Abstract
Modal-gating shifts represent an effective regulatory mechanism by which ion channels control the extent and time course of ionic fluxes. Under steady-state conditions, the K+ channel KcsA displays three distinct gating modes, high-Po, low-Po and a high-frequency flicker mode, each with about an order of magnitude difference in their mean open times. Here, we show that in the absence of C-type inactivation, mutations at the pore-helix position Glu71 unmask a series of kinetically distinct modes of gating in a side-chain-specific way. These gating modes mirror those seen in wild–type channels and suggest that specific interactions in the side-chain network surrounding the selectivity filter, in concert with ion occupancy, alter the relative stability of pre-existing conformational states of the pore. The present results highlight the key role of the selectivity filter in regulating modal gating behavior in K+ channels.
Keywords: Patch clamp, Kinetic Model single-channel, macroscopic currents, K+ channel, X-ray Crystallography
INTRODUCTION
Potassium channels are ubiquitous membrane proteins with a fundamental role in generation and modulation of the electrical excitability in cells1. Channel function is finely controlled by the interplay between activation gating at the stimulus-driven bundle crossing, and C-type inactivation gating at the selectivity filter2. Recent high resolution crystallographic analyses have provided atomic level details of K+ channels trapped with the activation gate in the closed3-5 or open conformation6-9 and the inactivation gate in the conductive or non-conductive conformations5,9.
A series of crystal structures of KcsA trapped in various degrees of gate opening and ion occupancy9 have shown that entry to the C-type inactivated state is associated with a sequential reduction in ion occupancy at the S2 and S3 binding sites correlated to the extent of opening at the inner bundle gate. Although these structures provide unique insights into the basic structural transitions underlying the K+ channel gating cycle, a cursory look at most single-channel recordings reveal that even the simplest ion channels exhibit kinetically complex behaviors beyond the present set of structures. This functional heterogeneity involves conductive and non conductive states10-13 as well as a variety of sub conductance levels12,14-17 for which there are no current structural correlates. We have provided evidence showing that under saturating stimulus conditions and at steady-state (when the activation gate is in its fully-open conformation), most of these gating fluctuations arise from conformational changes at the selectivity filter11,12,18,19. Not unexpectedly, the nature of the permeant ion20-24 as well as a variety of mutations near the filter17,25-28 have been shown to dramatically modulate the frequency and lifetimes of these gating events. In many cases, the structural consequences of these perturbations are reflected in changes in the ionic occupancy at the filter, which partly explains the divergence from normal functional behavior26,29.
Modal gating appears to be a characteristic feature of many K+ channels, where time-dependent single-channel activity can switch abruptly between periods of high and low open probability under fixed experimental conditions30-32. In several channels, differential inactivation rates underlie some of these gating regimes31-34. The KcsA selectivity filter and adjacent regions display a considerable amount of conformational flexibility, as revealed from a comparison of existing KcsA structures in high and low K+5, partial and fully-open states9, in the presence of blockers35 and in the (so-called) “flipped” structure observed in the E71A mutant11. It is easy to speculate that this intrinsic structural flexibility might underlie some of the heterogeneous functional behavior of the selectivity filter that leads to multiple gating modes 12.
Here, we have probed the functional and structural origins of modal gating in KcsA by studying a series of side-chain substitution at Glu71 position. These mutations sharply reduce entry into the C-type inactivated state while stabilizing three kinetically defined gating modes, depending on the type of side chain at position 71. These gating modes are reminiscent of those seen in wt KcsA, based on their distinct intra-burst open probability (Po), and were named high-Po mode (for mutants E71A/G/C/V/S/T), low-Po mode (E71I) and Flickery mode (E71Q). High-resolution closed-state crystal structures of some of these mutations, together with molecular dynamics (MD) simulations reveal changes in the ion profiles and water occupancy in and around the selectivity filter. These observations provide an initial rational to the origins of the conformational fluctuations occurring at the selectivity filter of the open-conductive channel.
RESULTS
Variable modal kinetic behavior of wt KcsA
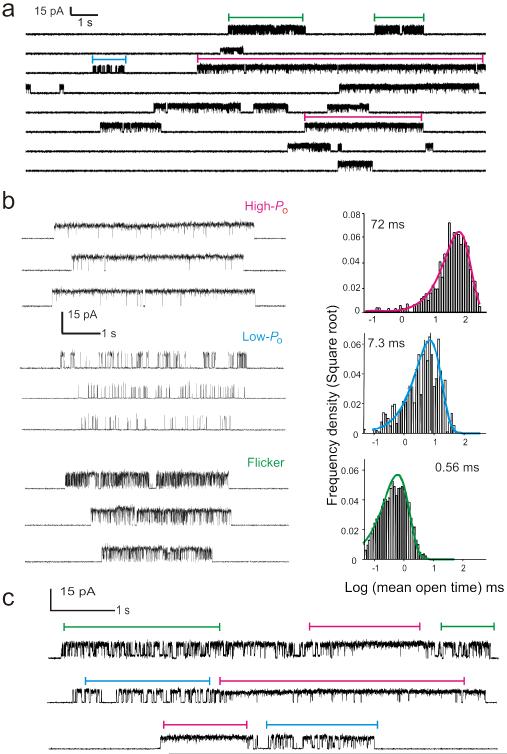
At steady-state, and under saturating proton concentrations, KcsA predominantly resides in the non-conductive C-type inactivated state11,12. These long silent periods are interrupted by brief sojourns into the conductive conformation, before transitioning back to the non-conductive inactivated state11 (Fig. 1a). KcsA has been reported to exhibit a highly variable single channel kinetic behavior, displaying at least three distinct patterns or “modes” of gating12 (Fig. 1b). These modes are characterized by set variations in mean open and mean closed times: a high-Po mode with long open times τo ~100 ms, a low-Po mode with intermediate open times τo ~ 10 ms and a Flickery mode characterized by very short open times τo < 1 ms. The distribution of these modes is found to be random in nature with no obvious evidence for pH or voltage dependence. The proportion of time spent in the individual modes varies greatly from one patch to another; however the predominant mode is that of the high-Po. Steady-state single-channel recordings show that transitions between modes can take place within the same burst (Fig. 1c), supporting the view that modal behavior in KcsA results from the conformational heterogeneity of individual channels. However, these transitions are infrequent and modal changes are typically observed after sojourns into the C-type inactivated state.
Figure 1.
Modal gating behavior of wt KcsA. (a) A continuous recording of KcsA single-channel currents measured under steady-state conditions at pH 3.0 and + 150 mV in 200 mM symmetric K+ solutions. (b) KcsA displays a highly variable kinetic behavior which arises from a combination of three distinct modes of channel activity, the high-Po, low-Po and the flickery mode (Left). Histograms show a distribution of open times within bursts for each of the three modes of channel activity with mean open times indicated in parenthesis (Right). (c) Channels occasionally switch between modes within a burst of activity, suggesting that modes arise from a homogenous population of channels.
