Abstract
The estimation of nondisplaceable binding from cerebellar white matter, rather than from whole cerebellum, was proposed for the PET tracer carbonyl-11C-WAY-100635 (N-{2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl}-N-(2-pyridyl)cyclohexane-carboxamidel]) because of the heterogeneity of total ligand binding in this region. For the 5-hydroxytryptamine receptor 1A (5-HT1A) antagonist 18F-N-{2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl}-N-2-pyridyl)trans-4-fluorocyclohexanecarboxamide (18F-FCWAY), the estimation of nondisplaceable binding from cerebellum (VND) may be additionally biased by spillover of 18F-fluoride activity from skull. We aimed to assess the effect of using cerebral white matter as reference region on detection of group differences in 5-HT1A binding with PET and 18F-FCWAY.
Methods
In 22 temporal lobe epilepsy patients (TLE) and 10 healthy controls, 18F-FCWAY distribution volume in cerebral white matter (VWM) was computed using an extrapolation method as part of a partial-volume correction (PVC) algorithm. To assess the feasibility of applying this method to clinical studies in which PVC is not performed, VWM was also calculated by placing circular, 6-mm-diameter regions of interest (ROIs) in the centrum semiovalis on parametric images. Binding potentials were BPF = (VT − VND)/fP and BPF-WM = (VT − VWM)/fP, where VT is total distribution volume and fP = 18F-FCWAY plasma free fraction. Statistical analysis was performed using t tests and linear regression.
Results
In the whole group, VWM was 14% ± 19% lower than VND (P < 0.05). VWM/fP was significantly (P < 0.05) lower in patients than in controls. All significant (P < 0.05) reductions of 5-HT1A receptor availability in TLE patients detected by BPF were also detected using BPF-WM. Significant (P < 0.05) reductions of 5-HT1A specific binding were detected by BPF-WM, but not BPF, in ipsilateral inferior temporal cortex, contralateral fusiform gyrus, and contralateral amygdala. However, effect sizes were similar for BPF-WM and BPF. The value of VWM calculated with the ROI approach did not significantly (P > 0.05) differ from that calculated with the extrapolation approach (0.67 ± 0.32 mL/mL and 0.72 ± 0.34 mL/mL, respectively).
Conclusion
Cerebral white matter can be used for the quantification of nondisplaceable binding of 5-HT1A without loss of statistical power for detection of regional group differences. The ROI approach is a good compromise between computational complexity and sensitivity to spillover of activity, and it appears suitable to studies in which PVC is not performed. For 18F-FCWAY, this approach has the advantage of avoiding spillover of 18F-fluoride activity onto the reference region.
Keywords: epilepsy, white matter, PET, 5-HT1A receptors, 18F-FCWAY
Pet studies of serotonergic 5-hydroxytryptamine receptor 1A (5-HT1A) are of interest in several neuropsychiatric disorders. Carbonyl-11C-WAY-100635 and 18F-N-{2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl}-N-2-pyridyl)trans-4-fluorocyclohexanecarboxamide (18F-FCWAY) are frequently used for quantitative measurements with PET of 5-HT1A receptor specific binding. Quantification of the binding potential requires the estimation of nondisplaceable (free plus nonspecific) binding. Autoradiographic studies showed that, among gray matter regions, 5-HT1A receptor concentration was lowest in the cerebellum (1,2). Therefore, this region traditionally has been used for the estimation of nondisplaceable binding for 5-HT1A tracers.
More recently, PET studies with carbonyl-11C-WAY-100635 indicated a significant concentration of 5-HT1A in cerebellar vermis and cortex of adult subjects and suggested that cerebellar white matter could be a better reference region for the estimation of nondisplaceable binding (3,4). The potential advantage of using cerebellar white matter has been addressed in a limited number of studies (3–5).
The possibility of using a reference region other than the cerebellum is of particular interest for the 5-HT1A tracer 18F-FCWAY (6). After intravenous injection, this tracer rapidly undergoes defluorination to 18F-fluoride, which irreversibly accumulates in the skull (7), causing substantial spillover of activity into the neighboring brain tissue. Despite the development of a correction strategy (8,9), tissue counts can be biased, especially in low-binding regions such as the cerebellum. Therefore, regions of interest (ROIs) have been drawn far from the cortical rim to avoid bias in binding potential measurement (10).
On the basis of results obtained for carbonyl-11C-WAY-100635 with cerebellar white matter and because of the distance of the centrum semiovalis from the extracerebral 18F-fluoride activity, we hypothesized that cerebral white matter could be a valid alternative to the cerebellum for the estimation of nondisplaceable binding for 18F-FCWAY. The aim of this study was to assess the impact on detection of differences in 5-HT1A receptor availability between temporal lobe epilepsy (TLE) patients and healthy controls using 18F-FCWAY binding in cerebral white matter as an estimate of nondisplaceable binding. The regional pattern of group differences obtained using the cerebellum was previously reported (10).
