Abstract
Activation of the phagocyte NADPH oxidase complex requires the assembly of the cytosolic factors p47PHOX, p67PHOX, p40PHOX, and Rac1 or Rac2, with the membrane-bound cytochrome b558. Whereas the interaction of p47PHOX with cytochrome b558 is well established, an interaction between p67PHOX and cytochrome b558 has never been investigated. We report here a direct interaction between p67PHOX and cytochrome b558. First, labeled p67PHOX recognizes a 91-kDa band in specific granules from a normal patient but not from a cytochrome b558-deficient patient. Second, p67PHOX binds to cytochrome b558 that has been bound to nitrocellulose. Third, GTP-p67PHOX bound to glutathione agarose is able to pull down cytochrome b558. Rac1-GTP or Rac1-GDP increased the binding of p67PHOX to cytochrome b558, suggesting that at least one of the oxidase-related functions of Rac1 is to promote the interaction between p67PHOX and cytochrome b558.
The NADPH oxidase of
phagocytes plays a pivotal role in host defense against microbial
infection. It catalyzes the reduction of oxygen to O at the expense of NADPH. The O
at the expense of NADPH. The O generated is the
precursor of potent oxidants that are used to kill the invading
microorganisms (1). The importance of the NADPH is evidenced by
patients with chronic granulomatous disease (CGD), in which an
inherited mutation in one of the components of the enzyme impairs its
ability to produce superoxide, leading to recurrent bacterial and
fungal infections (2).
generated is the
precursor of potent oxidants that are used to kill the invading
microorganisms (1). The importance of the NADPH is evidenced by
patients with chronic granulomatous disease (CGD), in which an
inherited mutation in one of the components of the enzyme impairs its
ability to produce superoxide, leading to recurrent bacterial and
fungal infections (2).
The NADPH oxidase is a highly regulated enzyme complex composed of a
number of proteins: in the cytosol, p67PHOX,
p47PHOX, p40PHOX, and
Rac1/Rac2, and in the membrane, gp91PHOX and
p22PHOX, which, together, comprise cytochrome
b558. When activation takes place, the
cytosolic components migrate to the membranes, where they associate
with the membrane-bound components to assemble the catalytically
active oxidase (3). Cytochrome b558,
which contains both flavin and heme groups and a putative consensus
sequence for the binding of NADPH (4), likely is responsible for
electron transfer from NADPH to oxygen upon activation of the cells,
although it was demonstrated that p67PHOX also
possesses an NADPH-binding site (5). The activation of electron flow in
cytochrome b558 probably is regulated
by the association with p47PHOX,
p67PHOX, and Rac1/Rac2.
P47PHOX, which is phosphorylated extensively
during oxidase activation (6), probably initiates enzyme assembly,
whereas p67PHOX appears to activate catalysis by
cytochrome b558, although the
mechanism of activation is obscure (7). The function of Rac remains
unclear, although it is absolutely required for NADPH oxidase
activation (8). The function of p40PHOX also is
not well defined. Some results suggest that
p40PHOX is an inhibitory subunit (9), but others
showed an increase of O production (10) or an
increased affinity for p47PHOX (11).
production (10) or an
increased affinity for p47PHOX (11).
During activation, multiple protein–protein interactions occur. Both p47PHOX and p67PHOX contain two SH3 domains, which mediate specific interactions between the factors: the C-terminal SH3 domain of p67PHOX interacts with p47PHOX, and the N-terminal SH3 domain of p47PHOX interacts with p22PHOX (12). Currently, it is proposed that in resting state, p47PHOX is folded in a masked conformation involving intramolecular interactions between the two SH3 domains. Upon activation, phosphorylation of p47PHOX disrupts the SH3-mediated intramolecular interaction and p47PHOX adopts a conformation that allows it to interact with the p22PHOX (13), bringing p67PHOX in proximity with cytochrome b558. Furthermore, the interaction of Rac with p67PHOX also is considered crucial for NADPH oxidase activation, because mutant Rac proteins with defective interactions cannot activate the oxidase (14). p40PHOX interacts with p67PHOX, although its functional role is not clear (15). Other data suggest a cytochrome b558–p67PHOX interaction. Studies with synthetic peptides of gp91PHOX suggest not only that p47PHOX associates with cytochrome b558 at multiple sites during the activation but that p67PHOX also associates with cytochrome b558. Indeed, some gp91PHOX peptides inhibit translocation of p67PHOX more than that of p47PHOX in a cell-free system (16). Furthermore, that NADPH oxidase could be activated by exposing cytochrome b558 to a high concentration of p67PHOX in the absence of p47PHOX (but in the presence of Rac) suggests a direct interaction between cytochrome b558 and p67PHOX (17).
