Abstract
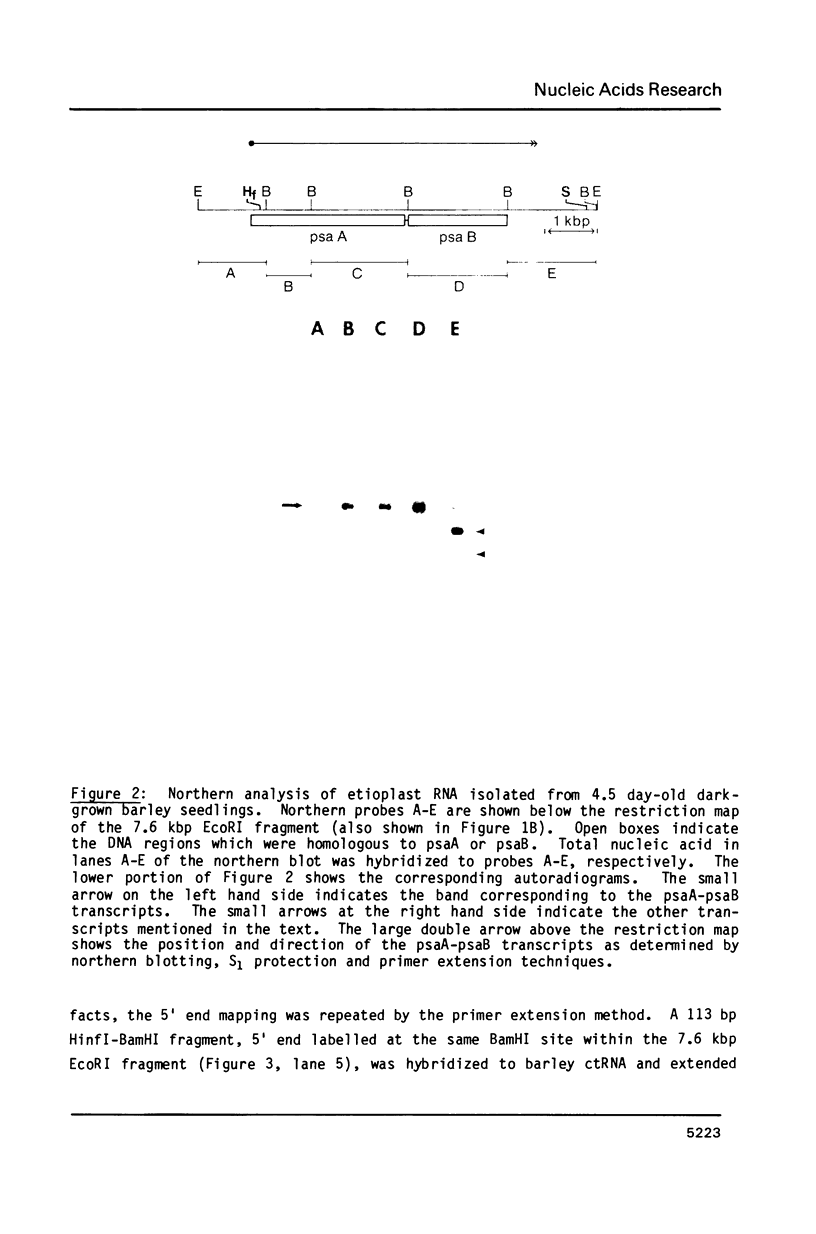
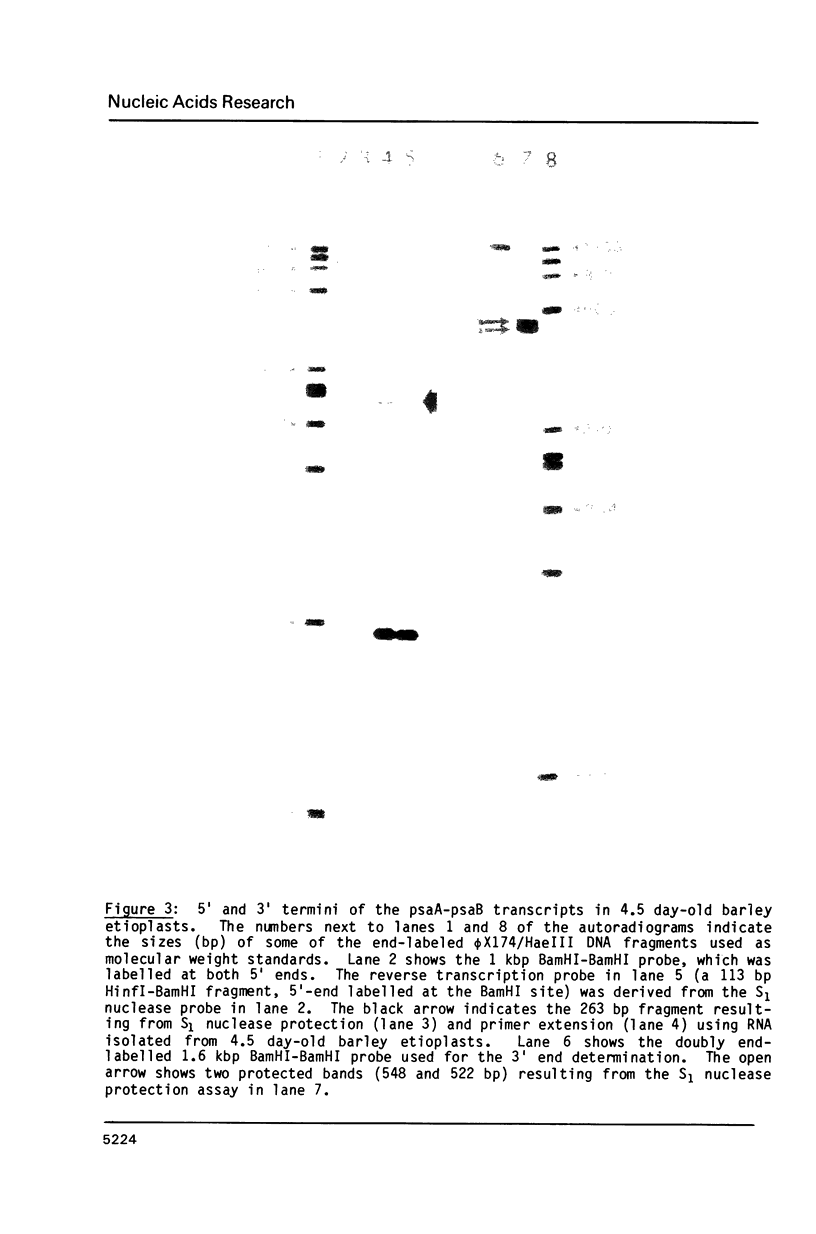
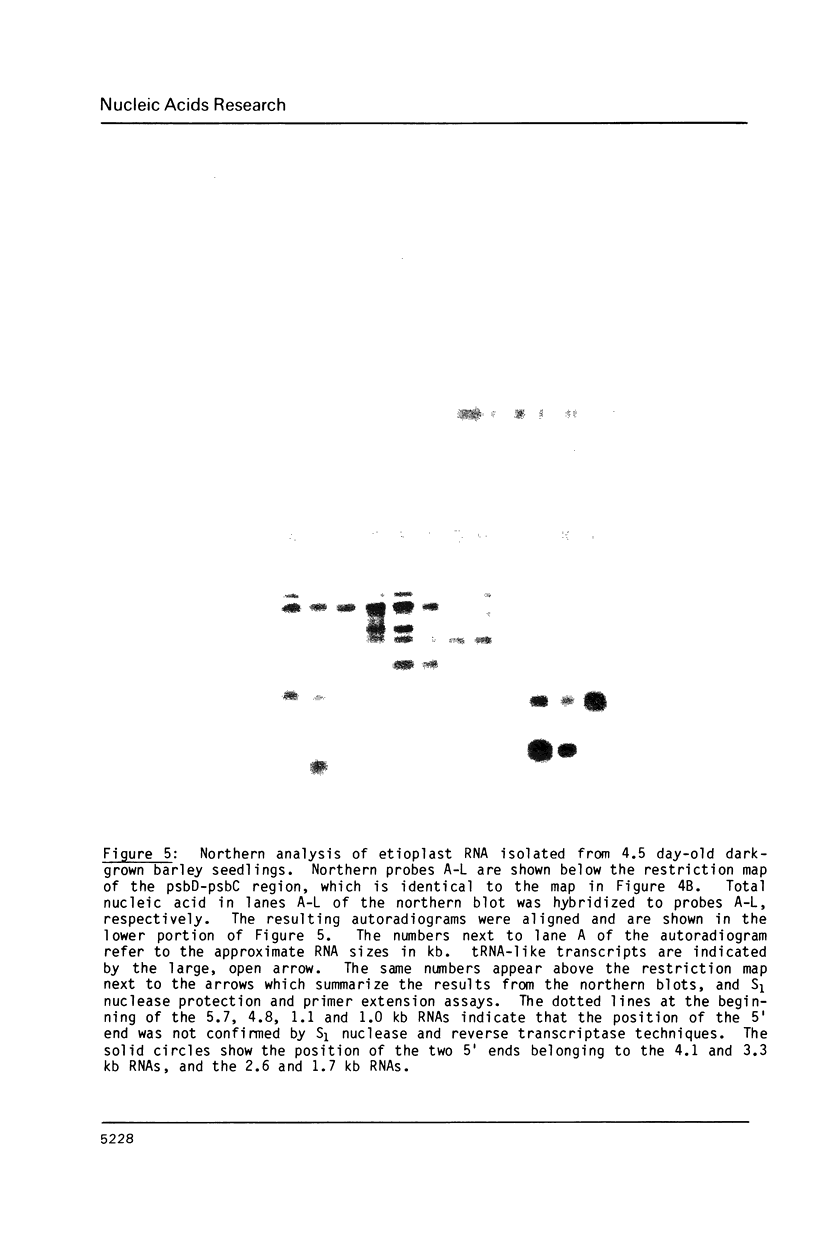
Four plastid genes, psaA, psaB, psbD and psbC, were localized on the barley plastid genome. PsaA was adjacent to psaB in one transcription unit and psbD was adjacent to psbC in a second transcription unit. The transcription units containing psaA-psaB and psbD-psbC are separated by approximately 25 kbp on the barley plastid genome and are transcribed convergently. Transcripts hybridizing to each transcription unit were characterized by northern blot analysis, S1 protection experiments and primer extension analysis. Two 5.3 kb transcripts hybridize to psaA-psaB. The two transcripts have a common 5' end but differ at their 3' ends by about 26 nucleotides. The transcription unit which contains psbD-psbC also includes trnS(UGA), trnG(GCC), and an open reading frame which codes for a 62 amino acid protein. Six large transcripts ranging from 5.7 kb to 1.7 kb hybridize to the psbD-psbC transcription unit as well as several RNAs of tRNA size. The large transcripts arise from three 5' ends and two clusters of 3' ends. The 3' ends map near trnG(GCC) and trnS(UGA) and could be generated by RNA processing or termination of transcription. Two of the six transcripts hybridize to psbC but not psbD suggesting that translation of psbD and psbC could occur on separate RNAs.
Full text
PDF




















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellemare G., Bartlett S. G., Chua N. H. Biosynthesis of chlorophyll a/b-binding polypeptides in wild type and the chlorina f2 mutant of barley. J Biol Chem. 1982 Jul 10;257(13):7762–7767. [PubMed] [Google Scholar]
