Abstract
Aims
This study used Ecological Momentary Assessment (EMA) data from smokers trying to quit to assess relations among coping, positive affect, negative affect, and smoking. The effects of stress coping on affect and smoking were examined.
Design
Data from a randomized clinical trial of smoking cessation treatments were submitted to multilevel modeling to test the effects of coping with stressful events on subsequent affect and smoking.
Participants
372 adult, daily smokers who reported at least one stressful event and coping episode and provided post-quit data.
Measurements
Participants’ smoking, coping, and affect were assessed in near real time with multiple EMA reports using electronic diaries pre-and post-quit.
Findings
Multilevel models indicated that a single coping episode did not predict a change in smoking risk over the next 4 or 48 hours, but coping in men was associated with concurrent reports of increased smoking. Coping predicted improved positive and negative affect reported within 4 hours of coping, but these affective gains did not predict reduced likelihood of later smoking. Pre-quit coping frequency and gender moderated post-quit stress coping relations with later positive affect. Men and those with greater pre-quit coping frequency reported greater gains in positive affect following post-quit coping.
Conclusions
Coping responses early in a quit attempt may help smokers trying to quit feel better but may not help them stay smoke-free.
Keywords: smoking cessation, stress, coping, affect, ecological momentary assessment, multilevel modeling
Introduction
Smoking remains the leading preventable cause of illness and death [1–2] and relapse remains the most common outcome of cessation attempts [3–4]. Stress and associated affective distress contribute to cessation failure and relapse [5–8]. Shiffman [9] found that most smokers reported that lapses were preceded by stress, a result replicated in other studies [10–11]. Similarly, within-subjects studies show that a third or more of smokers report that they lapsed during stressful events (see [12] for a review). However, Shiffman’s[9] retrospective study indicated that stressful experiences may be precursors to highly tempting situations, but not necessarily to lapses or relapses. In the Shiffman study, the use of coping to deal with relapse crisis was the only predictor of temptation outcome; any coping predicted greater likelihood of successful resolution of the temptation without smoking. Later studies found additional evidence that coping is closely associated with temptation outcomes [13–15].
Smoking cessation treatment programs were quick to integrate such findings and typically aim to promote coping in response to smoking temptations and warn would-be quitters about high-risk situations [3, 16]. For instance, the Public Health Service Clinical Practice Guideline Treating Tobacco Use and Dependence [3] recommends practical counseling that focuses on teaching problem-solving skills, such as cognitive coping strategies to regulate negative mood. Indeed, substantial evidence supports a positive relation between coping and successful resolution of temptations or relapses crises [7, 13–15, 17, 18]. For instance, a retrospective study [13] suggested that the number of temptation-coping strategies used positively predicted abstinence. Similarly, a community intervention trial [19] demonstrated that 7-day point prevalence abstinence at the six-month follow-up was associated with the average number of temptation-coping strategies used rather than the average number of temptations reported.
Although much research supports the consensus that stressful events and coping with temptations predict distal cessation outcomes, the effects of stress coping on affect and lapse vulnerability in the short-term remain unknown. Research has focused mainly on the effects of coping with temptations to smoke rather than coping with stressful events. The conceptual framework proposed by Wills and Shiffman [20] assumes a distinction between stress-coping, responses intended to deal with general life stressors, and temptation coping responses specific to temptations for substance use, and argued that stress coping and temptation coping make independent contributions to substance use. Affect is hypothesized to be a potent motivator of drug use [21] and frequent target of coping (i.e., emotion-focused coping) [22], and is therefore a candidate mediator of coping effects on smoking. Most studies have focused on negative affect, but not positive affect. Negative reinforcement models identify anhedonia (e.g., lack of positive affect) as an aversive state that prompts drug use [21]. Sustained or increased positive affect may indicate that this aversive state has been avoided or eliminated, and in turn, predicts abstinence.
Thus, the extent to which coping effectively improves affect, by either alleviating distress or increasing positive affect, has not been explored as a possible mediator of coping effects on abstinence. Previous research on coping has mainly studied post-quit coping and no studies have yet examined the role of pre-quit coping experience in cessation efforts. Pre-quit stress coping experience may help to automate coping so that it is less demanding and more effective, much like practice can make complex behaviors such as driving nearly effortless.
Moreover, most of the studies discussed above relied heavily on retrospective self-reports and between-subjects analyses. Stone et al. [23] cogently demonstrated that the correspondence between retrospective and EMA assessments was low; in retrospective assessments, cognitive coping was underreported while behavioral coping was over-reported, relative to EMA reports. Also, when coping efforts are assessed once per subject, the results from between-subjects comparisons only indicate differences between people who use a certain coping style and those who do not. Such differences may reflect stable individual differences rather than the effects of coping per se. Within-subject designs can disentangle individual differences and situation-specific coping effects. Such studies have yielded discrepant results [18, 24].
In the current study, we attempted to examine how coping with stress was related to subsequent affect and smoking status. We predicted that stress coping-smoking relations would depend on: (1) the efficacy of coping (as measured by affect improvement), and (2) prior coping experience (as measured by frequency of pre-quit coping with stress). Coping efficacy was assessed by decreases in negative affect and increases in positive affect. We also sought to explore gender differences in coping effects as a possible explanation for lower smoking cessation rates in women than men [25, 26]. Past research has shown that men and women tend to differ in terms of: coping likelihood and style, success in quitting [25, 26, 27], and how smoking risk is affected by stress [28]. In this study, we explored whether gender moderated coping effects on later affect and smoking.