In the present study, we show a direct interaction between p67PHOX and cytochrome b558 and find that this interaction increases when the proteins are incubated in the presence of Rac1-GTP/GDP.
Experimental Procedures
Expression and Purification of Recombinant p67PHOX and Rac1 in Escherichia coli.
P67PHOX was expressed in E. coli as a glutathione S-transferase (GST) fusion protein and purified with glutathione-Sepharose beads (18). The protein was separated from GST while on the beads by cleavage with PreScission protease (Amersham Pharmacia) or eluted from the beads by incubating for 20 min at 4°C with 1 ml of 50 mM Tris⋅HCl, pH 8/5 mM glutathione/150 mM NaCl. Rac 1 was expressed in E. coli as a GST fusion protein and purified with glutathione-Sepharose beads (Amersham Pharmacia) followed by thrombin cleavage.
Purification of Cytochrome b558.
Cytochrome b558 was purified from human neutrophil plasma membranes by heparin and hydroxyapatite chromatography and subsequently relipidated and reflavinated as described by Cross et al. (19).
Overlay, Dot–Blot, and Affinity Precipitation Studies.
For overlay studies, specific granules (2.5 × 107 cells equivalent), prepared as described (20) from a normal subject or a CGD patient deficient in gp91PHOX, were resolved by SDS/PAGE and transferred to nitrocellulose. The nitrocellulose was blocked in 50 mM Tris, pH 7.5/100 mM NaCl/2 mM EDTA/5% low-fat milk/0.1% Nonidet P-40 for 1 h at room temperature and then incubated in binding buffer (20 mM Hepes, pH 7.5/0.5% low-fat milk/1% Nonidet P-40/10 mM NaCl/1 mM EGTA) in the presence of p67PHOX (1.5 μg/ml), which had been phosphorylated (see below) in the presence of [γ32-P]ATP, for 2 h at room temperature. Subsequently, the nitrocellulose was washed five times with wash buffer (50 mM Tris⋅HCl, pH 7.6/50 mM NaCl/0.05% Tween 20/0.1% azide/0.2% low-fat milk), dried, and subjected to autoradiography. The same blots were then stripped in 62.5 mM Tris⋅HCl, pH 6.7/100 mM 2-mercaptoethanol/2% SDS at 50°C for 30 min, and a Western blot was performed by using a mAb directed against gp91PHOX (a kind gift of U. Knaus, The Scripps Research Institute, La Jolla, CA).
For dot-blot assays, 8.6 pmol each of pure cytochrome b558, p47PHOX, carbonic anhydrase, or BSA was applied directly to nitrocellulose filters, which then were incubated with p67PHOX (1.5 μg/ml) in binding buffer for 2 h at room temperature. Filters then were washed extensively with wash buffer and incubated with anti-p67PHOX antibody for detection of binding.
