Abstract
Background
Therapies for peanut allergy (PNA) are urgently needed. Food Allergy Herbal Formula -2 (FAHF-2) has profound therapeutic effects in a murine peanut allergy model and is safe for food allergic adults in clinical trials. However the large FAHF-2 pill-load is not conducive to clinical studies in children. Thus refining FAHF-2 to decrease pill-load is essential for the inclusion of children in clinical trials and to facilitate studying FAHF-2 as a clinically useful botanical drug.
Objectives
Testing long term efficacy and safety of a butanol-purified extract of FAHF-2 (B-FAHF-2) in a murine model of PNA, and to explore its immunological mechanisms of action.
Methods
FAHF-2 was purified by butanol extraction. C3H/HeJ mice with established PNA received the 1st course of B-FAHF-2 at 6 mg, twice daily for 7 weeks (PNA/B-FAHF-2) or water (PNA/Sham) and were then challenged immediately after completing the treatment and 6 more times every 1–2 months post treatment up to week 50. Mice then received a second course of B-FAHF-2 treatment at week 52 and were challenged at week 65. In vivo and in vitro immunological effects on T, B and mast cells were also determined.
Results
Butanol purification reduced the volume of the effective dose ~5 fold. All PNA/B-FAHF-2 mice were completely protected from peanut anaphylaxis until the 5th challenge after the 1st course of treatment, as compared to PNA/sham mice. Partial protection persisted up to 50 weeks. A 2nd treatment course restored complete protection. B-FAHF-2 significantly suppressed Th2 cytokine, IgE and histamine levels in vivo, and showed direct inhibition of Th2, IgE-producing B cells and mast cell activation in vitro. B-FAHF-2 had a high margin of safety.
Conclusion and clinical relevance
B-FAHF-2 produced long-lasting protection against PN anaphylaxis for approximately half of the murine lifespan without side effects. B-FAHF-2 exhibited direct effects on multiple food allergy effector cells.
Keywords: Chinese herbal medicine formula, FAHF-2, B-FAHF-2, Peanut Anaphylaxis, Th2 cytokines, Histamine, IgE
INTRODUCTION
Peanut allergy (PNA) is the most common cause of fatal and near-fatal food allergic reactions in the U.S. The prevalence of peanut or tree nut allergy in children younger than 18 years is 2.1%, compared with 1.2% in 2002 and 0.6% in 1997 in the U.S.[1]. Other than immediate access to post-anaphylactic rescue medications, strict avoidance is the only way to manage this condition. Unfortunately, accidental ingestion is common [2]. A clinical trial using monthly humanized recombinant anti-IgE injections showed some effect in preventing allergic responses to small amounts of PN protein in a majority of PN-sensitive human subjects[3]. However, the treatment effect was short-lived and continued protection by this approach would require monthly injections for an indefinite period of time. Several new therapies for food allergy, such as oral immunotherapy (OIT) and sublingual immunotherapy (SLIT) are under investigation. OIT and SLIT for egg, milk, hazelnut and PN allergy appear to desensitize the majority of patients while on therapy and allow them to ingest some amount of the allergen without allergic reactions, [4;5]. However, there is, as yet, no evidence that these therapies induce long-term tolerance. One study found that significant allergic reactions occurred when therapy was discontinued for 1 to 3 weeks and followed by additional therapy using the same dose, or by accidental ingestion of the food allergen [6]. Peanut OIT is not ready for clinical use in view of the risk to benefit ratio [7]. Effective, safe, convenient and long lasting therapies for food allergy are urgently needed.
Food-induced anaphylaxis is predominantly IgE-mediated [8]. A Th2-biased immune status, excessive IgE production and activation of mast cells/basophils are the key immunopathological mechanisms underlying food-induced anaphylaxis. We previously reported that the 9-herb preparation, Food Allergy Herbal Formula (FAHF)-2 completely blocks anaphylactic symptoms in the peanut-sensitized murine model[9;10] and the block persists for at least 6 months post-therapy following a single 7 wk course of treatment. These persistent effects were associated with lasting suppression of PN-specific Th2 responses and elevation in Interferon-γ levels [11]. This preclinical study provided the rationale for a clinical trial of FAHF-2. A recent completed dose cohort acute phase I study in patients, ages12–45 years with peanut and/or tree nut, fish, and shellfish allergies showed that FAHF-2 was safe and well tolerated, and suppressed Th2 cytokine secretion by cultured peripheral blood mononuclear cells from these patients [12]. However, as with other herbal products, the large number of pills required daily when moving from a traditional tea formulation to the present pill formulation makes adherence difficult, especially for children. Although no dose-limiting side effects were reported in the Phase 1 study, several participants in the highest dose group felt that 12 tablets 3-times daily posed a significant burden. FAHF-2 in its current form is not suitable as therapy for children. Thus, it is essential to refine the product for practical reasons of making it suitable for studies in food allergic children as well as adults and to enhance the clinical study of FAHF-2 as a botanical drug and further understand its immunological actions.
We previously reported that the FAHF-2 formula is superior to any of its individual herbal constituuents in protecting mice from PN anaphylaxis [13]. Herbal products contain both medicinal and inactive components. Concentration of active components by organic solvent extraction is widely used to refine aqueous extracts of herbal mixtures [14]. Ethanol purification of FAHF-2 (an aqueous extract) reduced the daily dose by approximately 30% while retaining efficacy and safety (current IND FAHF-2). Based on the known characteristics of FAHF-2, we hypothesized that the less polar solvent butanol [14] would further refine the product. We previously reported that a single FAHF-2 treatment course produced long term protection against anaphylaxis that declined 6 months post-therapy [15]. We hypothesized that full protection could be restored by additional treatment. We previously also found that FAHF-2 suppressed Th2 responsiveness, IgE production and histamine release. Although IFN-γ was shown to be important, it alone could not account for FAHF-2 effects on effector cells [15]. We therefore hypothesized that FAHF-2 may have direct effects on effector cells, including Th2 cells, B cells and mast cells/basophils.
The present study tested these hypotheses, and demonstrated that butanol extraction of FAHF-2 (B-FAHF-2) at approximately one fifth the dose of FAHF-2 also generated protection for 6 months in an animal model of PN allergy, and that a 2nd course of treatment, following a gradual decline 8.5 months following the 1st treatment course, fully restored protection. B-FAHF-2 also showed direct effects on Th2 cells, B cells and mast cells in vitro.