MATERIALS AND METHODS
Patient Selection
Data from 22 TLE patients (18 men; mean age ± SD, 37 ± 11 y) and 10 healthy volunteers (7 men; mean age, 35 ± 9 y) previously studied (10) were reexamined. Briefly, patients were referred for evaluation of medically refractory TLE. All patients were taking different combinations of antiepileptic drugs (10,11). None had experienced partial seizures for at least 2 d, or a secondarily generalized tonic clonic seizure for at least 1 mo, before PET studies. T1-weighted volumetric MR images were acquired for segmentation purposes, and T2-weighted and fluid-attenuated inversion recovery (FLAIR) images were obtained for the evaluation of mesial temporal sclerosis. The study was approved by the National Institute of Neurological Disorders and Stroke Institutional Review Board and the National Institutes of Health Radiation Safety Committee.
PET Procedure
Each patient was scanned with an Advance Tomograph (GE Healthcare). A bolus of 333 ± 74 MBq of 18F-FCWAY was injected intravenously, and dynamic scanning was performed for 120 min in 3-dimensional mode. Arterial samples were taken to quantify plasma 18F-FCWAY and 18F-fluorocyclohexanecarbox-ylic acid metabolite (18F-FC) concentrations and whole-blood activity. The 18F-FCWAY fraction unbound to plasma proteins was measured with ultracentrifugation (12).
PET Data Analysis
Radioactivity frames registered to MR images (13) were corrected for cerebral uptake of acid radioactive metabolite, intravascular radioactivity, and 18F-fluoride spillover onto the brain (8,9). The method to correct for 18F-fluoride spillover assumes that there is a known true skull time–activity curve, and each brain region receives a fractional contribution of this time–activity curve that is higher for areas that are closer to the skull (8,9). Next, a previously developed partial-volume correction (PVC) algorithm (10,14) was applied. Briefly, the PVC algorithm corrects on a frame-by-frame basis gray matter pixels for spill-out of gray matter activity and for spill-in of activity from white matter (15). No correction for partial-volume averaging between adjacent gray matter regions is performed (16,17). The procedure is based on the segmentation of MR images into binary masks (18), which are subsequently smoothed to the PET resolution; these smoothed masks are named sGM, sWM, and sCSF. The corrected activity gray matter values can be calculated as follows:
| Eq. 1 |
where CPVC represents the corrected activity value in a gray matter pixel after PVC, CORIG is the original uncorrected pixel value, CWM is the estimated white matter activity, and sGM and sWM are the pixel values (range, 0–1) from the smoothed masks for gray and white matter, respectively. Original and corrected pixel data were fitted to a 2-tissue-compartment model with 3 parameters using the metabolite-corrected input function to provide parametric images of 18F-FCWAY distribution volume (V).
18F-FCWAY distribution volume in cerebral white matter (VWM) was estimated directly on parametric images. To obtain an accurate estimate of VWM, pixel values that represent 100% white matter should be used. Such pixels have a high sWM value (close to the maximum value of 1) and are typically found in the centrum semiovalis. Therefore, pixels with sWM values between 0.986 and 0.995 were identified. The lower threshold of 0.986 was chosen to ensure a stable fit in all patients. Potential inaccuracies in white matter estimation introduce only marginal errors in PVC-corrected gray matter values (15,19), but the effect is expected to be more important if white matter is used as the estimate of nondisplaceable binding. An anatomic constraint was applied to avoid sampling from the basal ganglia and thalamus, in which gray matter is frequently misidentified as white matter (14). Values of these pixels were then fitted as a linear function of sWM, and the fitted value at sWM = 1 was used as an estimate of nondisplaceable binding.
The extrapolation method is used for studies in which PVC is performed. To assess the feasibility of using white matter for more routine clinical studies without PVC, VWM was also calculated with an ROI procedure on parametric PET images. First, 3 contiguous transverse slices for which the centrum semiovalis displayed minimal activity were identified. Next, a 6-mm ROI was centered on the area of lowest signal in both hemispheres. This operation was performed on each identified slice, using a total of 6 ROIs. The mean pixel value was calculated in each ROI, and the 6 regional values were averaged to obtain VWM.
Derivation of Binding Potential
Binding potentials were derived using both VND and VWM, as following:
| Eq. 2 |
| Eq. 3 |
where VT is the total distribution volume in the target ROI, VND is the distribution volume in the cerebellum, and fP is 18F-FCWAY plasma free fraction (20).
ROI Analysis
Temporal and extratemporal ROIs were drawn on MR images and applied to coregistered parametric images (10). Cerebellar ROIs were initially drawn along the outer cortical edge, then uniformly shrunk until, on visual inspection, no spillover of activity was observed. Final cerebellar ROIs were about 50% smaller than original ROIs (10). ROI measurements were computed using only gray matter pixels, as defined by the gray matter segment (18). For the cerebellum, for which gray matter segment definition is not accurate, the mean ROI value was computed from unmasked PET images coregistered to MRI volumes (10). The effect size was computed as the difference between patients and controls divided by the SD in the control group. Statistical analysis was performed using t test and linear regression, and statistical significance was set at uncorrected P < 0.05.
RESULTS
Figure 1 shows transverse images of 18F-FCWAY distribution volume in a TLE patient. In the cerebellum, the large spillover of 18F-fluoride activity masks the visualization of possible differences in specific binding between gray and white matter. At the cortical level, there is a clear difference in 18F-FCWAY binding between centrum semiovalis white matter and gray matter or mixed gray matter–white matter regions. Spillover of 18F-fluoride activity is observed in the left frontal and bilateral parietal cortex.
FIGURE 1.
