzza S, Klein RG, Moulton JL. Persistence of Attention-Deficit/Hyperactivity Disorder into adulthood: What have we learned from the prospective follow-up studies? Journal of Attention Disorders. 2003;7(2):93–100. doi: 10.1177/108705470300700203. [DOI] [PubMed] [Google Scholar]
- Mason DJ, Humphreys GW, Kent L. Insights into the control of attentional set in ADHD using the attentional blink paradigm. Journal of Child Psychology and Psychiatry. 2005;46:1345–1353. doi: 10.1111/j.1469-7610.2005.01428.x. [DOI] [PubMed] [Google Scholar]
- Milich R, Balentine AC, Lynam DR. ADHD combined type and ADHD predominantly inattentive type are distinct and unrelated disorders. Clinical Psychology: Science and Practice. 2001;8:463–488. [Google Scholar]
- Miller LM, Sun FT, Curtis CE, D'Esposito M. Functional interactions between oculomotor regions during prosaccades and antisaccades. Human Brain Mapping. 2005;26:119–127. doi: 10.1002/hbm.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTA Cooperative Group. Multimodal treatment study of children with ADHD. A 140 month randomized clinical trial of treatment strategies for attention deficit/hyperactivity disorder. Archives of General Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Armstrong IT, Hampton KA, Moore KD. Altered control of visual fixation and saccadic eye movements in attention-deficit hyperactivity disorder. Journal of Neurophysiology. 2003;90:503–514. doi: 10.1152/jn.00192.2003. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Blaskey LG, Huang-Pollack CL, Rappley MD. Neuropsychological executive functions and DSM–IV AD/HD subtypes. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:59–66. doi: 10.1097/00004583-200201000-00012. [DOI] [PubMed] [Google Scholar]
- O'Driscoll GA, Depatie L, Holahan AL, Savion-Lemieux T, Barr RG, Jolicoeur C, Douglas VI. Executive functions and methylphenidate response in subtypes of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1452–1460. doi: 10.1016/j.biopsych.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Persson J, Welsh KM, Jonides J, Reuter-Lorenz PA. Cognitive fatigue of executive processes: Interaction between interference resolution tasks. Neuropsychologia. 2007;45:1571–9. doi: 10.1016/j.neuropsychologia.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piek JP, Dyck MJ, Nieman A, Anderson M, Hay D, Smith LM, McCoy M, Hallmayer J. The relationship between motor coordination, executive functioning and attention in school adged children. Archives of Clinical Neuropsychology. 2004;19(8):1063–76. doi: 10.1016/j.acn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Müri RM, Nyffeler T, Milea D. The role of the human dorsolateral prefrontal cortex in oculomotor behavior. Annals of the New York Academy of Sciences. 2005;1039:239–51. doi: 10.1196/annals.1325.023. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Ryan N. The Schedule for Affective Disorders and Schizophrenia for School-Age Children (Kiddie-SADS)—1986. Pittsburgh, PA: Western Psychiatric Institute and Clinic; 1986. [Google Scholar]
- Querne L, Berquin P. Distinct response time distributions in attention deficit hyperactivity disorder subtypes. Journal of Attention Disorders. 2009;13(1):66–77. doi: 10.1177/1087054708323006. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: An attentional blink? Journal of Experimental Psychology: Human Perception and Performance. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Rowe DC, Stever C, Giedinghagen LN, Gard JM, Cleveland HH, Terris ST, Mohr JH, Sherman S, Abramowitz A, Waldman ID. Dopamine DRD4 receptor polymorphism and attention deficit hyperactivity disorder. Molecular Psychiatry. 1998;3:419–426. doi: 10.1038/sj.mp.4000432. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Huijbregts S, Sheres A, Oosterlaan J. The top and bottom of ADHD: A neuropsychological perspective. Neuroscience and Biobehavioral Reviews. 2003;27(7):583–92. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 1996;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Solanto MV, Gilbert SN, Raj A, Zhu J, Pope-Boyd S, Stepak B, Vail L, Newcorn JH. Neurocognitive functioning in AD/HD, predominantly inattentive and combined subtypes. Journal of Abnormal Child Psychology. 2007;35(5):729–44. doi: 10.1007/s10802-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior Research Methods, Instruments and Computers. 1999;31(1):137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Stawicki JA, Nigg JT, von Eye A. Family psychiatric history evidence on the nosological relations of DSM-IV ADHD combined and inattentive subtypes: New data and meta-analysis. Journal of Child Psychology and Psychiatry. 2006;47(9):935–45. doi: 10.1111/j.1469-7610.2006.01628.x. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Dockstader C, Tannock R. Temporal information processing in ADHD: findings to date and new methods. Journal of Neuroscience Methods. 2006;151(1):15–29. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- van Mourik R, Oosterlaan J, Sergeant JA. The Stroop revisited: A meta-analysis of interference control in AD/HD. Journal of Child Psychology and Psychiatry. 2005;46:150–165. doi: 10.1111/j.1469-7610.2004.00345.x. [DOI] [PubMed] [Google Scholar]
- Volk HE, Todorov AA, Hay DA, Todd RD. Simple identification of complex ADHD subtypes using current symptom counts. Journal of the Academy of Child and Adoloescent Psychiatry. 2009;48:441–50. doi: 10.1097/CHI.0b013e31819996ba. [DOI] [PMC free article] [PubMed] [Google Scholar]