MATERIALS and METHODS
Reagents and mice
Freshly ground, whole roasted PN (White Rose brand, NJ) were homogenized in phosphate buffered saline (PBS) (Mediatech, VA), and a crude PN extract (CPE) was prepared as previously described [16–18]. Cholera toxin (CT) was purchased from List Biological Laboratories, Inc (Campbell, CA). The RBL 2H3 (rat basophil cell line) and U266B cells (human myeloma cell line) were purchased from American Tissue culture American Type Culture Collection (Manassas, Virginia).
Five-week-old female C3H/HeJ mice purchased from the Jackson Laboratory (Bar Harbor, ME) were maintained on PN-free chow under specific pathogen-free conditions according to standard guidelines for the care and use of animals [19].
Butanol fractionation of FAHF-2
Dried aqueous extract of FAHF-2, produced in a GMP-certified facility was obtained from Beijing Shen Hua Shi Di Medical Technology, Beijing China, and stored at room temperature. All herbs used for manufacture were of Chinese origin. The quality of the raw herbs was established according to the standards of the Pharmacopoeia of People’s Republic of China [20] and as previous described [12;13]. Based on organoleptic and microscopic examination, raw herbs used in FAHF-2 were identified as follows: fruits of Prunus mume, skin of the fruits of Zanthoxylum schinifolium, roots of Angelica sinensis, rhizome of Zingiber officinalis, twigs of Cinnamomum cassiae, bark of Phellodendron chinense, rhizome of Coptis chinensis, roots of Panax ginseng and fruiting body of Ganoderma lucidum. The botanical information for the individual herbs including geographical location, harvest season, pre-processing, heavy metal and pesticide residues has been published previously [13].
Butanol extracts of FAHF-2 were prepared in our laboratory. Powdered FAHF-2 extract (5 g) was dissolved in distilled water (125 ml) with sonication for 15 min, transferred to a separatory funnel, and then extracted with butanol (125 ml, Fisher Scientific, Pittsburgh, PA, USA) at room temperature for 4h. The organic layer was collected and the aqueous layer re-extracted with butanol three additional times. The combined butanol extracts were then mixed with distilled water (3:1) and evaporated under reduced pressure. The dried extract (B-FAHF-2) was stored at room temperature. The yield of butanol extract was 22.37 %±1.55 from 5 separate preparations.
Endotoxin levels in B-FAHF-2 were measured using the Pyrogent Plus assay kit (Lonza, MA). The level was below the detectable limit (0.03 EU/ml).
HPLC fingerprint and chemical characteristics of B-FAHF-2
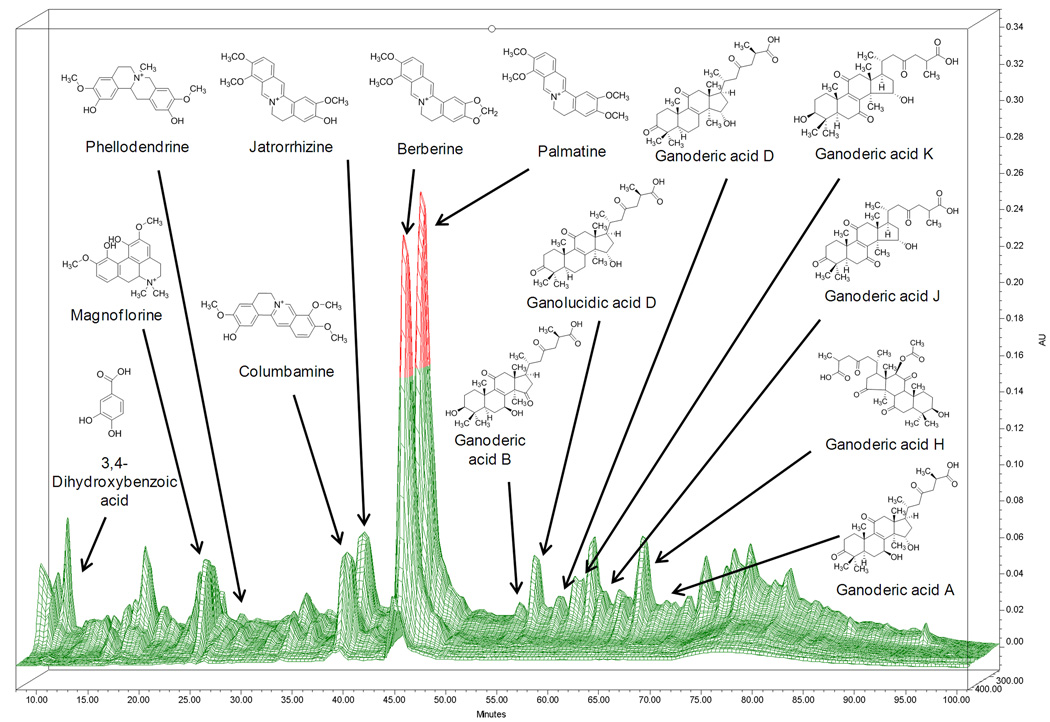
Liquid chromatography was performed using a Waters 2690 HPLC-PDA system (Waters, Milford, MA). 10µl of B -FAHF-2 (15 mg/ml) was separated on a ZORBAX SB-C18 (4.6× 150mm, 5µm) column (Agilent, Santa Clara, CA) using mobile phase consisted of 0.1% aqueous phosphoric acid (A) and acetonitrile (B) at a constant flow rate of 1ml/min. The linear gradient program started at 2– 25% B in 0–45 min, 25– 35% B in 45–70 min, 35–55% in 70–85min, 55–75% in 85–95 min, and maintained at 75% for 5 min [12]. Data was collected and processed with Waters’ Empower software. Mass spectra were measured using Waters Alliance 2695 HPLC system coupled with Waters LCT premier TOF mass spectrometer in positive mode was used to characteriz the known chemical constituents in FAHF-2. The HPLC fingerprint of B-FAHF-2 and known major chemical markers are shown in Figure 1.
Figure 1. A 3D- HPLC-DAD fingerprint of B-FAHF-2.
Thirteen components were identified using standard compounds and exact molecular weights, which were determined by LC-MS analysis. The mass range was from m/z 50 to 1000 in W optics mode. The parameters were set as follows: cone gas, 40 l/h; desolvation gas, 500l/h; source temperature, 110 °C. Lock mass was used with Leucine Enkephalin as the reference.
