Abstract
Background
Cognitive impairments are frequently observed in clients who enter treatment programs for substance abuse. The potential for early recovery of cognitive abilities is suggested by previous research; however, the extent of improvement and risk factors that may help predict individual differences in rates of recovery remain unclear. This study is a 6-week follow-up and retest of an original sample of 197 men and women who had received a broad neuropsychological assessment at addiction treatment entry. The aim was to examine the potential clinical significance of changes in cognitive functioning and the extent to which differential recovery was predictable from client background information.
Methods
Fifteen neuropsychological tests were readministered to 169 of 197 clients 6 weeks after treatment entry. Structural equation modeling was used to estimate separately the practice effects and recovery in four cognitive domains: executive function, memory, information processing speed, and verbal ability. Client background information included age, sex, education, substance use and consequences, psychopathology, medical problems, familial alcoholism history, and childhood behavior problems.
Results
A four-factor model of latent neuropsychological ability that was previously identified at treatment entry was replicated at follow-up. Statistically significant increases in the means of the four latent abilities were found. Memory showed a medium effect size improvement. Executive function, verbal ability, and information processing speed, however, showed only small effect size improvements, suggesting limited clinical significance. Substance use between treatment entry and follow-up, antisocial personality disorder, negative use consequences, less education, and medical problems were modestly predictive of less recovery.
Conclusion
Cognitive recovery in the first 6 weeks of treatment is possible, but, with the possible exception of memory, improvement may be minor in terms of clinical relevance.
Keywords: cognitive impairment, cognitive recovery, individual differences
INTRODUCTION
The negative impact of chronic, excessive alcohol and drug use on brain structure and function is supported by an abundance of neuropsychological and neuroimaging data (e. g. Rourke and Loberg, 1996; Volkow et al., 2003). Although recovery from alcohol- and drug-induced deficits is frequently observed, the extent and the rate of cognitive recovery are highly variable. Whereas some recovery occurs in a spontaneous manner as physical and neurological health improve through detoxification and abstinence (Goldman, 1995; Pfefferbaum et al., 1995), additional neuropsychological and behavioral improvements may be dependent on external factors, such as cognitive remediation (Forsberg and Goldman, 1987; Roehrich and Goldman, 1993). Moreover, the extent of recovery that does occur spontaneously is thought to be affected by client characteristics, such as age (Forsberg and Goldman, 1985). Accordingly, identification of demographic, behavioral, and psychological characteristics that have an impact on the time course of cognitive recovery could be useful to treatment providers by facilitating the development of a treatment plan that maximizes a client’s potential for positive substance use outcomes.
Cognitive improvements are commonly observed immediately after the acute withdrawal stages (Goldman, 1995). Further improvements, as defined by statistically significant increases in neuropsychological test performance, within a few weeks of entry into a treatment program have been reported (e.g. Goldman et al., 1983; Page and Linden, 1974), however, the clinical significance (i.e., how these improvements relate to changes in day-to-day functioning) of these positive changes in cognitive ability is unclear. The aim of this study was to assess the extent of early cognitive recovery in men and women who entered treatment for alcohol and drug use disorders and to examine whether demographic, behavioral, and psychological characteristics predicted individual differences in rates of recovery. Data for this study are from a 6-week follow-up and retest of clients who had been assessed previously 1 week after they entered treatment (after the acute withdrawal phase) (Bates et al., 2002b). We previously reported risk factors that explained 34 to 57% of the true variance in latent neuropsychological abilities of executive function, memory, verbal ability, and receptive verbal processing speed in this sample at treatment entry (Bates et al., 2002b). Age, education, and medical status had robust and generalized associations with ability levels, whereas diagnoses of drug use disorders, childhood behavior problems, familial alcoholism, and psychopathology showed unique relations to specific latent abilities.
