Abstract
1α,25-Dihydroxyvitamin D3 (1,25(OH)2D3) has shown strong promise as an anti-proliferative agent in several malignancies, yet its therapeutic use has been limited by its toxicity leading to search for analogs with anti-tumor property and low toxicity. In this study we evaluated the in vitro and in vivo properties of 1,25-dihydroxyvitamin D3-3-bromoacetate (1,25(OH)2D3-3-BE), an alkylating derivative of 1,25(OH)2D3 as a potential therapeutic agent for renal cancer. Dose-response of 1,25(OH)2D3-3-BE in two kidney cancer cell-lines was evaluated for its antiproliferative and apoptotic properties, and mechanisms were evaluated by Western Blot and FACS analyses. Therapeutic potential of 1,25(OH)2D3-3-BE was assessed by determining its stability in human serum, and evaluating its efficacy in a mouse xenograft model of human renal tumor. We observed that 1,25(OH)2D3-3-BE is significantly more potent than an equivalent concentration of 1,25(OH)2D3 in inhibiting growth of A498 and Caki 1 human kidney cancer cells. 1,25(OH)2D3-3-BE-mediated growth inhibition was promoted through inhibition of cell cycle progression by down-regulating cyclin A and induction of apoptosis by stimulating caspase activity. Moreover, 1,25(OH)2D3-3-BE strongly inhibited Akt phosphorylation and phosphorylation of its downstream target, caspase 9. 1,25(OH)2D3-3-BE appeared to be stable in human serum. In xenograft mouse model of human renal tumor, 1,25(OH)2D3-3-BE was more potent at reducing tumor size compared to 1,25(OH)2D3 which was accompanied by an increase in apopotosis and reduction of cyclin A staining in the tumors. These results suggest a translational potential of this compound as a therapeutic agent in renal cell carcinoma. Data from this study and extensive studies of vitamin D for the prevention of many malignancies support the potential of 1,25(OH)2D3-3-BE for preventing renal cancer and the development of relevant in-vivo prevention models for assessing this potential, which do not exist at present.
Keywords: vitamin D, renal cancer, anti-proliferative effect, apoptosis, anti-tumor effect
INTRODUCTION
Kidney cancer is among the ten most common cancers in men and women and its rate has been increasing steadily over the past three decades. The American Cancer Society estimates that there were approximately 57,760 new cases of kidney cancer in the United States in the year 2009, and approximately 12,980 people died from this disease (1). Renal cell carcinoma (RCC) accounts for an estimated 90–95% of all kidney cancer and has been increasing at a rate of approximately 3% per year in the United States and Europe. Approximately 50% of localized RCC develops into a metastatic disease within a relatively short time frame (2). In addition, RCC characteristically produces no symptoms during its initial growth, making early diagnosis difficult, and is generally resistant to conventional chemo- and radiation therapies (2, 3). Current therapeutic options include radical nephrectomy for early stage disease and immunotherapy for advanced and metastatic stages. Anti-angiogenic agents and Raf-kinase-inhibiting small molecules have also shown promise in treating RCC, but are not curative (4–6). Clearly, more effective therapies and novel approaches to treatment of this disease are needed.
Numerous epidemiological studies have demonstrated the importance of vitamin D, dietary or otherwise, in preventing various cancers (7). Additionally, the therapeutic potential of 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3), the biologically active metabolite of vitamin D, and its analogs in cancer is well-documented (8). However, the inherent calcemic toxicity of this hormone, particularly in pharmaceutical doses, has prevented its general use as an anticancer agent (9–11). Thus, development of vitamin D analogs exhibiting potent antiproliferative activity but reduced systemic toxicity has become an active area of research (8).
We have developed novel analogs of 1,25(OH)2D3 and its pre-hormonal form, 25-hydroxyvitamin D3 (25-OH-D3), that specifically bind and label the ligand-binding pocket of the nuclear receptor for 1,25(OH)2D3 (vitamin D receptor, VDR) (12, 13). Previously, we reported that 1α,25-dihydroxyvitamin D3-3-bromoacetate (1,25(OH)2D3-3-BE) and 25-hydroxyvitamin D3-3-bromoacetate (25-OH-D3-3-BE), VDR-alkylating derivatives of 1,25(OH)2D3 and 25-OH-D3, respectively, are more potent than 1,25(OH)2D3 in promoting antiproliferative effects on human cancer cell lines, including hormone-sensitive and hormone-insensitive prostate cancer cell lines (14–17). Lange et al. also reported antiproliferative and apoptotic effects of 25-OH-D3-3-BE in high risk neuroblastoma (18).
In the present study, we compared the in vitro and in vivo growth-inhibitory properties of 1,25(OH)2D3-3-BE to 1,25(OH)2D3 in human renal cancer cells and examined potential molecular mechanisms underlying its activities. We observed that 1,25(OH)2D3-3-BE is more potent than 1,25(OH)2D3 in inhibiting the growth of A498 and Caki 1 renal cancer cells. Mechanistically, 1,25(OH)2D3-3-BE-mediated growth inhibition of renal cancer cells was associated with an increase in apoptosis, arrest in the G2/M checkpoint in the cell cycle, and inhibition of Akt-phosphorylation. In nude mice 1,25(OH)2D3-3-BE was more potent at reducing xenografted tumor size compared to 1,25(OH)2D3 which was accompanied by an increase in apopotosis and reduction of cyclin A staining in the tumors.
MATERIALS AND METHODS
Materials
1,25(OH)2D3-3-BE was synthesized according to our published procedure (19). 1α,25-Dihydroxyvitamin D3-3-[1-14C]bromoacetate (14C-1,25(OH)2D3-3-BE, specific activity 14.3 mCi/mmol) was synthesized by replacing bromoacetic acid in the synthetic scheme with [1-14C]bromoacetis acid (sp. activity 14.3 mCi/mmol, DuPont, New England Nuclear, Boston, MA). Its radiochemical purity was ascertained by co-HPLC analysis with a standard sample of 1,25(OH)2D3-3-BE. 1,25(OH)2D3 was a generous gift from Dr. Milan Uskokovic, Hoffman La-Roche, Nutley, NJ. Concentrations of 1,25(OH)2D3 and 1,25(OH)2D3-3-BE were determined spectrophotometrically using an extinction coefficient of 18,400 at 265 nm. Purity of the compounds was determined by HPLC analysis (normal and reverse phases). LY294002 was from Cell Signaling Technology (Danvers, MA). A498 (HTB-44) and Caki 1 (HTB-46) cell lines were purchased from ATCC (Manassas, VA) and maintained in DMEM with 10% FBS. 3–4 weeks old athymic male mice (average weight 20 gm) were purchased from Taconic Farms (Germantown, NY) and maintained in an AALAC-approved animal care facility at Boston University School of Medicine.