In this study, EMA data were used to determine whether coping with stress predicted change in the likelihood of a smoking lapse over 48 hours, relative to stressful events subjects did not cope with. We predicted that coping with stress would protect against smoking lapses and would improve affect (i.e., decrease negative and increase positive affect) over 4 hours. We further hypothesized that the relation between coping (vs. no coping) and both later lapse and affect would be moderated by the frequency of stress coping pre-quit, such that those who reported more coping pre-quit would have improved affect and decreased lapse risk following coping post-quit. We also predicted that the relation between stress coping and subsequent lapses would be mediated by intervening changes in affect.
Method
Participants
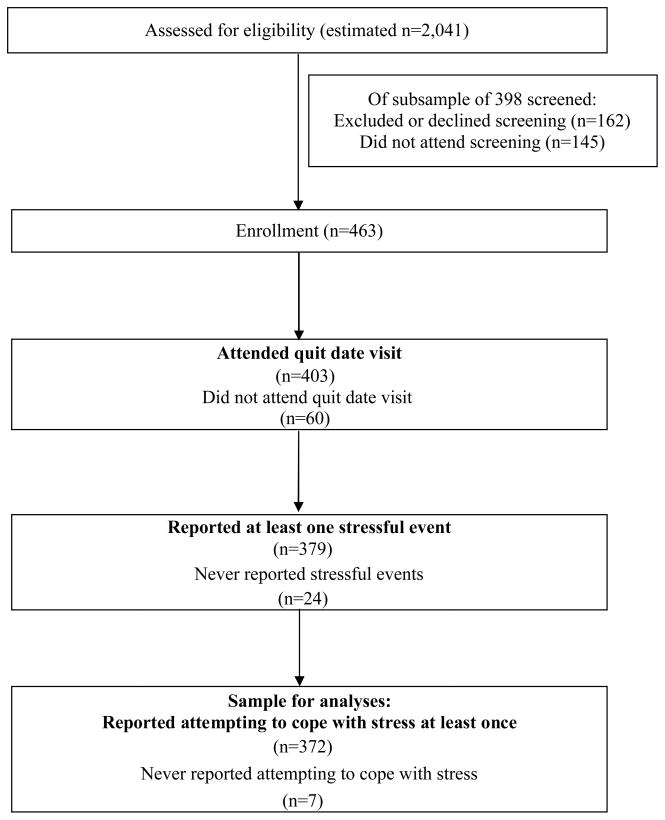
Data in the current study were collected for a double-blind, randomized, placebo-controlled clinical trial of bupropion SR and individual counseling for smoking cessation [29]. Participants were daily smokers (age 18 years and older) recruited in the Madison, Wisconsin area via mass media. Inclusion/exclusion criteria are shown in Table 1. A total of 463 participants enrolled. Of these, 372 (80.3%) attended the quit day visit and reported at least one stressful event and one stress-coping effort (Figure 1). Demographic characteristics of the subjects are summarized in Table 2. Subjects retained for analyses were equally distributed across treatment conditions χ2(3, N = 463) = 2.10, p = .552 and those dropped from the present analyses (due to attrition or lack of stress or coping reports) did not differ from those retained in terms of age, minority status, years smoking, CO level, or number of past quit attempts (all ps > .05). The dropped and retained samples differed in terms of cigarettes smoked per day, gender, and post-quit stressful event frequency, as shown in Table 2. The actual sample sizes for the analyses varied from 346 to 347 due to missing data.
Table 1.
Inclusion/exclusion criteria.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| 18 years of age or greater | History of bipolar disorder or psychosis |
| Able to read and write English | diagnosis or treatment |
| At least fairly motivated to quit smoking | Current depression (CES-D score over 16)* |
| Willing to fulfill study requirements | Current illegal drug use |
| Smoking at least 10 cigarettes per day | Use of other tobacco products in last 7 days |
| Baseline CO level of at least 10 parts per million | Current use of stop-smoking treatments |
| Participation in a study in the past 30 days | |
| Living with someone enrolled in the study | |
| Uncontrolled hypertension | |
| Current heavy drinking | |
| History of seizure | |
| Past negative reactions to bupropion, | |
| Pregnancy | |
| Breast feeding |
Except when an interview with a licensed clinical psychologist suggested another cause for elevated scores (such as an anxiety disorder, sleep disorder, or pain disorder associated with elevated ratings on specific relevant symptoms).
Figure 1.
Participation flow diagram depicting the number of subjects excluded from analyses due to drop out prior to the quit day, the absence of any reports of stressful events, and the absence of any reports of stress coping. The number of people screened for enrollment was estimated assuming a 22.86% enrollment rate based on two cohorts for which data were available, as data were not available for all cohorts.
Table 2.
