Abstract
We have previously shown that the lipooligosaccharide (LOS) from Neisseria meningitidis and N. gonorrhoeae engages the TLR4/MD-2 complex. In this study, we report that LOS from different meningococcal and gonococcal strains have different potencies to activate NF-κB through TLR4/MD-2, and that the relative activation can be correlated with ion abundances in MALDI-TOF mass spectrometry that are indicative of the number of phosphoryl substituents on the lipid A (LA) component of the LOS. The LOS from three of the strains, meningococcal strain 89I and gonococcal strains 1291 and GC56, representing high, intermediate and low potency on NF-κB activation, respectively, differently activated cytokine expression through the TLR4/MD-2 pathway in monocytes. In addition to induction of typical inflammatory cytokines such as TNF-α, IL-1β, and IL-6, MIP-1α and MIP-1β also were significantly higher in cells treated with 89I LOS which had the most phosphoryl substitutions on the LA compared to 1291 and GC56. We found that LOS activated both the MyD88- and TRIF-dependent pathways through NF-κB and IFN regulatory factor 3 (IRF-3) transcription factors, respectively. Moreover, LOS induced the expression of costimulatory molecule CD80 on the surface of monocytes via upregulation of IRF-1. These results suggest that phosphoryl moieties of LA from N. meningitidis and N. gonorrhoeae LOS play an important role in activation of both the MyD88- and TRIF-dependent pathways. Our findings are consistent with the concept that bacteria modulate pathogen-associated molecular patterns by expression of phosphoryl moieties on the LA to optimize interactions with the host.
INTRODUCTION
Infections due to Neisseria meningitidis and N. gonorrhoeae represent a major public health problem around the world. N. meningitidis is the leading cause of epidemic meningitis and fatal sepsis in otherwise healthy individuals (1). Worldwide more than 500,000 cases of meningococcal infection occur annually leading to approximately 50,000 deaths, and large epidemic outbreaks occur which can cause periodic spikes in the infection rate. N. gonorrhoeae is a major cause of sexually transmitted infections in the U.S. with over 700,000 cases in 2008, which can lead to pelvic inflammatory disease in 10–20 percent of infected women who can suffer secondary sequelae such as chronic pain, infertility, and ectopic pregnancy as a result (2). In addition, a growing number of epidemiologic and clinical studies provide strong evidence that gonococcal infection can facilitate the transmission of HIV (3). Control remains problematic due to increased antibiotic resistance and the lack of vaccines for infections caused by serogroup B N. meningitidis, the leading cause of meningococcal disease in the Americas and Europe, and by N. gonorrhoeae (1,4).
The mechanisms underlying the immune and inflammatory responses to Neisserial infections and how these protect against infection or contribute to clinical symptoms are incompletely understood. Inflammation is associated with many of the major complications exhibited by the clinical symptoms, but is generally considered to be a critical protective component of the host immune response to these pathogens. N. meningitidis is a frequent colonizer of the human upper respiratory tract, however in some individuals the bacterium spreads to the bloodstream causing meningitis and/or sepsis, which are serious conditions with high morbidity and mortality (5). N. gonorrhoeae most commonly causes uncomplicated endocervical infection in women which is characterized by vaginal discharge and sometimes by dysuria (6). However, approximately 50% of infected women are asymptomatic, rendering them at higher risk of developing pelvic inflammatory disease and disseminated gonococcal infection. Also, in contrast to N. meningitidis, N. gonorrhoeae does not appear to induce an effective state of adaptive immunity against repeated infection (7).
Lipooligosaccharide (LOS)3 is a major Neisserial virulence factor which is responsible for the induction of pro-inflammatory cytokines through the TLR4 pathway. The level of cytokine expression closely mimics the pathogenesis of both gonococcal and meningococcal infections observed in vivo (8–11). LOS is formed by a hydrophobic lipid A (LA) moiety that is anchored to the bacterial outer membrane and covalently linked to a hydrophilic oligosaccharide core which is exposed on the surface of the bacteria. The LOS, unlike LPS of Gram-negative bacteria, lacks the repeating O-polysaccharide component. The LA moiety portion of LOS has one major structure with variation in the number of phosphoesters and acyl chains. The LA structure consists of β-1', 6-linked disaccharide of glucosamine acylated with β-hydroxymyristate (2, 2') and β-hydroxylaurate (3, 3'), and acyloxyacyl linkages on the N-linked hydroxymyristates to two laurate residues (12). Our laboratory previously observed major differences in pro-inflammatory TLR4-mediated signaling elicited by LOS purified from various meningococcal and gonococcal strains resulting in significant variation in the cytokine responses of THP-1 monocytic cells (13). We also demonstrated that strain-specific binding of gonococcal LOS to TREM-2 was significantly reduced by O-deacylation (14). It remains unclear how LA structural heterogeneity affects activation of innate immune receptors resulting in a spectrum of immune responses and clinical symptoms in Neisserial infection.
It has been established that LPS from Escherichia coli induces innate immune responses by activating the TLR4/MD-2 receptor complex and that the activated TLR4/MD-2 subsequently recruits adapter molecules MyD88, TIRAP, TRIF, and TRAM through the cytoplasmic Toll-interleukin-1-receptor (TIR) domain of TLR4 (15). The recruited adapter molecules initiate signal transduction that leads to the secretion of pro-inflammatory cytokines and to the expression of surface molecules such as CD80 on APC (16). Recent studies suggest that these adapter molecules are selectively recruited to TLR4/MD-2 complexes and activate the MyD88 pathway through NF-κB leading to the expression of pro-inflammatory cytokines or the TRIF pathway through IFN regulatory factor 3 (IRF-3) leading to the expression of type I IFN, depending on the fine structure variation of the LA portion of ligand interacting with MD-2 (17–20). The mechanistic basis for differences in signal transduction due to LA structural variation remains unknown. In addition, activation of other signaling molecules, such as PI3K, may also be involved in the recruitment of either MyD88 or TRIF to TLR4 (19,20).
Recently, our laboratory demonstrated LA structural heterogeneity in the LOS from a variety of clinical isolates, and showed that LA structural variation was correlated with in vitro bioactivity in human monocytes based on the induction of the pro-inflammatory cytokines TNF-α, IL-6, and IL-1β (12,21). However, the impact of structural heterogeneity on TLR4/MD-2 activation, on the subsequent activation of the MyD88 and TRIF pathways, and on a broader cytokine production profile remains to be explored. It is unclear whether LA heterogeneity leads to variations in potency for activation of the two TLR4 pathways, which may underlie the variable immune responses and clinical symptoms that are observed in Neisserial infections.
In this study, we explore the relationship between the structures of various Neisserial LOS from clinical isolates and their engagement of the two branches of the TLR4/MD-2 pathway. We demonstrate that LOS from different strains of meningococci and gonococci have different efficacy in activation of NF-κB and that the difference in biooactivity is correlated with ion abundance ratios that are indicative of differing phosphoryl substitutions on the LA component. We then show that LOS variously induces cytokine expression by activation of the TLR4/MD-2 pathway in monocytes through both MyD88- and TRIF-dependent NF-κB and IRF-3 signaling, respectively. Moreover, we report that the LOS upregulates the expression of costimulatory molecule CD80 on the surface of monocytes through upregulation of the expression of IRF-1.
MATERIALS AND METHODS
Bacterial strains and LOS extraction
N. gonorrhoeae strains 1291, GC56, F62, DOV, and PID2, and N. meningitidis serogroup A strains 7880 and 7889 and serogroup C strain 89I are clinical isolates that have been described previously (12,13,22). LOS was extracted and purified by a modification of the hot phenol-water method (23,24). Hexaacyl LA fractions from 89I LOS containing phosphoryl and phosphoethanolamine (PEA) or phosphoryl substituents were prepared using TLC as described previously (21).
Preparation of intact LOS for mass spectrometry (MS)
Samples were prepared for MS using a method previously described (21,25). Briefly, purified LOS (1–2 mg/ml) was suspended in a solution of methanol: water (1:1) with 5 mM EDTA. An aliquot was desalted on Parafilm with a few cation-exchange beads (Dowex 50WX8-200) that had been converted to the ammonium form. After mixing the sample with an equal volume of dibasic ammonium citrate (20 mM), 0.5 μl was deposited on top of a thin layer of matrix formed on the sample plate by deposition of drops (0.3–0.9 μl) of a solution composed of 2,4,6-trihydroxyacetophenone (200 mg/ml; Sigma-Aldrich, St. Louis, MO) in methanol, with nitrocellulose transblot membrane (15 mg/ml; Bio-Rad, Hercules, CA) in acetone: isopropanol (1:1 v/v) mixed in a 4:1 v/v ratio.
MALD-TOF MS
MS was performed using MALDI in the linear mode on a Voyager-DE STR TOF instrument equipped with a 337-nm nitrogen laser and delayed extraction. Negative-ion spectra were obtained with an average of 500 pulses per spectrum and an acceleration voltage of 20 kV. Data Explorer software was used to digitally smooth and correct the baseline of the spectra. External calibration was performed with the average masses of the molecular ions of the peptides porcine renin substrate, bovine insulin, and oxidized insulin chain B (Sigma-Aldrich), and internal calibrations were performed using two- or three-points as previously described (12).