Cellular Proliferation Assay
Cellular proliferation was measured using the TACS MTT Cell Proliferation kit according to the manufacturer’s instructions (Trevigen, Gaithersburg, MD). Briefly, A498 and Caki 1 cells were plated in 96-well plates at 1000 cells per well. 16 hours later, cells were treated with 1,25(OH)2D3-3-BE, 1,25(OH)2D3 or ethanol (vehicle) control in media containing 5% FBS. The medium containing compounds was replenished every 2 days. After 7 days, MTT solution was added to each well, and the plates were incubated at 37°C for 3 hours followed by the addition of detergent reagent. The plates were incubated at 25°C for 15 h and absorbance at 570 nm measured on a microplate reader (Spectramax 190 Plate Reader, Molecular Devices, Sunnyvale, CA).
Caspase activity assay
Caspase activity was determined using the Apo-ONE Homogeneous Caspase-3/7 assay according to the manufacturer’s instructions (Promega, Madison, WI). Caspase-3/7 activity was determined following treatment of Caki 1 cells for 6 hours with 1,25(OH)2D3, 1,25(OH)2D3-3-BE or ethanol (vehicle) control. Fluorescence released following cleavage of the pro-fluorescent substrate, Z-DEVD-110 was measured at the emission maximum of 521 nm. The amount of fluorescent product generated is representative of the amount of active caspase-3/7 in the sample.
Cell cycle analysis and sub G0/G1 DNA content
A498 cells were plated at 5×105 cells in 10cm tissue culture dishes. 16 hr later the cells were treated with 1,25(OH)2D3-3-BE, 1,25(OH)2D3 or ethanol control for 6 hr. The cells were trypsinized, collected and centrifuged at 1500 rpm for 5 min. They were re-suspended in 1.5 ml saponin/PI solution (0.3% saponin (w/v), 2.5% PI (w/v), 0.1 mM EDTA, 10 μg/ml RNase in PBS) and incubated overnight in the dark. FACS analysis was performed using a Beckman Coulter FC500 flow cytometer. ModFit LT software (Verity Software House, Topsham, ME) was used for analysis.
Western blot analysis
A498 and Caki 1 cells were plated at 3×105 cells in 6 cm tissue culture dishes. 16 hr later the cells were treated with 1,25(OH)2D3-3-BE, 1,25(OH)2D3 or ethanol (vehicle control). At the indicated times, the cells were washed with PBS, scraped in PBS and collected by centrifugation. Total cellular extracts were prepared by re-suspending the cell pellets in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 7.5) containing 50 mM NaF, 1 mM sodium vanadate (Na3VO4) and protease inhibitors (Protease Inhibitor Cocktail, Sigma-Aldrich, St. Louis, MO). Following 10 min incubation of the samples on ice, the extracts were cleared by micro-centrifugation for 10 min at 14,000 rpm, supernatants were transferred to new tubes and protein concentration of each extract was determined by Bradford assay. Samples were separated on 4–12% MES NuPAGE gels (Invitrogen, Carlsbad, CA) and transferred to PVDF membrane. Signals were detected by enhanced chemiluminescence (Perkin Elmer, Boston, MA) and autoradiography. The antibodies used were anti-Akt and anti-phospho Akt (Ser 473) (Cell Signaling, #9272 and #9271 respectively), anti-phospho-caspase-9 (Ser 196) (Santa Cruz, #11755), anti cyclin A (Neomarkers, Rb-1548) and anti-β actin (Sigma, #A5441).
Serum-stability of 1,25(OH)2D3-3-BE
Pooled human serum (1 ml) was spiked with 14C-1,25(OH)2D3-3-BE (100,000 cpm) for one hr at 37°C followed by extraction with 5 × 1 ml of ethyl acetate. Combined organic extract was dried under argon and the residue was re-dissolved in a small volume of 5% H2O-MeOH, and analyzed in an Agilent 1100 Series HPLC system (Thermo-Fisher, Waltham, MA), connected to a Packard Flow Scintillation Analyzer (Model no. 150TR, Meriden, CT), using 5% H2O-MeOH as mobile phase, flow rate1.5 ml/min, detection 265 nm (for non-radioactive materials), Agilent C18 analytical column (Thermo-Fisher, Waltham, MA). Prior to the analysis of the radioactive sample a mixture of standard samples of 1,25(OH)2D3 and 1,25(OH)2D3-3-BE was analyzed under same conditions of HPLC-analysis.
Xenograft tumor growth in athymic mice
Caki 1 cells were grown, trypsinized and re-suspended in PBS to obtain a concentration of 5×106 cells/100 μL. Cell suspensions (100 μL aliquots) were injected subcutaneously in the flanks of athymic mice. When tumors grew to approximately 100 mm3 the animals were separated into 6 animals per group, and were treated with 1,25(OH)2D3-3-BE, 1,25(OH)2D3 (dissolved in 5% dimethylacetmide in sesame oil, 0.75 μg/kg body weight/100 μl), or vehicle by intraperiteneal injection every third day. Tumor size and body weights were measured on days of injection. Treatments stopped once the control group tumors reached an average volume of 1.5 cm3 when animals were killed. Tumors were excised and stored in 10% neutral buffered formalin, and blood samples were collected by cardiac puncture. Statistical analysis of tumor size was carried out by Students t-test. Serum-calcium of the treated animals was measured according to manufacturer’s instructions (Quantichrom Calcium Assay Kit, #DICA-500, BioAssay Systems, Hayward, CA)..