Mutations at position 71 stabilize diverse gating regimes
While in voltage-dependent channels modal gating has been associated with specific biochemical modifications36,37, the origin of KcsA’s kinetic heterogeneity and sub-conductance levels has remained unclear. Mutations near the selectivity filter have shown to substantially reduce this variability. In particular, the C-type inactivation-removing mutation E71A also unmasks a kinetically homogeneous high-Po behavior, indistinguishable from the high-Po gating mode seen in wt KcsA11,12. We therefore carried out an in-depth analysis of the role of different side-chain substitutions at position 71 on the steady-state single channel kinetics of KcsA. Out of a total fifteen substitutions, nine mutations: Ala, Cys, Thr, Ser, Val, Ile, Gln, His and Gly were well tolerated. Mutations to Arg, Lys, Leu, Asp, Asn and Phe severely compromised channel folding and stability and were not analyzed further.
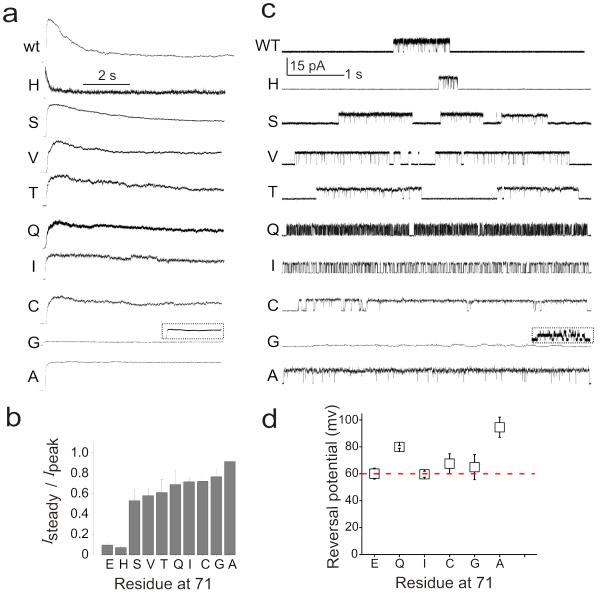
Analysis of macroscopic currents from the nine functional Glu71 mutants revealed that while there were no major effects on the time course of activation gating, all mutations directly affect the stability of the C-type inactivated state (Fig. 2a). As shown earlier11,38, E71A eliminates C-type inactivation, E71H severely enhanced it, and E71S stabilized an intermediate level. Mutants E71C, E71I, E71V, E71T and E71Q also slow down C-type inactivation with steady-state Po larger than 0.5 (Fig. 2b). The steady-state single channel activity faithfully reflected each of the mutants macroscopic behavior, where the long silent periods characteristic of wt KcsA recordings (>100 ms), were mostly absent (Fig. 2c).
Figure 2.
Glu71 mutants stabilize individual gating modes in a side-chain specific way (a) Macroscopic responses of wt and various Glu71 mutants elicited by pH jumps from 8.0 to 4.0 using a rapid solution exchanger in the presence of 200 mM KCl and the membrane potential held at +150 mV. The current trace for the E71G mutant is shown at a relative amplitude, compared with the other traces, the inset shows the same trace expanded in the current axis. (b) A plot of Isteady/ Ipeak for various Glu71 mutants (n>5) (c) Single-channel currents were recorded under steady-state conditions at pH 4.0 and +150 mV in 200 mM symmetric K+ solutions. Grey box highlights mutants that are focused in this study. (d) Selectivity versus Na+ estimated from single-channel I-V ramps under bi-ionic conditions. No detectable Na+ currents were seen in any of the mutants. Eapparent is the potential at which K+ currents can last be resolved. Error bars show s.d (n>5)
However, the key observation from this set of mutants is that, besides slowing down C-type inactivation, these side-chain substitutions displayed unique intra-burst kinetic patterns arising from differences in the duration of opening and closing dwell times. Substitution to Ala, Gly, Cys, Thr, Val and Ser resulted in a common phenotype with long opening bursts and few short intra-burst closures. Substitution to Ile displays very homogenous kinetics with similar mean open and closed times, while Gln leads to a sustained, high frequency flickering behavior with very short open and closed sojourns. Interestingly, substitution to Gly also reduced the single channel conductance by factor of 10 (to 1.2 pA), a feature that was also reflected in the small amplitude of its macroscopic currents.
Overall, mutations that slowed down inactivation displayed a very homogenous kinetic behavior (Supplementary Fig. 1). All of the tested Glu71 mutants were fully selective to K+ against Na+ under bi-ionic conditions (Fig. 2d). Given their kinetic homogeneity and the obvious similarities to the pre-existing gating modes in wt-KcsA, we chose the mutants that best represented each type of kinetic mode for further analysis: E71A for the high-Po mode, E71I for the low-Po mode and E71Q for the Flickery mode.
Kinetic analysis of Glu71 mutants
The high open probability of E71A, E71I and E71Q (>0.7) allowed us to easily target recordings arising from a single active channel. This way, experiments carried out at different proton concentrations confirmed that the effect of increasing pH lies mostly in decreasing the burst length, as a result of the closure of the activation gate, with no major effects on the behavior of the burst itself. This is a clear indication that the transitions within the burst fully reflect the conformational fluctuations at the selectivity filter (Supplementary Fig. 2).
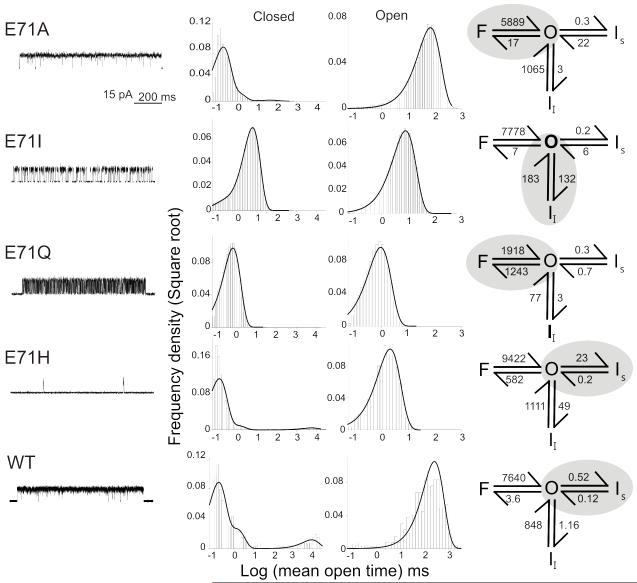
The rate constants to and from the conductive state for the various gating mode mutants were determined by fitting open and closed dwell times distributions to a model with one conductive and three nonconductive states (Fig. 3). These three non conductive states were defined as the “Slow” inactive state (Is), with dwell times (τc) around 100 ms; an Intermediate Inactive state (Ii) having dwell times between 1-10 ms; and the Flicker (F) state with dwell times in the 0.1-0.5 ms range. This classification is based on the lifetimes from single wt KcsA patches11,12. The behavior of the severely C-type inactivated mutant E71H was also analyzed to set a kinetic baseline for the transitions from a fully inactivated state. In Figure 3 (right panels) the kinetic schemes and the shaded region show the predominant transition in each of the mutants. Our key observation is that each mutation is associated with changes in the rate constants governing transitions to and from each of the states that define modal gating in KcsA. Thus, while the E71Q mutation mostly affects transitions into and out of the F state, E71I favors transitions to Ii and E71H is fundamentally biased towards Is. As expected, most Glu71 mutants had a profound influence on the lifetime of Is, a fact that might suggest that destabilization of Is is a precondition to the stabilization of Ii or F.