Demographic characteristics of final sample and differences in subsample of subjects who were retained (N=372) vs. excluded (N=91) from current analyses.
| Variable | Value | Retained Cases (N=372) | Excluded Cases (N=91) | χ2 | p |
|---|---|---|---|---|---|
| Sex (N=372) | Female | 198 (53.2%) | 35(38.5%) | 6.38 | .012* |
| Race/Ethnicity | Hispanic | 4 (1.1%) | 1 (1.1%) | .984 | |
| White | 332 | 80 (87.9%) | 5.02 | .285 | |
| African-American | 22 (5.9%) | 4 (4.4%) | |||
| Asian, Pacific Islander | 3 (0.8%) | 1 (1.1%) | |||
| American Indian | 1 (0.3%) | 2 (2.2%) | |||
| Other | 11 (3.0%) | 4 (4.4%) | |||
| Marital Status | Married | 162 (43.5%) | 36 (43.5%) | 1.18 | .946 |
| Divorced | 69 (18.5%) | 16 (17.6%) | |||
| Never married | 91 (24.5%) | 26 (28. 6%) | |||
| Cohabitating | 34 (9.1%) | 10 (11.0%) | |||
| Separated | 8 (2.2%) | 2 (2.2%) | |||
| Widowed | 6 (1.6%) | 1 (1.1%) | |||
| Education | < High school | 13 (3.5%) | 7 (7.7%) | 9.27 | .055 |
| High school graduate | 77 (20.7%) | 27(29.7%) | |||
| Some college | 184 (49.5%) | 40(44.0%) | |||
| College degree | 96 (25.8%) | 17 (18.7%) | |||
| Employment Status | Employed for wages | 273 (73.4%) | 50 (54.9%) | 11.70 | .111 |
| Self-employed | 36 (9.7%) | 8 (8.8%) | |||
| Unemployed <1 year | 18 (4.8%) | 7 (7.7%) | |||
| Homemaker | 17 (4.6%) | 1 (1.1%) | |||
| Student | 8 (2.2%) | 4 (4.4%) | |||
| Retired | 9 (2.4%) | 5 (5.5%) | |||
| Disabled | 6 (1.6%) | 4 (4.4%) | |||
| Household Income | < $25,000 | 105 (28.9%) | 36 (39.6%) | 9.95 | .127 |
| $25,00-$34,999 | 57 (15.3%) | 13 (14.2%) | |||
| $35,000-$49,999 | 71 (19.1%) | 17 (18.7%) | |||
| $50,000-$74.999 | 76 (20.4%) | 13 (14.2%) | |||
| >$75.000 | 55 (14.5%) | 11 (12.1%) | |||
| M (SD) | M (SD) | t or χ2 | p | ||
| Age | 38.94 (11.94) | 38.00 (13.06) | −.663 | .507 | |
| Cigarettes smoked per day | 21.44 (10.26) | 23.95 (10.79) | 2.06 | .040* | |
| Previous quit attempts | 6.19 (11.45) | 4.92 (4.68) | −.959 | .338 | |
| Baseline CO level | 24.54 (11.74) | 24.84 (10.80) | .211 | .833 | |
| Baseline FTND Score | 4.98 (2.33) | 5.63 (2.46) | 2.31 | .021* | |
| Years smoking | 21.19 (11.31) | 20.43 (12.09) | −.571 | .569 | |
| Pre-quit stressful event count | 9.06 (9.33) | 6.87 (9.72) | −1.84 | .067 | |
Note: May not sum to 372 (retained cases) or 91 (excluded cases) due to missing data.
Measures
Baseline Assessment
Participants provided demographic information (see Table 2), baseline depressive symptoms using the Center for Epidemiologic Studies-Depression scale (CES-D) [30], smoking history, and completed the Fagerström Test of Nicotine Dependence (FTND [31]), Wisconsin Smoking Withdrawal Scale (WSWS [32]), the Positive and Negative Affect Schedule (PANAS [33]), and the Negative Emotionality Scale (NES [34]).
Ecological Momentary Assessment
EMA data were collected via Electronic Diaries (EDs; Palm Vx Palmtop Computer, Palm, Inc., Santa Clara, CA) programmed by in vivo data Inc. (Pittsburgh, PA) to administer four to seven two-minute momentary reports (random reports) at pseudo-random times separated by at least 30-minute intervals daily for two weeks pre- and four weeks post-quit.
Random reports assessed the occurrence of stressful events (yes/no) since the last report, and, if so, whether participants tried to cope with stress (yes/no). Subjects were instructed to endorse the stress item when they felt subjectively stressed by an event, regardless of the magnitude of the precipitating event (i.e., there was no formal, a priori definition of a stressful event). The stress coping item was displayed only when stressful events were reported (which occurred on 8% of reports). In addition, the number of cigarettes smoked since the last report (0–20) was assessed and subjects rated affect and withdrawal just before the prompt on an 11-point scale ranging from 1 (No!!) to 11 (Yes!!). Two highly correlated positive affect P-PANAS items (“interested” and “enthusiastic”) (r=.83 [29]) were averaged to yield a positive affect summary score. The average of the items “tense or anxious” and “sad or depressed” (r = .45) was used as a measure of momentary negative affect. Positive and negative affect scores were negligibly related (β=−0.01, p=0.012, df=34,123).