TLR4 reporter cell signaling
TLR4 signaling was measured by using HEK293-blue reporter cells which were generated by stable transfection of cells with TLR4, MD-2 and CD14 genes (HEK-Blue™-4; InVivogen, San Diego, CA). The cells contain an optimized secreted embryonic alkaline phosphatase (SEAP) reporter gene placed under the control of an NF-κB-inducible promoter. This reporter gene allows the monitoring of signaling through TLR4 based predominantly on the activation of NF-κB. The phosphatase activity was detected by the use of HEK-Blue Detection medium. Briefly, HEK-Blue cells were diluted to 1×105 cells per ml and 200 μl (20,000 cells) of the cell suspension were added to the wells of a 96-well plate and treated with LOS (final concentration 10 ng/ml) for 18 h. To infect cells with bacteria, the concentration of viable bacteria was adjusted spectrophotometrically at 650 nm and bacteria were added to cells at a multiplicity of infection of 1. For some experiments, cells were treated with the lipid IVa at a concentration of 10, 100, or 500 ng/ml (Avanti Polar Lipids, Alabaster, AL) for 1 h prior to addition of bacteria at MOI of 1 or LOS at 100 ng/ml. For SEAP detection, 20 μl of supernatant from treated cells was transferred to a 96-well plate containing 200 μl of QUANTI-blue per well. Cell culture medium from untreated cells was used as the negative control. The reaction was incubated at 37ºC for 30 min and SEAP activity was assessed by reading the OD at 620 nm with a microplate reader.
Purification of peripheral blood monocytes
For human monocyte isolation, fresh buffy coat provided by Blood Centers of the Pacific (San Francisco, CA) was diluted 1:1 with PBS. The diluted buffy coat was overlaid on to Ficoll-Paque (GE Healthcare Biosciences, Piscataway, NJ) and centrifuged at 1000 rpm for 30 min. The interphase containing the monocytes was collected and washed twice with PBS. Cells were then seeded in T-75 cell culture flasks at 106 cells per ml of growth media. After incubation at 37ºC in 5% CO2 for two hours, lymphocytes were removed with PBS washing and the adherent monocytes were trypsinized, resuspended in culture media and reseeded into 24-well plates at 6.25 × 105 cells per well.
Pro-inflammatory cytokine response of human monocytes
The human monocytic leukemia cell line THP-1 was obtained from the American Type Culture Collection (Manassas, VA) and has been characterized previously (21). The cells were propagated in RPMI 1640 supplemented with 10% FBS at 37°C in a 5% CO2 atmosphere, and differentiated with 10 ng/ml PMA (Sigma-Aldrich) for 12–18 h. Stimulation of cells was performed in growth medium supplemented with 10% FBS as a source for LPS-binding protein. Differentiated THP-1 cells were seeded at 104 cells per well in 96-well plates and treated with LOS (100 ng/ml) or bacteria at a multiplicity of infection of 1 for 18 hours. The supernatants were collected and stored at −70°C for the subsequent cytokine assay. For primary human monocytes, adherent monocytes were reseeded at 105 cells per well (24-well plates) and then treated with 1 μg/ml of LOS for 18 h. Supernatants were collected and stored as described above. Control wells with THP-1 or primary monocytes were treated with culture medium only without bacteria or LOS. For some experiments, THP-1 cells were treated with lipid IVa at a concentration of 10, 100, or 500 ng/ml (Avanti Polar Lipids, Alabaster, AL) for 1 h prior to addition of 100 ng of LOS and incubate for additional 18 h. The cytokine levels in cell culture supernatants were assessed using bead-based singleplex (Bio-Rad, Hercules, CA) or multiplex assays (Invitrogen, Carlsbad, CA). Samples of cell supernatants (50 μl each) were processed as recommended by the manufacturers and analyzed using a Bio-Plex 200 system (Bio-Rad). For IFN-β, differentiated THP-1 cells were seeded at a concentration of 1×106 per well in 6-well plates and treated with LOS at 1 μg/ml for 18 h and supernatants were assayed for IFN-β by ELISA (PBL Interferon Source, Piscataway, NJ).
Flow cytometry
For studies of the expression of the costimulatory molecule CD80 on peripheral blood monocytes, LOS (1 μg/ml) or TLC-purified LA fractions were added to the cells (1×105 cells per well, 24-well plates) and incubated for 18 h. For some experiments, monocytes were treated with 10 μg/ml of neutralizing mouse anti-human TLR4 antibody or isotype-matched control antibody (eBioscience) for 30 min prior to addition of LOS. After washing, monocytes were harvested after treating cells with Accutase (Innovative Cell Technologies, San Diego, CA) for 5 min, washed with PBS, and then blocked by incubating on ice with one ml PBS containing 0.1 % BSA for 30 min. After removing the blocking buffer, cells were incubated on ice with 2 μg/ml of FITC-conjugated mouse anti-human CD80 (eBioscience) in a total volume of 100 μl of PBS containing 0.1% BSA for 60 min. FITC-labeled mouse IgG2b isotype antibody was used as a control. The cells were washed twice with PBS/BSA and analyzed by flow cytometry on a BD FACScan (Becton, Dickinson, Franklin Lakes, NJ).
TLR4 signaling and western blotting
PMA-differentiated THP-1 cells were distributed into 6-well plates (2 × 106 cells per well) and stimulated with LOS at 100 ng/ml for 0 to 240 min (to detect phosphor-p65 and phosphor-IRF-3), or 0 to 18 h (to detect IRF-1 and IRF-7). After washing with PBS, cells were lysed by directly adding SDS-containing sample buffer (Bio-Rad) to the wells and incubating on ice for 15 min with intermittent pipetting. After sonicating the cell lysates for 15 s, 20 μl aliquots of each sample was heated at 100°C for 5 min. After cooling on ice, samples were resolved in a 10% SDS polyacrylamide gel and transferred to a nitrocellulose membrane for immunoblotting. Phosphorylated and non-phosphorylated proteins were detected by antibodies directed against phosphor-p65, p65, phosphor-IRF-3, IRF-3, IRF-1, IRF-7, or actin (Cell Signaling, Beverly, MA). Secondary antibodies were HRP-conjugated rabbit anti-mouse IgG and detected proteins were visualized by ECL Plus (GE Healthcare Biosciences). The relative intensity of each immunoblot band was determined by densitometry using the Image J software available from the National Institutes of Health, after which the intensity values were normalized by calculating the ratio of the phosphorylated band intensity to that of the non-phosphorylated control band (for phosphorylation of NF-κB or IRF-3) or actin control band (for IRF-1 and IRF-7), and the data presented as fold changes compared to controls.
Statistical analysis
Statistical analyses were performed using SigmaStat for Windows version 3.11 (Systat Software, San Jose, CA). Groups of data were analyzed by the Tukey test for multiple pairwise comparisons. Values of p < 0.05 were considered significant for all comparisons.