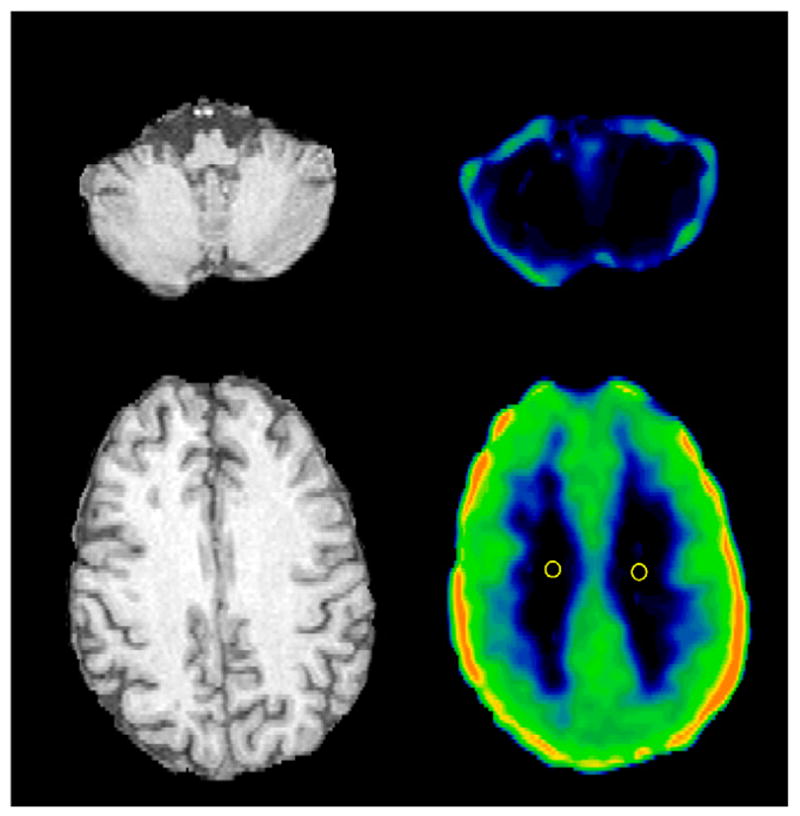
Transverse MR and 18F-FCWAY distribution volume images passing through centrum semiovalis and cerebellum in TLE patient. Negligible binding of tracer in cerebellar white matter and centrum semiovalis and large spillover of 18F-fluoride activity onto cerebellum were observed. Two 6-mm-diameter, circular ROIs used for calculation of VWM with manual approach are displayed. 18F-FCWAY distribution volume image (V/fP) is scaled to maximum of 80 mL/mL.
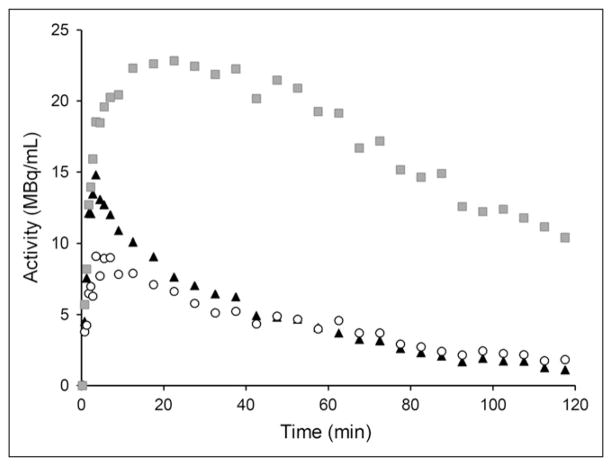
Figure 2 shows representative time–activity curves from a healthy control. In white matter, activity peaked at about 5 min after injection, then progressively decreased to 57%, 24%, and 21% of peak at 30, 60, and 120 min, respectively. The activity peak in white matter was about 40% lower than that in cerebellum. White matter and cerebellar time–activity curves overlapped, starting from about 25 min after injection. As expected, in both regions the time–activity curve was markedly different from the time–activity curve of the mesial temporal cortex.
FIGURE 2.
Representative time–activity curves from healthy control for white matter (○, 1.9 cm2), cerebellum (▴, 4.2 cm2), and mesial temporal cortex ( , 2.8 cm2).
, 2.8 cm2).
Figure 3 shows the extrapolation method used to automatically estimate VWM, as part of the PVC process. On the distribution volume images, values of voxels with smoothed white matter mask sWM between 0.986 and 0.995 were fitted to a straight line, and the extrapolated value for sWM = 1 was taken as an estimate of nondisplaceable binding. The volume of white matter voxels with an sWM between 0.986 and 0.995 was 19 ± 5 mL. The quality of fit, as quantified by r2 values, was good in the whole sample (r2 = 0.88 ± 0.11; range, 0.59–0.99).
FIGURE 3.