Figure 3.
Kinetic behavior of Glu71 mutants. Representative single-channel activity for Glu71 mutants (Left). Histograms show a distribution of closed and open channel lifetimes for the entire recordings (Middle). Single-channel current recordings were best fit by three closed and one open state for Glu. The closed states were defined as F, Ii and Is based on their lifetimes (Right). Rate constants of recovery from Is for wt and E71H are over estimated due to low Po and uncertainty in the number of channels in the patch.
Individual rate constants derived from the fitted experimental dwell time distributions in the context of our basic four state model (Table 1) were used to simulate single-channel and macroscopic ensemble currents. Since these mutants did not alter activation, we used rate constants previously determined for wt KcsA to describe the proton-dependent transitions12. As expected, the models reproduced all of the unitary characteristics of the different gating modes as well as the time course for the macroscopic responses to pH pulses (Supplementary Fig. 3).
Table 1.
Kinetic parameters of the model
| F → O | F ← O | Ii → O | Ii ← O | Is → O | Is ← O | N | |
|---|---|---|---|---|---|---|---|
| Wt | 7983 ± 1739 | 5.6 ± 1.34 | 526 ± 30 | 0.9 ± 0.3 | 0.09 ± 0.01 | 0.45 ± 0.07 | 3 |
| E71A | 6339 ± 268 | 10.9 ± 4.8 | 1055 ± 291 | 2.2 ± 0.9 | 14.0 ± 3.4 | 0.22 ± 0.10 | 3 |
| E71I | 6814 ± 287 | 21 ± 11.0 | 152 ± 59 | 151 ± 58 | 2.1 ± 1.9 | 0.33 ± 0.17 | 5 |
| E71Q | 1972 ± 115 | 1342 ±187 | 97 ± 66 | 3.5 ± 1.6 | 1.86 ± 1.2 | 0.34 ± 0.34 | 5 |
| E71H | 7906 ± 1853 | 325 ± 128 | 526 ± 30 | 44.2 ± 11.8 | 0.18 ± 0.02 | 24.0 ± 1.0 | 3 |
Crystal structures reveal subtle changes in ion occupancy
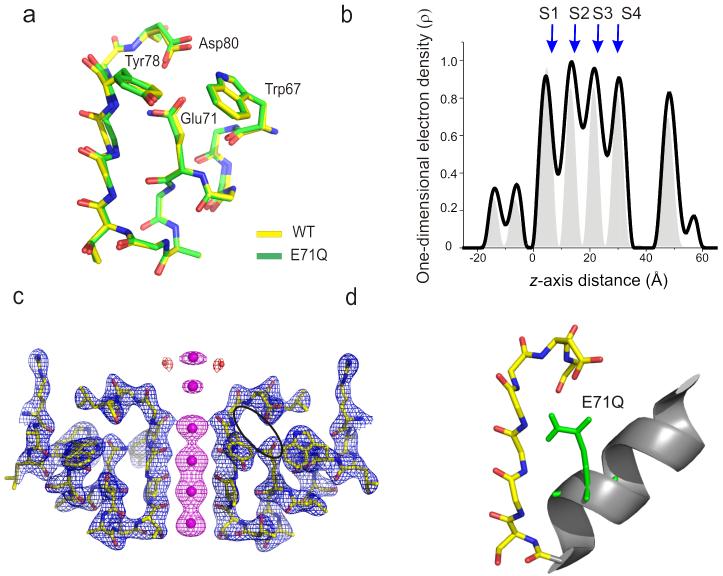
The discovery of individual mutants that greatly stabilized each of the major gating modes in KcsA provides a unique opportunity to probe the structural basis of each gating mode. As the E71A structure is already available in the putative conductive conformation (closed inner gate)11, we focused on mutations E71I, that favor the Ii state, and E71Q, favoring the F state. Crystals of mutants E71I and E71Q were obtained as Fab complexes, diffracting at resolutions of 2.3 Å and 2.7 Å, respectively, and were solved by molecular replacement methods5 using the structure of wt KcsA (1K4C) as search model.
Experimental 2Fo – Fc electron density maps corresponding to the filter region and Fo – Fc omit maps for the ion distribution profile are shown in Figures 4 for E71I and 5 for E71Q. Overall, the selectivity filter structures of all gating mode mutants (Figs. 4 and 5) showed no major changes in backbone conformation and thus, corresponds to the conductive conformation of the filter observed in most closed KcsA structures (RMSD with respect to 1K4C is 0.25 Å for E71I and 0.12 Å for E71Q). This result was not surprising, given that these mutations decrease the rate and extent of C-type inactivation and should indeed stabilize the conductive conformation. However, close observation of the electron density maps around the filter region revealed interesting differences in terms of the relative ion occupancy and the number of water molecules behind the filter (Figs. 4 and 5).
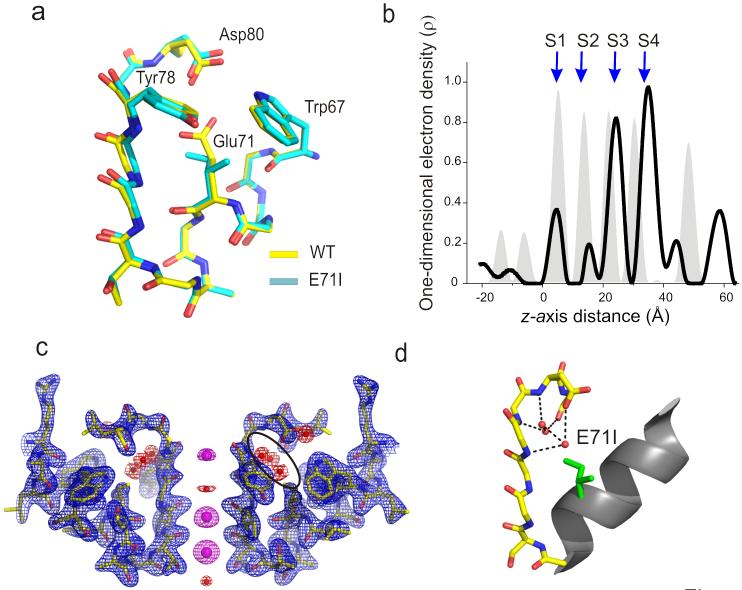
Figure 4.