RESULTS
Activation of NF-κB by LOS is correlated with LA phosphorylation
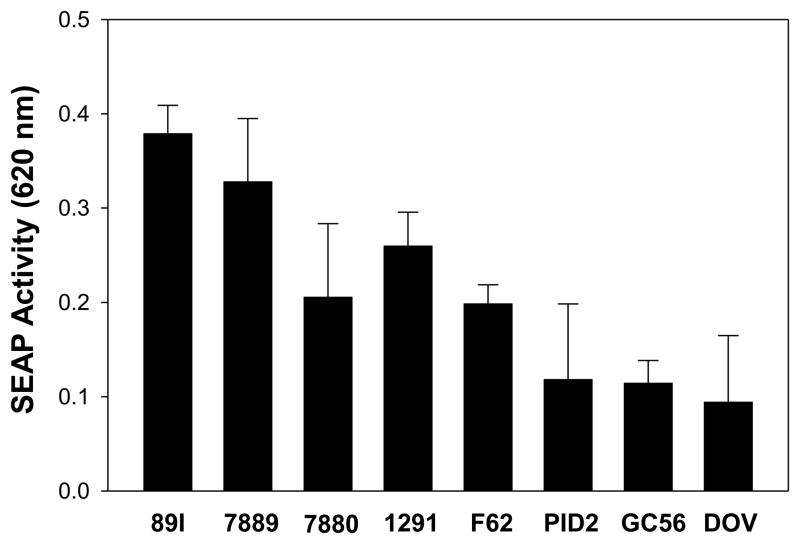
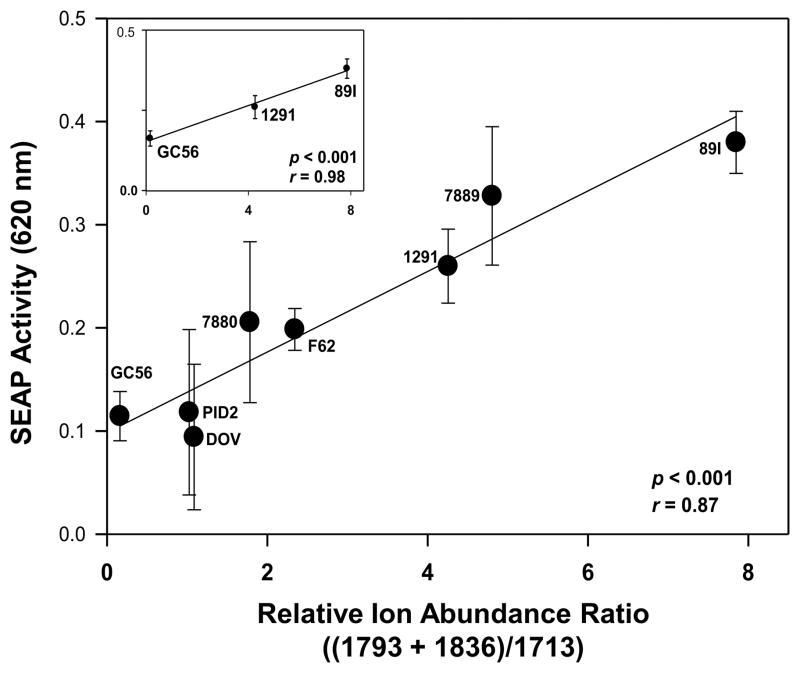
We have previously demonstrated that specific combinations of LA phosphorylation and acylation are associated with the potential of LA to induce pro-inflammatory cytokines in human monocytes (12,21). To investigate the impact of phosphorylation differences on the induction of cytokines by LA independent of acylation, we determined both the degree of phosphorylation of the LA from eight Neisserial strains using MALDI-TOF MS as well as their cytokine induction capability. Negative-ion MALDI-TOF spectra of the intact LOS from the strains were acquired, and fragment ion peaks were identified which corresponded to structures containing phosphoryl and PEA substitutions of hexaacyl LA. In particular, three prominent ion peaks were identified which corresponded to triphosphoryl LA at m/z 1793, diphosphoryl LA with a single PEA at m/z 1836, and a diphosphoryl LA without PEA substitution at m/z 1713. The strains varied in the relative abundances of each phosphorylated LA substituents contained within their LOS. To assess the bioactivity of the strains, we employed HEK-blue reporter cells that express the SEAP reporter gene under the control of an NF-κB-inducible promoter to analyze NF-κB activation in the TLR4/MD-2 signal transduction pathway. LOS was added to the reporter cells and SEAP activity was analyzed after incubation for 18 h. As shown in Fig. 1, the 89I LOS induced the highest level of SEAP activity, and DOV and GC56 LOS the lowest levels. To determine whether the degree of LA phosphorylation correlated with activation of NF-κB through TLR4/MD-2 signaling, we compared the relative ion abundance ratios for peaks indicative of the presence of three relative to two phosphoryl substituents on the LA to characterize their effect on biological potency. Specifically, the ratios of the sum of the relative ion abundances of the two peaks corresponding to LA containing three phosphate species (m/z 1793 and m/z 1836) to the abundance of diphosphoryl ions at m/z 1713 were regressed onto the induction of SEAP activity. As shown in Fig. 2, there was a significant positive correlation between the two values (r = 0.87, p < 0.001), indicating that the LOS which induced the highest levels of SEAP activity contained a greater degree of LA phosphate species than those which induced lower levels. Among the LOS, that from meningococcal strain 89I, which had the highest ratio of triphosphoryl compared to diphosphoryl substitution, was the most potent in NF-κB induction. The 1291 LOS had intermediate potency in NF-κB induction and a relative ion abundance ratio which fell between that of 89I and GC56, the latter of which had the lowest ion abundance ratio and also was the lowest potency inducer of NF-κB. These three were selected for further detailed functional analyses based on the significant correlation between their LA phosphorylation states and their ability to induce NF-κB as shown in Fig. 2 (r = 0.98, p < 0.001), and their representation of the entire range of bioactivity potencies that we observed.
Figure 1.
LOS variously activates NF-κB. HEK-293 NF-κB reporter cells (20,000 cells per well) were distributed in 96-well plates and treated with LOS (10 ng/ml) for 18 h as described in the Materials and Methods. SEAP activity was measured at OD 620 nm. The results presented are the mean ± SD from at least 3 independent experiments.
Figure 2.
The relative SEAP activity in HEK293 NF-κB reporter cells is positively correlated with increasing numbers of phosphoryl substituents on the LA. Negative-ion MALDI-TOF MS spectra were acquired of intact LOS from eight Neisserial strains. Ion abundance ratios were calculated from the relative intensity of fragment ion peaks at m/z 1793 for LA molecules with 3 phosphoryl substituents plus the relative intensity at m/z 1836 for LA molecules with 2 phosphoryls and a single PEA group, divided by the relative intensity at m/z 1713 for LA molecules with 2 phosphoryl groups lacking additional phosphoryl substitution. The ion abundance ratios were plotted versus the induction of NF-κB in the HEK293 cells, and the positive correlation (r = 0.87) between the two sets of values was statistically significant (p < 0.001). The inset presents the positive correlation between the ion abundance ratios for 3 strains selected as representative of low (GC56), intermediate (1291), and high (89I) induction potential for SEAP activity (r = 0.98; p < 0.001).
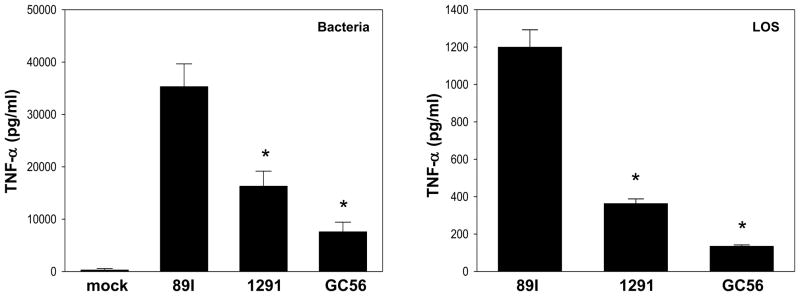
The three strains were tested as whole bacteria for their ability to induce pro-inflammatory cytokines. PMA-differentiated THP-1 cells were infected with bacteria and the production of TNF-α in the supernatant was analyzed. As presented in Fig. 3, infection with all of the strains significantly induced the expression of TNF-α. Similar to the induction of SEAP activity, meningococcal strain 89I induced a significantly higher amount of TNF-α than the two gonococcal strains (p < 0.001). A similar pattern was observed when using the corresponding LOS to treat the cells (Fig. 3). After an 18 h treatment with LOS, the supernatants were assayed for the levels of TNF-α. The 89I LOS induced a significantly higher level of TNF-α than 1291 and GC56 (p < 0.001). These results were also consistent with the NF-κB data using the SEAP reporter cells. We then examined whether lipid IVa, a tetraacylated intermediate in the LA biosynthetic pathway in E. coli, affected the ability of LOS to engage TLR4/MD-2 as it has recently been shown to inhibit activation of the TLR4 pathway by LPS through binding to the TLR4/MD-2 complex (26). To determine whether it can function as a TLR4 antagonist for LOS, THP-1 cells were pretreated with various concentrations of lipid IVa and incubated with 89I LOS for 18 hrs, and the supernatants were assayed for expression of TNF- α. Consistent with its antagonistic effect on LPS, lipid IVa also functioned as an antagonist for LOS by inhibiting LOS-induced TNF-α expression in THP-1 cells as well as LOS-induced NF-κB activation in HEK-blue reporter cells (data not shown).
Figure 3.
Cytokine induction in THP-1 cells by whole bacteria and LOS. Whole bacteria and LOS of strains 89I, 1291, and GC56 were used to infect THP-1 cells for 18 h. LOS was added at a final concentration of 100 ng/ml. Supernatants were harvested for the determination of TNF- α concentration. The results presented are the mean ± SD from at least 3 independent experiments. *Cytokine expression was significantly higher when cells were stimulated with 89I bacteria and LOS compared with that of 1291 and GC56 (p < 0.001).
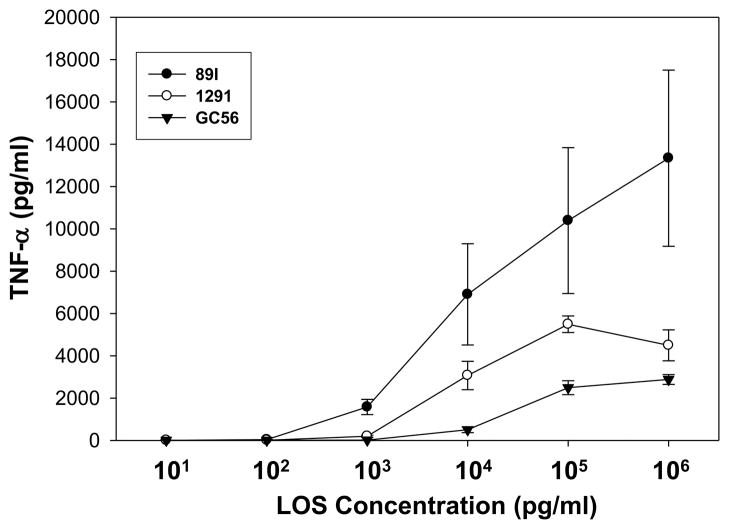
Previously, we have demonstrated in MD2-transfected HeLa cells that 1291 and GC56 LOS differentially stimulated TLR4 signaling in a dose-dependent manner (13). We next examined 89I, 1291, and GC56 LOS over a wide range of doses (10 pg/ml to 1 μg/ml) for their ability to stimulate cytokine expression through TLR4/MD-2 in THP-1 cells. As shown in Fig. 4, from concentration 1 ng/ml to 1 μg/ml 89I induced significantly higher TNF-α than did 1291 and GC56 (p<0.05). At 10 ng/ml 89I stimulated a higher level of TNF-α expression than did 1291 and GC56 at concentrations 10-100-fold higher, and the induction of TNF-α by both 1291 and GC56 LOS reached a plateau at 100 ng/ml whereas a plateau was not observed for 89I at a 10-fold higher concentration of 1 μg/ml. Taken together, the results suggest that LOS with different levels of phosphorylation have different potency for induction of pro-inflammatory cytokine expression through TLR4.