Although similar covariates of cognitive status have been consistently identified in other samples of individuals with alcohol and/or drug use disorders at treatment entry (Adams and Grant, 1986; Fein et al., 1990; Grant et al., 1978; Rourke and Loberg, 1996; Tarter and Edwards, 1987)evidence is conflicting regarding the extent to which factors such as age (Brandt et al., 1983; Ellenberg et al., 1980; Goldman et al., 1983; Munro et al., 2000) and family history of alcohol use disorders (Drake et al., 1995; Schafer et al., 1991) limit cognitive recovery. With age, for example, it is unclear whether the poorer cognitive performance observed in older alcoholics is restricted to specific domains of cognition such as memory (Munro et al., 2000) or visuospatial skills (Ellenberg et al., 1980), whether the rate of recovery varies with age (Goldman et al., 1983), and whether the poorer performance of older clients is more related to the effects of aging on the brain than the effects of alcohol use (Brandt et al., 1983). Even the impact of abstinence on cognitive recovery is not entirely straightforward. Whereas some studies report reversibility of neuropsychological and structural alterations as a result of abstinence (e.g. Adams et al., 1980; Carlen et al., 1978; Muuronen et al., 1989; Parsons et al., 1990), others found little association between length of abstinence and recovery (e.g. Brandt et al., 1983; Donovan et al., 1984; Medina et al., 2004). It should be noted, however, that these studies vary greatly in the length of time between initial testing and follow-up, as well as in their definitions of abstinence. Moreover, the role of abstinence in recovery is further complicated by the finding that abstinence may be of more importance for alcoholics who have family histories of alcoholism than for those who do not (Drake et al., 1995).
These equivocal results point to the complexity of defining cognitive impairment and recovery in relation to treatment for substance use disorders. The problem is compounded by the wide range of neuropsychological tests that are given to clients, as well as the timing of the initial and follow-up testing, the statistical approach to data analysis, and the nature of the research question under investigation. The size and the heterogeneity of the sample, the use of multiple drugs, diagnoses of multiple use disorders, and differences in primary drugs of abuse between clients further add to the complexity of evaluating cognitive recovery in most populations. Isolating neuropsychological recovery from practice effects (as a result of repeated exposure to the same testing materials) and determining the clinical significance of improvements are further research challenges. In a recent study of executive function recovery 15 months after treatment entry, statistically significant mean increases in executive ability over time had small effect sizes in two samples of individuals with alcohol use disorders, suggesting limited clinical significance of mean improvements (Bates et al., 2004). However, a combination of risk factors in direct and mediated pathways did predict between one quarter and one third of the variance (individual differences) in recovery. These results suggest that it would be clinically informative to examine the extent and predictors of short-term recovery in multiple, treatment-relevant neuropsychological domains.
In the present study, we used baseline cognitive abilities and risk factors associated with client demographics, physical and psychological status, and substance use to predict changes in abilities over 6 weeks. The contribution of practice effects to improvements in ability levels was estimated by comparing changes in the intercepts of individual neuropsychological tests. Recovery within the domains of executive function, memory, verbal ability, and information processing speed was estimated by changes in the means of underlying latent ability factors. The degree to which statistically significant observations of improvement might represent clinically relevant recovery was evaluated by calculating the effect sizes of mean changes. We hypothesized statistically significant mean increases in the latent abilities in the 6 weeks after treatment entry but anticipated that the clinical impact of these changes may be modest in view of the fact that the treatment programs from which participants were recruited were not designed to rehabilitate cognitive impairments (Goldman, 1995).
METHODS
Participants
Participants were 118 men and 79 women who volunteered to participate in a multi-site research program to assess the efficacy of addiction treatments and have been previously described in detail (Bates et al., 2002b). Briefly, participants were recruited from four treatment facilities, including two private, hospital-based chemical-dependency treatment programs that offer residential or intensive day treatment; an intensive day treatment program for older adults with alcohol problems; and an urban medical center, where clients were recruited by screening the medical records of inpatients or through self-report screening instruments that were administered to outpatients who sought care for medical problems. Exclusion criteria were history of organic brain dysfunction, Korsakoff’s syndrome or severe dementia, psychotic disorder, serious medical problems that precluded testing, methadone maintenance treatment, inability to read test materials, and age less than 18 years. The mean Full-Scale WAIS-R IQ score estimated from the Shipley Institute of Living Scale (Zachary, 1986) was 105.75 (SD = 13.19). Demographic characteristics of the participants are shown in Table 1. All participants met DSM-III-R criteria for a current psychoactive substance use disorder. Details of participants’ substance use are outlined in Table 2.
Table 1.
