Abstract
Neuroactive peptides and the intracellular calcium concentration ([Ca2+]i) play important roles in light-induced modulation of gene expression in the suprachiasmatic nucleus (SCN) neurons that ultimately control behavioral rhythms. Vasoactive intestinal peptide (VIP) and arginine vasopressin (AVP) are expressed rhythmically within populations of SCN neurons. Pituitary adenylate cyclase-activating peptide (PACAP) is released from retinohypothalamic tract (RHT) terminals synapsing on SCN neurons. Nociceptin/orphanin FQ (OFQ) receptors are functionally expressed in the SCN. We examined the role of several neuropeptides on Ca2+ signaling, simultaneously imaging multiple neurons within the SCN neural network. VIP reduced the [Ca2+]i in populations of SCN neurons during the day, but had little effect at night. Stimulation of the RHT at frequencies that simulate light input signaling evoked transient [Ca2+]i elevations that were not altered by VIP. AVP elevated the [Ca2+]i during both the day and night, PACAP produced variable responses, and OFQ induced a reduction in the [Ca2+]i similar to VIP. During the day, VIP lowered the [Ca2+]i to near nighttime levels while AVP elevated [Ca2+]i during both the day and night, suggesting that the VIP effects on [Ca2+]i were dependent, and the AVP effects independent of the action potential firing activity state of the neuron. We hypothesize that VIP and AVP regulate, at least in part, Ca2+ homeostasis in SCN neurons and may be a major point of regulation for SCN neuronal synchronization.
Keywords: Circadian Rhythm, Peptides, Vasoactive intestinal peptide, Vasopressin, Retinohypothalamic tract
Introduction
Several neuropeptides including VIP, AVP, PACAP, and OFQ have been shown to play important roles in the regulation of circadian clock function and entrainment (Piggins et al., 1996; Maywood et al., 2007; Miyakawa et al., 2007; Morin, 2007; Butler & Silver, 2009; Welsh et al., 2010). Calcium is important in the signal transduction pathways mediating photic entrainment and an essential component of the feedback loops that generate circadian rhythms (Albrecht et al., 1997; Shearman et al., 1997; Shigeyoshi et al., 1997; Morris et al., 1998; Lundkvist & Block, 2005; Lundkvist et al., 2005). Yet currently little is known of the role that Ca2+ plays in the neuropeptide regulation of the circadian clock.
The effect of neuropeptides on neuronal [Ca2+]i, typically involve G-protein coupled receptors resulting in alterations of the membrane potential or second messenger-mediated release from intracellular stores (Maywood et al., 2006; Pakhotin et al., 2006; O’Neill et al., 2008). Disruption of VIP signaling alters the action potential firing of SCN neurons, clock gene expression, behavioral circadian rhythms, and can be rescued by application of VIP or the VPAC2 receptor agonist RO 25-1553 (Aton et al., 2005; Maywood et al., 2006; Pakhotin et al., 2006; Brown et al., 2007). The role of Ca2+ in VIP-mediated signaling in SCN neurons is currently unknown. Vasopressin is rhythmically released in the SCN with a peak during the day (Yamase et al., 1991; Tominaga et al., 1992; Nakamura et al., 2001), which can be phase shifted by VIP (Watanabe et al., 2000), and may play a role in the generation of circadian rhythms (Gouzenes et al., 1999; Kuhlman et al., 2003; Li et al., 2009). Vasopressin agonists increase [Ca2+]i in neurons of the supraoptic nucleus, an effect that was attenuated by IP3-receptor blockers and PLC and PKA inhibitors, suggesting that both phospholipase C and adenylate cyclase pathways are involved (Sabatier et al., 2004). PACAP is localized with glutamate from RHT terminals (Hannibal et al., 2000; Hannibal et al., 2002). PACAP application produces phase delays early in the night and phase advances late in the night similar to a light pulse (Harrington et al., 1999; Piggins et al., 2001). PACAP potentiates the increased [Ca2+] produced by AMPA, but not NMDA receptor activation in populations of SCN neurons (Kopp et al., 2001; Dziema & Obrietan, 2002). Activation of OFQ receptors in the SCN reduces action potential firing, phase shifts the circadian clock, and down regulates mPER2 (Anton et al., 1996; Allen et al., 1999; Neal et al., 1999a; Teshima et al., 2005; Miyakawa et al., 2007). OFQ decreases calcium and increases a potassium conductance in multiple brain regions including the SCN (Allen et al., 1999; Meis, 2003; Gompf et al., 2005).
To clarify the role that Ca2+ plays in the neuropeptide regulation within the SCN network, we recorded Ca2+ from multiple neurons simultaneously examining the actions of VIP, AVP, PACAP, and OFQ on Ca2+ signaling.
Methods
Tissue preparation
Hypothalamic slices were prepared, imaged and test agents applied using previously published methods (Irwin & Allen, 2009). Briefly, male Spraque-Dawley rats (Charles River, Wilmington MA) were housed for at least 1 week on a 12:12-hr light:dark schedule. During the light phase, 4 to 6 week old rats were anesthetized with isofluorane (Novaplus, UK), their brains removed, and coronal hypothalamic slices (250 μm) containing the SCN were cut with a vibrating blade microtome (Leica VT1000S, Nussloch, Germany), while the tissue was surrounded by ice-cold artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 120, KCl 2.5, NaH2PO4 1.2, MgCl2 5, CaCl2 0.5, glucose 10, NaHCO3 26, adjusted to 300 mOsm with sucrose and saturated with 5% CO2 and 95% O2. The Institutional Animal Care and Use Committee of OHSU approved, in advance, all procedures involving animals.
Calcium Imaging
Multiple SCN neurons maintained in hypothalamic slices were loaded with the Ca2+-sensitive probe fura-2 AM for the simultaneous recording of Ca2+ responses using previously published methods (Irwin & Allen, 2009). Briefly, freshly prepared SCN slices were treated with fura-2 AM (50 μM) for 1 – 2 minutes (Invitrogen, CA; from stock 3.3 mM in DMSO) followed by incubation with fura-2 AM (10 – 25 μM) in ACSF for 1–2 hours (Irwin & Allen, 2009). The slices were washed for at least 30 min prior to recording to allow for fura-2-AM deesterification and maintained in a recording chamber (35°C) with a continuous laminar flow (1 – 2 ml/min) of an ACSF solution consisting of (in mM): NaCl 120, KCl 2.5, NaH2PO4 1.2, MgCl2 1.2, CaCl2 2.4, glucose 10, HEPES 10, NaHCO3 26; adjusted to 300 mOsm and bubbled with 5% CO2 and 95% O2 (Fig. 1). Calcium measurements were obtained by recording a pair of images at excitation wavelengths of 340 and 380 nm supplied via a monochronometer (Polychrome IV; Till Photonics, Martinsried, Germany) passing through a UG11 optical filter to restrict harmonic wavelengths above 400 nM, reflected via a 400 nm DCLP dichroic mirror, and through a Leica 40x/0.80 UV water immersion objective. Emitted light passed through a 510 ± 40 nm filter (Chroma Technology Corp, Rockingham, VT) and was recorded with a cooled charge-coupled device camera (CCD, ORCA-ER 12 bit level, Hamamatsu, Japan) with acquisition time and binning adjusted to minimize photobleaching and maximize recording speed via the digital imaging software Metafluor (Molecular Devices Corporation, Sunnyvale, CA). Optical data was converted to relative fluorescence intensity units for each cell. Data was presented as the emission following excitation at 340 and 380 nm and background subtraction at each wavelength as previously described (Irwin & Allen, 2009). Neurons were identified by an increase in the Ca2+ ratio in response to NMDA (200 μM for 5 sec). Cells that did not respond to NMDA or were dim were excluded. The Ca2+ response (an increase, decrease or no effect) to VIP, AVP or OFQ was defined by a ratio change ≥2 standard deviations from the mean of 6 – 11 baseline Ca2+ ratios recorded prior to the peptide application. The magnitude of the Ca2+ response to peptides having prolonged changes in Ca2+ was determined from the mean of six Ca2+ ratios at the apparent maximal response for each experiment. GABA, VIP(6-28) and PACAP produced transient neuronal Ca2+ responses, and were coded as an increase, decrease or no response by visual inspection. The magnitude of the transient Ca2+ response was determined as the maximum (or minimum for Ca2+ decreases) response within the first 30 sec of application. The SCN neurons were localized to the dorsomedial (DM) and ventrolateral (VL) regions of the SCN as previously described (Irwin & Allen, 2009).