Figure 4.
Dose-response of THP-1 monocytes to LOS. PMA-differentiated THP-1 cells were treated with 89I, 1291, or GC56 LOS at concentrations ranging from 10 pg/ml to 1 μg/ml. Cells were incubated for 18 h and supernatants were analyzed for TNF-α expression. The data points represent the mean ± SD of three independent experiments.
LOS differentially activates the MyD88- and TRIF-dependent pathways
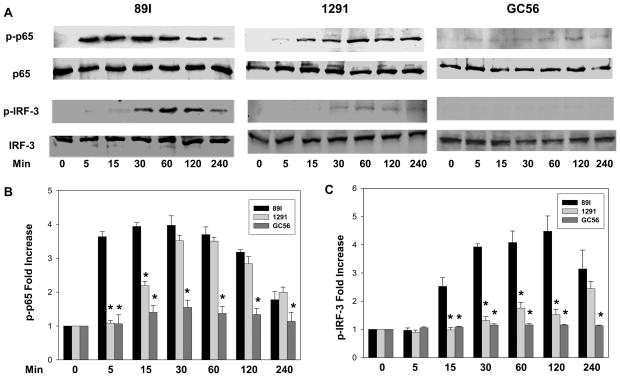
We next explored the question of whether LA phosphorylation and differential cytokine induction was related to differential activation of the MyD88- and TRIF-dependent pathways. After treatment with LOS for a time interval ranging from 0 to 240 minutes, THP-1 cells were harvested and the phosphorylation of NF-κB and IRF-3 was analyzed. As shown in Fig. 5A, 89I LOS induced significant NF-κB phosphorylation starting at 5 min, whereas the phosphorylation of NF-κB by LOS from gonococcal strains 1291 and GC56 was minimal until 15 and 120 min after treatment, respectively. For GC56 LOS, NF- κB phosphorylation was weak throughout the entire time period, and the ability of 1291 LOS to stimulate NF-κB phosphorylation was intermediate to that of 89I and GC56 LOS and did not approach that of 89I until 30 min. Densitometry analyses shown in Fig. 5B and 5C revealed that 89I LOS induced significantly higher levels of NF- κB activation than 1291 at 5 and 15 min, and more than that of GC56 from 5 min to 120 min. Similarly, IRF-3 was phosphorylated beginning 15 min after 89I LOS treatment, whereas it was not detected for 1291 LOS until 30 min following treatment. IRF-3 phosphorylation by GC56 was undetectable through the entire experimental timeframe (Fig. 5A and 5C). These results indicate that Neisserial LOS induce variable cytokine expression through differential activation of the MyD88- and TRIF-dependent pathways.
Figure 5.
Immunoblot for NF-κB and IRF-3 phosphorylation following LOS treatment. THP-1 cells (2 × 106) were distributed into each well of the 6-well plates and were stimulated with 100 ng/ml of 89I, 1291, or GC56 LOS for 0, 15, 30, 60, 120, and 240 min. After washing with PBS, cells were lysed by the direct addition of SDS sample buffer and applied to a 10% SDS polyacrylamide gel. The phosphorylated and non-phosphorylated proteins were detected by immunoblotting with antibody directed against either phosphor-p65, phosphor-IRF-3, p65 or IRF-3. Panel A, western immunoblots of NF-κB and IRF-3 phosphorylation following treatment by 89I, 1291, and GC56. Panels B and C, the relative intensity of each band in the immunoblots was determined using densitometry and the ratios of the phosphorylated bands to the non-phosphorylated bands were calculated. Data are presented as fold increases of treated compared with untreated samples, NF-κB (B) and IRF-3(C) phosphorylation. Data are representative of at least three independent experiments. * p < 0.05 indicates significance for comparisons between 89I and 1291 or GC56 LOS.
LOS-induced cytokine profile in human monocytes
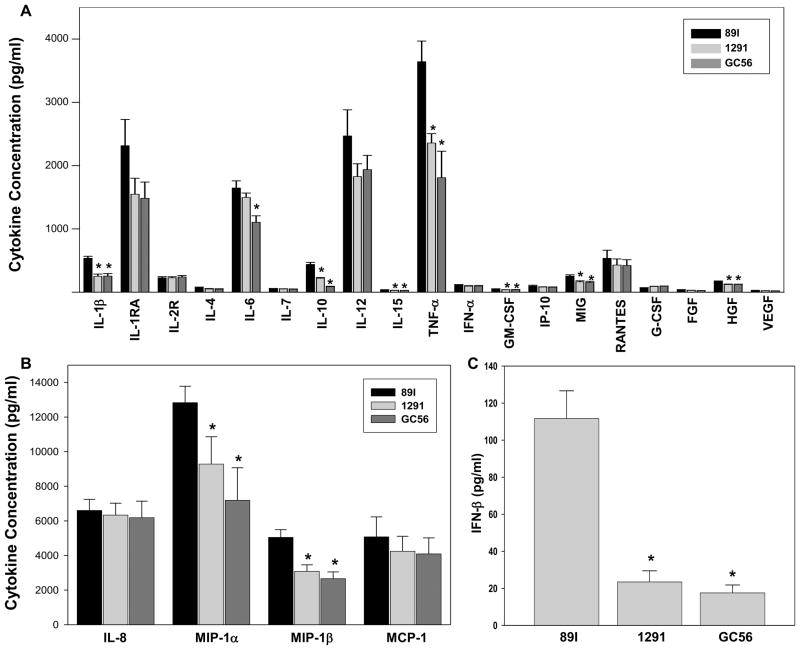
We further evaluated cytokine expression in primary human monocytes to determine whether LOS treatment will differentially stimulate the expression of a broad range of cytokines, and whether the cytokine profiles will correspond to that of NF-κB activation. For this purpose, 89I, 1291, and GC56 LOS were used to treat monocytes that were tested for cytokine expression following 18 hour stimulation. The results shown in Fig. 6 revealed that all three LOS induced the same 23 of the 30 cytokines assessed (Fig. 6A and B). The 7 cytokines that were not detectable included IL-2, IL-5, IL-13, IL-17, EGF, IFN-γ, and Eotaxin. Of the 23 cytokines induced, IL-1β, IL-10, IL-15, TNF-α, GM-CSF, MIG, MIP-1α and MIP-1β were induced to significantly higher levels by 89I LOS than by 1291 and GC56. The levels of IL-6 were similar between 89I and 1291, but significantly lower when cells were treated with GC56. The remaining 14 cytokines were induced to similar degrees by all three LOS. Among the cytokines activated by LOS, increased levels of TNF-α, IL-1β, and IL-6 have been reported previously by our group (12,13), however this is the first report of the upregulation in monocytes of MIP-1α and MIP-1β by Neisserial LOS. IFN-β, an important type I interferon induced via the TRIF-dependent pathway, was assayed using an ELISA as this cytokine was not included in the multiplex kit. As shown in Fig. 6C, all three LOS induced detectable levels of IFN-β, however 89I induced significantly higher levels compared to 1291 and GC56.
Figure 6.
Cytokine profiles of human monocytes after LOS treatment. Human monocytes freshly isolated from buffy coat were aliquoted at 1 × 106 cells per well in 6-well plates and treated with 1 μg/ml of LOS as described in the Materials and Methods. After 18 h stimulation, 50 μl of the supernatants were analyzed using a 30-plex kit for cytokine expression. A, 19 cytokines expressed at concentrations less than 4000 pg/ml. B, 4 cytokines expressed at concentrations greater than 4000 pg/ml. The results presented are the mean ± SEM of 3 independent experiments deriving from three individual donors. C, induction of IFN-β in THP-1 cells assayed by ELISA. The results presented are the mean ± SD of 3 independent experiments. * p < 0.05 for comparisons between 89I and 1291 or GC56 LOS.