Histochemistry
Mouse tumors were fixed in 10% neutral buffered formalin for 48 hours before tissue processing into paraffin wax. Five micron sections were cut and mounted onto positively charged slides. Hematoxylin and eosin (H & E) staining was performed using standard methods. Briefly, sections were deparaffinized with xylene, rinsed through graded alcohols and hydrated to water. The nuclei were stained for 5 minutes in Harris hematoxylin (Anatech, Battle Creek, MI), differentiated in acid alcohol (1% HCl in 70% alcohol by volume) and 1% ammonium hydroxide. The non nuclear elements were stained with alcoholic eosin (Anatech, Battle Creek, MI) for 3 dips and dehydrated through graded alcohols to xylene. The sections were covered with cover slips using Cytoseal 60 synthetic resin (Richard Allan via Thermo Fisher, Waltham, MA).
Immunohistochemistry
Antibodies for cyclin A (LabVision via Thermo, Waltham, MA) were optimized for immunohistochemistry on the Ventana NexES autostainer (Ventana Medical Systems, Tucson, AZ) at an operating temperature of 37°C. 5 μm fresh cut paraffin sections were deparaffinized in xylene, rinsed in graded alcohols and hydrated to water. Antigen retrieval was performed in a Decloaker chamber for 5 minutes at 125°C (22 psi). The retrieval solution was pH 9.5 BORG. Primary antibody for cyclin A was used at 1:100 and incubated for 30 minutes. A Ventana I-VIEW detection kit was modified to only detect rabbit antibodies by substituting a biotinylated goat anti-rabbit secondary (Jackson ImmunoResearch, West Grove, PA) diluted 1:50 in PBS pH 7.6. Sections were counterstained in Mayer’s hematoxylin for 2 minutes, dehydrated in alcohols, cleared in xylene and coverglass mounted as for histochemistry.
Pathological analysis
H & E stained and anti-cyclin A stained tissue sections of subcutaneous tumors were examined by a single pathologist (M.S.L.) blinded as to the treatment group. On H & E sections, the number of apoptotic bodies in the tumors per 10 random high power (400x) fields was recorded for each animal. For cyclin A analysis, the percent of brown cyclin A positive tumor nuclei was assessed for each tumor, counting 500 nuclei in multiple random fields.
RESULTS
1,25(OH)2D3-3-BE is more potent than 1,25(OH)2D3 in inhibiting the growth of renal carcinoma cells
In this study, we examined the effect of 1,25(OH)2D3-3-BE on the growth of the human renal cancer cell lines A498 and Caki 1. Cells were treated with 1,25(OH)2D3-3-BE or 1,25(OH)2D3 and cellular proliferation was quantitated by MTT assay. In both A498 and Caki-1 cells, treatment with 10−6M 1,25(OH)2D3-3-BE almost completely inhibited cellular proliferation, while an equivalent amount of 1,25(OH)2D3 inhibited growth by approximately 10 percent (Fig. 1). Caki 1 cells were more sensitive to 1,25(OH)2D3-3-BE than were A498 cells. Approximately 90% growth-inhibition was observed with 10−7M of 1,25(OH)2D3-3-BE in Caki 1 cells, while approximately 30% growth inhibition was observed in A498 cells (Fig. 1). These results demonstrate that 1,25(OH)2D3-3-BE elicits stronger antiproliferative effects in A498 and Caki 1 cells compared 1,25(OH)2D3 on an equimolar basis.
Fig. 1.
1,25(OH)2D3-3-BE is more potent than 1,25(OH)2D3 in inhibiting the growth of renal carcinoma cells. (A) A498 cells (left panel) and Caki 1 cells (right panel) were treated with various doses of 1,25(OH)2D3-3-BE or 1,25(OH)2D3 or ethanol (vehicle). After seven days, MTT solution was added to each well and absorbance read on a microplate reader. Absorbances from treated cells are plotted as percent of vehicle. Eight replicates for each treatment was performed. Error bars represent standard error of the mean (SEM). **, p<0.01; ***, p<0.001. (B) Morphologic appearance of Caki 1 and A498 cells following treatment with 1,25(OH)2D3-3-BE. Cells were treated for 4 hr (Caki 1) and 6 hr (A498) with 10−6M 1,25(OH)2D3, 1,25(OH)2D3-3-BE or ethanol control and phase contrast photomicrographs were obtained at 200x original magnification. The experiment was repeated three times and representative fields are shown.
Under microscopic visualization, we noted distinct morphological changes in the appearance of both A498 and Caki 1 cells in response to 1,25(OH)2D3-3-BE treatment. As shown in Figure 1B, after 6 hr of treatment with 1,25(OH)2D3-3-BE, both A498 and Caki 1 cells displayed cell rounding and began detaching from the plates. Interestingly, cells treated with 1,25(OH)2D3 did not display these characteristics and exhibited morphological features similar to vehicle treated control cells.
1,25(OH)2D3-3-BE promotes G2/M arrest of A498 cells
The cellular mechanism(s) leading to growth inhibition by 1,25(OH)2D3 are complex. In prostate cancer cells, 1,25(OH)2D3 causes cells to arrest in the G0/G1 phase of the cell cycle (20). This effect is thought to be mediated by increased expression of the cyclin-dependent kinase (CDK) inhibitors p21 and p27, and other cell-cycle regulators (21, 22). To examine the effect of 1,25(OH)2D3-3-BE on cell cycle progression in A498 cells, we measured cell cycle distribution by flow cytometry of propidium iodide stained cells following 6 hours of exposure to 1,25(OH)2D3-3-BE, 1,25(OH)2D3, or ethanol (vehicle control). As shown in Fig. 2A, the cell cycle distributions were similar in control and in 1,25(OH)2D3-treated cells. However, cells, treated with 1,25(OH)2D3-3-BE showed an approximately 2-fold increase in the relative proportion of cells in G2/M compared to control and 1,25(OH)2D3 treated cells. In addition, a population of cells with a sub-diploid DNA content appeared suggesting that 1,25(OH)2D3-3-BE may activate a G2/M checkpoint arrest in renal cancer cells, preventing progression through the cell cycle.
Fig. 2.
(A) 1,25(OH)2D3-3-BE arrests A498 cells in G2/M. FACS analysis was performed on PI-saponin-stained A498 cells treated for 6 hr with 10−6M 1,25(OH)2D3, 1,25(OH)2D3-3-BE or ethanol (vehicle) control. The percent of cells in G0/G1, S and G2/M phases of the cell cycle were calculated using Modfit software. (B) 1,25(OH)2D3-3-BE reduces cyclin A levels in Caki 1 and A498 cells. A498 and Caki 1 cells were treated with 10− 6M 1,25(OH)2D3 (D), 1,25(OH)2D3-3-BE (BE) or ethanol control (E) for 6 hr. Whole cell extracts were prepared and Western blot analysis performed for detection of levels of cyclin A. β-Actin was used as a loading control. The results are representative of two independent experiments.