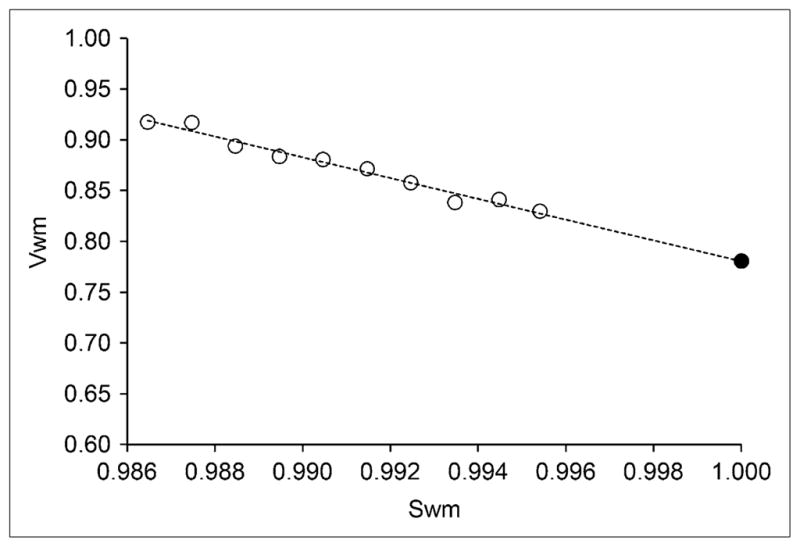
Estimation of 18F-FCWAY distribution volume in white matter (VWM) with extrapolation method. Values for VWM are plotted vs. smoothed white matter mask (sWM). VWM values of voxels with sWM between 0.986 and 0.995 (○) were fitted to straight line, and value at sWM = 1.0 (●) was used as estimate of nondisplaceable binding.
In the whole sample, VWM estimated with the extrapolation approach was significantly smaller than VND (0.72 ± 0.34 mL/mL and 0.84 ± 0.30 mL/mL; paired t test, P < 0.05). The percentage difference between VWM and VND was 14% ± 19%, without significant (P > 0.05) differences between TLE patients and controls. On a subject-by-subject basis, VWM was lower or of the same magnitude as VND in all but 3 subjects, in whom VWM was 11%, 14%, and 29% higher than VND. A significant relationship was found between VWM and VND (regression equation, VWM = −0.10 + 0.99 · VND [r2 = 0.76; P < 0.05]) (Fig. 4). VWM was significantly (P < 0.05) higher in patients than in controls (0.81 ± 0.35 mL/mL and 0.55 ± 0.25 mL/mL, respectively). 18F-FCWAY fP was significantly higher in patients than in controls (0.121 ± 0.033 vs. 0.066 ± 0.024, respectively; P < 0.05), a finding that may be attributed to antiepileptic drugs (10,11). VWM/fP, however, was significantly (P < 0.05) lower in patients than in controls (6.80 ± 2.56 mL/mL and 9.32 ± 3.16 mL/mL, respectively).
FIGURE 4.
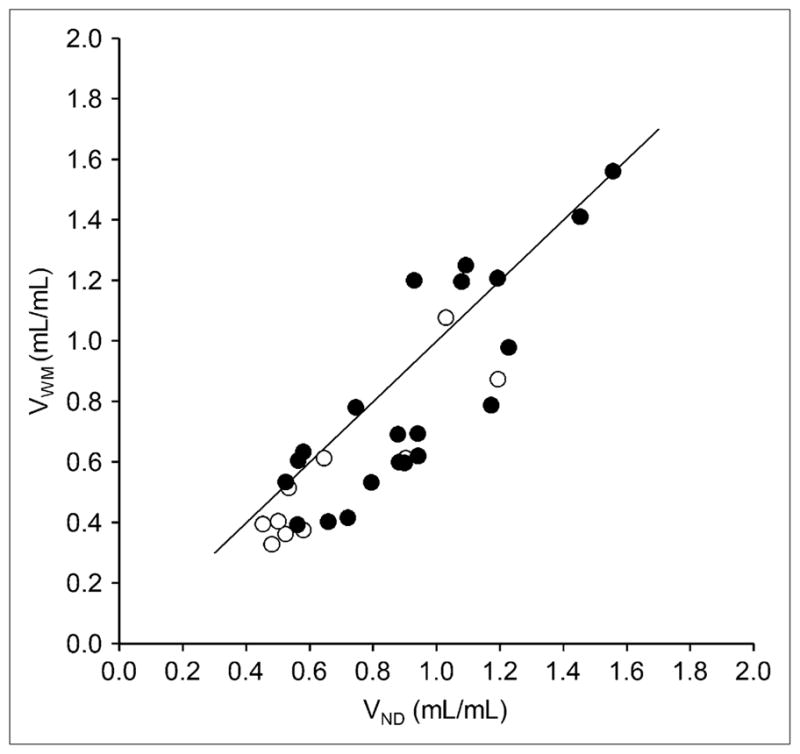
Correlation between 18F-FCWAY distribution volume in white matter (VWM) and in cerebellum (VND) in controls (○) and in TLE patients (●). For whole group, regression equation was VWM = −0.10 + 0.99 · VND (r2 = 0.76; P < 0.05).
The variability of VWM, as quantified by the percentage coefficient of variation (%CV), was greater than that of VND (45.2% vs. 39.5% in controls and 43.0% vs. 31.6% in patients, respectively). Nevertheless, the variability of BPF-WM was not significantly higher (P > 0.05) than that of BPF, respectively. In the whole group, mean values of %CV across ROIs were 36.8% ± 5.6 and 35.5% ± 6.0, respectively, for BPF and BPF-WM.