- Clayton D. A. Transcription of the mammalian mitochondrial genome. Annu Rev Biochem. 1984;53:573–594. doi: 10.1146/annurev.bi.53.070184.003041. [DOI] [PubMed] [Google Scholar]
- Fish L. E., Kück U., Bogorad L. Two partially homologous adjacent light-inducible maize chloroplast genes encoding polypeptides of the P700 chlorophyll a-protein complex of photosystem I. J Biol Chem. 1985 Feb 10;260(3):1413–1421. [PubMed] [Google Scholar]
- Gounaris K., Barber J., Harwood J. L. The thylakoid membranes of higher plant chloroplasts. Biochem J. 1986 Jul 15;237(2):313–326. doi: 10.1042/bj2370313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. C., Bird C. R., Courtice G. R., Hird S. M., Howe C. J., Huttly A. K., Phillips A. L., Smith A. G., Willey D. L., Bowman C. M. Chloroplast genes for photosynthetic membrane components of higher plants. Biochem Soc Trans. 1986 Feb;14(1):25–27. [PubMed] [Google Scholar]
- Gregory P., Bradbeer J. W. Plastid development in primary leaves of Phaseolus vulgaris. Development of plastid adenosine triphosphatase activity during greening. Biochem J. 1975 Jun;148(3):433–438. doi: 10.1042/bj1480433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Holschuh K., Bottomley W., Whitfeld P. R. Sequence of the genes for tRNACys and tRNAAsp from spinach chloroplasts. Nucleic Acids Res. 1983 Dec 20;11(24):8547–8554. doi: 10.1093/nar/11.24.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holschuh K., Bottomley W., Whitfeld P. R. Structure of the spinach chloroplast genes for the D2 and 44 kd reaction-centre proteins of photosystem II and for tRNASer (UGA). Nucleic Acids Res. 1984 Dec 11;12(23):8819–8834. doi: 10.1093/nar/12.