LOS differentially upregulates CD80 in monocytes
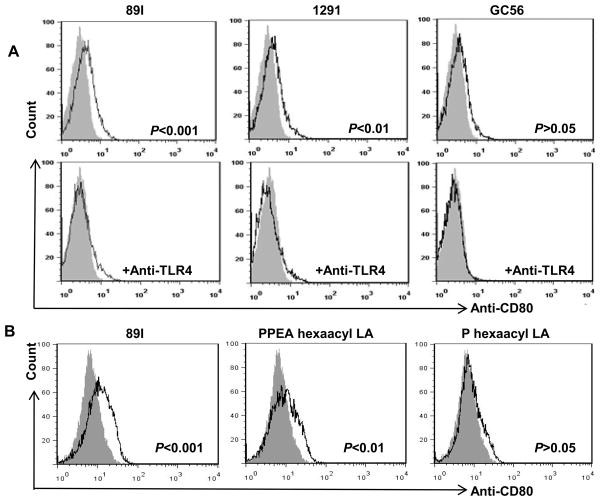
Type I IFN signaling is required for up-regulation of costimulatory molecule CD80, and the TRIF signaling pathway is thought to be the only route leading to production of type I IFN upon LPS stimulation (27,28). As shown in Fig. 5, our results demonstrated that LOS from different strains of Neisseria differentially activates the TRIF pathway. Thus, we hypothesized that LOS could differentially regulate the expression of CD80, an important marker for the functionality of APC and their subsequent activation of T cells. To test this hypothesis, monocytes were treated with LOS and the cells were analyzed for CD80 expression by flow cytometry. As predicted, the results presented in Fig. 7A show that LOS from 89I, 1291, and GC56, with differing numbers of phosphoryl and PEA moieties, differentially induced CD80 expression on the surface of monocytes. Both 89I and 1291 LOS upregulated CD80 expression with 89I inducing a higher level than 1291, whereas GC56 LOS did not upregulate CD80. To investigate whether the induction of CD80 by LOS was dependent on TLR4/MD-2 signaling, cells were pretreated with anti-TLR4 antibody prior to the addition of LOS. As shown in Fig. 7A, addition of the TLR4 antibody inhibited the induction of CD80 expression in monocytes by all the LOS tested, indicating that the induction of CD80 by LOS was TLR4-dependent.
Figure 7.
Differential induction of the costimulatory molecule CD80 on monocytes by LOS. Monocytes were aliquoted to 1 × 106 cells per well in 6-well plates and treated with LOS (1 μg/ml) with or without anti-TLR4 antibody (10 μg/ml). After 18 h stimulation, the cells were harvested and analyzed for CD80 expression by flow cytometry as described in Material and Methods. A, CD80 expression after 89I, 1291, and GC56 LOS treatment in the absence or presence of pretreatment with anti-TLR4 antibody for 30 min. Filled histogram indicates untreated cells and open histogram indicates cells treated with LOS or anti-TLR4 plus LOS. B, CD80 expression induced by 89I LOS, phosphoryl-PEA (PPEA) hexaacyl LA, and phosphoryl (P) hexaacyl LA. Filled histogram indicates untreated cells and open histogram indicates cells treated with LOS or LA.
We have previously subjected 89I LOS to mild acid hydrolysis to enable purification of individual components of LA by TLC, and showed that an increasing number of phosphoryl substituents from that on the diphosphoryl lipid A increases the potency of TNF-α induction (21). In particular, the TLC fraction which contained phosphoryl PEA hexaacyl LA was significantly more potent than fractions containing either di- or monophosphoryl hexaacyl lipid A. To examine whether the phosphoryl substituents play an important role in induction of CD80 through the TRIF-dependent pathway, we treated monocytes with phosphoryl PEA hexaacyl LA or phosphoryl hexaacyl LA from 89I and assessed CD80 expression. As shown in Figure 7B, similar to that of intact LOS, phosphoryl PEA hexaacyl LA induced significant CD80 expression (p < 0.01), while phosphoryl hexaacyl LA had no effect. This result demonstrated that phosphoryl substituents not only play an important role in inducing inflammatory cytokines, but they also effect the expression of costimulatory molecule CD80.
LOS regulates CD80 by differential activation of IRF-1
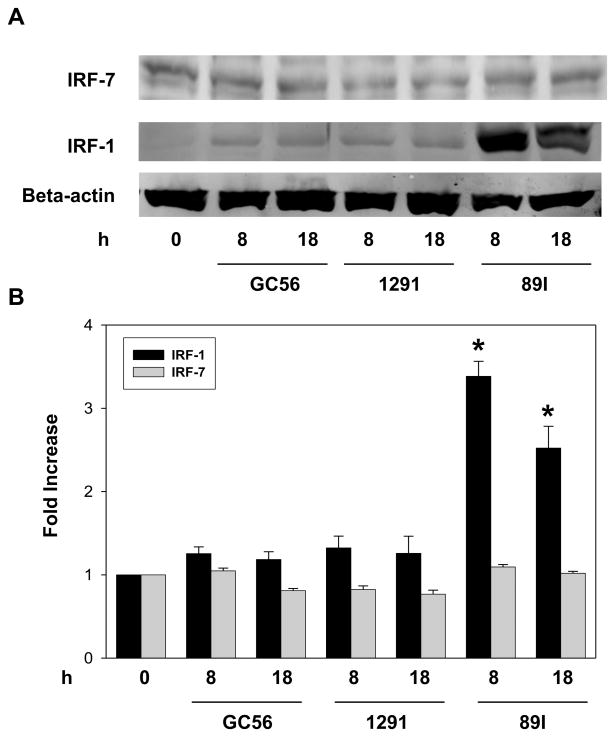
The expression of the CD80 is mediated by IRF-7 through activation of JNK in LPS-stimulated human monocytic cells (29). In addition, a recent study showed that both types I and II IFN upregulate CD80 in monocytes via IRF-1 (30). Since we found that LOS differentially activated CD80 expression, we wondered whether IRF-7 or IRF-1 or both play a role in regulating LOS-mediated CD80 expression. To this end, THP-1 cells were treated with LOS for a time period of 0 to 18 hours after which the expression of IRF-7 and IRF-1 was determined. As shown in Fig. 8A and 8B, regardless of the LOS used, the level of IRF-7 expression was unchanged. In contrast, the expression of IRF-1 was significantly enhanced by 8 h treatment with 89I LOS as indicated by a 3.5-fold increase from the baseline expression, and expression remained elevated for 18 h (2.7–fold increase). In contrast, the level of IRF-1 expression was only minimally increased when the cells were treated by 1291 and GC56 LOS. The relative amount of IRF-1 induction in THP-1 cells as the result of treatment with LOS is consistent with the relative potential for CD80 induction in monocytic cells. These data indicate that the differential activation of CD80 by LOS is correlated to their variable ability to activate the expression of IRF-1, but not IRF-7.
Figure 8.
IRF-1 and IRF-7 expression in THP-1 cells following LOS treatment. THP-1 cells were plated (1 × 106 cells per well) in a 6-well plate and stimulated with LOS at 100 ng/ml for 8 or 18 h. After washing with PBS, cells were lysed with SDS sample buffer and the cell lysates were applied to a 10% SDS polyacrylamide gel. Immunoblots were performed with antibody against IRF-1, IRF-7, or actin. A, western immunoblot detection of IRF-7 and IRF-1 in response to 89I, 1291, or GC56. B, the relative intensity of each band in the immunoblots was determined using densitometry and the ratios of the IRF-1 and IRF-7 bands to the corresponding actin bands were calculated. Data are presented as fold increases of treated compared with untreated samples. Data are representative of at least three independent experiments. * p < 0.05 indicates significance for comparisons between 89I and 1291 or GC56 LOS.
DISCUSSION
LOS is an important virulence factor in the pathogenesis of Neisserial infections, and is one of the primary components of the organisms that induce host pro-inflammatory cytokine responses (13,31). Specifically, the LA portion of the LOS molecule has been shown to be the inflammatory component of the Neisserial LOS (32,33). Previously, we observed that LOS from different strains of meningococci and gonococci variously induced cytokine responses in THP-1 cells (12,13). In this paper, we have presented data illustrating that NF-κB is differentially activated by LOS from different meningococcal and gonococcal strains. We have shown that the relative ability of LOS to activate NF-κB is positively correlated with ratios of the ion abundance in MALDI-TOF mass spectrometry of the intact LOS indicative of moieties with a greater number of phosphoryl substituents on the LA. Detailed analyses of the bioactivity of LOS from three strains, 89I, 1291, and GC56, was performed as these strains exhibited high, intermediate, and low potencies for NF-κB activation which correlated with similar high to low levels of LA phosphorylation. The results showed that LOS differentially induced cytokine expression by the activation of MyD88- and TRIF-dependent pathways induced by the TLR4/MD-2 receptor complex in monocytes, as demonstrated by the differential activation of NF-κB and IRF-3. Moreover, we found that LOS differentially induced the expression of CD80 on the surface of monocytes. Finally, we determined that the upregulation of CD80 expression by LOS was related to increased expression of IRF1. These results are consistent with the concept that Neisseria modulate pathogen-associated molecular patterns and that this modulation alters their interactions with the host in a manner that is likely to facilitate bacterial infection and survival.
The biological importance of the fine structure of LA has gained increasing recognition. This is evidenced by the study by Mata-Haro et al. which showed that the number of phosphoryl groups on the LA of LPS can affect its engagement of adapter molecules through the activation of enzymes (19). For example, monophosphoryl LA was shown to exclusively trigger the TRIF-dependent pathway by activating PI3K, which blocks the MyD88-Mal pathway. This suggests that the interaction of unique LA molecules with MD-2 can cause conformational changes in MD-2 and TLR4, and result in recruitment of specific adapter proteins and the subsequent activation of distinct pathways of signal transduction (19). In addition, structural analyses of the recognition of LPS by the TLR4-MD-2 complex by Park et al. showed that the differences in LPS could render important influences on the receptor complex (34). The shift of the phosphorylated glucosamine backbone by ~5 Å will allow phosphate groups of LPS to contribute to the receptor complex multimerization through interaction with the positively charged residues in TLR4 and MD-2. This study demonstrated that the TLR4-MD-2 complex can be remarkably versatile in the recognition of its ligand for defense against diverse microbial infections and formed the basis for our observation that the number of phosphate groups may affect the polarity of the receptor complex and subsequent signaling. In-depth analyses of the effects on inflammatory signaling of specific groups expressed on LA would benefit from the design of a set of ligands that could serve as molecular probes to more completely integrate the studies of function and structure, and eventually could aid in the development of novel vaccine adjuvants and therapeutic drugs for human inflammatory disorders associated with TLRs (19).