All significant differences detected using BPF (10) were also detected using BPF-WM. Significantly (P < 0.05) lower BPF-WM, but not BPF, was detected in ipsilateral inferior temporal cortex, contralateral fusiform gyrus, and contralateral amygdala. However, effect sizes were similar for BPF-WM and BPF (Table 1).
TABLE 1.
Group Differences Detected with BPF-WM and BPF
| Region | BPF-WM (mL/mL) | BPF (mL/mL)* | ||||
|---|---|---|---|---|---|---|
| Controls | Patients | P | Effect size | P | Effect size | |
| I. sup. temporal | 90 ± 26 | 77 ± 32 | 0.214 | −0.53 | 0.280 | −0.50 |
| C. sup. temporal | 86 ± 24 | 74 ± 27 | 0.199 | −0.52 | 0.264 | −0.49 |
| I. mid. temporal | 97 ± 28 | 80 ± 29 | 0.113 | −0.60 | 0.154 | −0.57 |
| C. mid. temporal | 93 ± 24 | 78 ± 25 | 0.096 | −0.65 | 0.135 | −0.63 |
| I. inf. temporal | 103 ± 29 | 80 ± 28 | 0.034 | −0.78 | 0.052 | −0.76 |
| C. inf. temporal | 100 ± 26 | 91 ± 32 | 0.423 | −0.32 | 0.528 | −0.29 |
| I. fusiform | 128 ± 35 | 82 ± 24 | <0.001 | −1.35 | <0.001 | −1.34 |
| C. fusiform | 122 ± 32 | 97 ± 32 | 0.041 | −0.78 | 0.058 | −0.76 |
| I. parahippocampus | 146 ± 38 | 84 ± 26 | <0.001 | −1.62 | <0.001 | −1.62 |
| C. parahippocampus | 146 ± 42 | 103 ± 29 | 0.002 | −1.02 | 0.003 | −1.02 |
| I. hippocampus | 129 ± 29 | 66 ± 31 | <0.001 | −2.20 | <0.001 | −2.22 |
| C. hippocampus | 126 ± 34 | 99 ± 30 | 0.025 | −0.79 | 0.035 | −0.78 |
| I. amygdala | 75 ± 17 | 51 ± 20 | 0.002 | −1.39 | 0.005 | −1.37 |
| C. amygdala | 77 ± 16 | 62 ± 22 | 0.045 | −0.94 | 0.078 | −0.89 |
| I. insula | 97 ± 28 | 64 ± 21 | 0.001 | −1.18 | 0.001 | −1.17 |
| C. insula | 97 ± 27 | 67 ± 20 | 0.001 | −1.11 | 0.002 | −1.10 |
| I. frontal | 70 ± 18 | 68 ± 23 | 0.643 | −0.16 | 0.820 | −0.11 |
| C. frontal | 67 ± 17 | 64 ± 23 | 0.648 | −0.16 | 0.828 | −0.11 |
| I. parietal | 65 ± 18 | 65 ± 22 | 0.914 | 0.01 | 0.890 | 0.06 |
| C. parietal | 64 ± 18 | 68 ± 24 | 0.761 | 0.20 | 0.593 | 0.26 |
| I. occipital | 41 ± 11 | 45 ± 13 | 0.524 | 0.39 | 0.298 | 0.51 |
| R. occipital | 39 ± 8 | 47 ± 16 | 0.235 | 1.02 | 0.130 | 1.10 |
Values obtained using VND as estimate of nondisplaceable binding (10).
I. = ipsilateral to focus; sup. = superior; C. = contralateral to focus; mid. = middle; inf. = inferior.
In the whole group, values of VWM calculated with the ROI approach did not significantly differ from those obtained with the extrapolation technique (0.67 ± 0.32 mL/mL and 0.72 ± 0.34 mL/mL, respectively; P > 0.05). Group differences in specific binding were detected without any regional discrepancy in comparison to those detected with the extrapolation approach.
DISCUSSION
The cerebellum has been used in several PET studies to estimate nondisplaceable binding of 5-HT1A. However, for both carbonyl-11C-WAY-100635 (21–23) and 18F-FCWAY (8), a 2-compartment model was necessary to accurately fit cerebellar time–activity curves. This finding was attributed to a slow component of nondisplaceable binding or to the progressive accumulation of radioactive metabolites (8,21–23). This unexpected finding motivated the search for nontraditional reference regions for the estimation of non-displaceable binding.
Using PET with carbonyl-11C-WAY-100635, Parsey et al. found that cerebellar white matter time–activity curves were well described by a 1-tissue-compartment model (3). Using cerebellar white matter, rather than the whole cerebellum, for the derivation of the binding potential improved the identifiability and time stability in all cortical regions. The authors concluded that for carbonyl-11C-WAY-100635, cerebellar white matter could be a better reference region than the whole cerebellum (3).
For 18F-FCWAY, the estimation of nondisplaceable binding from the cerebellum has the additional problem of spillover of 18F-fluoride activity. This applies to the cortex as well, even though specific binding is high in cortical regions. A method to correct for such spillover was developed. This method, however, does not accurately take into account intersubject variations in the skull shape and thickness (8,9). Disulfiram was used to inhibit defluorination of 18F-FCWAY in humans (24). The drug reduced the accumulation of 18F-fluoride in the skull and improved the visualization of radioligand cerebral binding. However, disulfiram also affected the clearance of 18F-FCWAY and acid radioactive metabolite and jeopardized the accuracy of the compartmental analysis (24).