Demographic Characteristics of the Participants.
| Characteristic | Value |
|---|---|
| N | 197 |
| Age in years (range) | 43.25 + 17.01a (18–81) |
| Education in years (range) | 12.94 ± 2.71 a (6–20) |
| Sex (% men) | 60 |
| Race/Ethnicity (%) | |
| White | 65 |
| African American | 26 |
| Hispanic/Latino | 5 |
| Other | 4 |
| Employed (%) | 50 |
| Married or living as married (%) | 46 |
| Medical Problemsc | |
| None | 54 (29.8%) |
| 1 | 42 (23.2%) |
| 2 | 43 (23.8%) |
| 3 | 27 (14.9%) |
| 4+ | 15 (8.3%) |
Mean ±Standard Deviation
Measured for 6 months prior to treatment entry
The number of reported Medical Problems was computed from the physical health portion of the Life Experiences and Social Resources Inventory (Moos and Moos, 1992). Patients were asked whether they had experienced any of the following during the year before treatment: stroke, diabetes, back problems, high blood pressure, anemia arthritis, diseases of the liver, heart or kidney, or hospitalization.
Table 2.
Substance Use Characteristics of the Participants
| Characteristic | Value |
|---|---|
| Use disorder diagnosis | |
| Alcohol only | 127 (64.47%) |
| Drug onlya | 33 (16.75%) |
| Alcohol & drug | 37 (18.78%) |
| Drinks per drinking dayb | 10.72 ± 10.18 |
| Percent of drinking daysb | 48.72 ± 35.65 |
| Percent of drug daysb, c | 19.41 ± 32.19 |
| Drug used | |
| Cocaine | 58 (83%) |
| Opiate | 23 (33%) |
| Marijuana | 18 (26%) |
| Abstinence at 6-week Follow-up | |
| Alcohol Only | 71/102 (69.9%) |
| Drug Only | 19/25 (76.0%) |
| Alcohol & Drug | 21/27 (77.8%) |
| Days abstinent prior to treatment | |
| Mean ± Standard Deviation | 18.9 ± 32.8 |
| Range | 0–184 |
All individuals exhibiting a drug use disorder received a diagnosis of either multiple drug use disorders or concurrent alcohol use disorders except for 17 persons with cocaine use disorder only, 6 with an opiate use disorder only and 1 with a marijuana use disorder only.
Measured for 6 months before treatment entry
These data include all participants, regardless of use disorder diagnosis
There was no difference between drug only and drug and alcohol use disordered groups in the proportion of current cocaine, opiate or cannabis use disorder diagnoses (see Bates et al., 2002). Four participants received an additional diagnosis of sedative, stimulant or hallucinogen disorders, or some combination of these.
Measures
The fifteen neuropsychological tests that had been administered at treatment entry were again administered at the 6-week follow-up. The neuropsychological tests and the literature sources are shown in Table 3. Tests were selected on the basis of the following criteria: (1) sensitivity to deficit, (2) sensitivity to individual differences in risk covariates, (3) sensitivity to a range of severity, (4) potential to relate to treatment outcome, and (5) standardization and time efficiency for administration in multiple treatment settings. Tests were predominantly from well-known batteries that assess abilities that often are impaired in individuals with alcohol and drug use disorders: abstraction and executive functioning, memory, visuomotor skills, and complex verbal abilities (Knight and Longmore, 1994; Nixon, 1995). These tests have been shown to have excellent psychometric properties and be highly sensitive in discriminating individuals with brain damage from normal controls [e.g., Trail Making, Booklet Category, Stroop, Digit Symbol Substitution, California Verbal Learning (Lezak, 1995; Spreen and Strauss, 1998)]. A small number of less well-known tests were included to enhance ecological validity of the memory assessment [Product Recall Task (Sussman et al., 1986)] and measure receptive verbal information processing speed [Active Passive Voice Test (Dennis and Kohn, 1985)]. Performance on the included tests has been conceptually (Bates and Convit, 1999; Goldman, 1983) and/or empirically (Sanchez- Craig and Walker, 1982) related to the acquisition of new information and skills during treatment.
Table 3.