1,25(OH)2D3-3-BE reduces the level of cyclin A in A498 and Caki 1 cells
Cyclins control progression through the cell cycle via their association with cyclin-dependent kinases. Cyclin A controls the transition from G2 to mitosis and its expression has been shown to have predictive value in the clinical stages of renal cancer (23, 24) Due to our observation that 1,25(OH)2D3-3-BE arrests cells in the G2/M checkpoint, and the importance of cyclin A in renal cancer, we investigated cyclin A expression in A498 and Caki 1 cells treated with either 1,25(OH)2D3 or 1,25(OH)2D3-3-BE We observed that 6 hour treatment of Caki 1 and A498 cells with 10−6M 1,25(OH)2D3-3-BE strongly reduced cyclin A, while the same concentration of 1,25(OH)2D3 failed to do so (Fig. 2B) indicating that 1,25(OH)2D3-3-BE may cause arrest at the G2/M checkpoint in these cells through down-regulation of cyclin A.
1,25(OH)2D3-3-BE treatment induces apoptosis in Caki 1 cells
Cellular growth inhibition mediated by 1,25(OH)2D3 correlates with increased apoptosis in some studies. For example, it is reported that 1,25(OH)2D3 induces apoptosis in LNCaP prostate cancer and MCF-7 breast cancer cells (25, 26), but this result is not universal (20). Previously, we reported that 1,25(OH)2D3-3-BE induces apoptosis in PC-3 prostate cancer cells (15, 16). Thus, we investigated the role of apoptosis in 1,25(OH)2D3-3-BE-mediated growth inhibition of renal cancer cells.
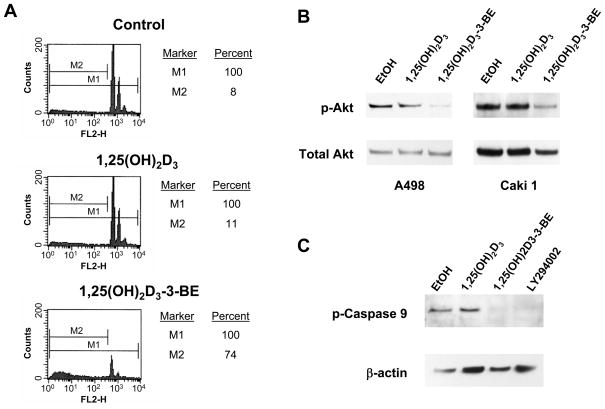
We observed rounding and sloughing of cells treated with 1,25(OH)2D3-3-BE, but not with 1,25(OH)2D3 or vehicle control in both Caki 1 and A498 cell lines (Fig. 1B). To determine if the striking morphological changes in kidney cancer cells in response to 1,25(OH)2D3-3-BE-treatment are related to induction of apoptosis, we performed flow cytometric analysis of nuclear DNA content following exposure to 1,25(OH)2D3-3-BE or 1,25(OH)2D3 in Caki 1 cells. As shown in Fig. 3A, the sub-G0/G1 (hypo-diploid) fraction, indicative of apoptotic cells, was equivalent between control and 1,25(OH)2D3-treated cells (8–11%). However, the 1,25(OH)2D3-3-BE-treated cells showed a large increase in this sub-G0/G1 population (74%).
Fig. 3.
1,25(OH)2D3-3-BE promotes apoptosis of Caki 1 cells. (A) Sub G0/G1 DNA FACS analysis of Caki 1 cells treated with 1,25(OH)2D3 or 1,25(OH)2D3-3-BE. Caki 1 cells were grown to 60–70% confluence, and then were incubated with 10−6M of either 1,25(OH)2D3 or 1,25(OH)2D3-3-BE for 6 hours. The cells were harvested and stained with propidium Iodide. Fluorescence was measured in a FACS analyzer (B) 1,25(OH)2D3-3-BE inhibits phosphorylation of Akt in A498 and Caki 1 cells. A498 and Caki 1 cells were incubated with 5×10−7M of 1,25(OH)2D3 and 1,25(OH)2D3-3-BE or ethanol control for 24 hours and Western analysis used to asses the levels of phosphorylated Akt (p-Akt). The blot was stripped and re- probed for total Akt as a loading control. The results are representative of two independent experiments. (C) 1,25(OH)2D3-3-BE inhibits Akt-mediated phosphorylation of caspase-9. Caki 1 cells were incubated with 5×10−7M of 1,25(OH)2D3, 1,25(OH)2D3-3-BE, the PI3K/Akt inhibitor LY294002 (10 μM) or ethanol control for 6 hours and Western analysis used to asses the levels of phosphorylated caspase-9 (p-Caspase 9). The blot was stripped and reprobed for β-actin as a loading control. The results are representative of three independent experiments.
Caspases are a family of proteases which play an essential role in apoptotic cell death, and caspase-activation is considered a hallmark of apoptosis. To examine the role of 1,25(OH)2D3-3-BE in caspase activation, we performed a caspase activity assay on Caki 1 cells following treatment with 1,25(OH)2D3 and 1,25(OH)2D3-3-BE. This assay detects activation of caspases 3 and 7 through cleavage of a fluorescent substrate specific for caspases 3 and 7. As seen in Table 1, no caspase activity was observed in cells treated with ethanol control or 1,25(OH)2D3. However, strong activation of caspase 3 and 7 activity was observed in cells treated with 1,25(OH)2D3-3-BE. Taken together, the results of sub G0/G1 DNA analysis and the caspase activation assay demonstrate the ability of 1,25(OH)2D3-3-BE to stimulate apoptosis in renal cancer cells.
Table 1. 1,25(OH)2D3-3-BE stimulates caspase-3/7 activity activity in Caki 1 cells.