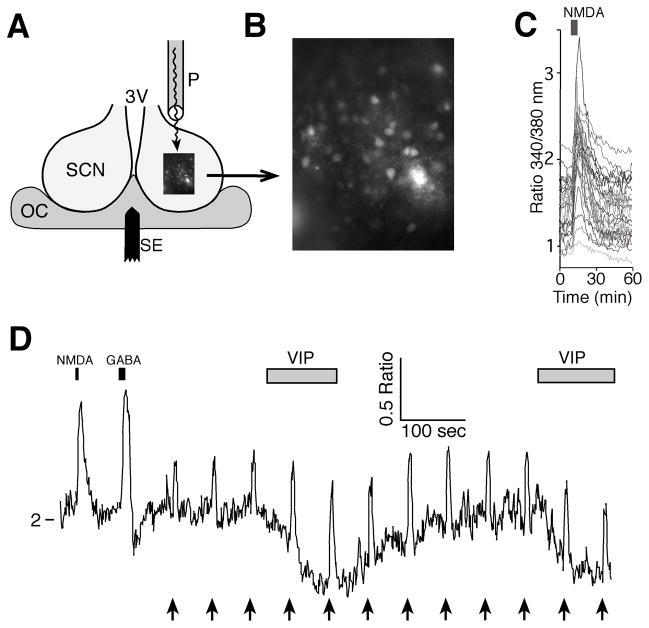
Figure 1. Measurement of intracellular Ca2+ simultaneously in multiple SCN neurons.
A. Schematic illustration of the recording setup showing a coronal hypothalamic slice containing the SCN and third ventricle (3V) with a bipolar stimulating electrode (SE) used to stimulate the RHT positioned in the optic chiasm (OC) and a perfusion pipette (P) positioned to flow solutions over the SCN. B. High power (40X objective) fluorescent image of fura-2 loaded SCN neurons. C. Example showing NMDA (200 μM, 5 sec) inducing a transient increase in the Ca2+ ratio (340nm/380nm) in SCN neurons during the day (ZT = 6 hours). D. Example showing changes in the Ca2+ ratio of a SCN neuron with responses to NMDA (200 μM, 5 sec), GABA (200 μM, 10 sec), RHT stimulation (arrows; 100 pulses, 200 μs at 20 Hz) and VIP (1 μM) application.
Chemicals
VIP, VIP(6-28), [Arg8]-Vasopressin, PACAP(1-38), GABA, and NMDA were obtained from Tocris Bioscience (Ellisville, MO). Fura-2 AM was purchased from Invitrogen (Carlsbad, California). Other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Data and statistical analysis
Igor Pro (Version 6.0.4, Wavemetrics, Lake Oswego, OR, USA), Statview 5.0.1 (SAS Institute, Cary, NC, USA) and Excel 11.5.1 (Microsoft, Redmond, WA) were used for curve fitting and data analysis. Unless otherwise stated data are presented as the mean ± S.E.M. and t-tests (two tailed). A p level of ≤0.05 was used to determine statistical significance.
Results
VIP reduces Ca2+ in SCN neurons during the day but not the night
The role of VIP in regulating Ca2+ was examined by simultaneously imaging the mean Ca2+ ratio of multiple neurons before and following VIP application during the day and night and at several locations within the SCN (Fig. 2). During the day VIP (250 nM) reduced the Ca2+ ratio [VIP(Ca−)] in 27% of neurons (ZT5-8 hours, n = 96), while VIP (1 μM) reduced the Ca2+ ratio in 54% of neurons (ZT3-9 hours, n = 152 neurons). The proportion of neurons responding to 1 μM VIP was significantly greater than at 250 nM (z = 4.2, p < 0.0001). Regression analysis of the baseline Ca2+ ratio in VIP(Ca−) neurons during the day plotted versus the change in the Ca2+ ratio induced by VIP (1 μM), results in a slope of −0.50 ± 0.07 (R2 = 0.41, n = 82, p < 0.0001) (Fig. 2C), suggesting that neurons with higher Ca2+ ratios and presumably faster action potentials firing frequencies, have a greater VIP-induced reduction of Ca2+ than quiescent neurons with low Ca2+ baseline ratios (Irwin & Allen, 2007; 2009). In contrast, but consistent with this hypothesis, at night, VIP (1 μM) reduced the Ca2+ ratio in only 7% of SCN neurons (ZT: 15–18 hours, n = 121 neurons) (Fig. 2A&B). The few neurons observed at night with a VIP(Ca−) response had a higher mean baseline Ca2+ ratio (1.30 ± 0.06, n = 8, Δ = −0.12 ± 0.04 ratio units) that differed significantly from the mean baseline Ca2+ ratio in neurons that did not respond to VIP [VIP(Ca0)] (1.16 ± 0.03, n = 105, Δ0.001 ± 0.005; t12 = 2.12, p = 0.028 one-tail with unequal variances). The Ca2+ ratio in 64% of neurons was not altered by treatment with VIP (250 nM) during the day. Similarly, VIP (1 μM) did not change the Ca2+ ratio of 39% of SCN neurons recorded during the day nor 87% during the night. VIP elevated the Ca2+ ratio in a small subset of neurons during both the day (9% at 250 nM; 7% at 1 μM) and night (7% at 1 μM).
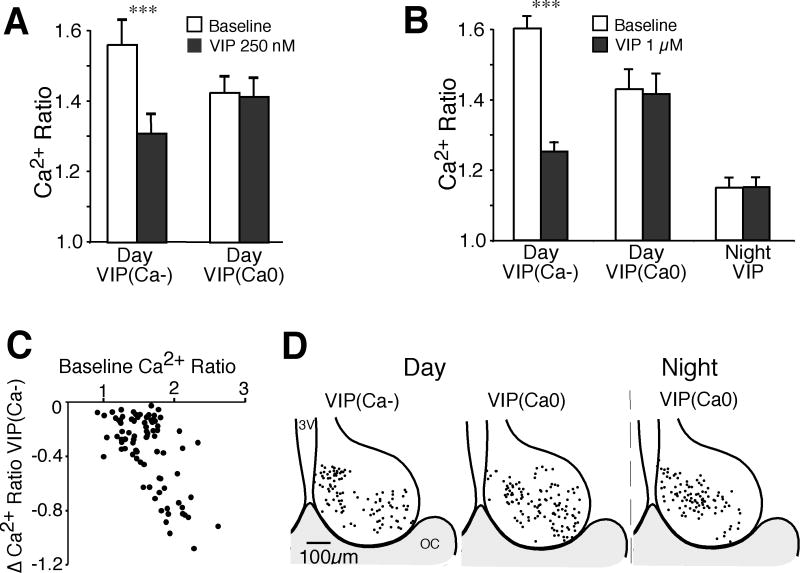
Figure 2. VIP induces a reduction of baseline [Ca2+]i during the day but not the night.
A. Comparison of the mean Ca2+ ratio of SCN neurons at baseline and following application of VIP (250 nM) during the day (n = 96). VIP (250 nM) induced a significant reduction from baseline in SCN neurons showing a VIP(Ca−) response (n = 26, t = 7.24, *** p < 0.0001), while most neurons had little or no response (VIP(Ca0), n = 61, t = 1.15, p = 0.25), and only 9 neurons had a Ca2+ increase (data not shown). B. Comparison of the mean Ca2+ ratio of SCN neurons at baseline and following application of VIP (1 μM) during the day (n = 152) and night (n = 121). During the day, VIP (1 μM significantly reduced the baseline Ca2+ ratio (mean ± SEM) in VIP(Ca−) neurons (n = 82, t = 11.9, *** p < 0.0001), but not in the VIP(Ca0) group (n = 59, t = 1.71, p = 0.093), while only 11 neurons showed a Ca2+ increase (data not shown). During the night, VIP reduced the Ca2+ ratio in 7% of neurons. The data shown is the mean ± SEM of all nighttime neurons (n = 121 neurons, t = 0.114, p = 0.91). Note the baseline Ca2+ ratios were higher during the day than the night, and that VIP reduced the Ca2+ ratios of VIP(Ca−) responding neurons to near nighttime levels. C. Plot of the baseline Ca2+ ratio versus the VIP (1 μM)-induced change in the Ca2+ ratio in VIP(Ca−) neurons during the day. Note that larger reductions in Ca2+ occur in neurons with higher baseline Ca2+ ratios (R2 = 0.41). D. Regional location of VIP-treated neurons during the day and night. The position for each VIP(Ca−) and VIP(Ca0) neuron was superimposed on a representative drawing of the SCN with the third ventricle (3V) on the left and optic chiasm (OC) at the bottom. Neither VIP(Ca−) or VIP(Ca0) neurons demonstrated a clear regional expression pattern.
In SCN neurons, the mean baseline Ca2+ ratio is higher during the day than the night (Colwell, 2000; Ikeda et al., 2003; Irwin & Allen, 2007; 2009). During the day, VIP (1 μM) lowered Ca2+ to near baseline nighttime levels (Fig. 2A). The reduction of the Ca2+ ratio in VIP(Ca−) neurons during the day was larger at 1 μM than at 250 nM (0.35 ± 0.029 vs. 0.25 ± 0.038 ratio units respectively, t106 = 1.75, p = 0.042 one-tail). These data demonstrate that while VIP can lower [Ca2+]i during both the day and night a much greater proportion of VIP(Ca−) responding neurons were observed during the day when the mean baseline [Ca2+]i is higher.