We previously analyzed the LA structures of LOS using negative-ion MALDI-TOF and showed that both the acylation and phosphorylation states of Neisserial LA affect the induction of TNF-α in THP-1 cells (21). Specifically, higher ratios of the relative ion abundance of hexaacylated diphosphoryl LA with a single PEA moiety at m/z 1836 as compared to either the pentaacylated diphosphoryl lipid A at m/z 1515 or hexaacylated diphosphoryl at m/z 1713 that were both lacking PEA was positively correlated with induction of TNF-α in THP-1 cells. Our current analyses revealed that a greater degree of phosphoryl substitution on the LA also is positively correlated with the activation of NF- κB through TLR4 signaling. The degree of phosphoryl substitution was represented by the ratio of the sum of the relative ion abundances of two peaks for hexaacylated LA, one at m/z 1793 for triphosphoryl LA and one at m/z 1836 for diphosphoryl LA with a single PEA, relative to the abundance of ions at m/z 1713 for a diphosphoryl hexaacylated LA without any PEA substitution. Our data on the different potency of activation of NF-κB and IRF-3 pathways by LOS provided additional direct evidence of the impact of the LA structure on engagement of the innate immune system. The inherent heterogeneity in the Neisserial LA from different strains of bacteria enables probing structural requirements for differential activation of specific biological pathways, such as induction of NF-κB and IRF-3. Additionally, an advantage of studying native LA over the use of synthetic LA or LA produced by genetic mutants is that the results with naturally occurring variants are more likely to reflect molecular differences that are pertinent to pathogenesis since the LA is thought to directly affect host immune responses, facilitate bacterial survival, and contribute to variability in disease outcome.
In general, TLR4 stimulation by LPS results in a branched downstream signaling pathway consisting of Mal/MyD88 and TRIF/TRAM branches which lead to production of different subsets of pro-inflammatory cytokines. For example, TNF-α, IL-1, and IL-6 are MyD88-dependent cytokines while IFN-β and IFN-inducible genes such as IFN-γ inducible protein 10 (IP-10), MCP-1, and RANTES are TRIF-dependent cytokines (35,36). Differences in the spectrum of cytokines induced by LOS with different LA structures could reflect variable activation of these distinct pathways. MyD88-activated NF-κB responses result in the most cytokine expression and TRIF-activated IRF-3 responses cause type I and II IFN expression, which play an important role in the regulation of adaptive immunity. The activation of the TLR4-TRIF branch activates IRF-3 and eventually promotes the expression of types I and II IFN as well as TNF-α, the latter of which leads to delayed NF-κB signaling (37). Our study demonstrated this concept with Neisseria LOS which is structurally different from LPS. We showed that 89I LOS represents a strong inducer of both the MyD88 and TRIF pathways. Treatment with 89I LOS strongly activated NF-κB within 5 min, consistent with its early activation and induction of inflammatory cytokine expression through the MyD88-dependent pathway. Additionally, through regulation of IRF-3 phosphorylation, 89I LOS activated the TRIF branch leading to the production of MyD88-independent cytokines that play important roles in adaptive immunity and in the maturation of dendritic cells. By stimulation of both pathways, 89I LOS induced a strong innate inflammatory response as demonstrated by its induction of the highest expression levels of TNF-α, IL-1β, and IL-6. In contrast to 89I LOS, GC56 LOS, which expresses an LA with a low amount of phosphorylation, induced a weak, delayed NF-κB activation. This weak induction of early NF-κB through the MyD88-dependent pathway and delayed activation of NF-κB through the TRIF-dependent activation of IRF-3 contributed to the overall effect on cytokine induction as demonstrated by the lower expression of inflammatory cytokines.
The results of the cytokine assays showed that all three LOS induced 23 of the 30 cytokines assessed, however nine of these were induced to significantly higher levels by 89I LOS than by either 1291 or GC56 LOS. These are mainly inflammatory cytokines whose expression levels can be correlated to the MyD88- dependent pathway which is strongly induced by 89I LOS. Among the pro-inflammatory cytokines, we observed the expression of significant amounts of IL-1β and its antagonist, IL-1RA. IL-1 (including IL-1α and IL-1β) is an important mediator of inflammation and tissue damage in multiple organs, both in experimental animal models of disease and in human diseases. IL-1RA on the other hand, is an antagonist of IL-1 receptor and therefore inhibits the function of IL-1 (38). The balance between IL-1 and IL-1RA has been suggested to play an important role in many diseases. In the normal physiological condition, IL-1RA levels can be elevated to as high as 100-fold more than that of IL-1 to maintain homeostasis. The imbalance indicates the relative physiologic or pathophysiologic effects of IL-1 (39-41). Interestingly, the ratios of LOS-induced IL-1RA to IL-1β in our study were higher when cells were treated with N. gonorrhoeae LOS than when treated with N meningitidis 89I LOS, which is consistent with the greater severity of meningococcal infection compared with gonococcal infection in general (7,21). Two inflammatory chemokines, MIP-1 α and MIP-1β, were induced to express at a high level by 89I LOS compared to that by 1291 or GC56 LOS. These two chemokines function by attracting leukocytes to the site of infections, and their overexpression could lead to tissue injury (42). The high level of induction of MIP-1α and MIP-1β by 89I LOS was related to the high potency of this LOS to stimulate TLR4 responses and likely reflects the high likelihood for serious illness and death from meningococcal disease (43).
Among TRIF-dependent cytokines, we observed a discordant expression level upon LOS stimulation. IFN-β, for example, an IRF-3-dependent cytokine which plays an important role in innate immunity, was correlated with the level of IRF-3 activation by 89I, 1291, and GC56 LOS, and has been shown to be related to meningococcal infection (44,45). However, IFN-α and MCP-1 showed similar levels of expression for the three LOS tested. There are two possible reasons for this discordant expression. First, although TRIF-associated gene expression requires the activation of IRF-3, both MyD88- and TRIF-activated NF-κB are required to induce maximal inflammatory cytokine expression, and IFN-β, a strong IRF-3 regulated gene (44,46), belongs in this category (46,47). Second, MCP-1 does not correlate to IRF-3 activation in our study, suggesting that the chemokines may also rely on additional pathways. This observation is supported by a recent study by Cekic et al. who demonstrated that MCP-1 as a TRIF-dependent gene also requires other signals, such as the p38 MAPK pathway, when induced by synthetic monophosphoryl LA (47). We did not detect IFN-γ, one of the TRIF-dependent genes, in our study. This is not surprising as we have observed a significant induction of IL-10 by LOS. The expression of IL-10, an anti-inflammatory cytokine and an important regulator of the host immune response, has been shown to inhibit the production of IFN-γ through the upregulation of SOCS1 (48). IFN-γ signaling is important in mediating MHC class II upregulation and enhancing the CD4+ T cell response to infection (49). Thus, IL-10-mediated inhibition of IFN-γ is likely to be crucial for protection against immune-mediated inflammatory disorders. It has been demonstrated in the case of bacterial pathogens such as Bordetella pertussis and B. bronchiseptica that the balance of IL-10 to IFN-γ is a means of limiting the host immune response to allow for persistence within the host by lowering the concentration of IFN-γ-induced chemokine IP-10 (50,51). This hypothesis is supported by studies showing the lack of adaptive immunity in gonococcal infection (7). In addition, our results are consistent with the clinical observation that a high concentration of IL-10 contributes to the low concentration of IFN-γ in fulminant meningococcal septicemia, and that IFN-γ plays a minor role in the pathophysiology of meningococcal septic shock (52). It is worth noting that not all cytokines were differentially induced in response to different LOS. The levels of MCP-1, IL-4, IL-8, IL-12, and G-CSF, for example, did not show significant differences between the three LOS treatments. It is not clear why this is the case, but most likely the expression of these cytokines is dependent on host signaling pathways which are redundant and therefore do not change significantly based on the variation of TLR4/MD-2 ligand recognition (34). Overall, the cytokine profile which was observed supports the concept that the phosphoryl contents of LA regulate cytokine expression through the MyD88- and TRIF-dependent pathways as proposed by Mata-Haro et al. (19).