Performance on Component Tests of the Neuropsychological Assessment Battery at Treatment Entry and 6-Week Follow-up: Overall Means and Standard Deviations
| Neuropsychological test (source) | Score | TE Mean ± SD (N) | 6W Mean ± SD (N) |
|---|---|---|---|
| Shipley Institute of Living Scale, Vocabulary (Zachary, 1986) | # correct | 27.46 ± 6.64 (196) | 28.10 ± 6.27 (158) |
| Shipley Institute of Living Scale, Verbal Abstraction (Zachary, 1986) | # correct | 20.01 ± 10.14 (196) | 21.64 ± 9.99 (161) |
| Word Fluency (FAS) Test (Benton and Hamsher, 1976) | # correct | 34.71 ± 12.41 (195) | 37.67 ± 13.52 (162) |
| Active-Passive Voice Test, Negative Syntax (Dennis and Kohn, 1975) | time (seconds) | 3.75 ± 1.74 (193) | 3.34 ± 1.36 (158) |
| Active-Passive Voice Test, Affirmative Syntax (Dennis and Kohn, 1975) | time (seconds) | 2.73 ± 1.00 (193) | 2.51 ± 0.93 (158) |
| Booklet Category Testa (De Fillippis & McCampbell, 1991) | # errors | 72.29 ± 30.72 (196) | 58.49 ± 31.53 (155) |
| Stroop Color and Word Test (Golden, 1978) | # correct C-W | 34.90 ± 12.46 (162) | 39.43 ± 12.25 (135) |
| Wisconsin Card Sort (Nelson, 1976) | # perseveration errors | 5.56 ± 6.23 (161) | 5.01 ± 6.34 (132) |
| Digit Symbol Substitution Test (Kaplan et al., 1991) | # correct | 47.24 ± 15.71 (166) | 50.37 ± 16.15 (136) |
| Trail Making Test B, Halstead-Reitan Battery (Reitan and Wolfson, 1985) | time (seconds) | 101.30 ± 72.03 (195) | 91.66 ± 58.41 (160) |
| Trail Making Test A, Halstead-Reitan Battery (Reitan and Wolfson, 1985) | time (seconds) | 37.55 ± 21.92 (196) | 34.46 ± 18.95 (162) |
| California Verbal Learning Test, Long and short delayb (Delis et al., 1987) | # correctly recalled | 9.05 ± 3.45 (190) | 10.33 ± 3.42 (158) |
| Product Recall Test (Sussman et al., 1986) | # correctly recalled | 8.05 ± 3.35 (196) | 10.35 ± 2.79 (161) |
| Digit Symbol Substitution, Symbols recall (Kaplan et al., 1991) | # correctly recalled | 5.21 ± 2.84 (166) | 5.96 ± 2.79 (137) |
| Tower of Hanoi – 4 block (Simon, 1975) | time (seconds) | 124.99 ± 88.25 (166) | 88.88 ± 66.43 (136) |
The brief version of this test was used (Russell and Levy, 1987).
These data represent a combined mean from the highly correlated long and short delay trials of the CVLT. This mean was then used as a measure of verbal memory in the structural equation model.
TE = treatment entry, 6W = 6-week follow-up.
Risk factors were assessed with instruments that had adequate reliability and validity (Bates et al., 2002b). Briefly, psychopathology and substance use diagnoses were made using the Structured Clinical Interview of DSM-III-R for axis I and axis II diagnoses (Spitzer et al., 1990). A modified version of the physical health portion of the Life Experiences and Social Resources Inventory (Moos and Moos, 1992) assessed medical problems. Familial alcoholism history on the part of first-degree relatives was identified by a structured interview, the Family History Research Diagnostic Criteria (Andreasen et al., 1977). Symptoms and behaviors related to hyperactivity, impulsivity, learning disability, aggressiveness, and attention-deficit/hyperactivity disorder before the age of 12 were identified using a retrospective checklist developed by Tarter et al. (1977).