Procedures
Study procedures are described in detail elsewhere [29]. Subjects were randomized to one of four conditions resulting from the full crossing of medication (9-weeks of bupropion SR vs. placebo) and counseling (8–10-minute counseling sessions vs. no counseling), attended a total of 13 study visits, carried EDs for 6 weeks, and received monthly follow-up phone calls through 1-year post-quit. Compensation for participation did not exceed $200.
Data Analysis
Data were analyzed using Hierarchical Linear Modeling (HLM) Version 6.04 software [35]. Models predicting smoking within 48 hours of an index episode and predicting affect within 4 hours of an index report were run. The following person-level covariates were tested in all models but were pruned if not significantly related to occasion-level coefficients: medication and counseling conditions; gender (1=female, 0=male); age; baseline FTND; P-PANAS (for positive affect models); N-PANAS and NES scores (for negative affect models); and initial cessation success vs. failure (1=quit smoking for at least 24 hours, 0=failed to quit on quit day). Pre-quit coping count was included as a moderator of coping-affect and coping-smoking relations. All models controlled for any smoking 48 hours before the index report (1=smoked at least once, 0=no smoking). As such, the models predicting smoking within 48 hours of an index report capture predictors of change in smoking status (i.e., lapse) from 48 hours before to 48 hours after the report.
Results
Smoking Risk Over 48 Hours
Results from Bernoulli models predicting smoking status 48 hours after an index report are shown in Table 3 (top panel). The trimmed model treats only the intercept reflecting the mean probability of smoking in the absence of stress and coping as random (reliability=.84) because allowing additional parameters to vary resulted in non-convergence. Smoking was modeled as a function of stress and coping at an index report (t0; treated as binary) and during the 48 hours following an index report (the same period smoking was assessed; with stress treated as a count variable and coping treated as binary). Results indicated that those taking bupropion (vs. placebo) and those who abstained on the quit day were less likely to smoke over 48-hours, whereas older subjects and women were more likely to smoke. A single episode of stress or stress coping had no relation with smoking 48 hours later overall, but the accumulation of stress and any coping over the next 48 hours were both positively related to concurrent smoking. The positive relation between concurrent stress coping and smoking was stronger for men than for women. Smoking in the 48 hours before the index report positively predicted smoking in the next 48 hours, particularly for men.
Table 3.
Trimmed multilevel model of stress, stress coping, and affect effects on smoking risk over 48 hours.
| Predictor Level-1 | Predictor Level-2 | Coefficient | Standard Error | T-ratio | Odds Ratio | 95% CI | df | p-value |
|---|---|---|---|---|---|---|---|---|
|
Coping-lapse relations | ||||||||
| Mean P (Smoking) 48 hrs after indexa | − 0.880 | 0.387 | − 2.276 | 0.415 | (0.194,0.886) | 342 | 0.023* | |
| Active bupropion SR | − 1.171 | 0.360 | − 3.251 | 0.310 | (0.153,0.629) | 342 | 0.002* | |
| Age | 0.047 | 0.015 | 3.173 | 1.048 | (1.018,1.080) | 342 | 0.002* | |
| Gender | 0.980 | 0.361 | 2.716 | 2.664 | (1.312,5.411) | 342 | 0.007* | |
| Quit at least 24 hours | − 3.141 | 0.374 | − 8.399 | 0.043 | (0.021,0.090) | 342 | 0.000* | |
| Index Stressful Event (Y/N) (48 hrs earlier) | − 0.266 | 0.168 | − 1.578 | 0.767 | (0.551,1.066) | 33,560 | 0.114 | |
| Index Stress Coping (Y/N) (48 hrs earlier) | 0.078 | 0.208 | 0.373 | 1.081 | (0.719,1.624) | 33,560 | 0.709 | |
| Smoking (Y/N) (48 hrs before index) | 0.890 | 0.085 | 10.447 | 2.434 | (2.060,2.876) | 33,560 | 0.000* | |
| Gender | − 0.316 | 0.103 | −3.055 | 0.729 | (0.596,0.893) | 33,560 | 0.003* | |
| Stressful Events (Count; in 48 hrs following index) | 0.318 | 0.027 | 11.585 | 1.375 | (1.303,1.451) | 33,560 | 0.000* | |
| Stress Coping (Y/N) (48 hrs after index) | 0.548 | 0.123 | 4.461 | 1.730 | (1.360,2.201) | 33,560 | 0.000* | |
| Gender | − 0.434 | 0.120 | − 3.623 | 0.648 | (0.512,0.819) | 33,560 | 0.001* | |
|
Affect-lapse relations | ||||||||
| Negative Affect (At index) | − 0.004 | 0.021 | −0.211 | 0.996 | (0.955, 1.038) | 21,271 | 0.839 | |
| Negative Affect (Within 4 hrs of index) | − 0.015 | 0.021 | −0.730 | 0.985 | (0.945, 1.026) | 21,271 | 0.465 | |
| Positive Affect (At index) | − 0.014 | 0.019 | − 0.754 | 0.986 | (0.950, 1.023) | 21,271 | 0.451 | |
| Positive Affect (Within 4 hrs of index) | − 0.026 | 0.018 | − 1.424 | 0.974 | (0.940, 1.010) | 21,271 | 0.155 | |
Random coefficient, reliability = .826. All other predictors were treated as fixed to facilitate model convergence.
p<.05
Separate models in which earlier levels of negative and positive affect were added to the predictors listed above were run (Table 3, bottom panel). Affect at the time of the index report and four hours after the index report (after stress and coping) were included. Neither positive nor negative affect was significantly predictive of smoking status within 44–48 hours.