Previous studies have demonstrated the importance of the TRIF pathway in the regulation of CD80 expression in macrophages and dendritic cells by LPS. For example, Shen et al showed that the upregulation of costimulatory molecules by LPS, including CD80, was TRIF-dependent in macrophages, however neither the MyD88 nor the TRIF pathway per se was critical for the upregulation of costimulatory molecules in dendritic cells (52). We postulated that differential activation of the TRIF-dependent pathway in monocytes might be related to the ability of LOS to induce the costimulatory molecule CD80, an important marker for antigen presentation and subsequent activation of adaptive immunity. Based on the level of IRF-3 activation, we predicted that 89I would strongly induce CD80 compared to 1291 and GC56 LOS. Indeed, we found that 89I LOS containing greater numbers of phosphoryl substituents induced greater CD80 expression in monocytes than did the LOS of 1291 and GC56. In addition, we showed that LA which contains phosphoryl PEA induced higher levels of inflammatory cytokines, as well as a higher degree of CD80 expression than LA containing phosphoryl only (19,21), thus further supporting the importance of the LA phosphoryl content on the differential induction of the MyD88 and TRIF pathways.
The molecular mechanisms involved in the transcriptional regulation of CD80 in response to types I and II IFN stimulation have been related to IRF-1 and IRF-7 expression (30), and their DNA binding ability (29). Bauvois et al. demonstrated that while IFN-α/β upregulates CD80 expression through the activation of IRF-1 and IRF-7, IFN-γ upregulates CD80 solely through IRF-1 (30). Interestingly, in monocytic U937 cells it was shown that only IFN-γ, and not IFN-α/β, induced CD80 expression. LPS, on the other hand, regulates CD80 expression in THP-1 cells through modulation of IRF-7 DNA binding ability to the CD80 promoter (29). Thus, the role of IRF-1 is not clearly defined in LPS-induced CD80 expression. Based on the results of our cytokine assays, we hypothesized that LOS would regulate CD80 through IFN-α/β as IFN-γ was not detectable, and therefore, both IRF-1 and IRF-7 would be required. Our results showed that while IRF-1 was differentially upregulated in THP-1 cells upon stimulation by different LOS, the level of IRF-7 did not change, suggesting that only IRF-1 upregulation is critical for LOS-induced CD80 expression. Despite the fact that the IRF-7 expression was not affected by LOS, the data does not preclude a role for IRF-7 in the regulation of CD80 upon stimulation by LOS. For example, treatment of THP-1 cells with LOS may enhance the DNA binding ability of IRF-7 and thereby increase the expression of CD80, rather than affecting the level of IRF-7 expression. Alternately, as demonstrated by Lim et al. (29), the DNA binding ability of IRF-7 may be a necessary but insufficient factor in the regulation of CD80 expression.
Understanding the impact of the structural variation of Neisserial LA on activation of innate immune responses is critical to our understanding of the pathogenesis of and immune responses to these bacterial infections. This has been exemplified recently by studies of the LPS from pathogenic Bordetella species, which express structural variation that results in wide-ranging TLR4-mediated innate immune responses (53,54). For example, the LPS of B. bronchiseptica is highly stimulatory of TLR4, and TLR4 is required for their clearance. In contrast, the LPS of B. pertussis and B. parapertussis are much less stimulatory of TLR4, and TLR4-deficiency does not render mice more susceptible to infection. Thus, these bacteria apparently adapt to their environment by independently modifying their LPS which alters TLR4-mediated responses, and may thereby compensate for slower growth rates and facilitate host colonization (54). Similar observations have been reported for the LPS of Salmonella, Yersinia, and Pseudomonas species (55,56). These Gram-negative bacteria tend to modify the structure of LPS through deacylating and palmitoylating LA, apparently allowing them to evade TLR4 responses. Similarly, we have now shown that differences in the phosphorylation of LA from LOS of different Neisseria strains are correlated with variable induction of the TLR4/MD-2 pathway resulting in variable cytokine and innate immune receptor responses. Our data are consistent with the overall concept that modification of the LA structure by Gram-negative bacteria is related to differences in the pathogenesis of infections.
In conclusion, this study showed that Neisserial LOS with structural variations have different potency to activate innate immunity through TLR4/MD-2 by differentially activating both the MyD88- and TRIF-dependent pathways. The presence of greater amounts of phosphoryl substituents on the LA was positively correlated with the greater ability of the LOS to induce innate immunity through the activation of NF- κB. In addition, the LOS smilarly variously activated the expression of CD80 through the upregulation of the transcription factor IRF-1 in monocytes. Furthermore, in addition to the typical inflammatory cytokines such as TNF-α, IL-1β, and IL-6, we found that MIP-1α and MIP-1β also were significantly higher in cells treated with 89I LOS, which contained more phosphoryl substitutions than that of 1291 and GC56. The results demonstrate that phosphoryl moieties of LA from N. meningitidis and N. gonorrhoeae LOS play an important role in the determination of the overall innate inflammatory response.
Acknowledgments
The authors would like to acknowledge the UCSF Mass Spectrometry Core Facility which is supported by an NCI Cancer Center Support Grant (P30 CA82103) and the Sandler Family Foundation, and the UCSF Mass Spectrometry Facility (A.L. Burlingame, Director), which is supported by the Biomedical Research Technology Program of the National Center for Research Resources, NIH, NCRR P41RR001614. This is paper number 106 from the Center for Immunochemistry.
Footnotes
This work was supported by the Research Service of the U.S. Department of Veterans Affairs and by Public Health Service Grant AI063927 from the National Institute of Allergy and Infectious Diseases.
Abbreviations used in this paper:
LOS lipooligosaccharide
LA lipid A
IRF IFN regulatory factor
PEA phosphoethanolamine
MS mass spectrometry
SEAP secreted embryonic alkaline phosphatase
References
- 1.Stephens DS. Conquering the meningococcus. FEMS Microbiol Rev. 2007;31:3–14. doi: 10.1111/j.1574-6976.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 2.Soper DE. Pelvic inflammatory disease. Obstet Gynecol. 2010;116:419–428. doi: 10.1097/AOG.0b013e3181e92c54. [DOI] [PubMed] [Google Scholar]
- 3.Farley TA, Cohen DA, Wu SY, Besch CL. The value of screening for sexually transmitted diseases in an HIV clinic. J Acquir Immune Defic Syndr. 2003;33:642–648. doi: 10.1097/00126334-200308150-00014. [DOI] [PubMed] [Google Scholar]
- 4.Tapsall JW. Neisseria gonorrhoeae and emerging resistance to extended spectrum cephalosporins. Curr Opin Infect Dis. 2009;22:87–91. doi: 10.1097/QCO.0b013e328320a836. [DOI] [PubMed] [Google Scholar]
- 5.Lo H, Tang CM, Exley RM. Mechanisms of avoidance of host immunity by Neisseria meningitidis and its effect on vaccine development. Lancet Infect Dis. 2009;9:418–427. doi: 10.1016/S1473-3099(09)70132-X. [DOI] [PubMed] [Google Scholar]
- 6.Holder NA. Gonococcal infections. Pediatr Rev. 2008;29:228–234. doi: 10.1542/pir.29-7-228. [DOI] [PubMed] [Google Scholar]
- 7.Fox KK, Thomas JC, Weiner DH, Davis RH, Sparling PF, Cohen MS. Longitudinal evaluation of serovar-specific immunity to Neisseria gonorrhoeae. Am J Epidemiol. 1999;149:353–358. doi: 10.1093/oxfordjournals.aje.a009820. [DOI] [PubMed] [Google Scholar]
- 8.Fichorova RN, Desai PJ, Gibson FC, III, Genco CA. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun. 2001;69:5840–5848. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramsey KH, Schneider H, Cross AS, Boslego JW, Hoover DL, Staley TL, Kuschner RA, Deal CD. Inflammatory cytokines produced in response to experimental human gonorrhea. J Infect Dis. 1995;172:186–191. doi: 10.1093/infdis/172.1.186. [DOI] [PubMed] [Google Scholar]
- 10.Zughaier SM, Tzeng YL, Zimmer SM, Datta A, Carlson RW, Stephens DS. Neisseria meningitidis lipooligosaccharide structure-dependent activation of the macrophage CD14/Toll-like receptor 4 pathway. Infect Immun. 2004;72:371–380. doi: 10.1128/IAI.72.1.371-380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGee ZA, Clemens CM, Jensen RL, Klein JJ, Barley LR, Gorby GL. Local induction of tumor necrosis factor as a molecular mechanism of mucosal damage by gonococci. Microb Pathog. 1992;12:333–341. doi: 10.1016/0882-4010(92)90096-7. [DOI] [PubMed] [Google Scholar]