Crystal structure of E71I. (a) Single-subunit line representation of the P-loop of E71I overlaid onto the wt structure5 (PDB entry 1K4C) highlights the conductive conformation of the selectivity filter backbone. (b) One-dimensional electron density profiles along the central symmetry (z) axis is shown. S1-S4 denotes the K+ binding sites. Gray peaks in the background correspond to one-dimensional electro density profile of the wt structure. (c) Electron density map of residues 60–84 from two diagonally symmetric subunits. Sticks, polypeptide chain; blue mesh, 2σ-contour of the 2Fo – Fc electron density map for the protein; magenta mesh, 6σ-contour of the Fo – Fc omit map for the ions ; red mesh, 4σ-contour of the Fo – Fc omit map for the waters. (d) A single-subunit P-loop is shown with side chains at Glu71 and Asp80 in stick representation. The H-bond interaction between the three crystallographic water molecules within the cavity behind the filter and the rest of the protein are represented by black dotted lines.
Figure 5.
Crystal structure of E71Q. (a) Single-subunit line representation of the P-loop of E71Q overlaid onto the wt structure5 (PDB entry 1K4C) highlights the conductive conformation of the selectivity filter backbone. (b) One-dimensional electron density profiles along the central symmetry (z) axis is shown. S1-S4 denotes the K+ binding sites. Gray peaks in the background correspond to one-dimensional electro density profile of the wt structure. (c) Electron density map of residues 60–84 from two diagonally symmetric subunits. Sticks, polypeptide chain; blue mesh, 2.5σ-contour of the 2Fo – Fc electron density map for the protein; magenta mesh, (4-6)σ-contour of the Fo – Fc omit map for the ions ; red mesh, 5σ-contour of the Fo – Fc omit map for the waters. (d) A single-subunit P-loop is shown with side chains at Glu71 and Asp80 in stick representation. At 2.7 Å resolution we observe no crystallographic waters within the cavity behind the filter.
The one-dimensional electron density profile along the pore axis of E71I points to a clear loss of ion occupancy at the S2 binding site, together with an apparent decrease in the occupancy at S1 (Fig. 4b). On the other hand, the E71Q mutant shows a modest increase in the occupancy at the S2 binding site in comparison to wt KcsA (Fig. 5b). Besides these changes, the most striking difference among the mutants is the number of water molecules buried between the pore-helix and the selectivity filter, bridging the filter region to the rest of the protein. In wt KcsA, a water molecule is coordinated by hydrogen bond interactions with Glu71, Asp80 and the backbone of Tyr78 in the selectivity filter. In the E71I structure, the “cavity” formed by the reduced side chain volume is filled by 3 crystallographic waters interacting with the filter through a network of hydrogen bonds connecting the backbone of Gly79, Asp80, Leu81 and Tyr78 and the carboxyl group in Asp 80 (Fig 4c, d). There was no observable coordinated water in this region in E71Q (Fig 5c, d) but this is likely due resolution issues (at 2.7 Å).
Glu71 mutants affect filter conformational dynamics
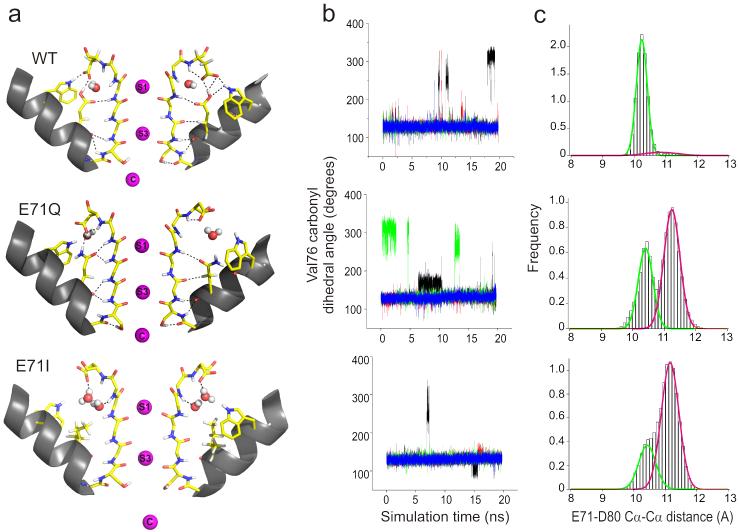
With the availability of individual crystal structures underlying each of the major gating modes, we then addressed the question of whether increased stability of Ii and F in the E71I and E71Q mutants might be reflected in the conformational fluctuations of the filter. Molecular dynamics (MD) simulation studies suggest that water molecules behind the selectivity filter can affect the filter’s conformational flexibility during ion permeation39. The timeframes of the single channel transitions observed for Ii and F (and of course, Is) can be orders of magnitude away from the dynamic window available to straightforward MD simulations, however the present structures offer a unique opportunity to evaluate short timeframe differences that might point to subsequent events directly linked to these gating transitions. We carried out a series of 20 ns MD simulations for mutants E71A, E71I, E71Q and E71G along with wt KcsA, in a fully explicit system embedded in a lipid bilayer.
From these MD runs, we observed two events of particular interest: First, the amide plane at Val76-Gly77 in the selectivity filter (in one of the subunits at a given time) underwent a 180° reorientation, pointing the backbone carbonyl oxygen of Val76 away from the conduction pathway (Fig. 6a). This type of transition has been observed in a number of MD simulations40-43 and leads to the loss of a favorable interaction at the S3 binding site, potentially affecting the free energy barrier associated to the translocation of K+ from S3 to S244. A plot of the backbone torsion angle as a function of the simulation time shows that the outward-facing (away from the conduction pathway) Val76 carbonyl is greatly stabilized in E71Q, where this conformation occurs ~40% of the total simulation time at least in a single subunit (Fig. 6b). In contrast, in wt KcsA the Val76 carbonyl remained outward-facing some 13% of the time, while for the E71I mutant it was only 3% (Fig. 6b). At the functional level, the dwell time ratio between the F and O states in the E71Q mutation was approximately 40:60 (Fig 3), essentially mirroring the outward-facing/straight conformations in the simulation above.
Figure 6.