Induction of PNA, B-FAHF-2 treatment and post therapy PN challenges
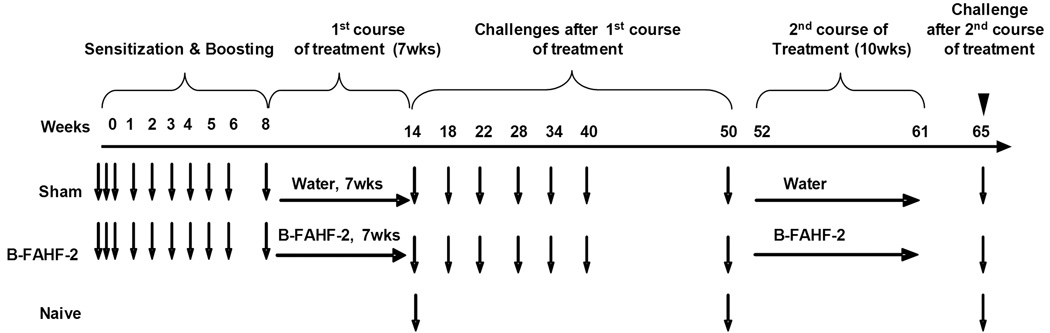
PNA was induced in C3H/HeJ mice as previously described [9] and the protocol is depicted in in Figure2. PNA mice received B-FAHF-2, 6 mg/ 0.5 ml of water i.g., twice daily for seven weeks (PNA/B-FAHF-2, n=8). The daily B-FAHF-2 dose was approximately one fifth of the daily dose of FAHF-2 (64 mg) previously found to be effective in the murine PNA model. Control PNA mice received an equal amount of water (PNA/sham, N=8). Mice were challenged i.g. with PN (200mg/mouse) 24 h after completion of treatment (at week 14 after initial PN sensitization). To determine prolonged protection, mice were re-challenged 6 additional times at wks 18, 22, 28, 34, 40, and 50 after the 1st treatment course. To determine whether FAHF-2 treated mice responded to a second course of treatment when complete protection began to decline in some PNA/B-FAHF-2 mice, treatment was repeated at week 52 and continued for 10 weeks. Mice were subsequently re-challenged 4 weeks later (wk 65, over 12 months after completing the first course of treatment). The mucosal adjuvant CT, 20 mg/ mouse, was co-administered with PN at all times except during the final challenge. Age-matched naive mice were included as controls. Blood was collected at the time of each challenge and body temperatures were recorded.
Figure 2. In vivo experimental protocol.
C3H/HeJ mice were subjected to weekly oral peanut (10mg with CT at 20 µg) sensitization from week 0 through week 5 and boosted thereafter at week 6 and week 8 with PN (50mg with CT at 20 µg). Twenty-four hours later, PN allergic mice were treated with B-FAHF-2 or water (sham) daily for seven weeks, and then were challenged at intervals indicated starting from week 14 and ending at week 50. A second course of B-FAHF-2 was administered for 10 weeks (wks 52–61) and mice were given a final challenge 4 weeks later at week 65 (PN 200µg).
Assessment of hypersensitivity reactions
Anaphylactic signs were evaluated 30 minutes following oral challenge utilizing the scoring system previously described[9]: 0 - no signs; 1 - scratching and rubbing around the snout and head (mild reaction); 2 - puffiness around the eyes and snout, diarrhea, pilar erecti, reduced activity, and/or decreased activity with increased respiratory rate (moderate reaction); 3 - wheezing, labored respiration, cyanosis around the mouth and the tail (severe reaction); 4 - no activity after prodding, or tremor and convulsion (near fatal reaction); 5 - death. Rectal temperatures were measured immediately after scoring using a rectal probe (Harvard Apparatus, NJ).
Measurement of plasma histamine levels
Blood was collected into chilled EDTA-coated tubes (Fisher Scientific, PA) by retro-orbital bleed after anaphylaxis scores and temperatures were recorded. Plasma was harvested and stored at −80°C until used. Histamine was measured using an enzyme immunoassay kit (ImmunoTECH Inc., Marseille, France) as described by the manufacturer and as reported previously [9;21]
Measurement of serum PN specific IgE, and IgG2a levels
Blood was collected by retro-orbital bleeding using heparinized tubes (Fisher Scientific, PA), one day prior to the wk 3 sensitization and then periodically one day prior to boosting, during treatment and challenges. Sera were stored at −80°C until used. PN-specific IgE and IgG2a levels were determined by ELISA as previously described. [9]
Determination of splenocyte cytokine profiles
Following the final challenge and immediately after completing the clinical evaluation, splenocytes (SPCs) and mesenteric lymph node (MLN) cells from each group were prepared as previously described [15]. Cells were cultured in 24 well plates (4 ×106/well/ml) in the presence or absence of crude peanut extract (CPE) (200 mg/ml) or Concanavalin A (2.5 µg/ml, Data not shown). Supernatants were collected after 72 hrs of culture. Cytokine levels were determined by ELISA in triplicate according to the manufacturer’s instructions (R&D systems, Minneapolis for IL-13), PharMingen, San Diego for all others).
Safety testing
The LD 50 protocol was designed as previously described [10] with slight modifications. Naive mice were fed 12 times the daily therapeutic mouse dose of B-FAHF-2 and observed for 12 hrs, 24 hrs and then for 14 days. Mice fed water served as controls (sham). Since, as expected, no deaths or clinical symptoms occurred, blood samples were collected 14 days after the feeding, and determinations of blood urea nitrogen (BUN), creatinine, and alanine aminotransferase (ALT) to assess kidney and liver functions respectively and CBC testing were performed by Antech Diagnostics, NY. Histological analysis of major organs was performed by a veterinary pathologist in a blinded manner at Mount Sinai School of Medicine, Center for Laboratory Animal Sciences.
Evaluation of the Effect of B-FAHF-2 in vitro
PN-primed polarized Th2 cell culture and cytokine measurement
To evaluate the direct effect of B-FAHF-2 on Th2 cytokine production by antigen-primed polarized Th2 cells, splenocytes (SPCs) were isolated from PNA mice [9], cultured in 24 well plates (4 ×106/well/ml) in the presence or absence of 200 mg/ml crude peanut extract (CPE) with or without B-FAHF-2 at different concentrations (14.4–115 µg/ml). Supernatants were collected after 72-hr culture. IL-4 and IL-5 levels were determined by ELISA according to the manufacturer’s instructions (PharMingen, San Diego), and as previously described.[9;10]. Cell viability was determined by trypan blue exclusion.