For affinity precipitation, 80 pmol of GST or full-length GST-p67PHOX was incubated in the presence of 4.3 pmol of cytochrome b558 in 200 μl of 20 mM Hepes, pH 7.5/1% Nonidet P-40/10 mM NaCl/1 mM EGTA for 2 h at room temperature. In some assays, phosphorylated GST-67PHOX was used instead. Forty microliters of glutathione-Sepharose 4B beads (Pharmacia Amersham) was then added followed by incubation for 2 h at 4°C. After washing five times with the same buffer, the beads were pelleted, resuspended in 40 μl of Laemmli sample buffer, and boiled for 5 min. Proteins were separated by SDS/PAGE on a 12% Tris-glycine gel (Bio-Rad) and transferred onto nitrocellulose. The cytochrome b558 on the nitrocellulose then was visualized by using a monoclonal anti-gp91PHOX antibody. To determine the effect of Rac1 and Cdc42 (a kind gift of U. Knaus) on p67PHOX binding to gp91PHOX, 800 pmol of Rac 1, Rac1-guanosine 5′-O-(3-thiotriphosphate) (GTP[γS]) or Rac1-guanosine 5′-O-(2-thiodiphosphate) (GDP[βS]), Cdc42, Cdc42-GTPγS, or Cdc42-GDPβS was added to the assay at the same time as p67PHOX. Rac1 and Cdc42 were loaded with GTP[γS] or GDP[βS] as described by Dang et al. (18).
Western Blotting.
Nitrocellulose membranes were blocked with 5% nonfat dry milk in borate-buffered saline, pH 8.4 (100 mM boric acid/25 mM borax/75 mM NaCl) for 1 h at room temperature and then incubated with 1/5,000 goat anti-p67PHOX antibody or 1/2,000 mouse monoclonal anti-gp91PHOX antibody overnight. The membranes then were washed extensively and incubated with alkaline phosphatase-conjugated 1/5,000 anti-rabbit IgG or horseradish peroxidase-conjugated 1/5,000 anti-mouse IgG for 1 h at room temperature. Blots were visualized by using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium phosphate substrate (Bio-Rad) or enhanced chemiluminescence Western blotting reagents (Amersham Pharmacia).
Phosphorylation.
Phosphorylation of p67PHOX by MAP kinase and protein kinase C (Calbiochem) in combination was performed by incubating 80 pmol of p67PHOX in a reaction mixture containing 40 mM Hepes (pH 7.5), 10 mM MgCl2, 10 mM DTT, 0.6 mM CaCl2, 5 μg/ml 1,2-dioleoyl-Sn-glycerol (Sigma), 50 μg/ml l-α-phosphatidylserine (Sigma), the indicated kinase, and 50 μM ATP in a total volume of 40 μl for 30 min at 30°C.
Statistical Analysis.
All of the experiments were repeated a minimum of three times. Results are expressed as means ± SE. Statistical analysis was performed by using one-way ANOVA. A P < 0.05 was considered to be statistically significant.
Results
P67PHOX Interacts Directly with Cytochrome b558.
Because previous studies have suggested the existence of an interaction between p67PHOX and cytochrome b558 (21), we aimed to demonstrate this interaction directly by different approaches. In a first approach, an overlay technique was used. Neutrophil-specific granules (used here as a source of cytochrome b558) from a normal subject and a CGD patient deficient in gp91PHOX were separated by SDS/PAGE and transferred to nitrocellulose. The blot then was incubated with p67PHOX that had been labeled by phosphorylation by using [γ-32P]ATP. As shown in Fig. 1 Left, the labeled p67PHOX recognized a major band whose molecular mass of ≈91 kDa corresponds to that of gp91PHOX. This band was not recognized in specific granules from a CGD patient deficient in gp91PHOX. To establish clearly the identity of this protein, the same blots were stripped as described under Experimental Procedures and incubated with a monoclonal anti-gp91PHOX antibody. The anti-gp91PHOX antibody detected a band that comigrated with the band recognized by the labeled p67PHOX in the normal specific granules but not in the specific granules from the CGD patient, indicating further that the band recognized by labeled p67PHOX was the gp91PHOX subunit of cytochrome b558
Figure 1.
Far Western blot with specific granules from normal or CGD patient overlaid with labeled p67PHOX. Specific granules (2.5 × 107 cell equivalents) from a normal subject or a CGD patient were resolved by 12% SDS/PAGE and blotted onto nitrocellulose. The blot then was incubated with p67PHOX labeled by phosphorylation with [γ-32P]ATP. After washing five times, the blot was exposed to x-ray film (Left). The same blot then was stripped as described in Experimental Procedures and incubated with a mAb against gp91PHOX (Right).