Underlying conformational dynamics of the selectivity filter and the fast gating kinetics. (a) Structural snapshots of outward-facing carbonyl conformations. (b) Dynamics of carbonyl reorientation in KcsA. Time traces of the Val76 carbonyl dihedral angle (N-CA-C-O) during 20 ns molecular dynamics trajectories. Different color lines correspond to different subunits. Potassium ions were initially placed in the cavity and sites S1 and S3. (c) Distribution of Glu71-Asp80 Cα-Cα distances. The green and magenta fits correspond to populations with Asp80 facing-down (centered at ~10.3Å) and “flipped”-outward (centered at ~11.1Å) respectively.
Given the known differences in the timescales of single channel kinetic and MD simulations, this comparison between relative populations of states in simulations with the single channel kinetics only represent part of a global sequence of events and at best, should be considered only as a qualitative piece of the filter dynamics puzzle. Still, we draw attention to the fact that out of five MD runs (wt, E71A, -I, -Q and -G), only the flicker-prone E71Q mutant showed a considerable increase in the frequency and lifetime of Val76 reorientation (Fig. 6 and Supplementary Fig. 4). Therefore, we would like to suggest that the reorientation of the Val76 carbonyl might indeed be associated to the conformational changes that eventually lead to short-lived flicker states in single-channel records.
The second observation involves the outward “flipping” of the Asp80 side-chain relative to its position in wt KcsA, a movement reminiscent of the conformation observed in one of the crystal forms of E71A11 (the so-called “flipped” structure) and in the selectivity filter of Kir 3.145. Monitoring the Cα-Cα distance between the Glu71 and Asp80 reveals a very narrow distribution in wt KcsA, indicative of a strong interaction between these two residues (Fig. 6c, top). On the other hand, E71Q and E71I display a broader distribution with a distinct second population that corresponds to channels with “flipped” Asp80 (Fig. 6c, bottom two). In fact, this dual-population behavior is observed in all gating mode mutants at position 71, suggesting that the overall mobility of the Asp80 side chain is enhanced in the absence of an interaction with Glu71 (Fig. 6 and Supplementary Fig. 5).
DISCUSSION
K+ channel stationary gating is known to involve non-conductive kinetic states with lifetimes ranging from sub millisecond to several seconds. Transitions between these and conductive states define burst properties at the single-channel level, while changes in the equilibrium between these gating events lead to gating mode-shifts. Modal gating is a common feature in a wide range of channels, particularly Kv30-32, Nav34, Cav46-48, BK49,50 channels as well as the AChR51-53, and NMDA receptors54,55. In some channels, the mechanism for modal inter-conversion is subject to cellular control via phosphorylation and other post-translational modifications30,31,36,37,56, but the molecular underpinning of these events has remained unknown in the majority of channels. In this study we show that kinetically diverse conformational states that give rise to modal gating shifts can also originate from conformational fluctuations at or near the filter. Conveniently, in KcsA each of the naturally occurring modes can be individually stabilized, depending on the nature of the side-chain at position 71 in the pore-helix. Given that a wide range of mutations converged on a limited set of gating modes, we suggest that the various side-chain substitutions at position 71 do not introduce new kinetic behaviors, but actually modulate the relative stability of pre-existing conformational states that are intrinsic to wt KcsA. These states were defined by their intrinsic dwell time distribution (each roughly tenfold faster) as the slow (or deep) inactivated state Is, and intermediate inactivated conformation (Ii) and the highly fluctuating F or flicker state.
The question still remains as to why the wt channel exhibits gating heterogeneity while mutants reveal a more homogenous behavior. One common feature among these mutations is a substantial loss of C-type inactivation and given that modes arise predominantly as channels recover from this inactivated state, it is likely that modes switches are associated with channels transitioning between the deep inactivated and a series of open conductive conformations. These transitions might have slightly different energy paths, therefore ensuing a heterogeneous behavior in wt KcsA.
The structural snapshots that underlie the molecular events leading to C-type inactivation (Is) have recently been defined crystallographically9. These include a sequential loss of ion binding sites S2 and S3, a pinching of the permeation pathway at Gly77 and a compression of the filter along the fourfold symmetry axis. The crystal structures and MD simulations of the E71I and E71Q mutations offer insights into the short-lived Ii and F states, respectively.
Ion occupancy in the E71I mutant filter is almost fully lost at S2 and partially decreased at S1, an ion profile that is distinctly different to that seen in either the fully conductive or the C-type inactivated filter9. We propose that this conformation of the filter is related to the intermediate Ii state for two reasons: First, even with the loss of external ion binding sites the backbone conformation is essentially that of the fully conductive filter. In comparison, loss of S2 and a partial loss of S3 in a incompletely C-type inactivated filter leads to obvious changes in the filter backbone9. Second, recovery from C-type inactivation has been shown to be sensitive to external permeant ion concentration and to the ability of the ion to move from one site to another57. Given its easy access to the external bulk K+, ion rebinding at S1 is expected to be more favorable than at S2 and S3. We therefore suggest that during permeation in wt KcsA, loss of ions at the external binding sites lead to the intermediate Ii states while sequential vacancies at the deeper S2 and S3 sites lead to the more stable, fully developed C-type inactivated (Is) state.
Another interesting feature of this structure relates to the variation in the number and location of water molecules coordinated in the cavity behind the selectivity filter. In Wt KcsA, a water molecule links the carboxyl groups on Glu71 and Asp80 with the backbone amides of Tyr78 and Gly79 in the conductive conformation, but two water molecules in the collapsed, low K+ structure5 that coordinate these residues. Substitution of Glu71 to Ile results in three water molecules that generate extensive H-bonding interactions with the backbones of residues Gly79, Leu81 and Tyr78, and the Asp80 side-chain. Although the precise effect of substituting the functionally important E71-D80 interaction for the three waters of coordination is as yet unknown, it is tempting to speculate that the resulting H-bond network promotes the stabilization of Ii with a relative destabilization of the conductive conformation of the filter (O).
Our molecular dynamics simulations suggest that the frequency of spontaneous reorientation transitions of the Val76 carbonyl group is greatly enhanced in the E71Q mutation while it is mostly unaffected in the rest of the mutants. However, given the wide differences in time-scale between our experimental and computational data, it is difficult to establish a direct correlation between the dwell-time of the outward-facing carbonyl conformation (ns timescale) and the kinetics of single-channel flicker events (μs timescale). Clearly, it is unlikely that one carbonyl Val76 reorientation corresponds to one flicker event observed in electrophysiology. However, we suggest that an increase in the frequency of flickers with parallel increases in the incidence of Val76 reorientation could accompany ion translocation from one binding site to the next during permeation, also triggering transitions that lead to short-lived flicker states. Thus, reorientation of Val76 might be an “initiating” event that leads to subsequent non-conductive conformations of the selectivity filter. These conformations would be metastable in the μs timescales, as has been suggested on the basis of equivalent MD runs44.
