Abstract
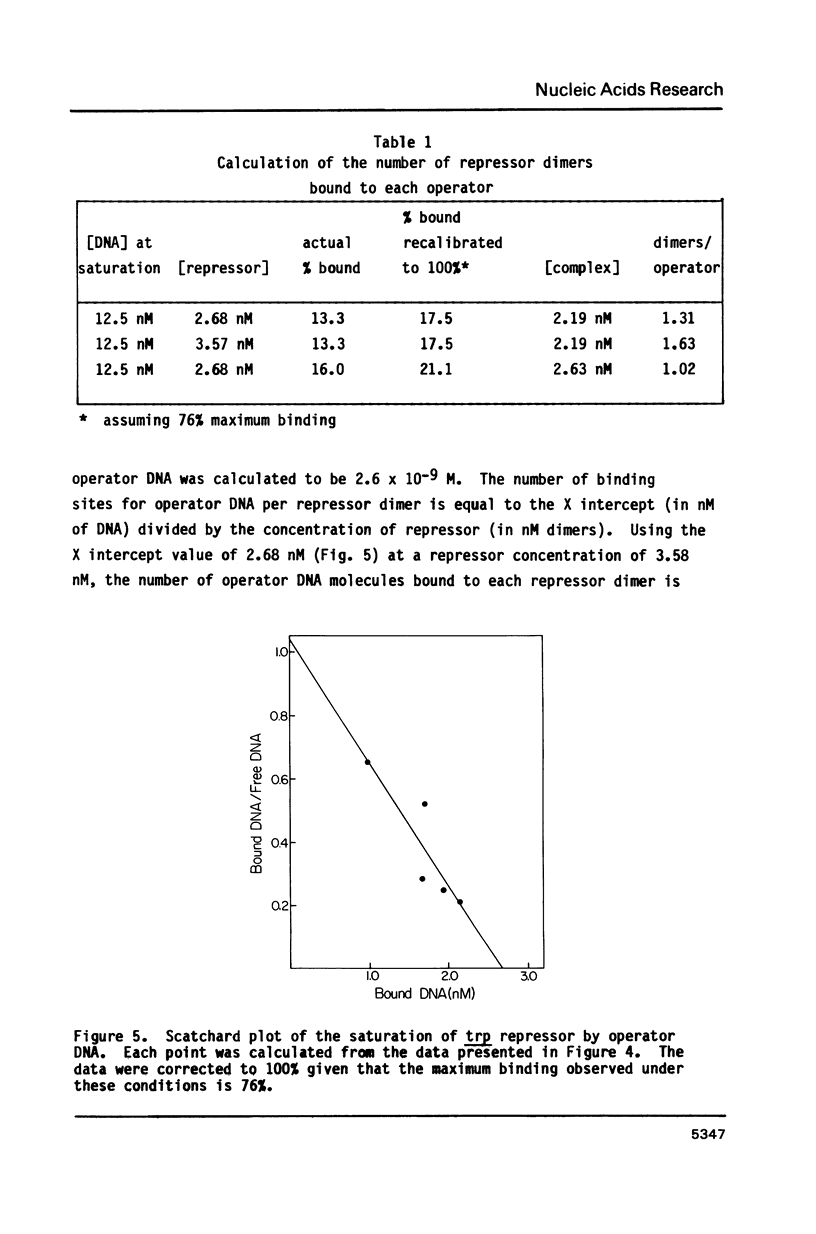
A filter binding assay was developed that allows measurement of specific binding of trp repressor to operator DNA. The most important feature of this procedure is the concentration and type of salt present in the binding buffer. Using this assay the dissociation constant of the repressor-operator complex was determined to be 2.6 X 10(-9) M, and 1.34 repressor dimers were found to be bound to each operator-containing DNA molecule. These values agree with those obtained by more complex methods. The dissociation constant of the repressor for the corepressor L-tryptophan in the presence of operator DNA was shown to be 2.5 X 10(-5) M. A synthetic 48 bp operator fragment was used to determine the repressor-operator dissociation constant in the presence of tryptophan or tryptophan analogs which have higher or lower affinities for aporepressor. The rate of dissociation of repressor from operator DNA also was determined. Our findings indicate that dissociation is influenced by the concentration of tryptophan or tryptophan analogs and suggest that release of the corepressor may be the first step in dissociation of the repressor-operator complex.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidson D. N., Bruce C., Gunsalus R. P. Interaction of the Escherichia coli trp aporepressor with its ligand, L-tryptophan. J Biol Chem. 1986 Jan 5;261(1):238–243. [PubMed] [Google Scholar]
- Bennett G. N., Schweingruber M. E., Brown K. D., Squires C., Yanofsky C. Nucleotide sequence of region preceding trp mRNA initiation site and its role in promoter and operator function. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2351–2355. doi: 10.1073/pnas.73.7.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Yanofsky C. Nucleotide sequence and expression of Escherichia coli trpR, the structural gene for the trp aporepressor. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7117–7121. doi: 10.1073/pnas.77.12.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachimiak A., Kelley R. L., Gunsalus R. P., Yanofsky C., Sigler P. B. Purification and characterization of trp aporepressor. Proc Natl Acad Sci U S A. 1983 Feb;80(3):668–672. doi: 10.1073/pnas.80.3.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley R. L., Yanofsky C. Mutational studies with the trp repressor of Escherichia coli support the helix-turn-helix model of repressor recognition of operator DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):483–487. doi: 10.1073/pnas.82.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorstein R. Q., Joachimiak A., Sprinzl M., Sigler P. B. The structural basis for the interaction between L-tryptophan and the Escherichia coli trp aporepressor. J Biol Chem. 1987 Apr 5;262(10):4922–4927. [PubMed] [Google Scholar]