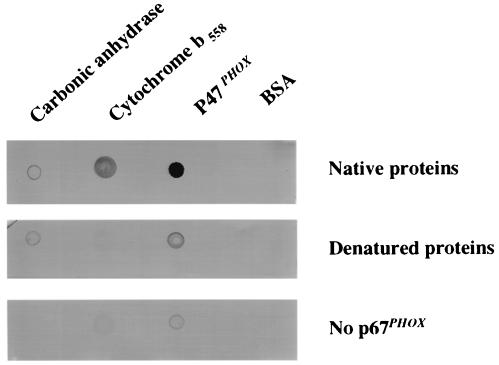
In a second approach, purified cytochrome b558, p47PHOX (positive control), BSA, and carbonic anhydrase (negative controls) were applied directly onto nitrocellulose, which then was incubated with p67PHOX (1.5 μg/ml). The dot–blot then was washed extensively and treated with p67PHOX antibody for detection of binding. As shown in Fig. 2 Top, p67PHOX bound purified cytochrome b558 and p47PHOX but not carbonic anhydrase or BSA. P67PHOX was not able to bind to denatured cytochrome b558 and bound only weakly to denatured p47PHOX (Fig. 2 Middle). Furthermore, the signal observed did not represent a nonspecific binding of the antibodies to cytochrome b558, because in the absence of p67PHOX (Fig. 2 Bottom), no signal was observed. Finally, the direct binding of p67PHOX to cytochrome b558 was confirmed by affinity-precipitation experiments. Cytochrome b558 was incubated in the presence of GST-p67PHOX or GST. Fig. 3 shows that cytochrome b558 was precipitated with GST-p67PHOX coupled to glutathione-Sepharose beads but not with GST coupled to glutathione-Sepharose beads.
Figure 2.
Binding of p67PHOX to immobilized cytochrome b558. Eight and six-tenths pmol each of pure cytochrome b558, p47PHOX (positive control), carbonic anhydrase, or BSA was applied directly to nitrocellulose filters, which were incubated with p67PHOX (1.5 μg/ml) as described in Experimental Procedures. The filters then were washed and incubated with anti-p67PHOX antibody. (Top) Native proteins. (Middle) Denatured proteins. (Bottom) No p67PHOX.
Figure 3.
Affinity precipitation of cytochrome b558 by GST-p67PHOX. Eighty picomoles of GST or full-length GST-p67PHOX were incubated in the presence of 4.3 pmol of cytochrome b558 in 200 μl of 20 mM Hepes, pH 7.5/1% Nonidet P-40/10 mM NaCl/1 mM EGTA for 2 h at room temperature. Forty microliters of glutathione-Sepharose 4B beads (Amersham Pharmacia) then were added, followed by incubation for 2 h at 4°C. After washing five times with the same buffer, the beads were pelleted, resuspended in 40 μl of Laemmli sample buffer (22), and boiled for 5 min. Proteins were resolved by 12% SDS/PAGE and transferred onto nitrocellulose. The proteins then were detected by using a monoclonal anti-gp91PHOX antibody.
Effect of Rac1 on the Interaction of p67PHOX with Cytochrome b558.
Of particular interest is the question of whether Rac is able to alter the interaction between cytochrome b558 and GST-p67PHOX. The results (Fig. 4A) showed a major increase in the amount of cytochrome b558 associated with p67PHOX when a larger amount of Rac1 was present. Of interest was the finding that this effect was observed with both Rac1-GTP[γS] and Rac1-GDP[βS]. Under the same conditions, Cdc42, a GTPase closely related to Rac1 but not capable of inducing NADPH oxidase activation, had no effect (Fig. 4B).
Figure 4.