MD runs further revealed a bimodal distribution of Asp80 side-chain positions for all of the Glu71 mutations (except E71H). The existence of these two conformations is not surprising if we consider that the loss of the interaction with Glu71 should enhance the mobility of Asp80. It is interesting to note that the enhanced flexibility of Asp80 is also reflected as an increase in the crystallographic B-factor of this region in the E71I crystal structure (Supplementary Fig. 6). We believe that the additional conformational freedom of Asp80, leads to a decrease in the backbone constrains at Tyr78 and Gly79, with obvious consequences to the overall conformational dynamics of the filter. However, it still remains unclear as to how this motional freedom relates to the different gating forms seen in wt KcsA. Since most voltage-dependent K+ channels have a valine at the position corresponding to Glu71 in KcsA, some of these conformational fluctuations of the filter might play a role in other members of the K+ channel family.
In conclusion, the pore helix, selectivity filter, and external vestibule are dynamic structures where small local conformational changes (that include motions of the carbonyl oxygens, small fluctuations of the filter backbone, or changes in the configuration and occupancy of water molecules behind the filter) can lead to drastic effects on gating. These transitions define the interplay between ions and the filter and thus underlie the diverse gating patterns observed in single channel recordings of most K+ channels. In KcsA, selectivity filter fluctuations are defined by a complex energy landscape that defines three kinetically distinct gating fluctuations. Transitions into each of the different gating modes depend on the relative depth of the energy wells associated with the three of pre-existing selectivity filter conformations.
Supplementary Material
Acknowledgements
We thank F. Bezanilla, L. Cuello, V. Vásquez and H. Raghuraman for insightful discussions; M. Wiener and M. Purdy for crystallographic data collection (for E71Q); the staff of the SER-CAT 22-ID and GM/CA-CAT 23-ID beamlines at the Advanced Photon Source for their invaluable assistance in data collection; R. MacKinnon (Rockefeller University) for providing the KcsA antibody hybridoma cell line; and the National Center for Supercomputing Applications (NCSA) and the Laboratory Computing Resource Center (LCRC) at Argonne National Laboratory for computer time. This work was supported by US National Institutes of Health grants to E.P. (R01GM057846, U54GM087519) and B.R (R01GM062342); and American Heart Association Postdoctoral Fellowship to S. C.
APPENDIX
Materials and Methods
Channel Expression and Purification
Wt and mutant KcsA, cloned in pQE32 vector, were expressed in E.coli XL1-blue cells. Membrane preparations were made by homogenizing the cells and spinning them down at 100,000g for 1 h. Membrane pellets were then solubilized by incubating them with PBS containing dodecyl maltoside at room temperature and then purified with a Co2+-based metal-chelate chromatography resin (Talon resin, Clontech). The quality of the purified protein was checked by gel-exclusion chromatography using a Superdex-200 column.
Electrophysiology and kinetic analysis
Electrophysiological measurements were made by patch clamp recordings in channel-reconstituted liposomes. Purified protein was reconstituted in asolectin vesicles by dilution with 200 mM KCl and 10 mM 4-morpholine propanesolfonic acid (MOPS) buffer at pH 7.0. Residual detergent was further removed by incubation with biobeads (Bio-Rad). Channel incorporated-liposome suspension was then centrifuged for 2 h at 100,000g and the pellet was resuspended in 60 μl of KCl/MOPS buffer. A drop of the proteoliposome was placed on a glass slide and dried over-night in a desiccator at 4°C. The sample was then re-hydrated with 20 μl of buffer, which yielded giant liposomes. This preparation was suitable for patch clamp recordings after ~ 2 hrs. For macroscopic currents, KcsA was reconstituted in 1:100 (mass:mass) of protein to lipid ratio, while for single channel studies we used a ratio of 1:10,000 (mass:mass). Currents were recorded under symmetrical conditions of 200 mM KCl and 10 mM MOPS buffer unless otherwise specified. Some of the critical experiments were also performed in succinate buffer to ensure that the fundamental gating properties are not affected by MOPS (data not shown). Recording pipettes were pulled from thin-walled borosilicate glass and heat polished such that they had a bath resistance of 1–2 MΩ when filled with 200mM KCl, 10 mM MOPS solution. All measurements in this study were conducted in the inside-out configuration of the patch clamp technique. Experiments were carried out at room temperature (20-22°C). Currents were elicited in response to pH jumps from 8.0 to 4.0 using an RCS-160 fast solution exchanger (Biologic) fed by gravity. During pH pulses, the membrane was held at +150 mV. Macroscopic currents were sampled at 5 kHz using an Axon 200-B patch-clamp amplifier. Single-channel currents were digitized at a sampling rate of 40 kHz and low-pass filtered to 5 kHz through an 8-pole Bessel filter.
Kinetic analysis
All kinetic analyses were done using the QuB suite of programs (www.qub.buffalo.edu). Drifts in baseline were adjusted using the baseline correction algorithms within the QuB preprocessing module. Current traces were idealized into noise-free open and close transitions using SKM, a segmental k-means algorithm (the Viterbi algorithm) based on a hidden Markov modeling procedure at full bandwidth 58. The number of closed and open states that best describe the data were identified by using a maximum likelihood criteria after imposing a dead time of 25 μs.
Crystallization of Glu71 mutants
Glu71 mutants were expressed and purified as described above. E71I and Q were crystallized in the presence of an antibody Fab fragment by the sitting drop method as described previously38. Crystals diffracted to Bragg spacing of 2.3 Å for E71I and of 2.7 Å for E71Q. Data was collected on beamlines 22ID-D (SERCAT) and 23ID (GMCA) at the Advanced Photon Source and processed with HKL200067.
Crystallographic analysis
Structures were solved by molecular replacement using only the Fab fragment and extracellular part of wt KcsA (PDB 1K4C) without the selectivity filter as a search model to reduce the biasing of model prediction, as the expected conformation is supposed to be different from the closed state. The selectivity filter was built with side-chain density corresponding to Val76, Tyr78 and Asp80 as markers. Multiple cycles of refinement using CNS and manual rebuilding using the program O, was carried out until the complete model was built into the electron density and the Rfactors were lowered. Data collection and refinement statistics are provided in Table 2.
Table 2.