We also tested models in which the dependent variable was any smoking reported within four hours of the index report. These models also failed to detect relations between stress coping and later smoking. Results are presented in the supplementary material online.
Affect within 4 Hours
To test the efficacy of coping in improving affect, we ran the models shown in Tables 4 and 5. Separate models predicted positive and negative affect at the next report within four hours of an index report to assess whether coping was associated with changes in affect. Models controlled for the level of affect at the index report, smoking in the 48 hours preceding an index report, and the person-level covariates listed above (if significantly related to model coefficients). Models also examined relations between recent smoking, stress, and stress coping reported at the same time as affect to examine concurrent relations between these variables.
Table 4.
Trimmed model of negative affect within 4 hours of an index report.
| Predictor Level-1 | Predictor Level-2 | Coefficient | Standard Error | T-ratio | df | P-value |
|---|---|---|---|---|---|---|
| Mean Negative Affect Rating 4 hrs after index a | 3.151 | 0.175 | 17.975 | 339 | 0.000* | |
| Active bupropion SR | 0.293 | 0.214 | 1.368 | 339 | 0.179 | |
| Counseling | 0.244 | 0.210 | 1.165 | 339 | 0.245 | |
| Active bupropion SR X Counseling | − 0.833 | 0.299 | − 2.786 | 339 | 0.006* | |
| FTND | 0.077 | 0.032 | 2.395 | 339 | 0.017* | |
| Negative PANAS | 0.034 | 0.014 | 2.457 | 339 | 0.015* | |
| NES | 0.116 | 0.024 | 4.832 | 339 | 0.000* | |
| Quit at least 24 hours | − 0.027 | 0.165 | − 0.166 | 339 | 0.868 | |
| Index Negative Affectb (4 hrs earlier) | 0.309 | 0.014 | 22.296 | 346 | 0.000* | |
| Index Stressful Event (Y/N) (4 hrs earlier) | 0.135 | 0.074 | 1.827 | 21,522 | 0.067 | |
| Index Stress-Coping (Y/N) (4 hrs earlier) | − 0.250 | 0.089 | − 2.799 | 21,522 | 0.006* | |
| Smoking (Y/N) (48 hrs before index) | 0.063 | 0.042 | 1.513 | 21,522 | 0.130 | |
| Quit at least 24 hours | − 0.170 | 0.058 | − 2.919 | 21,522 | 0.004* | |
| Smoking (Y/N) (contemporaneous) | − 0.018 | 0.034 | − 0.547 | 21,522 | 0.584 | |
| Stressful Eventc (Y/N)(contemporaneous) | 1.009 | 0.136 | 7.437 | 345 | 0.000* | |
| Gender | 0.411 | 0.146 | 2.806 | 345 | 0.006* | |
| Stress-Coping (YN) (contemporaneous) | − 0.239 | 0.128 | − 1.870 | 21,522 | 0.061 |
Random coefficient, Reliability=.980,
Random coefficient, Reliability = 0.644, Random coefficient, Reliability = 0.679.
All other predictors were treated as fixed to facilitate model convergence.
p<.05
Table 5.
Trimmed model of positive affect within 4 hours of an index report.
| Predictor Level-1 | Level-2 | Coefficient | Standard Error | T-ratio | df | P-value |
|---|---|---|---|---|---|---|
| Mean Positive Affect Rating 4 hrs after index a | 7.457 | 0.183 | 40.848 | 343 | 0.000* | |
| Age | 0.045 | 0.009 | 5.171 | 343 | 0.000* | |
| Positive PANAS | 0.095 | 0.015 | 6.386 | 343 | 0.000* | |
| Quit at least 24 hours | − 0.229 | 0.219 | − 1.041 | 343 | 0.299 | |
| Index Positive Affectb (Y/N) (4 hrs earlier) | 0.295 | 0.015 | 19.298 | 346 | 0.000* | |
| Index Stressful Event (Y/N) (4 hrs earlier) | − 0.172 | 0.090 | − 1.923 | 21,528 | 0.054 | |
| Index Stress-Coping (Y/N) (4 hrs earlier) | 0.298 | 0.124 | 2.402 | 21,528 | 0.016* | |
| Gender | − 0.262 | 0.092 | − 2.843 | 21,528 | 0.005* | |
| Pre-quit Stressful Events | − 0.071 | 0.027 | − 2.660 | 21,528 | 0.008* | |
| Pre-quit Stress-Coping Efforts | 0.072 | 0.028 | 2.540 | 21,528 | 0.011* | |
| Smoking (Y/N) (48 hrs before index) | − 0.028 | 0.036 | − 0.777 | 21,528 | 0.437 | |
| Smoking (Y/N) (contemporaneous) | 0.027 | 0.042 | 0.638 | 21,528 | 0.523 | |
| Stressful Eventc (Y/N) (contemporaneous) | − 0.558 | 0.127 | − 4.385 | 346 | 0.000* | |
| Stress-Coping (Y/N) (contemporaneous) | 0.185 | 0.148 | 1.251 | 21,528 | 0.211 | |
| Pre-quit Stressful Events | − 0.093 | 0.039 | − 2.391 | 21,528 | 0.017* | |
| Pre-quit Stress-Coping Efforts | 0.117 | 0.043 | 2.705 | 21,528 | 0.007* |
Random coefficient, Reliability= .986,
Reliability= .651,
Reliability= 0.607. All other predictors were treated as fixed to facilitate model convergence.