Effect of Rac1 and Cdc42 on the binding of p67PHOX to cytochrome b558. The experiments were performed as described in the text, except that 800 pmol of Rac-GTP[γS]/GDP[βS] or Cdc42-GTP[γS]/GDP[βS] was used. (A Upper) Affinity precipitation in the presence of Rac. Lanes: 2, plus 800 pmol Rac1; 3, plus 800 pmol Rac1-GTP[γS]; 4, plus 800 pmol Rac1-GDP[βS]. (A Lower) Quantification of the binding by scanning densitometry. The data are expressed as percentage of full-length GST-p67PHOX and are the mean ± SE of three experiments. (B Upper) Affinity precipitation in the presence of Cdc42. Lanes: 2, plus 800 pmol Cdc42; 3, plus 800 pmol Cdc42-GTP[γS]; 4, plus 800 pmol Cdc42-GDP[βS]. (B Lower) Quantification of the binding by scanning densitometry. The data are expressed as percentage of full-length GST-p67PHOX and are the mean ± range of two experiments.
Discussion
In the present study, we demonstrated that p67PHOX is able to bind directly to cytochrome b558. First, labeled p67PHOX was able to recognize a 91-kDa band, which, by Western blot, was shown to comigrate with gp91PHOX in specific granules from a normal subject but not from a CGD patient deficient in cytochrome b558. Second, p67PHOX was able to bind to pure cytochrome b558 that had been applied to nitrocellulose. Third, cytochrome b558 could be affinity-precipitated by GST-p67PHOX but not by GST when either had been coupled to glutathione-Sepharose beads. A direct interaction between p67PHOX and cytochrome b558 is in accord with the idea that p67PHOX regulates the transfer of electrons from NADPH to the flavin (7) because p67PHOX then would be in proximity to the flavin center, enabling it to perform a regulatory function in this part of the protein. Recently, one specific domain of p67PHOX, called the activation domain, has been shown to be involved in this regulation (23).
Perhaps the most interesting result of these experiments was the finding that Rac1, a small GTPase that is necessary for the activation of the oxidase in a cell-free system, was able to promote the interaction between cytochrome b558 and GST-p67PHOX. This finding was specific for Rac1, because Cdc42, a closely related small GTPase that, however, is unable to promote the activation of NADPH oxidase, had no effect on this interaction. The interaction was promoted by both Rac1-GTP[γS] and Rac1-GDP[βS], a finding reminiscent of earlier work by Gabig et al. (24), who provided evidence that the oxidase used both the GTP- and GDP-bound forms of a small GTPase, and more recently by Gorzalczany et al. (25), who found that both the GTP[γS]- and GDP[βS]-bound forms of prenylated Rac1 are active in a cell-free system using membrane or purified cytochrome b558 and p67PHOX in the absence of p47PHOX. These findings suggest that the function of Rac1 in the activation of NADPH oxidase is to promote the interaction between cytochrome b558 and p67PHOX. Alternatively, it might participate in the recruitment of cytosolic p67PHOX to the membrane environment, as shown by Gorzalczany et al. (25) and by our own results using a high concentration of Rac1, although simple recruitment to the membrane seems unlikely to explain the results of the affinity-precipitation experiments. We cannot exclude the possibility that the increase in the affinity precipitation of cytochrome b558 is mediated by a direct interaction of Rac1-GTP[γS] with cytochrome b558, binding the cytochrome to GST-p67PHOX indirectly; but this, too, would be a significant three-way interaction between the cytochrome, GST-p67PHOX, and Rac1-GTP[γS].
In conclusion, our data demonstrate a direct interaction between p67PHOX and cytochrome b558. Furthermore, the interaction is promoted by Rac1-GTP[γS] (and Rac1-GDP[βS]), but not by the small GTPase Cdc42.
Acknowledgments
We thank Dr. Ulla Knaus for kindly providing us with the gp91PHOX antibody and Cdc42. This work was supported in part by U.S. Public Health Service Grants AI-24227, AI-28479, and AI-24838. P.M.-C.D. is a recipient of a postdoctoral fellowship from the Arthritis Foundation and is a postdoctoral fellow from Institut National de la Santé et de la Recherche Médicale U479, CHU Xavier Bichat, Paris.