- Nelson H. C., Sauer R. T. Lambda repressor mutations that increase the affinity and specificity of operator binding. Cell. 1985 Sep;42(2):549–558. doi: 10.1016/0092-8674(85)90112-6. [DOI] [PubMed] [Google Scholar]
- Paluh J. L., Yanofsky C. High level production and rapid purification of the E. coli trp repressor. Nucleic Acids Res. 1986 Oct 24;14(20):7851–7860. doi: 10.1093/nar/14.20.7851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Squires C. L., Yanofsky C., Yang H. L., Zubay G. Regulation of in vitro transcription of the tryptophan operon by purified RNA polymerase in the presence of partially purified repressor and tryptophan. Nat New Biol. 1973 Oct 3;245(144):133–137. doi: 10.1038/newbio245133a0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Yanofsky C. Interaction of the operator of the tryptophan operon with repressor. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3134–3138. doi: 10.1073/pnas.71.8.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevitz R. W., Otwinowski Z., Joachimiak A., Lawson C. L., Sigler P. B. The three-dimensional structure of trp repressor. 1985 Oct 31-Nov 6Nature. 317(6040):782–786. doi: 10.1038/317782a0. [DOI] [PubMed] [Google Scholar]
- Squires C. L., Lee F. D., Yanofsky C. Interaction of the trp repressor and RNA polymerase with the trp operon. J Mol Biol. 1975 Feb 15;92(1):93–111. doi: 10.1016/0022-2836(75)90093-5. [DOI] [PubMed] [Google Scholar]
- Squires C. L., Rose J. K., Yanofsky C., Yang H. L., Zubay G. Tryptophanyl-tRNA and tryptophanyl-tRNA synthetase are not required for in vitro repression of the tryptophan operon. Nat New Biol. 1973 Oct 3;245(144):131–133. doi: 10.1038/newbio245131a0. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Ohlendorf D. H., Anderson W. F., Matthews B. W. DNA-binding proteins. Science. 1983 Sep 9;221(4615):1020–1026. doi: 10.1126/science.6308768. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Zurawski G., Gunsalus R. P., Brown K. D., Yanofsky C. Structure and regulation of aroH, the structural gene for the tryptophan-repressible 3-deoxy-D-arabino-heptulosonic acid-7-phosphate synthetase of Escherichia coli. J Mol Biol. 1981 Jan 5;145(1):47–73. doi: 10.1016/0022-2836(81)90334-x. [DOI] [PubMed] [Google Scholar]