Caspase-3/7 activity was determined following treatment of Caki 1 cells for 6 hours with 1,25(OH)2D3, 1,25(OH)2D3-3-BE or ethanol (vehicle) control. Fluorescence released following cleavage of the pro-fluorescent substrate, Z-DEVD-110 was measured at the emission maximum of 521 nm. The amount of fluorescent product generated is representative of the amount of active caspase-3/7 in the sample. SE= standard error
| Treatment | Fluorescence Units | +/− SE |
|---|---|---|
| EtOH | 0 | 0 |
| 1,25(OH)2D3 | 0 | 0 |
| 1,25(OH)2D3-3-BE | 17841 | 821 |
1,25(OH)2D3-3-BE inhibits Akt phosphorylation in A498 cells
To investigate the molecular mechanism of 1,25(OH)2D3-3-BE-induced apoptosis in renal cancer cells cells, we examined the activation status of the pro-survival kinase, Akt in A498 cells. Akt is activated by its phosphorylation at threonine 308 and serine 473, events which promote cell survival and proliferation (27). Therefore, we analyzed the activation status of Akt by immunoblot analysis with an antibody specifically recognizing phosphorylated Akt (ser 473) following treatment of A498 and Caki 1 cells with 1,25(OH)2D3 or 1,25(OH)2D3-3-BE. Results of this analysis are shown in Figure 3B. 1,25(OH)2D3-3-BE strongly reduced the level of phosphorylated Akt in both cell lines. An equimolar concentration of 1,25(OH)2D3 also reduced Akt phosphorylation, but to a much lower extent than did 1,25(OH)2D3-3-BE. These results suggest that the apoptotic function of 1,25(OH)2D3-3-BE in renal cancer cells may be mediated, at least partially, by inhibition of signaling through the Akt pathway.
Caspase-9 is a downstream target of Akt. Activated (phosphorylated) Akt phosphorylates caspase-9 on serine 196 and inhibits its protease activity leading to cell survival. Thus, a potential molecular mechanism whereby 1,25(OH)2D3-3-BE promotes apoptosis centers on the ability of 1,25(OH)2D3-3-BE to inhibit Akt activation resulting in increased caspase-9 activity. To address this hypothesis, we examined caspase-9 phosphorylation in Caki 1 cells following treatment with 1,25(OH)2D3-3-BE and 1,25(OH)2D3. As shown in Fig. 3C, 1,25(OH)2D3-3-BE, but not 1,25(OH)2D3, inhibited phosphorylation of caspase-9. As a control, we used the PI3K/Akt inhibitor LY294002 to confirm that inhibition of Akt activity leads to decreased phosphorylation of caspase-9. These results further implicate Akt and its downstream target, caspase-9, as targets for the molecular mechanism whereby 1,25(OH)2D3-3-BE promotes apoptosis in renal cancer cells.
1,25(OH)2D3-3-BE is stable in human serum
HPLC-profile of 14C-1,25(OH)2D3-3-BE, incubated in human serum for 60 min at 37°C shows a single peak (Fig. 4B) that matches with the peak for a standard sample of 14C-1,25(OH)2D3-3-BE (Fig. 4A), indicating that 14C-1,25(OH)2D3-3-BE is stable in human serum at 37°C for at least one hr.
Fig. 4.
HPLC profiles of (A) a standard sample of 14C-1,25(OH)2D3-3-BE, and (B) organic extract of a sample of human serum, spiked with 14C-1,25(OH)2D3-3-BE. Conditions: C18 column, 5% H2O-MeOH: mobile phase, on-line radioactivity-detection. The experiment was repeated three times and representative data shown.
1,25(OH)2D3-3-BE inhibits tumor-growth in a mouse xenograft model
The effect of 1,25(OH)2D3-3-BE on the growth of renal cell tumors was evaluated in xenografts in nude mice. Caki 1 cells were injected subcutaneously in athymic nude mice and allowed to grow until the tumors reached approximately 100 mm3 in size at which time 1,25(OH)2D3-3-BE, 1,25(OH)2D3 or vehicle control were administered. In comparison to 1,25(OH)2D3-3-BE and 1,25(OH)2D3 treatment, the vehicle-treated control animals generated tumors which grew rapidly throughout the time course. In contrast, the tumors in the 1,25(OH)2D3-3-BE-treated group showed a significant reduction in size compared to control animals tumors and 1,25(OH)2D3-3-BE was more effective than 1,25(OH)2D3 in inhibiting tumor growth (Fig. 5A). To examine potential toxic effects of 1,25(OH)2D3-3-BE treatment, the body weights of the mice were determined each time compounds were administered. As shown in Figure 5B, we did not observe a difference in body weights between any of the treatment groups. Importantly, serum calcium values of the 1,25(OH)2D3 and 1,25(OH)2D3-3-BE-treated animals were not significantly different from the vehicle-control (Fig. 5C) denoting lack of toxicity. Collectively, these results demonstrate that 1,25(OH)2D3-3-BE is an effective agent at reducing renal cancer xenografts an appears to be well tolerated at this dose and time course.
Fig. 5.
1,25(OH)2D3-3-BE inhibits tumor growth in a mouse xenograft growth in a mouse xenograft model. (A) Caki 1 xenografted tumor growth in response to administration of 1,25(OH)2D3 and 1,25(OH)2D3-3-BE (0.75 μg/kg body weight each). Tumor size was measured at the indicated days after injection of tumor cells. Inset: Graphical representation of tumor volumes at the completion of the experiment. *=p<0.01 by Students T test. (B) 1,25(OH)2D3 and 1,25(OH)2D3-3-BE do not induce toxicity in mice. At each time where tumor size was measured the mice were weighed as a measure of toxic effects of 1,25(OH)2D3 and 1,25(OH)2D3-3-BE. (C) Serum-calcium values of treated animals were determined by a calcium-assay kit using manufacturer’s procedure (BioAssay System). Statistical analysis was done by Students T test.
1,25(OH)2D3-3-BE reduces cyclin A levels and increases apoptosis in tumor samples
We observed significant inhibition of cyclin A levels in our cell culture models of 1,25(OH)2D3-3-BE action. Therefore, we examined cyclin A staining in tumors from mice treated with 1,25(OH)2D3-3-BE, 1,25(OH)2D3 and vehicle (control). Immunohistochemical analysis of cyclin A in the xenografts demonstrated significant reduction in the percentage of cells having nuclear cyclin A staining with both 1,25(OH)2D3 and 1,25(OH)2D3-3-BE (Fig. 6A). Importantly, the reduction in cyclin A was more pronounced in tumors derived from 1,25(OH)2D3-3-BE treated animals (Fig. 6C).
Fig. 6.