Alcohol and other drug use was measured for the 6 months before treatment entry and again for the 6 weeks between treatment entry and follow-up. The Timeline Follow-Back Interview (Sobell and Sobell, 1992), which was modified to assess both alcohol and drug use, measured the percentage of drinking days and percentage of drug use days. The Rutgers Consequence of Use Scale (Pople et al., 1994) measured the self-reported consequences of chronic alcohol and drug use in the areas of family and other social relationships, psychological and physical health, and legal situations. To assess the role of abstinence on cognitive recovery, percentage of drinking days and percentage of drug use days were combined into a single, dichotomous variable that indicated whether a participant had engaged in substance use behaviors between treatment entry and follow-up. A dichotomous rather than a continuous representation of substance use was used because of the extreme skew of substance use scores, with 75% of the participants reporting no use during the first 6 weeks of treatment. The validity of self-reported abstinence during treatment was supported by 88% agreement between reported substance use by the client and a knowledgeable collateral (kappa = 0.8) in a subset (n =118) of the present sample (Morgenstern and Bates, 1999).
Procedure
All testing was conducted in compliance with National Institutes of Health guidelines for the ethical treatment of human subjects and was approved by the Rutgers Institutional Review Board for the Protection of Human Subjects in Research. Neuropsychological evaluations were performed at all test sites by the same cadre of interviewers (usually pre-doctoral clinical psychology students) using highly standardized procedures. Extensive interviewer training included observation of videotaped training films, practice test administration with normal subjects and individuals with clinical diagnoses, and observation via a one-way mirror. Interviewers were re-evaluated every 6 months to prevent “drift.” Standardized test battery administration and test scoring manuals were developed, field-tested, and used by all interviewers. Participants provided informed consent and were individually retested 6 weeks after treatment entry (within a 1-week window). Each participant was administered the same neuropsychological battery that had been completed at treatment entry and the Timeline Follow-Back Interview. Zero blood alcohol concentration was confirmed by means of breath analysis before testing.
Data Analysis
Treatment entry and the 6-week follow-up data were used to examine (1) whether the latent structure of neuropsychological abilities identified at treatment entry was equivalent to that at 6 weeks after entry, (2) changes over 6 weeks in means of latent factor abilities and intercepts of manifest test scores, and (3) predictors of changes in abilities over time. To reduce bias as a result of attrition, Mplus (Muthén and Muthén, 1998) was used to estimate model parameters from raw data using a full information covariance matrix and a maximum likelihood approach with missing data assumed to be missing at random (Little and Rubin, 1987). Previously published confirmatory factor analysis of the treatment entry data yielded a four-factor model with discriminant and convergent validity (Bates et al., 2002b). It included latent constructs termed executive functioning, memory, verbal ability, and complex information processing speed. This model generally corresponded to an a priori hypothesized model.1
The equivalence of the latent structure of neuropsychological ability found at treatment entry versus that found at the 6-week follow-up was assessed by constraining the relevant measurement model parameters to be invariant over time and testing for significant increments in χ2 (indicative of model misfit) given these constraints. Practice effects were assumed to be test specific and based on an individual’s experience with a given task. Therefore, they were defined by significant increases in the intercepts of individual neuropsychological tests across time. Conversely, cognitive recovery was defined by significant increases in the means of the latent ability factors across time. Structural equation path modeling then was used to determine the amount of true variance in neuropsychological recovery that was predicted by the risk factors. Approximating from power tables developed by MacCallum et al. (1996), power was >0.955 (α = .05, n = 197) for rejecting the hypothesis of close fit and >0.870 for rejecting the hypothesis of not close fit based on 100 degrees of freedom.
RESULTS & DISCUSSION
Attrition Analyses
After the assessment completed at treatment entry, 14% (n = 28) of participants withdrew from the study. Dropouts and retained participants did not differ on baseline neuropsychological test scores, age, sex, education, alcohol and drug use problem severity, additional psychiatric diagnoses, medical conditions, childhood behavior problems, or type of treatment (all p > 0.05). Models that were estimated from full information covariance matrices with and without excluding the 28 dropouts yielded equivalent results. The present results are based on the full sample of 197 participants.
Practice Effects and Factor Invariance
One challenge of longitudinal studies involving repeated administrations of neuropsychological tests is to determine the relative extent to which changes in test performance over time represent substantive changes in brain function versus the influence of factors such as measurement unreliability or practice with specific tests. A related issue is whether the underlying abilities that supported performance during the first test administration are the same as or different from the abilities that were called upon when the tests were performed for a second time. Confirmatory factor analysis was used to examine these questions using data from the 15 neuropsychological tests (Table 3) that were given at treatment entry and 6 weeks later.