Abbreviations
- CGD
chronic granulomatous disease
- GST
glutathione S-transferase
- GTP[γS]
guanosine 5′-O-(3-thiotriphosphate)
- GDP[βS]
guanosine 5′-O-(2-thiodiphosphate)
References
- 1.Babior B M. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 2.Johnston, R. B. (2001) Curr. Opin. Hematol., in press. [DOI] [PubMed]
- 3.El Benna J, Ruedi J M, Babior B M. J Biol Chem. 1994;269:6729–6734. [PubMed] [Google Scholar]
- 4.Segal A W, West I, Wientjes F, Nugent J H A, Chavan A J, Haley B, Garcia R C, Rosen H, Scrace G. Biochem J. 1992;284:781–788. doi: 10.1042/bj2840781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith R M, Connor J A, Chen L M, Babior B M. J Clin Invest. 1996;98:977–983. doi: 10.1172/JCI118882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayakawa T, Suzuki K, Suzuki S, Andrews P C, Babior B M. J Biol Chem. 1986;261:9109–9115. [PubMed] [Google Scholar]
- 7.Nisimoto Y, Motalebi S, Han C H, Lambeth J D. J Biol Chem. 1999;274:22999–23005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- 8.Heyworth P G, Knaus U G, Uhlinger D J, Conroy L, Bokoch G M, Curnutte J T. Mol Biol Cell. 1993;4:261–269. doi: 10.1091/mbc.4.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Mendez I, Leto T L. Blood. 1995;85:1104–1110. [PubMed] [Google Scholar]
- 10.Tsunawaki S, Kagara S, Yoshikawa K, Yoshida L S, Kuratsuji T, Namiki H. J Exp Med. 1996;184:893–902. doi: 10.1084/jem.184.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cross A R. Biochem J. 2000;349:113–117. doi: 10.1042/0264-6021:3490113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumimoto H, Kage Y, Nunoi H, Sasaki H, Nose T, Fukumaki Y, Ohno M, Minakami S, Takeshige K. Proc Natl Acad Sci USA. 1994;91:5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang J, Kleinberg M E. J Biol Chem. 1999;274:19731–19737. doi: 10.1074/jbc.274.28.19731. [DOI] [PubMed] [Google Scholar]
- 14.Nisimoto Y, Freeman J L R, Motelebi S A, Hirshberg M, Lambeth J D. J Biol Chem. 1997;272:18634–18641. doi: 10.1074/jbc.272.30.18834. [DOI] [PubMed] [Google Scholar]
- 15.Fuchs A, Dagher M-C, Vignais P V. J Biol Chem. 1995;270:5695–5697. doi: 10.1074/jbc.270.11.5695. [DOI] [PubMed] [Google Scholar]
- 16.Park M-Y, Imajoh-Ohmi S, Nunoi H, Kanegasaki S. Biochem Biophys Res Commun. 1994;204:924–929. doi: 10.1006/bbrc.1994.2548. [DOI] [PubMed] [Google Scholar]
- 17.Koshkin V, Lotan O, Pick E. J Biol Chem. 1996;271:30326–30329. doi: 10.1074/jbc.271.48.30326. [DOI] [PubMed] [Google Scholar]
- 18.Dang P M C, Johnson J L, Babior B M. Biochemistry. 2000;39:3069–3075. doi: 10.1021/bi9919807. [DOI] [PubMed] [Google Scholar]
- 19.Cross A R, Erickson R W, Ellis B A, Curnutte J T. Biochem J. 1999;338:229–233. [PMC free article] [PubMed] [Google Scholar]
- 20.Kjeldsen L, Sengelov H, Lollike K, Nielsen M H, Borregaard N. Blood. 1994;83:1640–1649. [PubMed] [Google Scholar]
- 21.Park M Y, Imajoh-Ohmi S, Nunoi H, Kanegasaki S. Biochem Biophys Res Commun. 1997;234:531–536. doi: 10.1006/bbrc.1997.6672. [DOI] [PubMed] [Google Scholar]
- 22.Han C-H, Freeman J L R, Lee T, Motalebi S A, Lambeth J D. J Biol Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- 23.Gabig T G, Crean C D, Mantel P L, Rosli R. Blood. 1995;85:804–811. [PubMed] [Google Scholar]
- 24.Gorzalczany, Y., Sigal, N., Itan, M., Lotan, O. & Pick, E. (2001) J. Biol. Chem., in press. [DOI] [PubMed]
- 25.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]