1,25(OH)2D3-3-BE stimulates apoptosis and reduces cyclin A levels in renal cancer xenografts. (A) Immunohistochemical analysis of cyclin A levels in xenografts. The tumors derived from control, 1,25(OH)2D3 and 1,25(OH)2D3-3-BE treated mice were examined for cyclin A levels. Arrows indicate positive nuclear staining for cyclin A. (B) Apoptotic bodies are increased in tumors from 1,25(OH)2D3-3-BE treated mice. Circles indicate representative apoptotic bodies. (C) Quantification of cyclin A staining (left panel) and apoptotic bodies (right panel). Positive nuclear staining for cyclin A and the number of apoptotic bodies in control tumors (Con), tumors derived from 1,25(OH)2D3 treated mice (D) and tumors derived from 1,25(OH)2D3-3-BE treated mice were counted as described in Materials and Methods. For cyclin A statistical analysis (students T test), *=P<0.005 and **=P<0.0005. For the number of apoptotic bodies *=P<0.02.
Because we observed potent stimulation of apoptosis by 1,25(OH)2D3-3-BE in vitro, we examined the presence of apoptotic bodies, as an indication of apoptosis, in the xenografts. The number of apoptotic bodies per high power field was increased in tumors from the 1,25(OH)2D3-3-BE treated animals suggesting that 1,25(OH)2D3-3-BE stimulated apoptosis in vivo (Figs. 6B and 6C). However, 1,25(OH)2D3 did not significantly increase apoptosis in the xenografts. These findings are in concordance with our observations that, compared to 1,25(OH)2D3, 1,25(OH)2D3-3-BE is a more potent inducer of apoptosis in renal cancer cells in vitro.
DISCUSSION
There is a paucity of information about the effect of 1,25(OH)2D3 and its analogs in renal cancer. Nagakura et al. demonstrated that 1,25(OH)2D3 and some of its metabolites inhibited the growth of renal cancer cell line KU-2 (28). In addition, Fuzioka et al. demonstrated that 1,25(OH)2D3 inhibited the growth of murine Renca renal cancer cell line-induced tumor in a mouse model (29). These results suggest potential utility of 1,25(OH)2D3 and its analogs in treating renal cancer.
We observed that 1,25(OH)2D3-3-BE is a significantly stronger antiproliferative agent compared with equimolar amounts of 1,25(OH)2D3 both in vitro (Fig. 1) and in a mouse xenograft tumor model (Fig. 5). Greater efficacy of 1,25(OH)2D3-3-BE compared to 1,25(OH)2D3 can potentially be explained by its proposed ability to titrate and engage all VDR molecules, due to the kinetic nature of the alkylation process. This is an important consideration in cases where VDR level is low. For example, Trydal et al. determined VDR level in 23 primary renal cell carcinomas and compared these levels with autologous normal kidney tissue. They reported that VDR levels for the renal cell carcinomas were approximately three times lower than autologous normal kidney tissue (30). We evaluated VDR levels in Caki 1 and A-498 cells, treated with 1,25(OH)2D3, 1,25(OH)2D3-3-BE or vehicle, and observed comparable levels of VDR by immunoblot analysis suggesting that changes in VDR levels do not reflect response to 1,25(OH)2D3-3-BE (data not shown).
In Caki 1 cells, we observed 1,25(OH)2D3-3-BE induces apoptosis, in addition to cell-cycle arrest, as evidenced by sub G0/G1 DNA analysis and arrest at the G2/M checkpoint (Figs. 2 and 3). 1,25(OH)2D3-3-BE also strongly stimulated caspase 3/7 activity, a hallmark of apoptosis (Table 1). The induction of apoptosis by 1,25(OH)2D3 has been shown to involve up-regulation of pro-apoptotic Bax and Bcl-XL, Bcl-2 family proteins that regulate the intrinsic pathway for apoptotic induction (25, 26). However, in A498 cells 1,25(OH)2D3-3-BE (as well as 1,25(OH)2D3) failed to activate these proteins (data not shown), suggesting that activation of caspases (by 1,25(OH)2D3-3-BE) in kidney cancer cells may follow a different pathway.
Akt is a serine/threonine kinase which is activated by many signals in a phophatidylinositol-3′-kinase (PI3K)-dependent manner (31, 32). Akt is involved in a variety of normal and tumorigenic functions such as cell proliferation, growth and survival. Hara et al. screened 45 tumor samples from renal cell carcinoma patients and reported that phosphorylated Akt expression increased significantly in comparison to associated normal kidney tissue and that an Akt inhibitor induced apoptosis in KU19-20 and Caki-2 cells which have high Akt activity (33). We observed that 1,25(OH)2D3-3-BE strongly inhibited Akt-phosphorylation in A498 and Caki 1 cells (Fig. 3B), indicating that the ability of 1,25(OH)2D3-3-BE to inhibit Akt activation may be critical in the molecular mechanism of its action.
Caspase 9 is a downstream effector of Akt-activity. As presented in Figure 3B, we observed complete inhibition of caspase-9 phosphorylation following 1,25(OH)2D3-3-BE treatment of Caki 1 cells. Interestingly, 1,25(OH)2D3 did not inhibit caspase-9 phosphorylation, potentially revealing a key mechanism explaining the observed differences in the ability of 1,25(OH)2D3- 3-BE and 1,25(OH)2D3 to promote apoptosis in renal cancer cells.
The stability of a drug in serum is a key pharmacokinetic property. Serum stability is particularly important for 1,25(OH)2D3-3-BE, because it contains an ester bond which may be prone to hydrolysis by esterases. Therefore, we determined the stability of 1,25(OH)2D3-3-BE in human serum. HPLC-profile of an organic extract of a serum sample, spiked with 14C-1,25(OH)2D3-3-BE showed the intact peak of 14C-1,25(OH)2D3-3-BE after one hr incubation at 37° C (Fig. 4). This result attests to the stability of 1,25(OH)2D3-3-BE in serum, and enhances its potential as a therapeutic agent.