A simple approach to minimize the effect of 18F-fluoride spillover onto the cerebellum is to draw ROIs far from the cerebellar edge (10). However, some residual spillover from the bone or mixing of regions with different 5-HT1A receptor concentrations cannot be excluded. Alternatively, a different reference region could be identified.
Suitability of Cerebral White Matter for Quantification of 18F-FCWAY Nondisplaceable Binding
The suitability of a region to provide an estimate of nondisplaceable binding can be inferred from kinetic analysis or preblocking studies. Adequate fitting with a 1-tissue-compartment model and negligible k3 and k4 values are consistent with a lack of significant concentration of specific receptors. In preblocking studies, the baseline regional distribution volume is compared with that obtained after the administration of a specific receptor cold ligand. If a region is truly devoid of specific binding, the regional value should be comparable in the 2 conditions. Carson et al. performed preblocking 18F-FCWAY PET studies in monkeys (8). The administration of unlabelled WAY-100635 in advance produced a significant reduction in total binding in the cerebellum by about 44%. This result is inconsistent with the assumption that the cerebellum is a region with only nondisplaceable binding sites for 18F-FCWAY. Unfortunately, changes of the distribution volumes in white matter were not evaluated (8). Admittedly, the lack of preblocking studies and of formal kinetic analysis, that is, analysis of goodness of fit and calculation of rate constants, represents a significant limit of this study.
Cerebral white matter would be expected to be suitable for the quantification of nondisplaceable binding. White matter is composed of bundles of myelinated axons. The myelin sheath, which is produced by glial cells, is a bimolecular layer of lipids interspersed between protein layers. No significant expression of 5-HT1A or receptor messenger RNA was found in glial cells (25–27).
Visual inspection of both carbonyl-11C-WAY-100635 (3,28) and 18F-FCWAY (8,10,24) images shows low activity in the centrum semiovalis.
Finally, starting from about 25 min after the injection of 18F-FCWAY, the cerebral white matter time–activity curve is similar to the cerebellar time–activity curve, and both time–activity curves have characteristics consistent with negligible concentration of specific binding sites, compared with the cortex.
Group Differences Using White Matter
We failed to show a substantial gain in statistical power using white matter. In TLE patients, lower 5-HT1A receptor specific binding was detected in ipsilateral inferior temporal cortex, contralateral fusiform gyrus, and contralateral amygdala using VWM but not VND (Table 1). However, by comparing P values and effect sizes, it is evident that the lack of significant group differences in these regions using BPF (10) is likely a false-negative finding. These results are not unexpected given that regional values of specific binding are much greater than those of nondisplaceable binding, independently of the reference region. Our findings are consistent with results obtained with carbonyl-11C-WAY-100635, indicating no substantial advantage in the detection of sex differences in the hippocampus using BPF-WM, compared with using BPF (3).
Comparison Between VWM and VND
We found lower VWM than VND. Although this difference was statistically significant, the magnitude of the difference (14%) was low, and several factors could contribute to this finding. Admittedly, smaller 18F-FCWAY distribution volume in white matter per se does not constitute evidence of less specific binding. Because of the low parent accumulation in these regions, differences in radioligand delivery, blood volume, radiometabolite accumulation, spillover of 18F-fluoride onto the cerebellum, and partial-volume averaging could contribute to this difference.
The lower peak of the white matter time–activity curve than of the cerebellar time–activity curve likely reflects lower flow-mediated radioligand delivery. The lower peak of the cerebellar white matter time–activity curve than of the whole cerebellum time–activity curve was reported by Parsey et al. (3) and by Hirvonen et al. (4). Starting from about 25 min after injection, the 2 time–activity curves had similar height and time dependence.
Group Differences in Nondisplaceable Binding
The absence of significant group differences in non-displaceable binding is a prerequisite for determining that measured differences of interest reflect only specific binding. Such differences can be assessed only in arterial line–based models, which are now favored less than less invasive reference tissue models (21,29–32). However, when arterial line–based models were used, significant group differences in nondisplaceable binding with 5-HT1A tracers were reported. A higher VND (not corrected for fp) was found in healthy women than in healthy men (3,33), whereas a lower uncorrected VND was found in depressed patients than in healthy controls (5). A lower VND, after correction for fp, was previously reported in these TLE patients (10,11). If a region is devoid of specific binding and fp drives the kinetics of the tracer, group differences in nondisplaceable binding estimates might be expected in values uncorrected for fp, but they should be canceled out after fp correction. These group differences could be attributed to actual differences in 5-HT1A receptor availability, nondisplaceable binding, blood volume, or acid corrections. For 18F-FCWAY, an additional artificial cause might be the spillover of 18F-fluoride activity.