Data collection and refinement statistics
| E71Q | E711 | |
|---|---|---|
| Data collection | ||
| Space group | I4 | I4 |
| Cell dimensions | ||
| a=b, c (Å) | 155.7,76.1 | 156.4, 76.0 |
| α=β=γ(°) | 90 | 90 |
| Resolution (Å) | 40-2.7 | 40-2.3 |
| Rsym or Rmerge (%) | 6.9(36.3) | 8.0(32.4) |
| I/σI | 17.5(3.7) | 20.0(2.7) |
| Completeness (%) | 94.0(93.2) | 96.1(94.3) |
| Redundancy | 6.1(3.1) | 3.9(3.1) |
| Refinement | ||
| Resolution (Å) | 40-2.7 | 40-2.3 |
| No. reflections | 23689 | 39318 |
| Rwork/Rfree (%) | 22.6/26.8 | 26.4/27.1 |
| No. atoms | ||
| Protein | 4074 | 4073 |
| Ligand/ion | 7 | 5 |
| Water | 4 | 7 |
| B-factors | ||
| Protein | 59.2 | 45.7 |
| Ligand/ion | 41 | 51 |
| Water | 52 | 82.4 |
| R.m.s deviations | ||
| Bond lengths (Å) | 0.007 | 0.005 |
| Bond angles (°) | 1.36 | 1.11 |
Molecular dynamics simulations
The simulation system was represented by an atomic model of the various mutants of the closed KcsA channel embedded in a membrane bilayer solvated by an aqueous solution of KCl. The model contained the KcsA tetramer, dipalmitoylphosphatidylcholine (DPPC) molecules, water molecules and K+ ions in the cavity and at sites S1 and S3. More potassium and chloride ions were added to ensure electrical neutrality and mimic a 150 mM KCl concentration. The system was set up using the CHARMM program59 following a previously described methodology41. Constant-pressure molecular dynamics simulation were carried out using the NAMD program60. Each mutant was simulated for 20 ns.
Footnotes
Accession Codes
The atomic coordinates of the mutants E71I and E71Q have been deposited in the Protein Data Bank under accession codes 3OR7 and 3OR6, respectively.
References
- 1.Hille B. Ion channels of excitable membranes. Sinauer Associates Inc.; 2001. [Google Scholar]
- 2.Yellen G. The moving parts of voltage-gated ion channels. Q Rev Biophys. 1998;31:239–95. doi: 10.1017/s0033583598003448. [DOI] [PubMed] [Google Scholar]
- 3.Doyle DA, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 4.Kuo A, et al. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–6. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414:43–8. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–22. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 7.Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 8.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–82. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 9.Cuello LG, Jogini V, Cortes DM, Perozo E. Crystal structure of open-inactivated KcsA and the mechanism of C-type inactivation in K+ channels. Nature. 2010;466:203–208. doi: 10.1038/nature09153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoppa NE, Sigworth FJ. Activation of shaker potassium channels. I. Characterization of voltage-dependent transitions. J Gen Physiol. 1998;111:271–94. doi: 10.1085/jgp.111.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cordero-Morales JF, et al. Molecular determinants of gating at the potassium-channel selectivity filter. Nat Struct Mol Biol. 2006;13:311–8. doi: 10.1038/nsmb1069. [DOI] [PubMed] [Google Scholar]
- 12.Chakrapani S, Cordero-Morales JF, Perozo E. A quantitative description of KcsA gating II: single-channel currents. J Gen Physiol. 2007;130:479–96. doi: 10.1085/jgp.200709844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zagotta WN, Hoshi T, Aldrich RW. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J Gen Physiol. 1994;103:321–62. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrempf H, et al. A prokaryotic potassium ion channel with two predicted transmembrane segments from Streptomyces lividans. Embo J. 1995;14:5170–8. doi: 10.1002/j.1460-2075.1995.tb00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuello LG, Romero JG, Cortes DM, Perozo E. pH-dependent gating in the Streptomyces lividans K+ channel. Biochemistry. 1998;37:3229–36. doi: 10.1021/bi972997x. [DOI] [PubMed] [Google Scholar]
- 16.Chapman ML, VanDongen AM. K channel subconductance levels result from heteromeric pore conformations. J Gen Physiol. 2005;126:87–103. doi: 10.1085/jgp.200509253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng J, Sigworth FJ. Selectivity changes during activation of mutant Shaker potassium channels. J Gen Physiol. 1997;110:101–17. doi: 10.1085/jgp.110.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blunck R, Cordero-Morales JF, Cuello LG, Perozo E, Bezanilla F. Detection of the opening of the bundle crossing in KcsA with fluorescence lifetime spectroscopy reveals the existence of two gates for ion conduction. J Gen Physiol. 2006;128:569–81. doi: 10.1085/jgp.200609638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakrapani S, Cordero-Morales JF, Perozo E. A quantitative description of KcsA gating I: macroscopic currents. J Gen Physiol. 2007;130:465–78. doi: 10.1085/jgp.200709843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pusch M, Bertorello L, Conti F. Gating and flickery block differentially affected by rubidium in homomeric KCNQ1 and heteromeric KCNQ1/KCNE1 potassium channels. Biophys J. 2000;78:211–26. doi: 10.1016/S0006-3495(00)76586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demo SD, Yellen G. Ion effects on gating of the Ca(2+)-activated K+ channel correlate with occupancy of the pore. Biophys J. 1992;61:639–48. doi: 10.1016/S0006-3495(92)81869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeMasurier M, Heginbotham L, Miller C. KcsA: it’s a potassium channel. J Gen Physiol. 2001;118:303–14. doi: 10.1085/jgp.118.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choe H, Sackin H, Palmer LG. Gating properties of inward-rectifier potassium channels: effects of permeant ions. J Membr Biol. 2001;184:81–9. doi: 10.1007/s00232-001-0076-3. [DOI] [PubMed] [Google Scholar]
- 24.Lu T, Wu L, Xiao J, Yang J. Permeant ion-dependent changes in gating of Kir2.1 inward rectifier potassium channels. J Gen Physiol. 2001;118:509–22. doi: 10.1085/jgp.118.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu T, et al. Probing ion permeation and gating in a K+ channel with backbone mutations in the selectivity filter. Nat Neurosci. 2001;4:239–46. doi: 10.1038/85080. [DOI] [PubMed] [Google Scholar]