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L. S., Thompson W. F., Briggs W. R. Different Red Light Requirements for Phytochrome-Induced Accumulation of cab RNA and rbcS RNA. Science. 1984 Dec 21;226(4681):1447–1449. doi: 10.1126/science.226.4681.1447. [DOI] [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Control of gene expression during higher plant chloroplast biogenesis. Protein synthesis and transcript levels of psbA, psaA-psaB, and rbcL in dark-grown and illuminated barley seedlings. J Biol Chem. 1987 Mar 25;262(9):4341–4348. [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Regulation of chloroplast-encoded chlorophyll-binding protein translation during higher plant chloroplast biogenesis. J Biol Chem. 1986 Aug 25;261(24):11138–11145. [PubMed] [Google Scholar]
- Kreuz K., Dehesh K., Apel K. The light-dependent accumulation of the P700 chlorophyll a protein of the photosystem I reaction center in barley. Evidence for translational control. Eur J Biochem. 1986 Sep 15;159(3):459–467. doi: 10.1111/j.1432-1033.1986.tb09908.x. [DOI] [PubMed] [Google Scholar]
- Mackender R. O. Etioplast Development in Dark-grown Leaves of Zea mays L. Plant Physiol. 1978 Oct;62(4):499–505. doi: 10.1104/pp.62.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Plesnicar M., Bendall D. S. The photochemical activities and electron carriers of developing barley leaves. Biochem J. 1973 Nov;136(3):803–812. doi: 10.1042/bj1360803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley F., Weil J. H. Organization and sequence of five tRNA genes and of an unidentified reading frame in the wheat chloroplast genome: evidence for gene rearrangements during the evolution of chloroplast genomes. Curr Genet. 1985;9(6):495–503. doi: 10.1007/BF00434054. [DOI] [PubMed] [Google Scholar]