Choice of Binding Outcome
Previous PET studies with carbonyl-11C-WAY-100635 found that BPF is the most sensitive binding outcome for the accurate quantification of 5-HT1A receptor availability (3,5,23,33). On the basis of this finding and to account for group differences in VND, we had chosen BPF as primary binding outcome (10,11). For 18F-FCWAY, the magnitude of nondisplaceable binding is small, as evidenced by the low value of VND or VWM, compared with VT, in cortical and limbic areas. Thus, VT primarily reflects specific binding. In this sample, regional group differences detected with BPF were similar to those detected with VT/fp without significant loss of statistical power (data not shown). Thus, if spillover of 18F-fluoride onto the cerebellum is a concern and scientific evidence supporting the use of white matter as an estimate of nondisplaceable binding is not sufficient, the small group differences in nondisplaceable binding could be neglected and VT/fp could be used as primary binding outcome in future studies in TLE patients with 18F-FCWAY (34).
Variability of VWM
The variability of VWM was higher than that of VND. Comparable %CV for cerebellar white matter and whole cerebellum was detected by both Parsey et al. (3,33) and Hirvonen et al. (4). Because of the higher specific binding of cortical regions and large interregion variability, ultimately no variability is added to 18F-FCWAY binding potential measurements.
Cerebellum and TLE
In TLE patients, the use of cerebral white matter as reference region may be preferable because of cerebellar atrophy. Epilepsy itself—and some antiepileptic drugs, particularly phenytoin—may induce cerebellar structural change and hypometabolism on 18F-FDG PET (35,36). These effects may be more severe in patients with long epilepsy duration and uncontrolled seizures, who are most likely to have PET studies (35). Moreover, changes in 5-HT concentration in the cerebellum have been reported because of antiepileptic drugs and the effects of uncontrolled epilepsy itself (37).
CONCLUSION
Using cerebral white matter, rather than the cerebellum, as an estimate of 18F-FCWAY nondisplaceable binding, we found regional reductions of 5-HT1A receptor specific binding in TLE patients, without loss of statistical power. These findings add to results obtained with carbonyl-11C-WAY-100635 that support the use of white matter for the estimation of nondisplaceable binding for 5-HT1A tracers in clinical studies and expand the emerging topic of non-traditional reference regions.
Acknowledgments
We thank Richard Carson for helpful comments and Charles Fraser for technical assistance.
References
- 1.Hall H, Lundkvist C, Halldin C, et al. Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]WAY-100635. Brain Res. 1997;745:96–108. doi: 10.1016/s0006-8993(96)01131-6. [DOI] [PubMed] [Google Scholar]
- 2.Burnet PW, Eastwood SL, Harrison PJ. [3H]WAY-100635 for 5-HT1A receptor autoradiography in human brain: a comparison with [3H]8-OH-DPAT and demonstration of increased binding in the frontal cortex in schizophrenia. Neurochem Int. 1997;30:565–574. doi: 10.1016/s0197-0186(96)00124-6. [DOI] [PubMed] [Google Scholar]
- 3.Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, John Mann J. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- 4.Hirvonen J, Kajander J, Allonen T, Oikonen V, Nagren K, Hietala J. Measurement of serotonin 5-HT1A receptor binding using positron emission tomography and [carbonyl-11C]WAY-100635: considerations on the validity of cerebellum as a reference region. J Cereb Blood Flow Metab. 2007;27:185–195. doi: 10.1038/sj.jcbfm.9600326. [DOI] [PubMed] [Google Scholar]
- 5.Hirvonen J, Karlsson H, Kajander J, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol. 2008;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- 6.Lang L, Jagoda E, Schmall B, et al. Development of fluorine-18-labeled 5-HT1A antagonists. J Med Chem. 1999;42:1576–1586. doi: 10.1021/jm980456f. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins RA, Choi Y, Huang SC, et al. Evaluation of the skeletal kinetics of fluorine-18-fluoride ion with PET. J Nucl Med. 1992;33:633–642. [PubMed] [Google Scholar]
- 8.Carson RE, Lang L, Watabe H, et al. PET evaluation of [18F]FCWAY, an analog of the 5-HT1A receptor antagonist, WAY-100635. Nucl Med Biol. 2000;27:493–497. doi: 10.1016/s0969-8051(00)00118-9. [DOI] [PubMed] [Google Scholar]
- 9.Carson RE, Wu Y, Lang L, et al. Brain uptake of the acid metabolites of F-18-labeled WAY 100635 analogs. J Cereb Blood Flow Metab. 2003;23:249–260. doi: 10.1097/01.WCB.0000046145.31247.7A. [DOI] [PubMed] [Google Scholar]
- 10.Giovacchini G, Toczek MT, Bonwetsch R, et al. 5-HT1A receptors are reduced in temporal lobe epilepsy after partial volume correction. J Nucl Med. 2005;46:1128–1135. [PMC free article] [PubMed] [Google Scholar]
- 11.Theodore WH, Giovacchini G, Bonwetsch R, et al. The effect of antiepileptic drugs on 5-HT-receptor binding measured by positron emission tomography. Epilepsia. 2006;47:499–503. doi: 10.1111/j.1528-1167.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 12.Carson RE, Channing MA, Blasberg RG, et al. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13:24–42. doi: 10.1038/jcbfm.1993.6. [DOI] [PubMed] [Google Scholar]