Localization of VIP-induced Ca2+ response
We examined whether the VIP induced Ca2+ changes differed regionally within the SCN during the day and night. The location of each VIP(Ca−) and VIP(Ca0) neuron was superimposed over a representative drawing of the SCN (Fig. 2D). VIP(Ca−) responding neurons demonstrated no clear regional pattern.
VIP modulation of RHT signaling in the SCN network
Since VIP reduced the daytime baseline [Ca2+]i, it may also play an important role in modulating light-responsive retinal ganglion cell signaling via the RHT to the SCN. Electrical stimulation of the RHT evokes divergent Ca2+ responses in SCN neurons that were dependent on the action potential firing frequency and modulation by endogenous GABAA activity (Irwin & Allen, 2007; 2009). Stimulation of the RHT via a bipolar electrode (100 pulses at 20 Hz) placed in the optic chiasm induced transient Ca2+ elevations, reductions or no responses in the postsynaptic SCN neurons (Fig. 3A). Application of VIP (1 μM) did not alter the magnitude of the Ca2+ elevations induced by RHT stimulation during the day or the night.
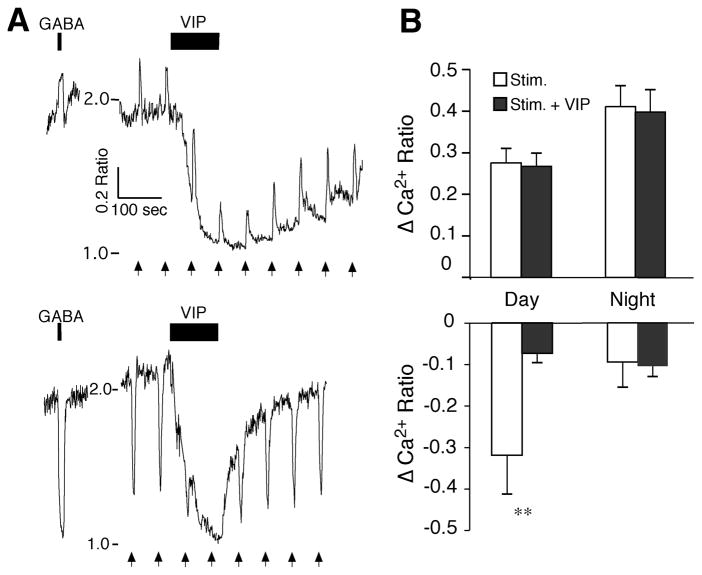
Figure 3. VIP modulation of RHT signaling in the SCN network.
A. The postsynaptic Ca2+ ratio in two SCN neurons with similar reductions in response to VIP (1 μM), but opposing responses to both GABA (200 μM, 10 sec) and stimulation of the RHT (arrows, 100 pulses, 200 μs at 20 Hz). B. VIP (1 μM) had little or no effect on RHT-evoked elevations of Ca2+ (top) during the day (n = 36 neurons, t = 0.43, p = 0.67) and night (n = 22, t = 0.39, p = 0.70), but attenuated RHT-evoked Ca2+ reductions during the day (n = 10, t = 2.82, ** p < 0.020), but not the night (n = 3, t = 1.06, p = 0.40). Data is the mean ± SEM of maximum (Ca2+ elevations) and minimum (Ca2+ reductions) responses to RHT-evoked changes in the Ca2+ ratio before and during VIP (1 μM) application. Note that since VIP reduced the baseline Ca2+, the magnitudes of RHT-evoked Ca2+ reductions were also reduced, while at night the baseline Ca2+ ratio was low with few RHT-evoked Ca2+ transient reductions.
During the day when [Ca2+]i is generally high and neurons are rapidly firing, a RHT-induced reduction of [Ca2+]i reflects an activation of GABAergic neurons and subsequent release of GABA; while at night when baseline [Ca2+]i is low, a RHT-induced reduction of the [Ca2+]i occurs in only a small number of neurons, suggesting that [Ca2+]i cannot be reduced further (Irwin & Allen, 2009). Therefore, since VIP reduced the baseline Ca2+ ratios during the day to near nighttime levels, the daytime RHT-evoked Ca2+ reductions were also reduced (Fig. 3B); at night the baseline Ca2+ ratios were generally low and VIP had little effect on RHT-evoked Ca2+ reductions. These data suggest that, while VIP induces a reduction of [Ca2+]i, it does not impair the ability of RHT input to elevate [Ca2+]i in SCN neurons.
The role of GABAA receptor activation in the VIP-induced reduction of Ca2+
VIP increases the frequency of GABA-mediated synaptic currents (Itri & Colwell, 2003; Itri et al., 2004). Additionally, GABAA receptor activation can either raise or lower [Ca2+]i in SCN neurons (Choi et al., 2008; Irwin & Allen, 2009). We therefore examined the relationship between VIP- and GABA-induced Ca2+ responses in 122 SCN neurons. No association was observed between VIP responsive (VIP(Ca−), n = 71) and non-responsive (VIP(Ca0), n = 51) neurons, in relation to GABA- (200 μM, 10 sec) -evoked Ca2+ ratio increases (GABA(Ca+), n = 47), decreases (GABA(Ca−), n = 45) or no responses (GABA(Ca0), n = 30; χ2 = 0.23, p = 0.89). Since GABAA antagonists can alter baseline [Ca2+]i (Irwin & Allen, 2009) and VIP(Ca−) responses are dependent on baseline [Ca2+]i (Fig. 2C), experiments with GABAA antagonists were not performed. These data suggest that the VIP-induced reduction of [Ca2+]i is not mediated via GABA activity.
A VIP antagonist, VIP(6-28) modifies [Ca2+]i in SCN neurons
Since VIP reduced [Ca2+]i in SCN neurons during the day, we tested the hypothesis that endogenous VIP facilitates the [Ca2+]i reduction observed during the night using the VIP antagonist VIP(6-28). Application of VIP(6-28) (2 – 4 μM, ZT = 14 – 18 hrs, n = 53) induced a variety of Ca2+ responses in SCN neurons including transient and sustained elevations (41.5%), reductions (18.9%), and no responses (39.6%) (Fig. 4). VIP(6-28) elevated the Ca2+ ratio in the majority (~69%, n = 32) of responding neurons. Further, VIP(6-28) evoked larger Ca2+ elevations than reductions (Fig. 4B). During the day (ZT = 4– 6 hrs, n = 33), the pattern of VIP(6-28) induced Ca2+ responses was similar to that observed during the night (39.4% elevations, 12.1% reductions, 48.5% no response; χ2 = 0.96, p = 0.62). The magnitude of the VIP(6-28) evoked Ca2+ excursions were similar during the day and night (elevation day 0.31 ± 0.05, n = 13, night 0.30 ± 0.06, n = 22, t33 = 0.11, p = 0.91; reduction day 0.11 ± 0.07, n = 4, night 0.15 ± 0.02, n = 10; t12 = 0.74, p = 0.47) (Fig. 4B). These data suggest that VIP may have a role in lowering [Ca2+]i during the night.
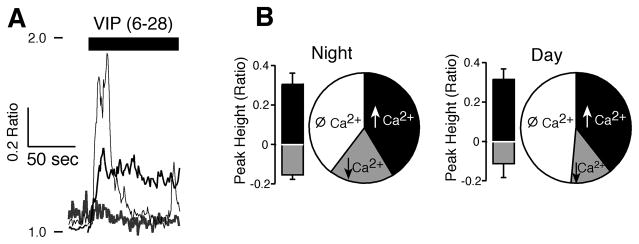
Figure 4. The VIP antagonist VIP(6-28) modified [Ca2+]i in SCN neurons at night.
A. Example showing three SCN neurons during the night with different responses to application of VIP(6-28) (2 μM), a transient rise, a prolonged elevation and a reduction in the Ca2+ ratio. B. Pie chart of VIP(6-28) data showing the proportion of neurons with elevations (↑, n = 22) reductions (↓, n = 10) and no responses (Ø, n = 21) in Ca2+. The bars represent the mean ± SEM of the maximal (black) and minimum (gray) responses (0–30 sec) of neurons showing VIP(6–28) (2–4 μM) evoked Ca2+ changes.