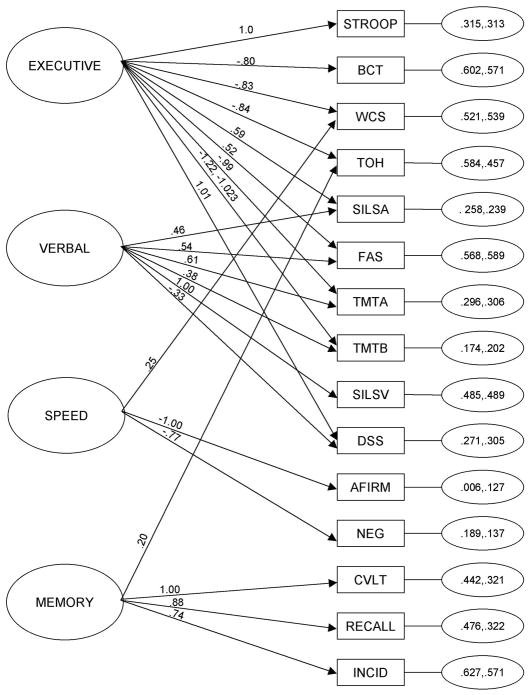
Factor loadings represent the degree to which the underlying ability factor supports performance on each test. They were constrained across the two assessment times to examine the extent to which individuals drew on the same underlying latent abilities to perform each test. Distinctions subsequently can be made between test performances that are improving on the basis of more efficient use of the same mental strategies from those that draw on different strategies. Constraining the factor pattern and all but one factor loading to be equivalent across time did not substantively change the model fit as compared with the unconstrained model (unconstrained model: χ2 = 469.925, df= 348, p=0.0000, RMSEA = 0.042, 90% CI = 0.032 – 0.052, SRMR = 0.040, CFI = 0.974, TLI = 0.968; constrained model: χ2 = 523.829, df= 372, p=0.0000, RMSEA = 0.046, 90% CI = 0.036 – 0.054, SRMR = 0.049, CFI = 0.968, TLI = 0.962)2. The one exception was that the Trail Making Test Part B loaded significantly more strongly on the executive ability factor at baseline (−1.220) than at follow-up (−1.023), suggesting that executive ability was slightly less involved in determining performance on this test during its second administration. That 14 of the 15 tests could have constrained factor loadings across 6 weeks without causing substantive model misfit points to the relative stability of the measurement model of neuropsychological abilities over time and suggests that participants were calling on essentially the same underlying abilities to complete the tests at both assessment times. The constrained model is shown in Fig. 1.
Figure 1. Constrained Measurement Model of 15 Neuropsychological Tests at Treatment Entry and 6 Weeks.
Latent factors are located on the left side of the diagram. We labeled the factors Executive, Speed, Verbal and Memory, however these names are not meant to encompass a complete definition of the specific abilities being drawn upon. Unstandardized factor loadings are shown on the arrows. For Trail Making Test, Part B (TMTB), the first listed loading is from the assessment at treatment entry. Residual variances for the indicators (treatment entry, 6-week follow up) are in the small ovals on the right side of the diagram. Neuropsychological tests, shown in squares, included: STROOP = Stroop Color and Word Test; BCT = Booklet Category Test; WCS = Wisconsin Card Sorting Test; TOH = Tower of Hanoi; SILSA = Shipley Institute of Living Scale, Abstraction; FAS = Word Fluency Test; TMTA = Trail Making Test, Part A; TMTB = Trail Making Test, Part B; SILSV = Shipley Institute of Living Scale, Vocabulary; DSS = Digit Symbol Substitution Test of the WAIS-R, NR; AFIRM = Affirmative Syntax from the Active-Passive Voice Test; NEG = Negative Syntax from the Active-Passive Voice Test; CVLT = California Verbal Learning Test; RECALL = Product Recall Test; INCID = Incidental Memory Test from the Digit Symbol Substitution Test of the WAIS-R, NR.