RBL-2H3 culture and degranulation
To evaluate the effect of B-FAHF-2 on mast cell/basophil activation, 5×105 /ml of RBL-2H3[22] were seeded in 24 well plates in culture medium (α-MEM supplemented with 10% fetal bovine serum and 100 U/ ml penicillin-100 µg/ml streptomycin) overnight. Medium was then replaced with fresh medium with or without B-FAHF-2 at different concentrations (7.2–115µg/ml). After 24 hr of culture, anti-DNP IgE (72.2 ng/ ml) (Sigma Aldrich. St. Louis, MO) was added to the wells. After overnight culture, cells were washed twice and DNP–BSA (150 ng /ml) in 500 µl Tyrode’s buffer was added and incubated for 60 min at 37°C. The degree of degranulation was measured by measuring the release of β-hexosaminidase, as previously described [22].
U266 human B cell culture and IgE measurement
To evaluate the direct effect of B-FAHF-2 on B cell IgE production, 2×105 /ml of human myeloma cells (U266 B cell line) were cultured in 24-well tissue culture plates in RPMI 1640 medium, supplemented with 10% FBS, 1mM sodium pyruvate [23], 1× 10−5 M 2-ME, 50 U/ ml penicillin G and 50 mg/ml streptomycin, with or without B-FAHF-2 (7.2–115µg/ml). Six days later, supernatant IgE levels in these and control cultures were measured using the ImmunoCAP (Phadia, Uppsala, Sweden) according to the manufacturer’s instructions.
Statistical analysis
Data were analyzed using the SigmaStat statistical software package (SPSS Inc. Chicago, IL). Symptom score, body temperature, plasma histamine and immunoglobulin data were analyzed by Kruskal Wallis One Way Anova on Ranks followed by Dunn’s method for multiple comparisons. Data for cytokines, RBL-2H3 degranulation and IgE from in vitro experiments were analyzed using One-Way ANOVA followed by the Bonferroni t-test for multiple comparisons. Tests were two tailed, and p values≤ 0.05 were considered significant.
RESULTS
B-FAHF-2 produced prolonged protection against anaphylaxis despite multiple peanut challenges
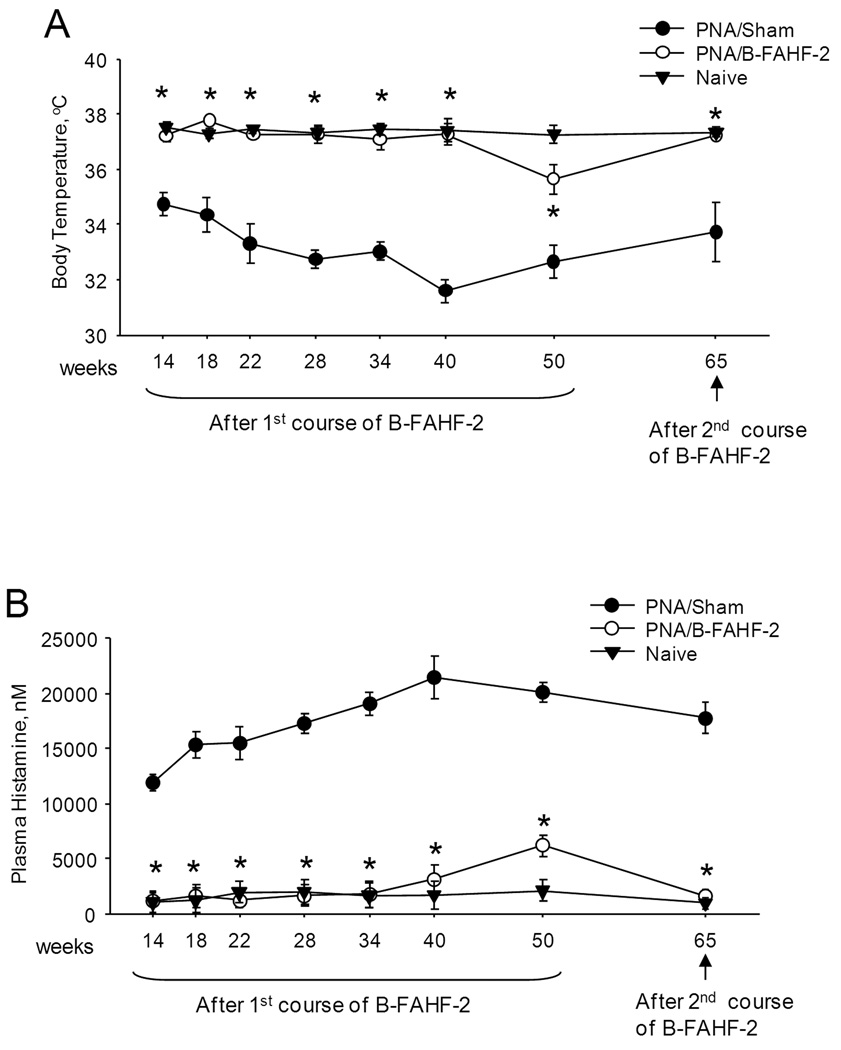
Eight of 8 PNA/Sham mice developed anaphylaxis following the first post-therapy challenge at week 14 (Table 1). These mice continued to respond to 7 subsequent challenges following the 1st course of treatment and the final challenge at wk 65 following the 2nd course of treatment. In sharp contrast, all PNA/B-FAHF-2-treated mice were completely protected up until wk 34 (5th challenge), and then 1 of 8 and 4 of 8 of B-FAHF-2 treated mice showed mild reactions (score 2) at wk 40 and 50 (6th and 7th challenges) respectively. The median scores of B-FAHF-2-treated mice were significantly lower than sham-treated mice following the 1st course of therapy (p<0.05). A 2nd course of B-FAHF-2 treatment (8.5 months after the first course of treatment) restored complete protection at the final challenge at week 65 (8th challenge). Therefore, mice were significantly protected for approximately 12 months, approximately half of the mouse lifespan. Systemic anaphylaxis in mice is accompanied by decreased body temperature. Compared to naive mice, PNA/Sham mice had significantly lower body temperature following each challenge (Figure 3A, p<001). Mean body temperatures of PNA/B-FAHF-2 mice were essentially the same as in naive mice and significantly higher than in PNA/Sham mice (p<0.05), at each of 7 challenges following the 1st course, and the challenge after the 2nd course of treatment.