In order to evaluate the potential of 1,25(OH)2D3-3-BE in renal cancer, we carried out an in vivo study with an athymic mouse model of human renal cancer. In this study we observed that tumors in vehicle-treated, control, animals grew rapidly throughout the time course. Significantly, 1,25(OH)2D3-3-BE, but not 1,25(OH)2D3 inhibited tumor-growth (Fig. 5A), reflecting the in vitro growth-inhibitory property of 1,25(OH)2D3-3-BE in kidney cancer cells. In addition, higher efficacy of 1,25(OH)2D3-3-BE in inhibiting tumor growth compared to 1,25(OH)2D3 was reflected by decreased cyclin A nuclear staining and increased apoptosis in the tumors (Figs. 6A-C). It is noteworthy that the molecular weights of 1,25(OH)2D3-3-BE and 1,25(OH)2D3 are 537.80 kD and 416.65 kD respectively. Therefore, if we consider equimolar concentrations of these compounds, 1,25(OH)2D3-3-BE is actually approximately 1.3–fold more active than 1,25(OH)2D3.
1,25(OH)2D3-3-BE did not show significant toxicity as reflected in the gross body weights of the animals throughout the study (Fig. 5B). As indicated in Fig. 5B 1,25(OH)2D3 apparently caused some weight-gain. But, upon statistical analysis body-weights of 1,25(OH)2D3 -treated animals were not significantly different from other groups (vehicle-control and 1,25(OH)2D3-3-BE). Furthermore, serum calcium values were not significantly different among the groups (Fig. 5C).
We have demonstrated that 1,25(OH)2D3-3-BE covalently attaches to the ligand-binding pocket of VDR (13), thus possibly making it less prone to catabolic degradation and higher activation of VDR. It can be argued that such a process may lead to ‘apparent higher dose of 1,25(OH)2D3’ and enhance toxicity. But, increasing the effective dose of 1,25(OH)2D3 by covalent labeling also means that we will require less of 1,25(OH)2D3-3-BE to bring about significant effect. We chose a dose of 0.75 μg/kg for both 1,25(OH)2D3 and 1,25(OH)2D3-3-BE at which level none of them showed any toxicity (Fig. 5B and 5C).
In summary, the results presented herein demonstrate that 1,25(OH)2D3-3-BE strongly suppresses growth of kidney cancer cells in vitro and tumor growth in vivo. These studies suggest further pre-clinical investigations, and continued mechanistic investigations of 1,25(OH)2D3-3-BE in inhibiting renal cancer tumorigenesis are warranted to evaluate its translational potential as a therapeutic agent in renal cell carcinoma. Considered together with extensive data on vitamin D in various cancer-prevention settings, these results also have important implications for renal-cell–cancer prevention. There are, however, no preclinical in-vivo models, for example genetically engineered models, for prevention research in this setting, and so such models should be developed for studying the vitamin D analog reported here as well as for studying other potentially effective preventive agents. Such prevention study would be especially relevant for people at a high risk of renal cell cancer (34–36).
Acknowledgments
This work was supported by National Cancer Institute grant CA 127629, Department of Defense Grant PC 051136, National Cancer Institute CA126317 (sub-contract) and Community Technology Fund, Boston University to R.R., American Cancer Society Research Scholar Grant RSG-04-170-01-CNE to J.R.L. and Department of Defense contract grant DAMD 17-03-1-0213 and National Cancer Institute CA 101992 to D.V.F.
Reference List
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.van Spronsen DJ, Mulders PF, de Mulder PH. Novel treatments for metastatic renal cell carcinoma. Crit Rev Oncol Hematol. 2005;55:177–91. doi: 10.1016/j.critrevonc.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Lam JS, Leppert JT, Figlin RA, Belldegrun AS. Role of molecular markers in the diagnosis and therapy of renal cell carcinoma. Urology. 2005;66:1–9. doi: 10.1016/j.urology.2005.06.112. [DOI] [PubMed] [Google Scholar]
- 4.Mancuso A, Sternberg CN. New treatments for metastatic kidney cancer. Can J Urol. 2005;12 (Suppl 1):66–70. [PubMed] [Google Scholar]
- 5.Ko YJ, Atkins MB. Chemotherapies and immunotherapies for metastatic kidney cancer. Curr Urol Rep. 2005;6:35–42. doi: 10.1007/s11934-005-0065-7. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Small EJ. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J Clin Oncol. 2005;23:1028–43. doi: 10.1200/JCO.2005.01.186. [DOI] [PubMed] [Google Scholar]
- 7.Giovannucci E. The epidemiology of vitamin D and cancer incidence and mortality: a review (United States) Cancer Causes Control. 2005;16:83–95. doi: 10.1007/s10552-004-1661-4. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda S, Jones G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol Cancer Ther. 2006;5:797–808. doi: 10.1158/1535-7163.MCT-05-0539. [DOI] [PubMed] [Google Scholar]
- 9.Muindi JR, Johnson CS, Trump DL, Christy R, Engler KL, Fakih MG. A phase I and pharmacokinetics study of intravenous calcitriol in combination with oral dexamethasone and gefitinib in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;65:33–40. doi: 10.1007/s00280-009-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fakih MG, Trump DL, Muindi JR, Black JD, Bernardi RJ, Creaven PJ, Schwartz J, Brattain MG, Hutson A, French R, Johnson CS. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13:1216–23. doi: 10.1158/1078-0432.CCR-06-1165. [DOI] [PubMed] [Google Scholar]
- 11.Trump DL, Potter DM, Muindi J, Brufsky A, Johnson CS. Phase II trial of high-dose, intermittent calcitriol (1,25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer. 2006;106:2136–42. doi: 10.1002/cncr.21890. [DOI] [PubMed] [Google Scholar]
- 12.Ray R, Swamy N, MacDonald PN, Ray S, Haussler MR, Holick MF. Affinity labeling of the 1 alpha,25-dihydroxyvitamin D3 receptor. J Biol Chem. 1996;271:2012–17. doi: 10.1074/jbc.271.4.2012. [DOI] [PubMed] [Google Scholar]