To the extent that practice effects are relatively test specific, the SEM approach allows practice effects to be partialled out from the clinically relevant improvement in latent abilities. This method of estimating practice effects is an alternative to the use of control groups, offsetting of initial testing and alterative versions of the tests (Goldman, 1995) and, although imperfect, more fully utilizes the strengths of the SEM technique. This is accomplished by examining the intercepts of manifest variables, which would be expected to increase as a result of changes associated with experience with specific tests rather than changes associated with underlying abilities. Constrained intercepts indicate that scores on individual tests fully co-vary with the means of the underlying latent factors. If intercepts cannot be constrained, then it raises the possibility that performance is being enhanced on these particular tests by unique practice effects or by recovery of an underlying ability other than the four represented in the model. In the present study, intercepts could not be constrained across time on 4 of the 15 neuropsychological tests—Product Recall, Stroop Color-Word, Booklet Category, and Tower of Hanoi—without significantly decreasing model fit (p < 0.05). Considering the short interval of time between tests, practice effects were not unusual on these executive functioning and memory tests (e.g., Spreen and Strauss, 1998).
Cognitive Improvements
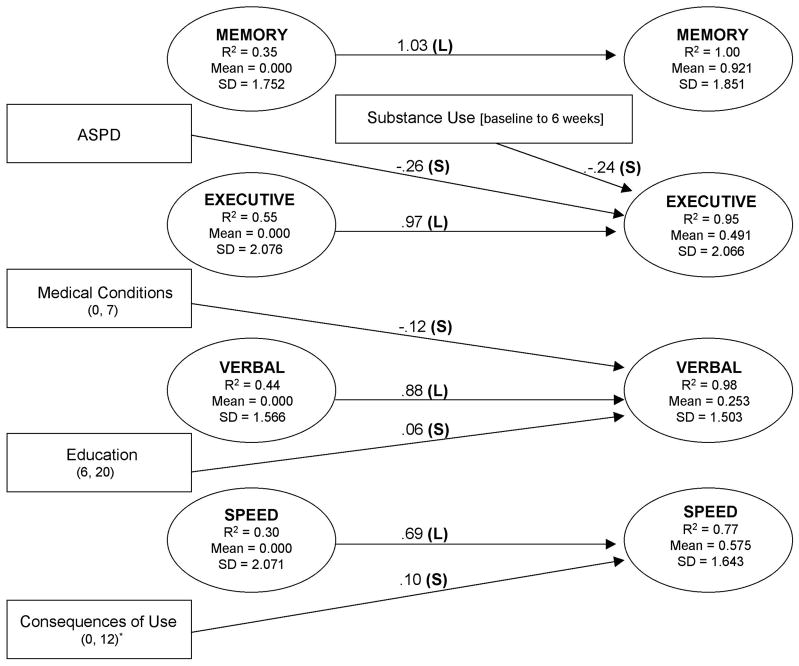
There were statistically significant (p < 0.05) mean improvements in the Executive (mean = 0.000, 0.491), Verbal (mean = 0.000, 0.253), Memory (mean = 0.000, 0.921), and Speed (mean = 0.000, 0.575) latent abilities across the 6 weeks (Fig. 2). These improvements in ability were distinct from practice effects or increases in performance on individual tests and are noteworthy in that they occurred after acute withdrawal but during the treatment phase of recovery. These increases in the means of latent factors are consistent with previous findings that improvements on individual neuropsychological tests occur during the first 4 to 6 weeks of treatment (for review, see Rourke and Loberg, 1996). However, these data alone do not clearly speak to the potential clinical relevance of cognitive improvements. It is possible that although statistically significant, recovery of this magnitude may not translate into functional improvements in clients’ ability to learn, retain, or use treatment-related information (e.g., Cohen, 1988; Jacobson et al., 1999; Rutledge and Loh, 2004). Therefore, we considered the likelihood that these increases in the latent ability means were of practical significance in terms of an effect size (ES) measurement (Finney et al., 1996; Ottenbacher and Barrett, 1989) that was calculated as SDs of change (Murphy and Myors, 2004). The greatest improvement was seen in the Memory factor (0.50 SD, medium effect size). Small effect size improvements were seen in the Speed, Executive, and Verbal factors (0.35, 0.24, and 0.17 SD change, respectively). Statistically significant yet small effect size increases likely represent relatively modest improvements from a clinical perspective (Cohen, 1988).