Table 1. B-FAHF-2 provided prolonged protection against anaphylaxis to multiple peanut challenges in vivo.
Anaphylactic reactions were scored 30 min following each oral peanut challenge. Data are shown as number of reactions/total and group score medians with range in parenthesis. Challenges up to wk 50 indicate responses after the 1st course of treatment.
| Course of Treatment |
Challenge (Time– Point) |
PNA/Sham | PNA/B-FAHF-2 | Naive | |||
|---|---|---|---|---|---|---|---|
| N/total | Score [Median (range)] |
N/total | Score [Median (range)] |
N/total | Score [Median (range)] |
||
|
1st |
1st (W14) | 8/8 | 3 (2–4) | 0/8 | 0 (0), * | 0/10 | 0, (0) |
| 2nd (W18) | 8/8 | 3.5 (3–4) | 0/8 | 0 (0), * | NC | NS | |
| 3rd (W22) | 8/8 | 3.5 (3–4) | 0/8 | 0 (0), * | NC | NS | |
| 4th (W28) | 8/8 | 4 (3–4) | 0/8 | 0 (0), * | NC | NS | |
| 5th (W34) | 8/8 | 4 (3–4) | 0/8 | 0 (0), * | NC | NS | |
| 6th (W40) | 8/8 | 4 (3–4) | 1/8 | 0 (0–2), * | NC | NS | |
| 7th (W50) | 8/8 | 4 (3–4) | 4/8 | 0 (0–2), * | 0/10 | 0 (0) | |
| 2nd | 8th (W65) | 8/8 | 4(3–4) | 0/8 | 0 (0), * | 0/10 | 0 (0) |
p<0.05 vs sham.
NC: Not Challenged
NS : Not scored
Challenge at week 65 was after the 2nd course of B-FAHF-2 treatment.
Figure 3. B-FAHF-2 is effective in preventing hypothermia and histamine release following multiple peanut challenges in vivo.
Body temperatures were measured 30 minutes after completion of oral challenge (A). Histamine in plasma harvested after completion of challenge was determined by ELISA (B). Data from naive mice at wk 14, 50 and 65 were from challenged mice and at wks 22–40 were from unchallenged mice. Initial B-FAHF-2 therapy was administered from week 8 to week 14, and retreatment was given from week 52 to week 62. Data shown as means ±SEM of each group n=8–10 as in Table 1. *, P<0.05vs sham.
Plasma histamine levels were markedly elevated in PNA/sham mice as compared to the naive controls following each challenge (Figure 3B). Histamine levels in PNA/B-FAHF-2 mice were similar to naive mice following 6 challenges. After the 7th challenge, histamine levels were slightly, but significantly higher than in naive controls, but still significantly lower than sham-treated mice (Figure3B, p<0.05). Following the second course of treatment with B-FAHF-2, histamine levels in PNA/B-FAHF-2 mice were indistinguishable from naive mice following the final challenge (Figure 3B).
B-FAHF-2 treatment produced persistent reduction of allergen-specific IgE levels and increased IgG2a levels
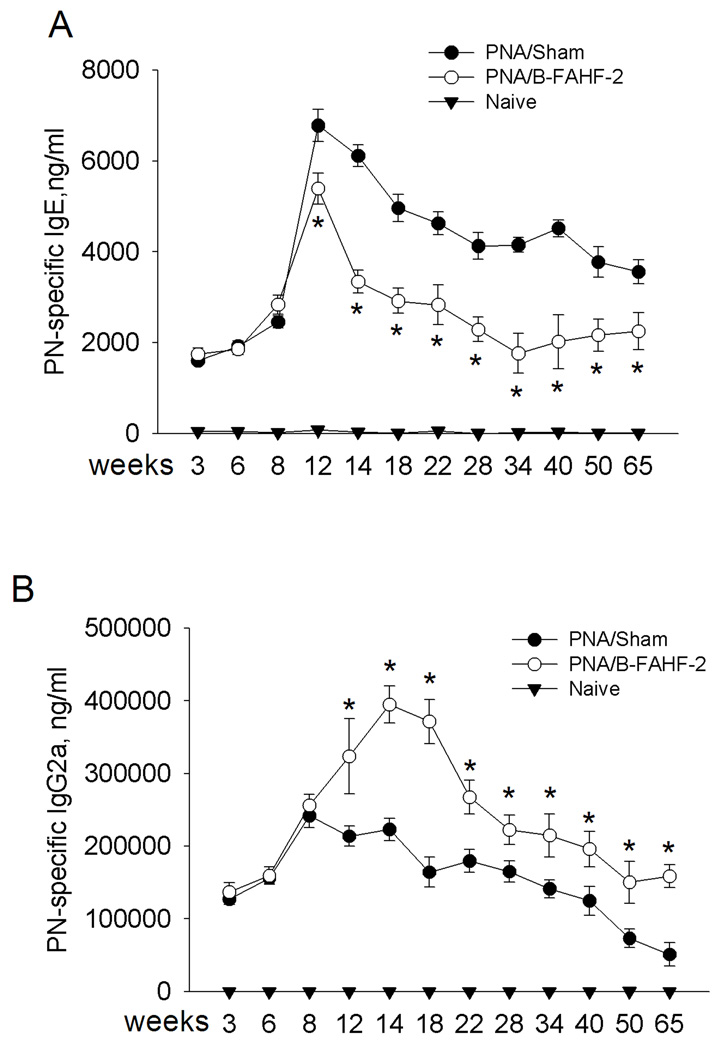
We next determined whether humoral immunological alterations were associated with B-FAHF-2 protection, focusing on PN-specific IgE and IgG2a levels. One day prior to treatment (wk 8) PN-specific IgE levels were equally elevated in all PNA mice (Figure 4A). IgE levels peaked at wk 12 and remained elevated through wk 65 in PNA/sham mice. PN-specific IgE levels of PNA/B-FAHF-2 mice were significantly lower than in PNA/sham mice by the 4th wk of treatment (wk 12 p<0.05), and immediately after completing treatment at wk 14 (p<0.05), and remained lower through wk 65 (p<0.05). Conversely, PN-specific IgG2a levels were significantly increased following 4 wks of B-FAHF-2 treatment and remained significantly elevated through wk 65 (Figure 4B, p<0.05).
Figure 4. B-FAHF-2 treatment mediated persistent reduction in allergen-specific IgE and increase in IgG2a levels.