AVP induces elevations of Ca2+ during the day and night in SCN neurons
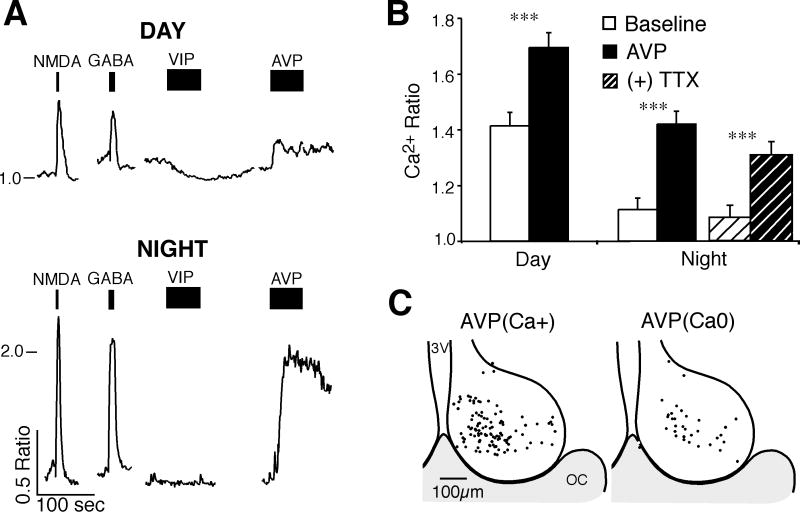
The effect of AVP on [Ca2+]i of SCN neurons was evaluated with applications of AVP (1 μM) during the day and night (Fig. 5). During the day, AVP elevated the Ca2+ ratio [AVP(Ca+)] in 74%, generated no response [AVP(Ca0)] in 22% and lowered the Ca2+ ratio [AVP(Ca−)] in 4% of SCN neurons (n = 72). Similarly, during the night the [Ca2+]i was increased in 75% and generated no response in 25% of SCN neurons (n = 73). While the baseline Ca2+ ratios of AVP(Ca+) neurons differed between the day (1.41 ± 0.05, n = 53) and night (1.11 ± 0.42, n = 55, t104 = 4.78, p < 0.0001), the magnitudes of the Ca2+ elevations were similar (day 0.28 ± 0.03, n = 53; night 0.31 ± 0.04, n = 55, t106 = 0.54, p = 0.59). Further, blocking action potential firing with tetrodotoxin (TTX; 0.5 μM) did not block the AVP-induced Ca2+ elevation (Fig. 5B). The AVP-induced change in [Ca2+]i was slow to develop with a mean time to peak (± SEM) of 27.0 ± 1.2 seconds.
Figure 5. AVP elevates the [Ca2+]i of SCN neurons during the day and night.
A. Example showing SCN neurons responding to AVP during the day and night. Tracings show treatments with NMDA (200 μM), GABA (200 μM), VIP (1 μM) and AVP (1 μM). Note that VIP lowered the Ca2+ ratio during the day but not at night, while AVP elevated the Ca2+ ratio [AVP(Ca+)] during both the day and night. B. Bar graph of AVP(Ca+) responding neurons showing the mean ± SEM Ca2+ ratio before and during AVP application during the day (n = 53, t = 8.25, *** p < 0.001) and night (n = 55, t = 8.44, *** p < 0.001), and during night with TTX (ANOVA, F3,54 = 28.6, p < 0.001, Bonferroni adjusted p = 0.48 [night-Baseline] and p = 0.09 [night-AVP]). C. Regional location of AVP-treated neurons (day and night combined). The position for each AVP(Ca+) and non-responding [AVP(Ca0)] neuron was superimposed on a representative drawing of the SCN with the third ventricle (3V) on the left and optic chiasm (OC) at the bottom. AVP(Ca+)-responding neurons appeared to be located primarily in the dorsomedial region.
While AVP induced [Ca2+]i elevations during both day and night, VIP-induced [Ca2+]i reductions almost exclusively during the day. There was however an association between responses to AVP and VIP (χ2 = 17.5, n = 66, p < 0.0001) during the day in SCN neurons. In 41% of neurons where AVP induced a Ca2+ ratio elevation (n = 51), VIP induced a Ca2+ ratio reduction (n = 21). And 87% of neurons that did not respond to AVP during the day (n = 15) also did not respond to VIP (n = 13). The baseline Ca2+ ratio of AVP(Ca+) responding neurons showed little correlation to the magnitude of the AVP-induced change in the Ca2+ ratio during the day (R2 = 0.03, n = 53, p = 0.24) or night (R2 = 0.09, n = 55, p = 0.03). These data suggest that the Ca2+ response to AVP was independent of the firing activity state of the SCN neuron, and that AVP(Ca+) sensitive neurons were more likely to be located in the dorsomedial region (Fig. 5C).
The modulation of SCN neuronal [Ca2+]i by PACAP
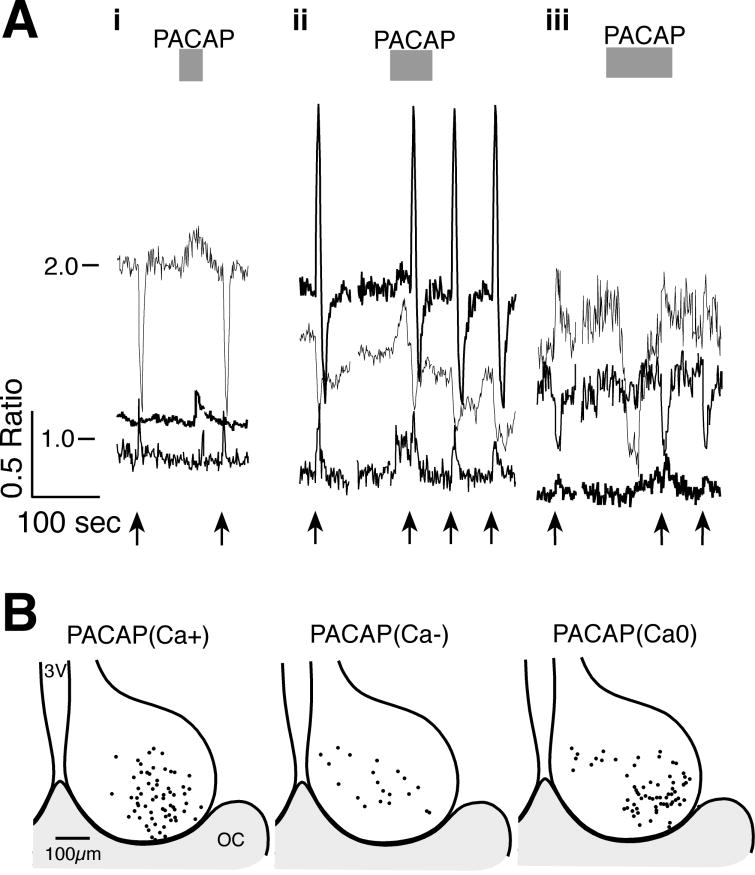
PACAP is thought to be co-released with glutamate from RHT terminals (Hannibal et al., 2000; Hannibal & Fahrenkrug, 2004). PACAP(1-38) applied to SCN neurons during the day, activated a variety of transient changes in [Ca2+]i without obvious alteration of RHT stimulation-induced transients (Fig. 6). PACAP (250 nM) elevated the Ca2+ ratio in 45.7%, reduced the Ca2+ ratio in 10.6% and had no change of the Ca2+ ratio in 43.6% of 94 neurons. PACAP (500 nM) induced similar (χ2 = 2.12, p = 0.35) changes, with an elevation of Ca2+ in 35.6%, a reduction in 16.9% and no-response in 47.5% of 59 neurons. Note that PACAP induced a response in only about 50% of neurons, and that reapplication often did not evoke subsequent Ca2+ transients (data not shown). Consistent with previous studies (Kopp et al., 2001; Dziema & Obrietan, 2002; Michel et al., 2006), these data suggest that PACAP acts to modulate the response of SCN neurons to input pathways.
Figure 6. The Effects of PACAP on [Ca2+]i in SCN neurons.
A. Examples of the variety of Ca2+ responses observed with PACAP (1–38) application onto SCN neurons during the day. i & ii) PACAP (250 nM) evoked transient Ca2+ elevations without altering the RHT stimulation-induced transients (arrows, 100 pulses at 20 Hz). iii) PACAP (500 nM) evoked a Ca2+ reduction in one neuron and an elevation in another. B. Regional localization of SCN Ca2+ responses to PACAP. The position of each neuron where PACAP elevated [PACAP(Ca+)], reduced [PACAP(Ca−)] or produced no response [PACAP(Ca0)] was superimposed on a representative drawing of the SCN with the third ventricle (3V) on the left and optic chiasm (OC) at the bottom.
The modulation of SCN neuronal [Ca2+]i by OFQ
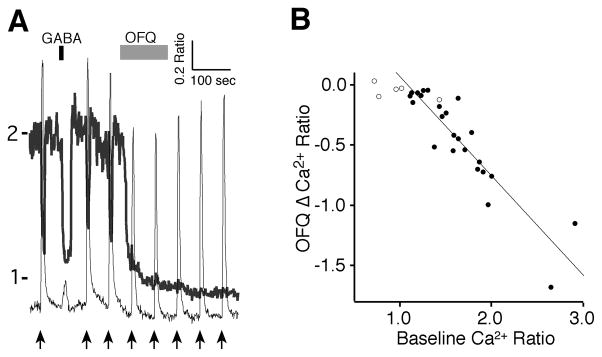
Orphanin-FQ (OFQ) (1 μM) produced the most dramatic and prolonged effect on the Ca2+ ratio of SCN neurons during the day, reducing the Ca2+ ratio in 83% of the neurons tested (n = 29; Fig. 7A). In these neurons, the magnitude of the OFQ-induced reduction (−0.45 ± 0.08 ratio units) correlated linearly with the baseline Ca2+ ratio (1.63 ± 0.09 ratio units, n = 24, slope = −0.83, R2 = 0.82, p < 0.0001) (Fig. 7B). Non-responding neurons already had lower baseline ratios (0.98 ± 0.13, n = 5). Unlike VIP, the OFQ effect was extremely long lasting and did not wash out during the remainder of the recording session. Five minutes after OFQ application, the Ca2+ ratio remained low and even continued to slightly decline (−0.03 ± 0.01 ratio units, t = 2.84, p = 0.0094). These data demonstrated that OFQ induces a robust and prolonged reduction of [Ca2+]i and suggests, that like VIP, this reduction is dependent on the action potential firing activity state of the SCN neuron.