- 13.Swamy N, Xu W, Paz N, Hsieh JC, Haussler MR, Maalouf GJ, Mohr SC, Ray R. Molecular modeling, affinity labeling, and site-directed mutagenesis define the key points of interaction between the ligand-binding domain of the vitamin D nuclear receptor and 1α,25-dihydroxyvitamin D3. Biochemistry. 2000;39:12162–71. doi: 10.1021/bi0002131. [DOI] [PubMed] [Google Scholar]
- 14.Chen ML, Ray S, Swamy N, Holick MF, Ray R. Mechanistic studies to evaluate the enhanced antiproliferation of human keratinocytes by 1α,25-dihydroxyvitamin D3-3-bromoacetate, a covalent modifier of vitamin D receptor, compared to 1α,25-dihydroxyvitamin D3. Arch Biochem Biophys. 1999;370:34–44. doi: 10.1006/abbi.1999.1353. [DOI] [PubMed] [Google Scholar]
- 15.Swamy N, Persons KS, Chen TC, Ray R. 1α,25-Dihydroxyvitamin D3-3beta-(2)-bromoacetate, an affinity labeling derivative of 1α,25-dihydroxyvitamin D3 displays strong antiproliferative and cytotoxic behavior in prostate cancer cells. J Cell Biochem. 2003;89:909–16. doi: 10.1002/jcb.10585. [DOI] [PubMed] [Google Scholar]
- 16.Swamy N, Chen TC, Peleg S, Dhawan P, Christakos S, Stewart LV, Weigel NL, Mehta RG, Holick MF, Ray R. Inhibition of proliferation and induction of apoptosis by 25-hydroxyvitamin D3-3β-(2)-Bromoacetate, a nontoxic and vitamin D receptor-alkylating analog of 25-hydroxyvitamin D3 in prostate cancer cells. Clin Cancer Res. 2004;10:8018–27. doi: 10.1158/1078-0432.CCR-04-0881. [DOI] [PubMed] [Google Scholar]
- 17.Lambert JR, Young CD, Persons KS, Ray R. Mechanistic and pharmacodynamic studies of a 25-hydroxyvitamin D3 derivative in prostate cancer cells. Biochem Biophys Res Commun. 2007;361:189–95. doi: 10.1016/j.bbrc.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Lange TS, Singh RK, Kim KK, Zou Y, Kalkunte SS, Sholler GL, Swamy N, Brard L. Anti-proliferative and pro-apoptotic properties of 3-bromoacetoxy calcidiol in high-risk neuroblastoma. Chem Biol Drug Des. 2007;70:302–10. doi: 10.1111/j.1747-0285.2007.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ray R, Ray S, Holick MF. 1α,25-Dihydroxyvitamin D3-3-bromoacetate, an affinity labeling analog of 1α,25-dihydroxyvitamin D3 receptor. Bioorg Chem. 1994;22:276–83. [Google Scholar]
- 20.Zhuang SH, Burnstein KL. Antiproliferative effect of 1alpha,25-dihydroxyvitamin D3 in human prostate cancer cell line LNCaP involves reduction of cyclin-dependent kinase 2 activity and persistent G1 accumulation. Endocrinology. 1998;139:1197–1207. doi: 10.1210/endo.139.3.5770. [DOI] [PubMed] [Google Scholar]
- 21.Yang ES, Burnstein KL. Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J Biol Chem. 2003;278:46862–868. doi: 10.1074/jbc.M306340200. [DOI] [PubMed] [Google Scholar]
- 22.Moffatt KA, Johannes WU, Hedlund TE, Miller GJ. Growth inhibitory effects of 1alpha, 25-dihydroxyvitamin D(3) are mediated by increased levels of p21 in the prostatic carcinoma cell line ALVA-31. Cancer Res. 2001;61:7122–29. [PubMed] [Google Scholar]
- 23.Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–71. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aaltomaa S, Lipponen P, la-Opas M, Eskelinen M, Syrjanen K, Kosma VM. Expression of cyclins A and D and p21(waf1/cip1) proteins in renal cell cancer and their relation to clinicopathological variables and patient survival. Br J Cancer. 1999;80:2001–7. doi: 10.1038/sj.bjc.6690634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blutt SE, McDonnell TJ, Polek TC, Weigel NL. Calcitriol-induced apoptosis in LNCaP cells is blocked by overexpression of Bcl-2. Endocrinology. 2000;141:10–7. doi: 10.1210/endo.141.1.7289. [DOI] [PubMed] [Google Scholar]
- 26.Simoli-Campbell M, Narvaez CJ, Tenniswood M, Welsh J. 1,25-Dihydroxyvitamin D3 induces morphological and biochemical markers of apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 1996;58:367–76. doi: 10.1016/0960-0760(96)00055-6. [DOI] [PubMed] [Google Scholar]
- 27.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- 28.Nagakura K, Abe E, Suda T, Hayakawa M, Nakamura H, Tazaki H. Inhibitory effect of 1–alpha,25-dihydroxyvitamin D3 on the growth of the renal carcinoma cell line. Kidney Intl. 1986;29:834–40. doi: 10.1038/ki.1986.74. [DOI] [PubMed] [Google Scholar]
- 29.Fuzioka T, Hasegawa M, Ishikura K, Matsuhita Y, Sato M, Tanji S. Inhibition of tumor growth and angiogenesis by vitamin D3 agents in murine renal cell carcinoma. J Urol. 1988;160:247–51. [PubMed] [Google Scholar]
- 30.Trydal T, Bakke A, Aksnes L, Aarskog D. 1,25-Dihydroxyvitamin D3 receptor measurement in primary renal cell carcinomas and autologous normal kidney tissue. Cancer Res. 1988;48:2458–61. [PubMed] [Google Scholar]
- 31.Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16:797–803. doi: 10.1097/01.cad.0000173476.67239.3b. [DOI] [PubMed] [Google Scholar]
- 32.Gills JJ, Dennis PA. The development of phosphatidylinositol ether lipid analogues as inhibitors of the serine/threonine kinase. Akt, Expert Opin Investig Drugs. 2004;13:787–97. doi: 10.1517/13543784.13.7.787. [DOI] [PubMed] [Google Scholar]
- 33.Hara S, Oya M, Mizuno R, Horiguchi A, Marumo K, Murai M. Akt activation in renal cell carcinoma: contribution of a decreased PTEN expression and the induction of apoptosis by an Akt inhibitor. Ann Oncol. 2005;16:928–33. doi: 10.1093/annonc/mdi182. [DOI] [PubMed] [Google Scholar]
- 34.Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–67. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 35.Purdue MP, Johansson M, Zelenika D, et al. Genome-wide association study of renal cell carcinoma identifies two susceptibility loci on 2p21 and 11q13.3. Nat Genet. doi: 10.1038/ng.723. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chow W-H, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol. 2010;7:245–57. doi: 10.1038/nrurol.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]