Serum was harvested after blood collection by retro-orbital bleeding at indicated time-points. Serum peanut-specific IgE (A) and IgG2a levels (B) were determined by ELISA. Data from naive mice at wk 14, 50 and 65 were from challenged mice and at wks 22–40 were from unchallenged mice. Initial B-FAHF-2 therapy was administered from week 8 to week 14 and retreatment was given from week 52 to week 62. Data are shown as means ± SEM for each group, n=8–10 as in Table 1. *, p<0.05vs sham.
B-FAHF-2 treatment suppressed antigen-specific Th2 cytokine secretion and increased Th1 cytokine IFN-γ secretion to recall PN allergen stimulation
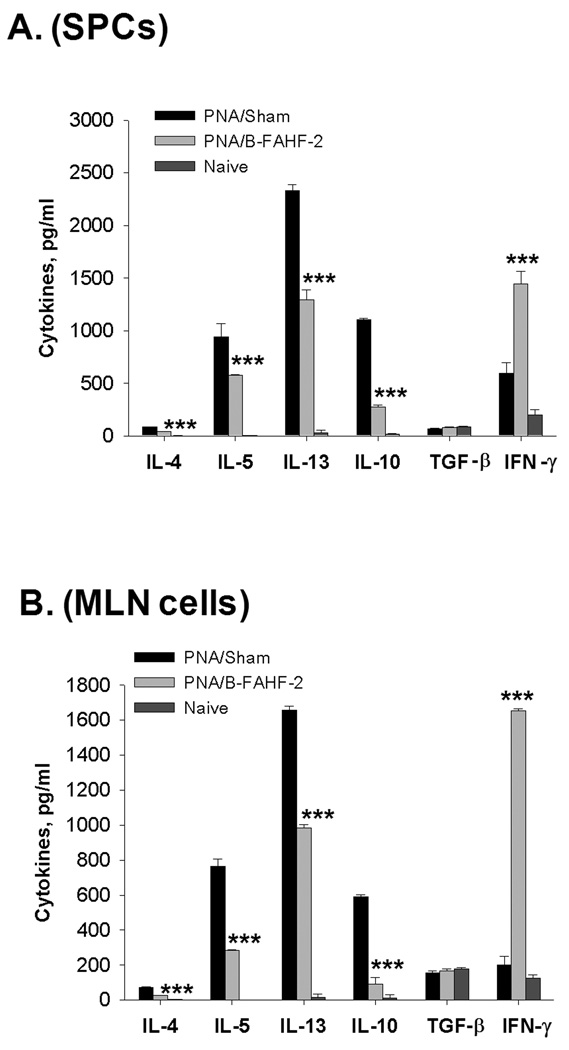
SPCs and MLN cells were isolated following the final challenge. IL-4, IL-5, IL-13 and IL-10 production by cultured SPCs (Figure 5A) and MLN cells (Figure 5B) from B-FAHF-2 treated mice were significantly reduced as compared to cells from sham treated mice) in response to recall antigen (CPE). In contrast, IFN-γ was significantly increased when compared to cells from sham-treated mice (p<0.001). TGF-β levels did not differ in cultured SPCs or MLN cells between groups (Figure 5A and B).
Figure 5. B-FAHF-2 treatment decreases PN-stimulated Th2 cytokines but increases PN-specific-IFNγ.
Splenocyte and MLN cultures were prepared from mice immediately after evaluation of anaphylactic reactions following the final challenge. Cells were stimulated with CPE for 72 hours and supernatant cytokines were measured by ELISA. Data are shown as Means ± SEM of pooled cultures measured in triplicate (N=8–10 mice /group). ***, p<0.001 vs Sham;
B-FAHF-2 safety profile
No mortality or morbidity was observed 24 hrs after feeding twelve times the daily dose of B-FAHF-2 used for the PNA study, and no signs of altered physical appearance or activity over a two week observation period were detected. Blood cell counts and serum liver/kidney function test results were all within the normal range two weeks after feeding (Table. 2). Histological examination of tissue samples from the heart, lung, liver, kidney, stomach and spleen from B-FAHF-2 mice showed no unusual findings (data not shown). In addition, B-FAHF-2-fed mice in the PNA study, showed no signs of altered physical appearance or activity over a 65 week observation period.
Table 2. Summary of complete blood count testing and biochemical assays.
Mice were euthanized with CO2, and blood was collected by cardiac puncture 2 weeks following feeding B-FAHF-2 (144 mg, 12 times effective mouse daily dose) or water. Biochemical assays and CBC testing were conducted as described in Methods. Results are mean ± SD of 5–10 mice from each group.
| B-FAHF-2, N=10 | Water, N=5 | Reference range | |
|---|---|---|---|
| ALT, U/L | 30.0 8.60 | 27 4.7 | 22–400 |
| AST, U/L | 75.0 22.84 | 80.4 21 | 24–320 |
| BUN, mg/dL | 25.2 5.51 | 21.6 2.5 | 9–36 |
| WBC, 10−3/dL | 4.5 0.70 | 3.84 1.3 | 1.8–10.7 |
| RBC, 10−6/dL | 9.13 0.32 | 8.7 0.8 | 6.36–9.42 |
| Hemoglobin, g/dL | 14.7 1.24 | 14.1 1.2 | 11.0–15.1 |
| Platelets | 808.1 135 | 728.1 174 | 592–2972 |
| Total Protein, g/dL | 6.09 0.84 | 5.4 0.57 | 5.9–6.9 |
| Albumin, g/DL | 2.82 0.56 | 2.52 0.50 | 2.4–4.4 |
| Creatine, mg/dL | 0.28 0.06 | 0.3 0 | 0.4–1.0 |
| Bilirubin, mg/dL | 0.22 0.10 | 0.3 0.2 | 0.2–0.5 |
| Alb/Glob ratio | 0.83 0.09 | 0.86 0.15 | 0.6–1.2 |
| Globulin, g/dL | 3.3 0.31 | 3.0 0.21 | 2.1–4.3 |
WBC = White Blood Cell; RBC= red blood cells; ALT=alanine aminotransferase (ALT), AST=aspirate aminotransferase; BUN=blood urea nitrogen.