Figure 7. The Effects of orphanin-FQ (OFQ) on [Ca2+]i in SCN neurons.
A. Example showing the effect of OFQ (1 μM) on two SCN neurons during the day with different responses to GABA (200 μM) and RHT stimulation (arrows, 100 pulses at 20 Hz). B. The magnitude of the OFQ reduction of Ca2+ was linearly correlated with the baseline Ca2+ ratio (filled circles, n = 24, slope = −0.83, R2 = 0.82). Non-responding neurons already had low baseline ratios (open circles, n = 5). Note that the effect of OFQ on the [Ca2+]i was long-lasting.
Discussion
Neuroactive peptides are released from distinct neuronal populations and act on selective receptors to activate a variety of intracellular signaling pathways that ultimately may alter gene transcription (Maywood et al., 2006; Maywood et al., 2007; Pfeffer et al., 2009). Changes of [Ca2+]i are linked to alterations of gene expression by neurotransmission and is one signal that may mediate the actions of some neuropeptides. Neuropeptides may change the [Ca2+]i by multiple mechanisms, including opening Ca2+ permeable channels, membrane hyperpolarization and depolarization with activation of voltage-gated Ca2+ channels, and sequestration or release of Ca2+ from intracellular stores. Neuroactive peptides present in the SCN were found to have distinct effects on [Ca2+]i. VIP and OFQ produced a greater reduction of [Ca2+]i in neurons with an elevated baseline [Ca2+]i, such as occurs during the day and is consistent with a reduction of the action potential firing frequency. While VIP appears to reduce the activity of SCN neurons, the Ca2+ transient increases induced by RHT stimulations were not altered, suggesting that an SCN neuron “quieted” by VIP can still respond to photic input as occurs following light applied during the dark period. Conversely, AVP slowly elevated the [Ca2+]i without an apparent dependence on the firing activity state of the neuron. PACAP application resulted in transient and variable effects on [Ca2+]i, conceivably this peptide may be acting as a modulator of glutamate co-released from RHT terminals in the SCN. Calcium perturbations resulting from voltage-(firing activity) dependent processes and from intracellular stores can produce both transient or prolonged changes of [Ca2+]i. However, in general voltage-dependent processes are more rapid and Ca2+ release from intracellular stores more gradual, yet both appear to make contributions to the overall circadian oscillation of [Ca2+]i (Colwell, 2000; Ikeda, 2004; Irwin & Allen, 2007; 2009). We hypothesize that induction of Ca2+-mediated transcriptional and translational changes to the clock are dependent on the activity state of the neuron and its ability to rapidly alter [Ca2+]i and are facilitated by neuropeptide modulation of both external and internal sources of Ca2+.
VIP has significant effects on the circadian clock and the activity of SCN neurons (Cutler et al., 2003; Pakhotin et al., 2006). VIP acting on the VPAC2 receptor synchronizes the circadian clocks of individual SCN neurons (Cutler et al., 2003; Piggins & Cutler, 2003; Aton et al., 2005). In smooth muscle, VIP has been thought to reduce Ca2+ via membrane hyperpolarization, sequestration into intracellular stores, and activation of G-proteins (Ohta et al., 1991; Kawasaki et al., 1997). VIP reduced the [Ca2+]i in populations of SCN neurons during the day but has little effect at night. The day-night difference may reflect the action potential firing activity of SCN neurons at different parts of the circadian cycle. The magnitude of the Ca2+ change was dependent on the baseline [Ca2+]i, such that VIP (and OFQ) induced large reductions in Ca2+ in neurons with high baseline [Ca2+]i, as occurs in fast firing neurons, and had little or no effect on neurons with low baseline [Ca2+]i, associated with low firing rates. Blocking action potentials with TTX similarly reduces [Ca2+]i in more neurons during the day than the night (Colwell, 2000; Irwin & Allen, 2009). Our findings are consistent with the observation that VIP reduces the action potential firing of SCN neurons by inhibiting voltage-dependent Na+ and K+ channels (Pakhotin et al., 2006). Since the firing activity of an SCN neuron contributes to the overall [Ca2+]i (Irwin & Allen, 2007; 2009), these data suggest that VIP alters the physiological action potential firing activity of the neuron to reduce the [Ca2+]i.
A key question is whether VIP is contributing to the lower [Ca2+]i and action potential firing rates observed during the night. VIP is released rhythmically in the SCN with higher levels at night than the day (Morin et al., 1993; Shinohara & Inouye, 1995; Nakamura et al., 2001; Aton & Herzog, 2005), and stimulated release of VIP in rat hypothalamus occurs only during the night (Nicholson et al., 1983). We found that the VIP antagonist VIP(6-28) induced transient elevations of [Ca2+]i in a proportion of SCN neurons during the night, suggesting a potential role for VIP activity in regulation of the SCN firing activity. However, while VIP(6-28) is frequently used as a VIP antagonist, it is only moderately effective even at large concentrations, may not be a competitive antagonist and may interact with other receptors (Markos et al., 2002). While these data suggest that VIP may have a role in lowering the [Ca2+]i of SCN neurons during the night, highly competitive antagonists are needed to confirm this mechanism.
While VIP expression is localized heavily in the ventral part of the SCN (Belenky et al., 2008), we did not observe regional differences in the VIP-induced reductions in [Ca2+]i. This is consistent with the wide distribution of VPAC2 expression, including about half of AVP-positive neurons (Kallo et al., 2004b) in the SCN, and consistent with a role of VIP in SCN synchronization. The SCN neurons with higher levels of [Ca2+]i that did not respond to VIP, may represent neurons without VIP receptors, different VPAC2 receptor signaling pathways, quiescent neurons with high [Ca2+]i due release from intracellular stores, or other mechanisms (Kallo et al., 2004b; Irwin & Allen, 2007; 2009).
Since VIP reduces the excitability of SCN neurons, and VIP release, VIP mRNA, and VPAC2 expression are higher during the night (Albers et al., 1990; Rea, 1990; Morin et al., 1993; Shinohara et al., 1994; Shinohara et al., 1999), an important question was whether light-induced glutamate release from RGC terminals in the SCN would be less likely to trigger the post-synaptic action potentials required to phase shift the clock. Stimulating the RHT, evokes divergent Ca2+ responses in neurons within the SCN (Irwin & Allen, 2009). VIP did not alter the magnitude of RHT-evoked Ca2+ elevations during either the day or night. These data suggest a model in which VIP reduces the firing of populations of SCN neurons, without altering the ability of the glutamatergic RHT input to elicit action potentials and elevate [Ca2+]i. This may be important in the ability for light to induce phase changes during the night.
GABA- and VIP- expressing neurons in the SCN network are known to frequently interconnect and colocalize (Francois-Bellan et al., 1990; Francois-Bellan & Bosler, 1992; Buijs et al., 1995; Castel & Morris, 2000). VIP regulates GABA release in the SCN and modulates SCN GABAergic signaling to subparaventricular zone neurons (Itri & Colwell, 2003; Itri et al., 2004; Hermes et al., 2009). Conceivably VIP might reduce the [Ca2+]i by evoking GABA release and lowering excitability in SCN neurons. However, GABA may lower or elevate [Ca2+]i in SCN neurons (Choi et al., 2008; Irwin & Allen, 2009). We found no association between the VIP and GABA activity, and observed that VIP-induced reductions of Ca2+ occurred in many neurons where GABA-induced an elevation (Fig. 3A), suggesting that GABA does not mediate the VIP-induced reductions of [Ca2+]i.