- 12.John CM, Liu M, Jarvis GA. Profiles of structural heterogeneity in native lipooligosaccharides of Neisseria and cytokine induction. J Lipid Res. 2009;50:424–438. doi: 10.1194/jlr.M800184-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pridmore AC, Jarvis GA, John CM, Jack DL, Dower SK, Read RC. Activation of toll-like receptor 2 (TLR2) and TLR4/MD2 by Neisseria is independent of capsule and lipooligosaccharide (LOS) sialylation but varies widely among LOS from different strains. Infect Immun. 2003;71:3901–3908. doi: 10.1128/IAI.71.7.3901-3908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan DN, Cooper MD, Potter JL, Roberts MH, Cheng H, Jarvis GA. TREM-2 binds to lipooligosaccharides of Neisseria gonorrhoeae and is expressed on reproductive tract epithelial cells. Mucosal Immunol. 2008;1:229–238. doi: 10.1038/mi.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brikos C, O'Neill LA. Signalling of toll-like receptors. Handb Exp Pharmacol. 2008;183:21–50. doi: 10.1007/978-3-540-72167-3_2. [DOI] [PubMed] [Google Scholar]
- 16.Palsson-McDermott EM, O'Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Z, Georgel P, Du X, Shamel L, Sovath S, Mudd S, Huber M, Kalis C, Keck S, Galanos C, Freudenberg M, Beutler B. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 18.Georgel P, Jiang Z, Kunz S, Janssen E, Mols J, Hoebe K, Bahram S, Oldstone MB, Beutler B. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4- dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–1632. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- 20.Ohto U, Fukase K, Miyake K, Satow Y. Crystal structures of human MD-2 and its complex with antiendotoxic lipid IVa. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 21.John CM, Liu M, Jarvis GA. Natural phosphoryl and acyl variants of lipid A from Neisseria meningitidis strain 89I differentially induce TNF-α in human monocytes. J Biol Chem. 2009;284:21515–21525. doi: 10.1074/jbc.M109.004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kogan G, Uhrin D, Brisson JR, Jennings HJ. Structural basis of the Neisseria meningitidis immunotypes including the L4 and L7 immunotypes. Carbohydr Res. 1997;298:191–199. doi: 10.1016/s0008-6215(96)00305-9. [DOI] [PubMed] [Google Scholar]
- 23.Westphal O, Jann K. Bacterial Lipopolysaccharides. Extraction with phenol-water and further applications of the procedure. In: Whistler RL, editor. Methods in Carbohydrate Chemistry. Academic Press; New York: 1965. pp. 83–91. [Google Scholar]
- 24.Apicella MA, Griffiss JM, Schneider H. Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A. Methods Enzymol. 1994;235:242–252. doi: 10.1016/0076-6879(94)35145-7. [DOI] [PubMed] [Google Scholar]
- 25.Sturiale L, Garozzo D, Silipo A, Lanzetta R, Parrilli M, Molinaro A. New conditions for matrix-assisted laser desorption/ionization mass spectrometry of native bacterial R-type lipopolysaccharides. Rapid Commun Mass Spectrom. 2005;19:1829–1834. doi: 10.1002/rcm.1994. [DOI] [PubMed] [Google Scholar]
- 26.Walsh C, Gangloff M, Monie T, Smyth T, Wei B, McKinley TJ, Maskell D, Gay N, Bryant C. Elucidation of the MD-2/TLR4 interface required for signaling by lipid IVa. J Immunol. 2008;181:1245– 1254. doi: 10.4049/jimmunol.181.2.1245. [DOI] [PubMed] [Google Scholar]
- 27.Ashtekar AR, Zhang P, Katz J, Deivanayagam CC, Rallabhandi P, Vogel SN, Michalek SM. TLR4-mediated activation of dendritic cells by the heat shock protein DnaK from Francisella tularensis. J Leukoc Biol. 2008;84:1434–1446. doi: 10.1189/jlb.0308215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoebe K, Janssen EM, Kim SO, Alexopoulou L, Flavell RA, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol. 2003;4:1223–1229. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- 29.Lim W, Gee K, Mishra S, Kumar A. Regulation of B7.1 costimulatory molecule is mediated by the IFN regulatory factor-7 through the activation of JNK in lipopolysaccharide-stimulated human monocytic cells. J Immunol. 2005;175:5690–5700. doi: 10.4049/jimmunol.175.9.5690. [DOI] [PubMed] [Google Scholar]
- 30.Bauvois B, Nguyen J, Tang R, Billard C, Kolb JP. Types I and II interferons upregulate the costimulatory CD80 molecule in monocytes via interferon regulatory factor-1. Biochem Pharmacol. 2009;78:514–522. doi: 10.1016/j.bcp.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Pridmore AC, Wyllie DH, Abdillahi F, Steeghs L, van der Ley P, Dower SK, Read RC. A lipopolysaccharide-deficient mutant of Neisseria meningitidis elicits attenuated cytokine release by human macrophages and signals via toll-like receptor (TLR) 2 but not via TLR4/MD2. J Infect Dis. 2001;183:89–96. doi: 10.1086/317647. [DOI] [PubMed] [Google Scholar]
- 32.Roth RI, Yamasaki R, Mandrell RE, Griffiss JM. Ability of gonococcal and meningococcal lipooligosaccharides to clot Limulus amebocyte lysate. Infect Immun. 1992;60:762–767. doi: 10.1128/iai.60.3.762-767.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Ley P, Steeghs L, Hamstra HJ, ten Hove J, Zomer B, van Alphen L. Modification of lipid A biosynthesis in Neisseria meningitidis lpxL mutants: influence on lipopolysaccharide structure, toxicity, and adjuvant activity. Infect Immun. 2001;69:5981–5990. doi: 10.1128/IAI.69.10.5981-5990.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park BS, Song DH, Kim HM, Choi BS, Lee H, Lee JO. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 35.O'Neill LA. The role of MyD88-like adapters in Toll-like receptor signal transduction. Biochem Soc Trans. 2003;31:643–647. doi: 10.1042/bst0310643. [DOI] [PubMed] [Google Scholar]
- 36.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN- regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 37.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-κB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 38.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 39.Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest. 1997;99:2930–2940. doi: 10.1172/JCI119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabay C, Porter B, Guenette D, Billir B, Arend WP. Interleukin-4 (IL-4) and IL-13 enhance the effect of IL-1β on production of IL-1 receptor antagonist by human primary hepatocytes and hepatoma HepG2 cells: differential effect on C-reactive protein production. Blood. 1999;93:1299–1307. [PubMed] [Google Scholar]
- 41.Gabay C, Gigley J, Sipe J, Arend WP, Fantuzzi G. Production of IL-1 receptor antagonist by hepatocytes is regulated as an acute-phase protein in vivo. Eur J Immunol. 2001;31:490–499. doi: 10.1002/1521-4141(200102)31:2<490::aid-immu490>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 42.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–2210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 44.Masatani T, Ito N, Shimizu K, Ito Y, Nakagawa K, Sawaki Y, Koyama H, Sugiyama M. Rabies virus nucleoprotein functions to evade activation of the RIG-I-mediated antiviral response. J Virol. 2010;84:4002–4012. doi: 10.1128/JVI.02220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Helfgott DC, Tatter SB, Santhanam U, Clarick RH, Bhardwaj N, May LT, Sehgal PB. Multiple forms of IFN- β2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol. 1989;142:948–953. [PubMed] [Google Scholar]
- 46.Xu HG, Ren W, Lu C, Zhou GP. Characterization of the human IRF-3 promoter and its regulation by the transcription factor E2F1. Mol Biol Rep. 2009 doi: 10.1007/s11033-009-9880-0. [DOI] [PubMed] [Google Scholar]
- 47.Cekic C, Casella CR, Eaves CA, Matsuzawa A, Ichijo H, Mitchell TC. Selective activation of the p38 MAPK pathway by synthetic monophosphoryl lipid A. J Biol Chem. 2009;284:31982–31991. doi: 10.1074/jbc.M109.046383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 49.Zhong G, Fan T, Liu L. Chlamydia inhibits interferon γ-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. J Exp Med. 1999;189:1931–1938. doi: 10.1084/jem.189.12.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolfe DN, Karanikas AT, Hester SE, Kennett MJ, Harvill ET. IL-10 induction by Bordetella parapertussis limits a protective IFN-γ response. J Immunol. 2010;184:1392–1400. doi: 10.4049/jimmunol.0803045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bussmeyer U, Sarkar A, Broszat K, Lüdemann T, Möller S, van Zandbergen G, Bogdan C, Behnen M, Dumler JS, von Loewenich FD, Solbach W, Laskay T. Impairment of gamma interferon signaling in human neutrophils infected with Anaplasma phagocytophilum. Infect Immun. 2010;78:358–363. doi: 10.1128/IAI.01005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen H, Tesar BM, Walker WE, Goldstein DR. Dual signaling of MyD88 and TRIF is critical for maximal TLR4-induced dendritic cell maturation. J Immunol. 2008;181:1849–1858. doi: 10.4049/jimmunol.181.3.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolfe DN, Buboltz AM, Harvill ET. Inefficient Toll-like receptor-4 stimulation enables Bordetella parapertussis to avoid host immunity. PLoS One. 2009;4:e4280. doi: 10.1371/journal.pone.0004280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann PB, Wolfe D, Latz E, Golenbock D, Preston A, Harvill ET. Comparative toll-like receptor 4-mediated innate host defense to Bordetella infection. Infect Immun. 2005;73:8144–8152. doi: 10.1128/IAI.73.12.8144-8152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Netea MG, van Deuren M, Kullberg BJ, Cavaillon JM, Van der Meer JW. Does the shape of lipid A determine the interaction of LPS with Toll-like receptors? Trends Immunol. 2002;23:135–139. doi: 10.1016/s1471-4906(01)02169-x. [DOI] [PubMed] [Google Scholar]
- 56.Knirel YA, Dentovskaya SV, Senchenkova SN, Shaikhutdinova RZ, Kocharova NA, Anisimov AP. Structural features and structural variability of the lipopolysaccharide of Yersinia pestis, the cause of plague. J Endotoxin Res. 2006;12:3–9. doi: 10.1179/096805105X67283. [DOI] [PubMed] [Google Scholar]