p<.05
Negative affect results indicated that those who received the combination of active (vs. placebo) bupropion SR and active counseling (vs. assessment control) and those who quit successfully for 24 hours reported lower negative affect, whereas those who had higher FTND, N-PANAS, and NES scores reported higher negative affect overall, in the absence of stress, coping, and smoking. Stressful events were marginally related to increased negative affect at the next report, but stress coping was significantly related to decreases in negative affect, controlling for negative affect at the index report. Stressful events reported at the same time as negative affect were positively related to negative affect, especially for women. Concurrently reported stress coping was marginally associated with lower negative affect. Smoking in the 48 hours before an index report was significantly related to reduced negative affect four hours post-index for those who quit successfully, but not for those who failed to quit. Recent smoking was not significantly related to negative affect.
Positive affect results indicated that older subjects and those with higher P-PANAS baseline scores had higher positive affect in the absence of stress, coping, and smoking post-quit. Stressful events at the index report were marginally predictive of reduced positive affect within four hours, whereas stress coping was predictive of improved positive affect, at least for men and those with more frequent coping prior to quitting. Those with more pre-quit stress reported less positive affect benefit from an index coping effort. Positive affect was also negatively related to concurrently reported stressful events, but positively related to concurrently reported stress coping for those with more practice coping pre-quit. Stress coping was less positively related to concurrently reported positive affect among those with more pre-quit stressful events, however. Positive affect was not significantly related to smoking in the 48 hours before the index report or in the four hours between the index report and rating of positive affect.
Coping Strategy Analyses
See supplementary material online for the results for the exploratory analyses of relations between specific coping strategies (i.e., cognitive, behavioral, and acceptance-based coping) and later affect and smoking.
Discussion
The purpose of this study was to use EMA data to test the hypothesis that stress coping improves affect and decreases smoking during a quit attempt. A secondary aim was to determine whether individual differences in prior coping moderated such relations. Results indicated that stress coping did not protect against smoking during a quit attempt. Instead, any stress coping in a 48 hour period was associated with increased risk of smoking during the same 48 hour period, particularly for men. Results also showed that coping was associated with short-term improvements in affect. Negative affect was lower within four hours of coping with stress, and positive affect was higher after coping, particularly in men and those who coped more frequently prior to quitting. So, despite the fact that there was some evidence that coping was effective (i.e., it appeared to have intended affective consequences), neither coping nor these affective gains were protective against smoking over the next 44–48 hours.
The finding that contemporaneous stress coping was associated with a greater likelihood of smoking, after controlling for any smoking in the past 48 hours, runs counter to our hypothesis. It is important to note that we did not detect a link between a single, index episode of stress coping and smoking in the short term (4 hours) or longer term (48 hours), but found instead that contemporaneous reports of coping and smoking were positively related. Because coping and smoking were assessed in the same 48 hour period, it is possible that smoking preceded coping (i.e., that smoking triggered coping efforts rather than the reverse) or that smoking prompted recall of coping efforts. The gender difference observed in this coping-lapse relation may, therefore, reflect a gender difference in smoking effects on coping or recall of coping. On the other hand, the results may indicate that men experience a greater increase in the likelihood of smoking after attempting to cope with stress than do women. Perhaps this reflects gender differences in thresholds for coping (that men engage in active coping for only the most stressful events that are likely to increase smoking risk, whereas women cope with less intense risk). This may also reflect gender differences in coping efficacy, although men seemed to benefit more from coping in terms of positive affect than did women and benefitted equally in terms of negative affect. Alternatively, this gender difference may reflect differences in expectancies about what coping will do to smoking urges. Research suggests that men are more motivated to smoke by nicotine effects than women, whereas women are more motivated by non-nicotine (e.g., sensory) effects than men [25, 36]. Perhaps this leads men to view stress coping as unlikely to reduce urges to smoke or withdrawal, and they are especially likely therefore to turn to smoking per se to achieve these effects.
Although stress coping did not protect against smoking, such coping predicted improved mood. Negative affect was lower within four hours of a coping episode, compared with non-coping episodes (controlling for stressful event occurrence). This effect was not moderated by cessation status on the quit day, gender, or pre-quit stress coping frequency. Positive affect improvements within four hours of coping, however, were greater for those who coped more often prior to quitting (i.e., those more practiced in coping), as anticipated, and for men. Men may experience a greater boost in positive affect following active coping because men may be socialized to use more active and instrumental coping strategies than are women; this type of coping may be more social-role congruent for men than women [27]. Stress coping was less effective in boosting positive affect among those who reported stressful events more frequently prior to quit controlling for pre-quit coping frequency, however. This suggests that those with the greatest stress burden pre-quit, perhaps due to chronic stressors or persistent hassles, benefit less from stress coping bouts post-quit.