V1a and V1b-type AVP receptors are expressed in the SCN and couple to Gq-type G proteins, which activates phospholipase C activity and subsequently increases inositol-1,4,5 trisphosphate-induced Ca2+ release from intracellular stores and diacylglycerol-mediated activation of protein kinase C (Majewski & Iannazzo, 1998; Birnbaumer, 2000). While the rhythmic release of AVP by SCN neurons peaks during the day (Yamase et al., 1991; Tominaga et al., 1992), the expression of V1a-type receptor mRNA, but not the V1b-type, peaks during the night (Young et al., 1993; Kalamatianos et al., 2004). We found that AVP can induce elevations of [Ca2+]i in the majority of SCN neurons during both the day and night. We observed a lag of approximately 30 sec from the onset of AVP application to the maximal response, suggesting that that the increased Ca2+ was not a result of the rapid opening of voltage dependent Ca2+ channels, but rather required second messenger-mediated process likely involving at least in part Ca2+ release from intracellular stores. During the night AVP evoked similar elevations of Ca2+ in the presence of TTX, further suggesting a mechanism of Ca2+ release from intracellular stores. However, the closing of G protein-coupled inwardly-rectifying potassium channels inducing membrane depolarization may also make a contribution to the rise in [Ca2+]i (Zhang et al., 2006). In the supraoptic nucleus [Ca2+]i is elevated by a V1a-type AVP receptor agonist and attenuated by blocking IP3 receptors. Similarly, Ca2+ increases induced by V1a- and V2- type, but not V1b-type, AVP receptor agonists were attenuated by PLC and PKA inhibitors, suggesting that both phospholipase C and adenylate cyclase pathways are involved (Gouzenes et al., 1999; Sabatier et al., 2004). This supports our observations that AVP elevates [Ca2+]i in SCN neurons during both the day and night. Further, the regional location of AVP-sensitive SCN neurons appearing to overlap regions with known AVP immunoreactivity (Yamase et al., 1991; Moore et al., 2002) and the slightly higher dorsomedial expression of V1a receptor mRNA (Young et al., 1993; Kalamatianos et al., 2004) suggest that AVP may be autoregulating its own release.
In addition to VIP and AVP, which appear to play key roles in generating and maintaining circadian timing, other neuropeptides modify the activity of the SCN (Teshima et al., 2005; Miyakawa et al., 2007). While the application of PACAP and VIP produced different types of Ca2+ responses, both peptides have been reported to have activity at the VPAC2 receptor while PACAP is more selective for the PAC1 receptor, and both receptors are expressed in the SCN (Ajpru et al., 2002; Kallo et al., 2004a; Yang et al., 2010). PACAP’s effect on [Ca2+]i was variable with the Ca2+ elevations tending to be transient and located more ventrolaterally, consistent with regions receiving greater RHT and PACAP associated input (Hannibal & Fahrenkrug, 2004). The different responses observed using PACAP may be a consequence of PACAP modulating the activity of neurotransmission pathways (Kopp et al., 1999; Kopp et al., 2001; Dziema & Obrietan, 2002; Michel et al., 2006). We found little or no change was observed in the Ca2+ transients induced by RHT stimulation before and after application of PACAP. Previously, we have shown that RHT stimulation induces glutamate receptor-mediated excitatory postsynaptic potentials (EPSPs), but does not produce a change in somatic [Ca2+]i without subsequently triggering an action potential and opening of voltage dependent Ca2+ channels (Irwin & Allen, 2007). This suggests that if endogenous PACAP is also released with RHT stimulation, it does alter somatic [Ca2+]i, and is consistent with the relatively small, variable and often transient effects on [Ca2+]i associated with pharmacologic applications of PACAP.
While SCN neurons have little or no OFQ mRNA expression (Neal et al., 1999b), the OFQ receptor mRNA is expressed along with high levels of radioligand binding (Anton et al., 1996; Neal et al., 1999a). Further, OFQ can functionally alter circadian rhythms (Teshima et al., 2005; Leggett et al., 2007). However, the anatomy of OFQ input to the SCN remains unclear. Several brain regions expressing OFQ mRNA also have neurons that project to the SCN including the raphe, the periaqueductal gray, and the lateral geniculate (Boom et al., 1999; Neal et al., 1999b; Hay-Schmidt et al., 2003; Horowitz et al., 2004). We found OFQ application induced a sustained reduction of Ca2+ that was strongly dependent (R2 = 0.82) on the baseline [Ca2+]i and consistent with prior observations (Allen et al., 1999; Ikeda et al., 2003). Like VIP, OFQ appeared to quiet SCN neurons during the day with a reduction of the [Ca2+]i to levels and variances more typical of neurons during the night (Irwin & Allen, 2009), and this is consistent with a reduction in baseline action potential firing by OFQ in SCN neurons (Allen et al., 1999; Teshima et al., 2005). While OFQ is known to reduce, it does not eliminate presynaptic glutamate release from RHT terminals (Gompf et al., 2005), and RHT stimulation can still trigger postsynaptic action potential firing consistent with the RHT-induced Ca2+ transients observed in a number of neurons. Thus these data demonstrate that OFQ, like VIP lowers the [Ca2+]i in populations of SCN neurons consistent with a reduction in the state of action potential firing activity.
The neuropeptides expressed in the SCN demonstrated unique effects on the [Ca2+]i. VIP lowers [Ca2+]i in SCN neurons in an activity state-dependent manner. Conversely, AVP elevated [Ca2+]i independent of the activity state of the neuron. For a threshold change in [Ca2+]i to subsequently evoke phase changes to the clock, the initial Ca2+ concentration, amplitude and duration are likely critical factors. We hypothesize that VIP facilitates photic entrainment of SCN neurons by lowering the action potential firing frequency and [Ca2+]i, which may facilitate the RHT input to induce a threshold change in [Ca2+]i. Conceivably AVP, which appears to raise [Ca2+]i may impede this process. While the contribution, significance and proportions of Ca2+ originating from extracellular and intracellular sources to the [Ca2+]i remain unclear, these data suggest that the activity state of the neuron, autoregulated at least in part by neuropeptides, may be a major point of regulation for SCN synchronization and modulation of light-input induced changes to the clock.
Acknowledgments
Support contributed by Grant MH 70922 (CNA).
References
- Ajpru S, McArthur AJ, Piggins HD, Sugden D. Identification of PAC1 receptor isoform mRNAs by real-time PCR in rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 2002;105:29–37. doi: 10.1016/s0169-328x(02)00387-x. [DOI] [PubMed] [Google Scholar]
- Albers HE, Stopa EG, Zoeller RT, Kauer JS, King JC, Fink JS, Mobtaker H, Wolfe H. Day-night variation in prepro vasoactive intestinal peptide/peptide histidine isoleucine mRNA within the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1990;7:85–89. doi: 10.1016/0169-328x(90)90077-q. [DOI] [PubMed] [Google Scholar]
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- Allen CN, Jiang ZG, Teshima K, Darland T, Ikeda M, Nelson CS, Quigley DI, Yoshioka T, Allen RG, Rea MA, Grandy DK. Orphanin-FQ/nociceptin (OFQ/N) modulates the activity of suprachiasmatic nucleus neurons. J Neurosci. 1999;19:2152–2160. doi: 10.1523/JNEUROSCI.19-06-02152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton B, Fein J, To T, Li X, Silberstein L, Evans CJ. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. The Journal of comparative neurology. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nature neuroscience. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Herzog ED. Come together, right...now: synchronization of rhythms in a mammalian circadian clock. Neuron. 2005;48:531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of gamma-aminobutyric acid and gamma-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. The Journal of comparative neurology. 2008;506:708–732. doi: 10.1002/cne.21553. [DOI] [PubMed] [Google Scholar]
- Birnbaumer M. Vasopressin receptors. Trends in endocrinology and metabolism: TEM. 2000;11:406–410. doi: 10.1016/s1043-2760(00)00304-0. [DOI] [PubMed] [Google Scholar]
- Boom A, Mollereau C, Meunier JC, Vassart G, Parmentier M, Vanderhaeghen JJ, Schiffmann SN. Distribution of the nociceptin and nocistatin precursor transcript in the mouse central nervous system. Neuroscience. 1999;91:991–1007. doi: 10.1016/s0306-4522(98)00683-6. [DOI] [PubMed] [Google Scholar]
- Brown TM, Colwell CS, Waschek JA, Piggins HD. Disrupted neuronal activity rhythms in the suprachiasmatic nuclei of vasoactive intestinal polypeptide-deficient mice. Journal of neurophysiology. 2007;97:2553–2558. doi: 10.1152/jn.01206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buijs RM, Wortel J, Hou YX. Colocalization of gamma-aminobutyric acid with vasopressin, vasoactive intestinal peptide, and somatostatin in the rat suprachiasmatic nucleus. The Journal of comparative neurology. 1995;358:343–352. doi: 10.1002/cne.903580304. [DOI] [PubMed] [Google Scholar]