- Robertson D., Laetsch W. M. Structure and function of developing barley plastids. Plant Physiol. 1974 Aug;54(2):148–159. doi: 10.1104/pp.54.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodermel S. R., Bogorad L. Maize plastid photogenes: mapping and photoregulation of transcript levels during light-induced development. J Cell Biol. 1985 Feb;100(2):463–476. doi: 10.1083/jcb.100.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak H. F., Grivell L. A., Borst P. Transcription of mitochondrial DNA. CRC Crit Rev Biochem. 1983;14(4):297–317. doi: 10.3109/10409238309102797. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Vierling E., Alberte R. S. Regulation of synthesis of the photosystem I reaction center. J Cell Biol. 1983 Dec;97(6):1806–1814. doi: 10.1083/jcb.97.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff P., Alt J., Herrmann R. G. Localization of the genes for the two chlorophyll a-conjugated polypeptides (mol. wt. 51 and 44 kd) of the photosystem II reaction center on the spinach plastid chromosome. EMBO J. 1983;2(12):2229–2237. doi: 10.1002/j.1460-2075.1983.tb01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurawski G., Bottomley W., Whitfeld P. R. Junctions of the large single copy region and the inverted repeats in Spinacia oleracea and Nicotiana debneyi chloroplast DNA: sequence of the genes for tRNAHis and the ribosomal proteins S19 and L2. Nucleic Acids Res. 1984 Aug 24;12(16):6547–6558. doi: 10.1093/nar/12.16.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]