B-FAHF-2 directly inhibited PN-specific Th2 cells, B cells and mast cells in vitro
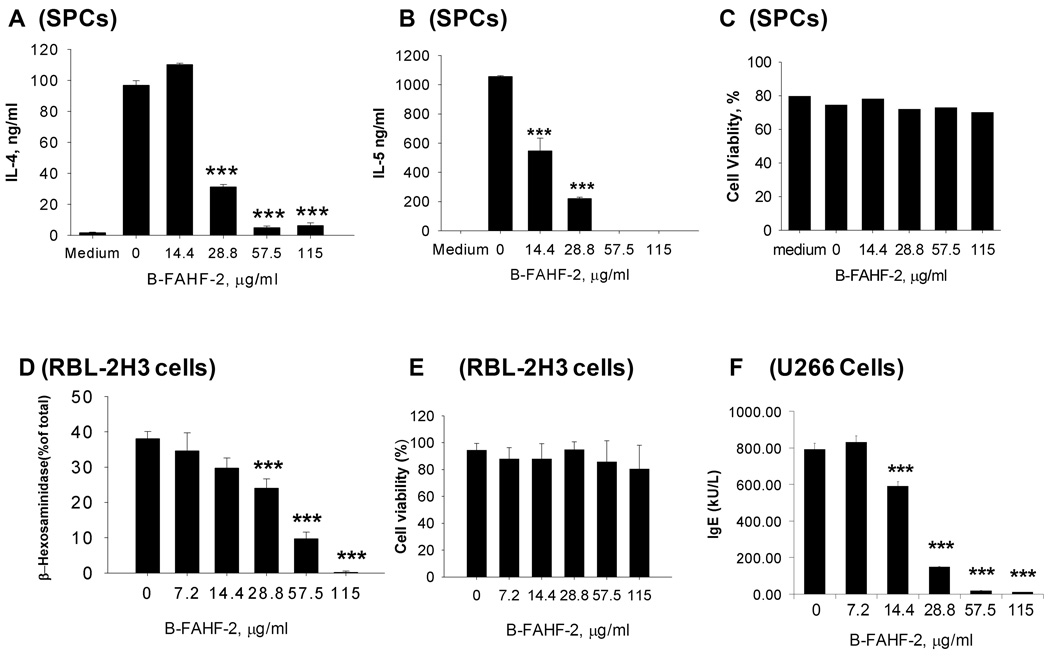
B-FAHF-2 produced dose-dependent suppression of PN-induced IL-4 (Figure 6A) and IL-5 (Figure 6B) production by PN-polarized SPCs, dose-dependent inhibition of mast cell degranulation as evidenced by significant reduction of percent β-hexosaminidase release by RBL-2H3 cells (Figure 6D), and dose-dependent inhibition of IgE production by human B cells (Figure 6F). No cytotoxicity was observed in PN-primed SPC (Figure 6 C), RBL 2H3 cells (Figure 6 E) or B cells (data not shown) at any dose tested.
Figure 6. Effect of B-FAHF-2 on effector cells in vitro.
Effects of B-FAHF-2 on IL-4 (A) and IL-5(B) production by PN-primed SPCs were measured by ELISA. Cell viability (C) was assessed by Trypan Blue exclusion. Effect of B-FAHF-2 on degranulation of RBL 2H3 cells (D) was measured by β-hexoseaminidase release and cell viability (E) was assessed as above. Effect of B-FAHF-2 on IgE production by U266 cells (F) was measured by ImmunoCap. Data shown as Mean ± SD of triplicate cultures. ***, p<0.001 vs untreated
DISCUSSION
Growing concern over the increased incidence and prevalence of PNA in children in westernized countries has intensified attempts to find novel and long lasting treatments. We previously showed that FAHF-2 treatment is effective, safe, and has persistent effects in a murine model of PNA [9–11]. Although our phase I study found no safety concerns, the high tablet load makes patient adherence very difficult during chronic treatment, especially by children. In this study, we used butanol purification to generate the refined product B-FAHF-2, which was about 20% of the weight of FAHF-2. To test efficacy, we conducted an in vivo study using an established murine model of PNA. We showed that immediately following a single 7-week treatment course, PNA/BAHF-2 mice were completely protected against anaphylaxis. If tolerance is defined as unresponsiveness to peanut exposure without ongoing treatment [7] all B-FAHF-2-treated PNA mice, but no sham-treated PNA mice, developed full tolerance that persisted for up to 34 wk post-treatment and partial tolerance for up to 50 weeks following a single course of treatment. A 2nd course of treatment restored complete tolerance. PNA is considered to be an “incurable condition”. Although B-FAHF-2 did not cure PNA, it did induce long-term tolerance. Two treatments produced tolerance to PN challenge for half of the mouse life span. Furthermore, there was sustained suppression of IgE levels following B-FAHF-2 therapy despite additional peanut challenges. Such long lasting protection has not been demonstrated by other approaches.
Peanut allergy is a type I hypersensitivity disorder. Th2 cells, B cells and mast cells/basophils are the key cells involved in the immunopathological mechanisms. In this study B-FAHF-2 produced sustained suppression of histamine release following repeated peanut challenges, as well as sustained suppression of PN-specific IgE and increased PN IgG2a levels, which was associated with up-regulation of IFN-γ and down-regulation of Th2 cytokines. Our previous study showed that administration of anti-IFN-γ antibody during FAHF-2 treatment significantly attenuated FAHF-2-mediated PN-specific IgE and Th2 cytokine production, but did not affect FAHF-2-mediated clinical protection 4 wks following therapy, suggesting that IFN-γ is an important, but not the sole mechanism underlying FAHF-2 long-term protection. In this study, we provided direct evidence that, in vitro, B-FAHF-2 directly suppressed PN-primed Th2 cell, B cell and mast cell activities, suggesting that multiple mechanisms underlie B-FAHF-2’s clinical effects. Further studies are required to elucidate the precise mechanisms responsible for B-FAHF-2 modulation of Th1/Th2 and effector cells.
In conclusion, we demonstrated an effective approach to improve the potency of a complex herbal formula product by using butanol extraction. B-FAHF-2 markedly reduced the effective daily dose in a murine model of PNA as compared to the aqueous extract- FAHF-2, while retaining an excellent efficacy and safety profile. Of particular importance is that the therapeutic effect is long lasting, treatment did not generate resistance and B-FAHF-2 acts on multiple effector cells involved in food allergy mechanisms. B-FAHF-2 may prove to be an effective, safe and convenient therapy for peanut and other food allergies. Although, animal models are not identical to human PNA, this preclinical study suggests that clinical investigation of B-FAHF-2 in peanut-allergic patients, including children, is warranted.