- 13.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 14.Giovacchini G, Lerner A, Toczek MT, et al. Brain incorporation of [11C]arachidonic acid, blood volume, and blood flow in healthy aging: a study with partial volume correction. J Nucl Med. 2004;45:1471–1479. [PubMed] [Google Scholar]
- 15.Muller-Gartner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–583. doi: 10.1038/jcbfm.1992.81. [DOI] [PubMed] [Google Scholar]
- 16.Labbe C, Froment JC, Kennedy A, Ashburner J, Cinotti L. Positron emission tomography metabolic data corrected for cortical atrophy using magnetic resonance imaging. Alzheimer Dis Assoc Disord. 1996;10:141–170. doi: 10.1097/00002093-199601030-00005. [DOI] [PubMed] [Google Scholar]
- 17.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med. 1998;39:904–911. [PubMed] [Google Scholar]
- 18.Pham DL, Prince JL. Adaptive fuzzy segmentation of magnetic resonance images. IEEE Trans Med Imaging. 1999;18:737–752. doi: 10.1109/42.802752. [DOI] [PubMed] [Google Scholar]
- 19.Meltzer CC, Kinahan PE, Greer PJ, et al. Comparative evaluation of MR-based partial-volume correction schemes for PET. J Nucl Med. 1999;40:2053–2065. [PubMed] [Google Scholar]
- 20.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 21.Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ. Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage. 1997;6:279–287. doi: 10.1006/nimg.1997.0303. [DOI] [PubMed] [Google Scholar]
- 22.Farde L, Ito H, Swahn CG, Pike VW, Halldin C. Quantitative analyses of carbonyl-carbon-11-WAY-100635 binding to central 5-hydroxytryptamine-1A receptors in man. J Nucl Med. 1998;39:1965–1971. [PubMed] [Google Scholar]
- 23.Parsey RV, Slifstein M, Hwang DR, et al. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tissue input functions. J Cereb Blood Flow Metab. 2000;20:1111–1133. doi: 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 24.Ryu YH, Liow JS, Zoghbi S, et al. Disulfiram inhibits defluorination of [18F]FCWAY, reduces bone radioactivity, and enhances visualization of radio-ligand binding to serotonin 5-HT1A receptors in human brain. J Nucl Med. 2007;48:1154–1161. doi: 10.2967/jnumed.107.039933. [DOI] [PubMed] [Google Scholar]
- 25.Burnet PW, Eastwood SL, Lacey K, Harrison PJ. The distribution of 5-HT1A and 5-HT2A receptor mRNA in human brain. Brain Res. 1995;676:157–168. doi: 10.1016/0006-8993(95)00104-x. [DOI] [PubMed] [Google Scholar]
- 26.Gerard C, Langlois X, Gingrich J, et al. Production and characterization of polyclonal antibodies recognizing the intracytoplasmic third loop of the 5-hydroxytryptamine1A receptor. Neuroscience. 1994;62:721–739. doi: 10.1016/0306-4522(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 27.Hall MD, el Mestikawy S, Emerit MB, Pichat L, Hamon M, Gozlan H. [3H]8-hydroxy-2-(di-n-propylamino)tetralin binding to pre- and postsynaptic 5-hydroxytryptamine sites in various regions of the rat brain. J Neurochem. 1985;44:1685–1696. doi: 10.1111/j.1471-4159.1985.tb07155.x. [DOI] [PubMed] [Google Scholar]
- 28.Andree B, Halldin C, Pike VW, Gunn RN, Olsson H, Farde L. The PET radioligand [carbonyl-11C]desmethyl-WAY-100635 binds to 5-HT1A receptors and provides a higher radioactive signal than [carbonyl-11C]WAY-100635 in the human brain. J Nucl Med. 2002;43:292–303. [PubMed] [Google Scholar]
- 29.Hume SP, Myers R, Bloomfield PM, et al. Quantitation of carbon-11-labeled raclopride in rat striatum using positron emission tomography. Synapse. 1992;12:47–54. doi: 10.1002/syn.890120106. [DOI] [PubMed] [Google Scholar]
- 30.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 31.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Ichise M, Cohen RM, Carson RE. Noninvasive estimation of normalized distribution volume: application to the muscarinic-2 ligand [18F]FP-TZTP. J Cereb Blood Flow Metab. 2008;28:420–430. doi: 10.1038/sj.jcbfm.9600530. [DOI] [PubMed] [Google Scholar]
- 33.Parsey RV, Oquendo MA, Simpson NR, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954:173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- 34.Toczek MT, Carson RE, Lang L, et al. PET imaging of 5-HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003;60:749–756. doi: 10.1212/01.wnl.0000049930.93113.20. [DOI] [PubMed] [Google Scholar]
- 35.Sandok EK, O’Brien TJ, Jack CR, So EL. Significance of cerebellar atrophy in intractable temporal lobe epilepsy: a quantitative MRI study. Epilepsia. 2000;41:1315–1320. doi: 10.1111/j.1528-1157.2000.tb04611.x. [DOI] [PubMed] [Google Scholar]
- 36.Theodore WH, Fishbein D, Dietz M, Baldwin P, Deitz M. Complex partial seizures: cerebellar metabolism. Epilepsia. 1987;28:319–323. doi: 10.1111/j.1528-1157.1987.tb03650.x. [DOI] [PubMed] [Google Scholar]
- 37.Meshkibaf MH, Subhash MN, Lakshmana KM, Rao BS. Effect of chronic administration of phenytoin on regional monoamine levels in rat brain. Neurochem Res. 1995;20:773–778. doi: 10.1007/BF00969688. [DOI] [PubMed] [Google Scholar]