Short-term improvements in affect related to stress coping did not reduce lapse risk over the next 44–48 hours after controlling for additional stressful events encountered during that period. This is consistent with past research indicating no relation between negative affect and ad lib smoking in non-treatment seeking smokers [37, 38]. The time frame used to detect affect-lapse relations may have been suboptimal, as past research suggests that negative affect influences first lapses over the course of hours rather than days [5]. This time interval may be too long to detect such effects, but it is important to note that coping did not reduce smoking risk in even a four-hour window in this study (see supplementary material). In any event, stress coping appears to improve affect more than it protects against a return to smoking during a quit attempt.
Limitations
Results from this study may have been influenced by sampling bias, measurement problems, and the fact that we did not manipulate the causal variables of interest (e.g., coping). The sample retained for analyses may differ from the general population of smokers trying to quit. Although reports were randomly prompted to minimize sampling biases, missing reports may be associated with certain situations or states of interest (e.g., severe stress or demanding coping). In addition, although past research supports the validity and reliability of brief EMA affect measures, measures of coping and stress occurrence are less well validated [39]. Assessments were brief to reduce subject burden, and so did not assess subjective coping efficacy, the severity or chronicity of stressful events, or the events that triggered smoking episodes. Past research has found that negative affect predicts smoking only when the smoking is triggered by stress [40]. If we limited analyses to smoking following stress, we might similarly find a relation with negative affect. The fact that our data replicated some known relations (e.g., the relation between any past smoking and increased risk of future smoking; the relations between stressful events and affect and smoking; bupropion SR reduction of smoking risk; relations among baseline affect and momentary affect [3, 9, 41, 42] suggests that our measures were somewhat valid and reliable, however. Finally, stress coping might, in theory, work via other means than affective change (e.g., altered self-efficacy). However, this would not change the fact that such coping was not related to smoking in the current data set.
Conclusions
Results of the present study of relations among coping, affect, and smoking suggest that stress coping in the early phases of a quit attempt may be more effective in improving affect than in reducing smoking risk, and may even increase smoking. Additional analyses should address the extent to which stress (vs. temptation events) moderates relations between coping and later smoking and may identify the types of coping that are effective and should be promoted in treatment.
Supplementary Material
Acknowledgments
This work was supported by Transdisciplinary Tobacco Use Research Center grants P50CA084724 from the National Cancer Institute and P50 DA019706 from the National Institute of Drug Abuse, and by grant NCI 1K05CA139871 (Baker).
GlaxoSmithKline provided complimentary active and placebo medication used in this study. GlaxoSmithKline was not involved in the design, data collection, analysis, or reporting of this study.
Footnotes
Conflict of Interest Statement
Douglas E. Jorenby has received research support from Nabi Biopharmaceutical and Pfizer, Inc. and consulting fees from Nabi Biopharmaceutical. Timothy B. Baker has served as a consultant, given lectures sponsored by, or has conducted research sponsored by GlaxoSmithKline, Nabi Biopharmaceuticals, Pfizer, and Sanofi-Synthelabo.
References
- 1.Centers for Disease Control and Prevention. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses—United Statesm 1997–2001. Morbidity and Mortality Weekly Report. 2002;51(14):300–303. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Health United States, 2003, With Chartbook on Trends in the Health of Americans. (PDF–225KB) Hyattsville, MD: CDC, National Center for Health Statistics; 2003. [Google Scholar]
- 3.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville, MD: U.S.: Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 4.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 5.Shiffman S. Dynamic influences on smoking relapse process. J Pers. 2005;73(6):1–34. doi: 10.1111/j.0022-3506.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 6.Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, et al. J Abnorm Psychol. 1997;106(1):104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- 7.Shiffman S, Gnys M, Richards TJ, Paty JA, Hickcox M, Kassel JD. Temptations to smoke after quitting: A comparison of lapsers and maintainers. Health Psychol. 1996;15(6):455–461. doi: 10.1037//0278-6133.15.6.455. [DOI] [PubMed] [Google Scholar]
- 8.Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, et al. J Abnorm Psychol. 2002;111(4):531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- 9.Shiffman S. Relapse following smoking cessation: A situational analysis. J Consult Clin Psych. 1982;50(1):71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Marlatt GA, Gordon JR. Determinants of relapse: Implications for the maintenance of behavior change. In: Davidson PO, Davidson SM, editors. Behavioral medicine: Changing health lifestyles. New York: Bunner/Mazel; 1980. [Google Scholar]