- 26.Zhou M, MacKinnon R. A mutant KcsA K+ channel with altered conduction properties and selectivity filter ion distribution. J Mol Biol. 2004;338:839–46. doi: 10.1016/j.jmb.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Haug T, Olcese R, Toro L, Stefani E. Regulation of K+ flow by a ring of negative charges in the outer pore of BKCa channels. Part II: Neutralization of aspartate 292 reduces long channel openings and gating current slow component. J Gen Physiol. 2004;124:185–97. doi: 10.1085/jgp.200308950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proks P, Capener CE, Jones P, Ashcroft FM. Mutations within the P-loop of Kir6.2 modulate the intraburst kinetics of the ATP-sensitive potassium channel. J Gen Physiol. 2001;118:341–53. doi: 10.1085/jgp.118.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morais-Cabral JH, Zhou Y, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 30.Dreyer I, Michard E, Lacombe B, Thibaud JB. A plant Shaker-like K+ channel switches between two distinct gating modes resulting in either inward-rectifying or “leak” current. FEBS Lett. 2001;505:233–9. doi: 10.1016/s0014-5793(01)02832-0. [DOI] [PubMed] [Google Scholar]
- 31.Singer-Lahat D, Dascal N, Lotan I. Modal behavior of the Kv1.1 channel conferred by the Kvbeta1.1 subunit and its regulation by dephosphorylation of Kv1.1. Pflugers Arch. 1999;439:18–26. doi: 10.1007/s004249900139. [DOI] [PubMed] [Google Scholar]
- 32.Cooper E, Shrier A. Inactivation of A currents and A channels on rat nodose neurons in culture. J Gen Physiol. 1989;94:881–910. doi: 10.1085/jgp.94.5.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armstrong CM, Gilly WF. Fast and slow steps in the activation of sodium channels. J Gen Physiol. 1979;74:691–711. doi: 10.1085/jgp.74.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Howe JR, Ritchie JM. Multiple kinetic components of sodium channel inactivation in rabbit Schwann cells. J Physiol. 1992;455:529–66. doi: 10.1113/jphysiol.1992.sp019315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenaeus MJ, Vamvouka M, Focia PJ, Gross A. Structural basis of TEA blockade in a model potassium channel. Nat Struct Mol Biol. 2005;12:454–9. doi: 10.1038/nsmb929. [DOI] [PubMed] [Google Scholar]
- 36.Bockenhauer D, Zilberberg N, Goldstein SA. KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat Neurosci. 2001;4:486–91. doi: 10.1038/87434. [DOI] [PubMed] [Google Scholar]
- 37.Levin G, et al. Phosphorylation of a K+ channel alpha subunit modulates the inactivation conferred by a beta subunit. Involvement of cytoskeleton. J Biol Chem. 1996;271:29321–8. doi: 10.1074/jbc.271.46.29321. [DOI] [PubMed] [Google Scholar]
- 38.Cordero-Morales JF, et al. Molecular driving forces determining potassium channel slow inactivation. Nat Struct Mol Biol. 2007;14:1062–9. doi: 10.1038/nsmb1309. [DOI] [PubMed] [Google Scholar]
- 39.Capener CE, Proks P, Ashcroft FM, Sansom MS. Filter flexibility in a mammalian K channel: models and simulations of Kir6.2 mutants. Biophys J. 2003;84:2345–56. doi: 10.1016/S0006-3495(03)75040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domene C, Klein ML, Branduardi D, Gervasio FL, Parrinello M. Conformational changes and gating at the selectivity filter of potassium channels. J Am Chem Soc. 2008;130:9474–80. doi: 10.1021/ja801792g. [DOI] [PubMed] [Google Scholar]
- 41.Berneche S, Roux B. Molecular dynamics of the KcsA K(+) channel in a bilayer membrane. Biophys J. 2000;78:2900–17. doi: 10.1016/S0006-3495(00)76831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shrivastava IH, Sansom MS. Simulations of ion permeation through a potassium channel: molecular dynamics of KcsA in a phospholipid bilayer. Biophys J. 2000;78:557–70. doi: 10.1016/S0006-3495(00)76616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guidoni L, Torre V, Carloni P. Water and potassium dynamics inside the KcsA K(+) channel. FEBS Lett. 2000;477:37–42. doi: 10.1016/s0014-5793(00)01712-9. [DOI] [PubMed] [Google Scholar]
- 44.Berneche S, Roux B. A gate in the selectivity filter of potassium channels. Structure. 2005;13:591–600. doi: 10.1016/j.str.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Nishida M, Cadene M, Chait BT, MacKinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. Embo J. 2007;26:4005–15. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delcour AH, Lipscombe D, Tsien RW. Multiple modes of N-type calcium channel activity distinguished by differences in gating kinetics. J Neurosci. 1993;13:181–94. doi: 10.1523/JNEUROSCI.13-01-00181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luvisetto S, et al. Modal gating of human CaV2.1 (P/Q-type) calcium channels: I. The slow and the fast gating modes and their modulation by beta subunits. J Gen Physiol. 2004;124:445–61. doi: 10.1085/jgp.200409034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yue DT, Herzig S, Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990;87:753–7. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McManus OB, Magleby KL. Kinetic states and modes of single large-conductance calcium-activated potassium channels in cultured rat skeletal muscle. J Physiol. 1988;402:79–120. doi: 10.1113/jphysiol.1988.sp017195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothberg BS, Bello RA, Song L, Magleby KL. High Ca2+ concentrations induce a low activity mode and reveal Ca2(+)-independent long shut intervals in BK channels from rat muscle. J Physiol. 1996;493(Pt 3):673–89. doi: 10.1113/jphysiol.1996.sp021414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auerbach A, Lingle CJ. Heterogeneous kinetic properties of acetylcholine receptor channels in Xenopus myocytes. J Physiol. 1986;378:119–40. doi: 10.1113/jphysiol.1986.sp016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Milone M, et al. Mode switching kinetics produced by a naturally occurring mutation in the cytoplasmic loop of the human acetylcholine receptor epsilon subunit. Neuron. 1998;20:575–88. doi: 10.1016/s0896-6273(00)80996-4. [DOI] [PubMed] [Google Scholar]
- 53.Naranjo D, Brehm P. Modal shifts in acetylcholine receptor channel gating confer subunit-dependent desensitization. Science. 1993;260:1811–4. doi: 10.1126/science.8511590. [DOI] [PubMed] [Google Scholar]
- 54.Popescu G, Auerbach A. Modal gating of NMDA receptors and the shape of their synaptic response. Nat Neurosci. 2003;6:476–83. doi: 10.1038/nn1044. [DOI] [PubMed] [Google Scholar]
- 55.Patlak JB, Gration KA, Usherwood PN. Single glutamate-activated channels in locust muscle. Nature. 1979;278:643–5. doi: 10.1038/278643a0. [DOI] [PubMed] [Google Scholar]
- 56.Huganir RL, Delcour AH, Greengard P, Hess GP. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. Nature. 1986;321:774–6. doi: 10.1038/321774a0. [DOI] [PubMed] [Google Scholar]
- 57.Levy DI, Deutsch C. Recovery from C-type inactivation is modulated by extracellular potassium. Biophys J. 1996;70:798–805. doi: 10.1016/S0006-3495(96)79619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J. 1996;70:264–80. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brooks BR, et al. CHARMM:A program for macromolecular energy, minimization and dynamics calculations. J Comput Chem. 1983;4:187–217. [Google Scholar]
- 60.Phillips JC, et al. Scalable molecular dynamics with NAMD. J Comput Chem. 2005;26:1781–802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.