- Butler MP, Silver R. Basis of robustness and resilience in the suprachiasmatic nucleus: individual neurons form nodes in circuits that cycle daily. Journal of biological rhythms. 2009;24:340–352. doi: 10.1177/0748730409344800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel M, Morris JF. Morphological heterogeneity of the GABAergic network in the suprachiasmatic nucleus, the brain’s circadian pacemaker. Journal of anatomy. 2000;196 ( Pt 1):1–13. doi: 10.1046/j.1469-7580.2000.19610001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Lee CJ, Schroeder A, Kim YS, Jung SH, Kim JS, Kim do Y, Son EJ, Han HC, Hong SK, Colwell CS, Kim YI. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci. 2008;28:5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. The European journal of neuroscience. 2000;12:571–576. doi: 10.1046/j.1460-9568.2000.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler DJ, Haraura M, Reed HE, Shen S, Sheward WJ, Morrison CF, Marston HM, Harmar AJ, Piggins HD. The mouse VPAC2 receptor confers suprachiasmatic nuclei cellular rhythmicity and responsiveness to vasoactive intestinal polypeptide in vitro. The European journal of neuroscience. 2003;17:197–204. doi: 10.1046/j.1460-9568.2003.02425.x. [DOI] [PubMed] [Google Scholar]
- Dziema H, Obrietan K. PACAP potentiates L-type calcium channel conductance in suprachiasmatic nucleus neurons by activating the MAPK pathway. Journal of neurophysiology. 2002;88:1374–1386. doi: 10.1152/jn.2002.88.3.1374. [DOI] [PubMed] [Google Scholar]
- Francois-Bellan AM, Bosler O. Convergent serotonin and GABA innervation of VIP neurons in the suprachiasmatic nucleus demonstrated by triple labeling in the rat. Brain research. 1992;595:149–153. doi: 10.1016/0006-8993(92)91466-r. [DOI] [PubMed] [Google Scholar]
- Francois-Bellan AM, Kachidian P, Dusticier G, Tonon MC, Vaudry H, Bosler O. GABA neurons in the rat suprachiasmatic nucleus: involvement in chemospecific synaptic circuitry and evidence for GAD-peptide colocalization. Journal of neurocytology. 1990;19:937–947. doi: 10.1007/BF01186821. [DOI] [PubMed] [Google Scholar]
- Gompf HS, Moldavan MG, Irwin RP, Allen CN. Nociceptin/orphanin FQ (N/OFQ) inhibits excitatory and inhibitory synaptic signaling in the suprachiasmatic nucleus (SCN) Neuroscience. 2005;132:955–965. doi: 10.1016/j.neuroscience.2004.11.057. [DOI] [PubMed] [Google Scholar]
- Gouzenes L, Sabatier N, Richard P, Moos FC, Dayanithi G. V1a- and V2-type vasopressin receptors mediate vasopressin-induced Ca2+ responses in isolated rat supraoptic neurones. The Journal of physiology. 1999;517 ( Pt 3):771–779. doi: 10.1111/j.1469-7793.1999.0771s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Fahrenkrug J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell and tissue research. 2004;316:99–113. doi: 10.1007/s00441-004-0858-x. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Hindersson P, Knudsen SM, Georg B, Fahrenkrug J. The photopigment melanopsin is exclusively present in pituitary adenylate cyclase-activating polypeptide-containing retinal ganglion cells of the retinohypothalamic tract. J Neurosci. 2002;22:RC191. doi: 10.1523/JNEUROSCI.22-01-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Moller M, Ottersen OP, Fahrenkrug J. PACAP and glutamate are co-stored in the retinohypothalamic tract. The Journal of comparative neurology. 2000;418:147–155. [PubMed] [Google Scholar]
- Harrington ME, Hoque S, Hall A, Golombek D, Biello S. Pituitary adenylate cyclase activating peptide phase shifts circadian rhythms in a manner similar to light. J Neurosci. 1999;19:6637–6642. doi: 10.1523/JNEUROSCI.19-15-06637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay-Schmidt A, Vrang N, Larsen PJ, Mikkelsen JD. Projections from the raphe nuclei to the suprachiasmatic nucleus of the rat. Journal of chemical neuroanatomy. 2003;25:293–310. doi: 10.1016/s0891-0618(03)00042-5. [DOI] [PubMed] [Google Scholar]
- Hermes ML, Kolaj M, Doroshenko P, Coderre E, Renaud LP. Effects of VPAC2 receptor activation on membrane excitability and GABAergic transmission in subparaventricular zone neurons targeted by suprachiasmatic nucleus. Journal of neurophysiology. 2009;102:1834–1842. doi: 10.1152/jn.91261.2008. [DOI] [PubMed] [Google Scholar]
- Horowitz SS, Blanchard JH, Morin LP. Intergeniculate leaflet and ventral lateral geniculate nucleus afferent connections: An anatomical substrate for functional input from the vestibulo-visuomotor system. The Journal of comparative neurology. 2004;474:227–245. doi: 10.1002/cne.20125. [DOI] [PubMed] [Google Scholar]
- Ikeda M. Calcium dynamics and circadian rhythms in suprachiasmatic nucleus neurons. Neuroscientist. 2004;10:315–324. doi: 10.1177/10738584031262149. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sugiyama T, Wallace CS, Gompf HS, Yoshioka T, Miyawaki A, Allen CN. Circadian dynamics of cytosolic and nuclear Ca2+ in single suprachiasmatic nucleus neurons. Neuron. 2003;38:253–263. doi: 10.1016/s0896-6273(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. Calcium response to retinohypothalamic tract synaptic transmission in suprachiasmatic nucleus neurons. J Neurosci. 2007;27:11748–11757. doi: 10.1523/JNEUROSCI.1840-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RP, Allen CN. GABAergic signaling induces divergent neuronal Ca2+ responses in the suprachiasmatic nucleus network. The European journal of neuroscience. 2009;30:1462–1475. doi: 10.1111/j.1460-9568.2009.06944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri J, Colwell CS. Regulation of inhibitory synaptic transmission by vasoactive intestinal peptide (VIP) in the mouse suprachiasmatic nucleus. Journal of neurophysiology. 2003;90:1589–1597. doi: 10.1152/jn.00332.2003. [DOI] [PubMed] [Google Scholar]
- Itri J, Michel S, Waschek JA, Colwell CS. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. Journal of neurophysiology. 2004;92:311–319. doi: 10.1152/jn.01078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalamatianos T, Kallo I, Coen CW. Ageing and the diurnal expression of the mRNAs for vasopressin and for the V1a and V1b vasopressin receptors in the suprachiasmatic nucleus of male rats. Journal of neuroendocrinology. 2004;16:493–501. doi: 10.1111/j.1365-2826.2004.01196.x. [DOI] [PubMed] [Google Scholar]
- Kallo I, Kalamatianos T, Piggins HD, Coen CW. Ageing and the diurnal expression of mRNAs for vasoactive intestinal peptide and for the VPAC2 and PAC1 receptors in the suprachiasmatic nucleus of male rats. Journal of neuroendocrinology. 2004a;16:758–766. doi: 10.1111/j.1365-2826.2004.01232.x. [DOI] [PubMed] [Google Scholar]
- Kallo I, Kalamatianos T, Wiltshire N, Shen S, Sheward WJ, Harmar AJ, Coen CW. Transgenic approach reveals expression of the VPAC2 receptor in phenotypically defined neurons in the mouse suprachiasmatic nucleus and in its efferent target sites. The European journal of neuroscience. 2004b;19:2201–2211. doi: 10.1111/j.0953-816X.2004.03335.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki J, Kobayashi S, Miyagi Y, Nishimura J, Fujishima M, Kanaide H. The mechanisms of the relaxation induced by vasoactive intestinal peptide in the porcine coronary artery. British journal of pharmacology. 1997;121:977–985. doi: 10.1038/sj.bjp.0701206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp MD, Meissl H, Dehghani F, Korf HW. The pituitary adenylate cyclase-activating polypeptide modulates glutamatergic calcium signalling: investigations on rat suprachiasmatic nucleus neurons. Journal of neurochemistry. 2001;79:161–171. doi: 10.1046/j.1471-4159.2001.00553.x. [DOI] [PubMed] [Google Scholar]
- Kopp MD, Schomerus C, Dehghani F, Korf HW, Meissl H. Pituitary adenylate cyclase-activating polypeptide and melatonin in the suprachiasmatic nucleus: effects on the calcium signal transduction cascade. J Neurosci. 1999;19:206–219. doi: 10.1523/JNEUROSCI.19-01-00206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman SJ, Silver R, Le Sauter J, Bult-Ito A, McMahon DG. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci. 2003;23:1441–1450. doi: 10.1523/JNEUROSCI.23-04-01441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett JD, Jessop DS, Fulford AJ. The nociceptin/orphanin FQ antagonist UFP-101 differentially modulates the glucocorticoid response to restraint stress in rats during the peak and nadir phases of the hypothalamo-pituitary-adrenal axis circadian rhythm. Neuroscience. 2007;147:757–764. doi: 10.1016/j.neuroscience.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Li JD, Burton KJ, Zhang C, Hu SB, Zhou QY. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. American journal of physiology. 2009;296:R824–830. doi: 10.1152/ajpregu.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist GB, Block GD. Role of neuronal membrane events in circadian rhythm generation. Methods in enzymology. 2005;393:623–642. doi: 10.1016/S0076-6879(05)93033-4. [DOI] [PubMed] [Google Scholar]