- 11.O’Connell KA, Martin EJ. Highly tempting situations associated with abstinence, temporary lapse, and relapse among participants in smoking cessation programs. J Consult Clin Psych. 1987;55(3):367–371. doi: 10.1037//0022-006x.55.3.367. [DOI] [PubMed] [Google Scholar]
- 12.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129(2):270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 13.Bliss RE, Garvey AJ, Heinold JW, Hitchcock JL. The influence of situation and coping on relapse crisis outcomes after smoking cessation. J Consult Clin Psych. 1989;57(3):443–449. doi: 10.1037//0022-006x.57.3.443. [DOI] [PubMed] [Google Scholar]
- 14.Curry SG, Marlatt GA. Unaided quitters’ strategies for coping with temptations to smoke. In: Shiffman S, Wills TA, editors. Coping and substance use. New York: Academic Press; 1985. pp. 243–265. [Google Scholar]
- 15.Shiffman S. Coping with temptations to smoke. J Consult Clin Psychol. 1984;52(2):261–267. doi: 10.1037//0022-006x.52.2.261. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein E, Glasgow RE. Smoking cessation: What have we learned over the past decade? J Consult Clin Psychol. 1992;60(4):518–527. doi: 10.1037//0022-006x.60.4.518. [DOI] [PubMed] [Google Scholar]
- 17.Katz RC, Singh NN. Reflections on the ex-smoker: Some findings on successful quitters. J Behav Med. 1986;9(2):191–202. doi: 10.1007/BF00848477. [DOI] [PubMed] [Google Scholar]
- 18.Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. J Consult Clin Psych. 1996;64(2):366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- 19.Stoffelmayr B, Wadland W, Pan W. An examination of the process of relapse prevention therapy designed to aid smoking cessation. Addict Behav. 2003;28(7):1351–1358. doi: 10.1016/s0306-4603(02)00250-2. [DOI] [PubMed] [Google Scholar]
- 20.Wills TA, Shiffman S. Coping and substance use: A conceptual framework. In: Shiffman S, Wills TA, editors. Coping and substance use. San Diego, CA: Academic Press; 1985. pp. 3–24. [Google Scholar]
- 21.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111(1):33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 22.Folkman S, Lazarus RS. Coping as a mediator of emotion. J Pers Soc Psychol. 1988;54(3):466–75. [PubMed] [Google Scholar]
- 23.Stone AA, Schwartz JE, Neale JM, Shiffman S, Marco CA, Hickcox M, et al. J Per Soc Psychol. 1998;74:1670–1680. doi: 10.1037//0022-3514.74.6.1670. [DOI] [PubMed] [Google Scholar]
- 24.O’Connell KA, Hosein VL, Schwartz JE, Leibowitz RQ. How does coping help people resist lapses during smoking cessation? Health Psychol. 2007;26(1):77–84. doi: 10.1037/0278-6133.26.1.77. [DOI] [PubMed] [Google Scholar]
- 25.Perkins KA. Sex differences in nicotine vs. non-nicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharmacol. 1996;4(2):166–177. [Google Scholar]
- 26.Wetter D, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consul Clin Psychol. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- 27.Ptacek JT, Smith RE, Zanas J. Gender, appraisal, and coping: A longitudinal analysis. J Pers. 1992;60:747–770. [Google Scholar]
- 28.McKee SA, Maciejewski PK, Falba T, Mazure CM. Sex differences in the effects of stressful life events on changes in smoking status. Addiction. 2003;98(6):847–855. doi: 10.1046/j.1360-0443.2003.00408.x. [DOI] [PubMed] [Google Scholar]
- 29.McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine Tob Res. 2008;10(4):717–729. doi: 10.1080/14622200801968343. [DOI] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 31.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. Brit J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 32.Welsch SK, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Development and validation of the Wisconsin Smoking Withdrawal Scale. Exp Clin Psychopharmacol. 1999;7(4):354–361. doi: 10.1037//1064-1297.7.4.354. [DOI] [PubMed] [Google Scholar]
- 33.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Per Soc Psychol. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 34.Tellegen A. Unpublished manuscript. University of Minnesota; Minneapolis: 1982. Brief manual for the Multidimensional Personality Questionnaire. [Google Scholar]
- 35.Raudenbush S, Bryk A, Congdon R. HLM for Windows (Version 6.04) Lincolnwood IL: Scientific Software International; 2007. [Google Scholar]
- 36.Perkins KA, Gerlach D, Vender J, Grobe J, Meeker J, Hutchison S. Sex differences in the subjective and reinforcing effects of visual and olfactory cigarette smoke stimuli. Nicotine Tob Res. 2001;3(2):141–50. doi: 10.1080/14622200110043059. [DOI] [PubMed] [Google Scholar]
- 37.Shiffman S, Gwaltney CJ, Balabanis M, Liu KS, Paty JA, Kassel JD, et al. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–54. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- 38.Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: An analysis of unrestricted smoking patterns. J Abnorm Psycol. 2004;113:166–171. doi: 10.1037/0021-843X.113.1.166. [DOI] [PubMed] [Google Scholar]
- 39.Folkman S, Moskowitz JT. Coping: Pitfalls and promise. Annu Rev Psychol. 2004;55:745–774. doi: 10.1146/annurev.psych.55.090902.141456. [DOI] [PubMed] [Google Scholar]
- 40.Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- 41.Westman EC, Behm FM, Simel DL, Rose JE. Smoking Behavior on the First Day of a Quit Attempt Predicts Long-term Abstinence. Arch Intern Med. 1997;157:335–340. [PubMed] [Google Scholar]
- 42.Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev. 2003;22:203–220. doi: 10.1080/09595230100100642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.