Acknowledgements
This work was supported by the Food Allergy Initiative, Winston Wolkoff Integrative Medicine for Allergy and Immunology Foundation, and by National Institutes of Health grant # 1 R01AT001495-01A1 and 2R01AT001495-05A1 awarded to Dr X-M Li.
Abbreviations
- Ab
antibody
- B-FAHF-2
butanol-extracted aqueous FAHF-2
- CAM
Complementary and Alternative Medicine
- CPE
crude peanut extract
- CT
cholera toxin
- FAHF-2
Food Allergy Herbal Formula -2PNA: peanut allergy
- i.g.
intragastrically
- IND
investigational new drug
- MLN
mesenteric lymph node
- OIT
oral immunotherapy
- PN
peanut
- SP
spleen
- SPC (s)
splenocyte (s)
- SLIT
sublingual immunotherapy
- TCM
Traditional Chinese Medicine
- Wk (s)
week or weeks
Footnotes
Author Disclosure Statement
Kamal D Srivastava, MPhil, Nan Yang, PhD, Yuming Chen, PhD, Ivan Lopez-Exposito, PhD, Ying Song, MD, Joseph Goldfarb, PhD, Jixun Zhan, PhD have no competing financial interests to disclose. Hugh A. Sampson MD and Xiu-Min Li MD hold US Patent PCT/US 05/08600 on FAHF-2, and have shares of Herbal Springs, LLC, which acquires and distributes herbal products
References
- 1.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010 doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–1207. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 3.Leung DY, Sampson HA, Yunginger JW, Burks AWJ, Schneider LC, Wortel CH, Davis FM, Hyun JD, Shanahan WRJ. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007;120:491–503. doi: 10.1016/j.jaci.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, Shreffler WG, Steele P, Henry KA, Adair M, Francis JM, Durham S, Vickery BP, Zhong X, Burks AW. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. doi: 10.1016/j.jaci.2009.05.022. 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rolinck-Werninghaus C, Staden U, Mehl A, Hamelmann E, Beyer K, Niggemann B. Specific oral tolerance induction with food in children: transient or persistent effect on food allergy? Allergy. 2005;60:1320–1322. doi: 10.1111/j.1398-9995.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- 7.Thyagarajan A, Varshney P, Jones SM, Sicherer S, Wood R, Vickery BP, Sampson H, Burks AW. Peanut oral immunotherapy is not ready for clinical use. J Allergy Clin Immunol. 2010;126:31–32. doi: 10.1016/j.jaci.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2003;111:S540–S547. doi: 10.1067/mai.2003.134. [DOI] [PubMed] [Google Scholar]
- 9.Qu C, Srivastava K, Ko J, Zhang TF, Sampson HA, Li XM. Induction of tolerance after establishment of peanut allergy by the food allergy herbal formula-2 is associated with up-regulation of interferon-gamma. Clin Exp Allergy. 2007;37:846–855. doi: 10.1111/j.1365-2222.2007.02718.x. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, Goldfarb J, Sampson HA, Li XM. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115:171–178. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009;123:443–451. doi: 10.1016/j.jaci.2008.12.1107. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Patil S, Yang N, Ko J, Lee J, Noone S, Sampson H, Li X-M. Safety, tolerability, and immunologic effects of a food allergy herbal formula (FAHF-2) in food allergic individuals: a randomized, double-blinded, placebo-controlled, dose escalation phase I study. Ann Allergy Asthma Immunol. 2010;105:75–84. doi: 10.1016/j.anai.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kattan JD, Srivastava KD, Zou ZM, Goldfarb J, Sampson HA, Li XM. Pharmacological and immunological effects of individual herbs in the Food Allergy Herbal Formula-2 (FAHF-2) on peanut allergy. Phytother Res. 2008;22:651–659. doi: 10.1002/ptr.2357. [DOI] [PubMed] [Google Scholar]
- 14.Xao CH, Lu XR. Chemistry of Traditional Chinese Medicine: Text Book for School of Medicine or Pharmocology. 447 Edn. Shanghai: Shanghai Science and Technolegy Publisher; 1999. [Google Scholar]
- 15.Srivastava K, Qu C, Zhang T, Goldfarb J, Sampson H, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009;123:443–451. doi: 10.1016/j.jaci.2008.12.1107. [DOI] [PubMed] [Google Scholar]
- 16.Burks AW, Williams LW, Connaughton C, Cockrell G, O'Brien TJ, Helm RM. Identification and characterization of a second major peanut allergen, Ara h II, with use of the sera of patients with atopic dermatitis and positive peanut challenge. J Allergy Clin Immunol. 1992;90:962–969. doi: 10.1016/0091-6749(92)90469-i. [DOI] [PubMed] [Google Scholar]
- 17.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, Stanley JS, Burks AW, Bannon GA, Sampson HA. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106:150–158. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 18.Beyer K, Morrow E, Li XM, Bardina L, Bannon GA, Burks AW, Sampson HA. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001;107:1077–1081. doi: 10.1067/mai.2001.115480. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Laboratory Animal Resources Commission of Life Sciences NRC. Guide for the Care and Use of Laboratory Animals. National Academy Press; 1996. [Google Scholar]
- 20.The State Pharmacopoeia Commission of The People's Republic of China. Pharmacopoeia of the People's Republic of China. English Edn. Beijing: Chemical Industry Press; 2005. [Google Scholar]
- 21.Srivastava KD, Zhang TF, Qu C, Sampson HA, Li XM. Silencing Peanut Allergy: A Chinese Herbal Formula, FAHF-2, Completely Blocks Peanut-induced Anaphylaxis for up to 6 Months Following Therapy in a Murine Model Of Peanut Allergy. J Allergy Clin Immunol. 2006;117:S328. (Abstract) [Google Scholar]
- 22.Passante E, Ehrhardt C, Sheridan H, Frankish N. RBL-2H3 cells are an imprecise model for mast cell mediator release. Inflamm Res. 2009;58:611–618. doi: 10.1007/s00011-009-0028-4. [DOI] [PubMed] [Google Scholar]
- 23.Kim HM, Lee JH, Won JH, Park EJ, Chae HJ, Kim HR, Kim CH, Baek SH. Inhibitory effect on immunoglobulin E production in vivo and in vitro by Siegesbeckia glabrescens. Phytother Res. 2001;15:572–576. doi: 10.1002/ptr.749. [DOI] [PubMed] [Google Scholar]