- Lundkvist GB, Kwak Y, Davis EK, Tei H, Block GD. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci. 2005;25:7682–7686. doi: 10.1523/JNEUROSCI.2211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski H, Iannazzo L. Protein kinase C: a physiological mediator of enhanced transmitter output. Progress in neurobiology. 1998;55:463–475. doi: 10.1016/s0301-0082(98)00017-3. [DOI] [PubMed] [Google Scholar]
- Markos F, Hennessy BA, Fitzpatrick M, O’Sullivan J, Snow HM. An evaluation of the efficacy of vasoactive intestinal polypeptide antagonists in vivo in the anaesthetized dog. Pharmacology. 2002;66:206–210. doi: 10.1159/000065535. [DOI] [PubMed] [Google Scholar]
- Maywood ES, O’Neill JS, Chesham JE, Hastings MH. Minireview: The circadian clockwork of the suprachiasmatic nuclei--analysis of a cellular oscillator that drives endocrine rhythms. Endocrinology. 2007;148:5624–5634. doi: 10.1210/en.2007-0660. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Meis S. Nociceptin/orphanin FQ: actions within the brain. Neuroscientist. 2003;9:158–168. doi: 10.1177/1073858403252231. [DOI] [PubMed] [Google Scholar]
- Michel S, Itri J, Han JH, Gniotczynski K, Colwell CS. Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus. BMC neuroscience [electronic resource] 2006;7:15. doi: 10.1186/1471-2202-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa K, Uchida A, Shiraki T, Teshima K, Takeshima H, Shibata S. ORL1 receptor-mediated down-regulation of mPER2 in the suprachiasmatic nucleus accelerates re-entrainment of the circadian clock following a shift in the environmental light/dark cycle. Neuropharmacology. 2007;52:1055–1064. doi: 10.1016/j.neuropharm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Moore RY, Speh JC, Leak RK. Suprachiasmatic nucleus organization. Cell and tissue research. 2002;309:89–98. doi: 10.1007/s00441-002-0575-2. [DOI] [PubMed] [Google Scholar]
- Morin AJ, Denoroy L, Jouvet M. Daily variations in concentration of vasoactive intestinal peptide immunoreactivity in hypothalamic nuclei of rats rendered diurnal by restricted-schedule feeding. Neuroscience letters. 1993;152:121–124. doi: 10.1016/0304-3940(93)90498-a. [DOI] [PubMed] [Google Scholar]
- Morin LP. SCN organization reconsidered. Journal of biological rhythms. 2007;22:3–13. doi: 10.1177/0748730406296749. [DOI] [PubMed] [Google Scholar]
- Morris ME, Viswanathan N, Kuhlman S, Davis FC, Weitz CJ. A screen for genes induced in the suprachiasmatic nucleus by light. Science. 1998;279:1544–1547. doi: 10.1126/science.279.5356.1544. [DOI] [PubMed] [Google Scholar]
- Nakamura W, Honma S, Shirakawa T, Honma K. Regional pacemakers composed of multiple oscillator neurons in the rat suprachiasmatic nucleus. The European journal of neuroscience. 2001;14:666–674. doi: 10.1046/j.0953-816x.2001.01684.x. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Akil H, Watson SJ., Jr Opioid receptor-like (ORL1) receptor distribution in the rat central nervous system: comparison of ORL1 receptor mRNA expression with (125)I-[(14)Tyr]-orphanin FQ binding. The Journal of comparative neurology. 1999a;412:563–605. [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. The Journal of comparative neurology. 1999b;406:503–547. [PubMed] [Google Scholar]
- Nicholson SA, Adrian TE, Bacarese-Hamilton AJ, Gillham B, Jones MT, Bloom SR. 24-hour variation in content and release of hypothalamic neuropeptides in the rat. Regulatory peptides. 1983;7:385–397. doi: 10.1016/0167-0115(83)90110-6. [DOI] [PubMed] [Google Scholar]
- O’Neill JS, Maywood ES, Chesham JE, Takahashi JS, Hastings MH. cAMP-dependent signaling as a core component of the mammalian circadian pacemaker. Science. 2008;320:949–953. doi: 10.1126/science.1152506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Ito S, Ohga A. Effects of vasoactive intestinal peptide (VIP) on contractile responses of smooth muscle in rat stomach. British journal of pharmacology. 1991;102:621–626. doi: 10.1111/j.1476-5381.1991.tb12222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhotin P, Harmar AJ, Verkhratsky A, Piggins H. VIP receptors control excitability of suprachiasmatic nuclei neurones. Pflugers Arch. 2006;452:7–15. doi: 10.1007/s00424-005-0003-z. [DOI] [PubMed] [Google Scholar]
- Pfeffer M, Muller CM, Mordel J, Meissl H, Ansari N, Deller T, Korf HW, von Gall C. The mammalian molecular clockwork controls rhythmic expression of its own input pathway components. J Neurosci. 2009;29:6114–6123. doi: 10.1523/JNEUROSCI.0275-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggins HD, Cutler DJ. The roles of vasoactive intestinal polypeptide in the mammalian circadian clock. The Journal of endocrinology. 2003;177:7–15. doi: 10.1677/joe.0.1770007. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Marchant EG, Goguen D, Rusak B. Phase-shifting effects of pituitary adenylate cyclase activating polypeptide on hamster wheel-running rhythms. Neuroscience letters. 2001;305:25–28. doi: 10.1016/s0304-3940(01)01796-7. [DOI] [PubMed] [Google Scholar]
- Piggins HD, Stamp JA, Burns J, Rusak B, Semba K. Distribution of pituitary adenylate cyclase activating polypeptide (PACAP) immunoreactivity in the hypothalamus and extended amygdala of the rat. The Journal of comparative neurology. 1996;376:278–294. doi: 10.1002/(SICI)1096-9861(19961209)376:2<278::AID-CNE9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rea MA. VIP-stimulated cyclic AMP accumulation in the suprachiasmatic hypothalamus. Brain research bulletin. 1990;25:843–847. doi: 10.1016/0361-9230(90)90179-4. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Shibuya I, Dayanithi G. Intracellular calcium increase and somatodendritic vasopressin release by vasopressin receptor agonists in the rat supraoptic nucleus: involvement of multiple intracellular transduction signals. Journal of neuroendocrinology. 2004;16:221–236. doi: 10.1111/j.0953-8194.2004.01155.x. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF, Jr, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Kimura F. Temporal profiles of vasoactive intestinal polypeptide precursor mRNA and its receptor mRNA in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1999;63:262–267. doi: 10.1016/s0169-328x(98)00289-7. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma K. Circadian rhythms in the release of vasoactive intestinal polypeptide and arginine-vasopressin in organotypic slice culture of rat suprachiasmatic nucleus. Neuroscience letters. 1994;170:183–186. doi: 10.1016/0304-3940(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Inouye ST. Photic information coded by vasoactive intestinal polypeptide and neuropeptide Y. Neuroscience and biobehavioral reviews. 1995;19:349–352. doi: 10.1016/0149-7634(94)00048-6. [DOI] [PubMed] [Google Scholar]
- Teshima K, Minoguchi M, Tounai S, Ashimori A, Eguchi J, Allen CN, Shibata S. Nonphotic entrainment of the circadian body temperature rhythm by the selective ORL1 receptor agonist W-212393 in rats. British journal of pharmacology. 2005;146:33–40. doi: 10.1038/sj.bjp.0706311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K, Shinohara K, Otori Y, Fukuhara C, Inouye ST. Circadian rhythms of vasopressin content in the suprachiasmatic nucleus of the rat. Neuroreport. 1992;3:809–812. doi: 10.1097/00001756-199209000-00022. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Vanecek J, Yamaoka S. In vitro entrainment of the circadian rhythm of vasopressin-releasing cells in suprachiasmatic nucleus by vasoactive intestinal polypeptide. Brain research. 2000;877:361–366. doi: 10.1016/s0006-8993(00)02724-4. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annual review of physiology. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamase K, Takahashi S, Nomura K, Haruta K, Kawashima S. Circadian changes in arginine vasopressin level in the suprachiasmatic nuclei in the rat. Neuroscience letters. 1991;130:255–258. doi: 10.1016/0304-3940(91)90409-m. [DOI] [PubMed] [Google Scholar]
- Yang K, Lei G, Jackson MF, Macdonald JF. The Involvement of PACAP/VIP System in the Synaptic Transmission in the Hippocampus. J Mol Neurosci. 2010 doi: 10.1007/s12031-010-9372-7. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Kovacs K, Lolait SJ. The diurnal rhythm in vasopressin V1a receptor expression in the suprachiasmatic nucleus is not dependent on vasopressin. Endocrinology. 1993;133:585–590. doi: 10.1210/endo.133.2.8344200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Doroshenko P, Cao XY, Irfan N, Coderre E, Kolaj M, Renaud LP. Vasopressin induces depolarization and state-dependent firing patterns in rat thalamic paraventricular nucleus neurons in vitro. American journal of physiology. 2006;290:R1226–1232. doi: 10.1152/ajpregu.00770.2005. [DOI] [PubMed] [Google Scholar]