Figure 2.
Risk factor path model for latent factors at 6-week follow-up. The timeline of the measures proceeds from left to right: latent factors (ovals) and risk correlates (rectangles) assessed at treatment entry on the left, substance use between treatment entry and follow-up in the center, and latent factors identified at follow-up shown on the right. Only significant paths from risk factors to latent factors at follow-up are shown, with the unstandardized path coefficients on the arrows. For dichotomous variables, the unstandardized path coefficients represent the difference between the ability means of the two groups. For continuous variables, point ranges are shown in parentheses. The consequences of use scale was rescaled (divided by 3) to facilitate iterative estimation. R2 of the latent factors refers to the proportion of true variance in latent abilities accounted for by the risk covariates. Means and SDs of the latent factors are provided because the effect sizes of unstandardized path coefficients need to be interpreted with respect to the SDs of continuous variables. For the readers’ convenience, paths in which estimated ESs (unique proportion of variance explained) were <0.10 were coded as small (S), ES = 0.10 to 0.24 as medium (M), and ES = ≥0.25 as large (L) (Murphy and Myors, 2004).
Predictors of Individual Differences in Recovery
Although improvement in neuropsychological abilities was subtle at the level of the group mean, certain demographic, behavioral, and psychological client characteristics (termed “risk factors”) may be informative for predicting individual differences in extent of recovery. The likelihood of meaningful individual differences is supported by the relatively large SDs of the latent factors at treatment entry and follow-up (Fig. 2). To examine whether client characteristics predicted changes in abilities, independent of their association to baseline ability levels, we fit to the data a model that incorporated direct paths from the parallel factor at treatment entry and from each risk factor to the four latent factors at both treatment entry and 6 weeks. This model was sequentially simplified by eliminating small and nonsignificant paths. At each step in the test of the hierarchical models, the trimmed model was compared by the χ2 difference test with the preceding model (Kline, 1998). The model was no longer trimmed when elimination of the smallest path caused a significant increment in χ2. Although the χ2 was significant in the final model, all other fit indices exceeded the recommended cut-offs that indicate close fit to the data (Hu and Bentler, 1999; Yu and Muthén, 2002), with the 90% confidence interval for the RMSEA falling below 0.05 (χ2 = 986.628, df= 753, p=0.0000, RMSEA = 0.040, 90% CI = 0.032 – 0.046, SRMR = 0.054, CFI = 0.954, TLI = 0.950). Figure 2 shows the four-factor model, at treatment entry and 6-week follow-up, and the client characteristics that affect changes in performance at 6 weeks. A measure of effect size that estimates the proportion of variance uniquely accounted for by a given risk factor (Murphy and Myors, 2004) was computed for each significant path. For ease of interpretation, ES <0.10 were coded as small, ES = 0.10 to 0.24 as medium, and ES = ≥0.25 as large (Murphy and Myors, 2004).
Across time, the latent factors were highly correlated, indicating that executive, verbal, memory, and processing speed abilities were relatively stable across 6 weeks. The treatment entry latent factor scores explained the majority of the unique variance (62–88%) in the corresponding latent factors at follow-up. The addition of client characteristics measured at treatment entry and substance use during the intervening 6 weeks accounted for additional variance in latent ability at 6 weeks (R2 = 0.95 for Executive, R2 = 1.00 for Memory, R2 = 0.77 for Speed, and R2 = 0.98 for Verbal; Fig. 2) 3. Higher education and fewer medical conditions contributed to recovery of Verbal ability. Diagnosis of antisocial personality disorder and lack of abstinence from alcohol and drugs predicted less recovery of latent Executive ability at 6 weeks. An unpredicted path indicated that self-reported negative consequences of drug and alcohol use were associated with greater recovery on the Speed factor (Fig. 2), suggesting that clients who reported more severe consequences of use at entry experienced slightly enhanced recovery of information processing speed than others. The significance of this observation is unclear but may indicate a more complex relationship between these variables than could be determined from our model.
Drug use disorder diagnoses were related to decreased Speed and Verbal abilities at treatment entry in this sample (Bates et al., 2002b). However, neither single nor polydrug diagnoses, in place of or in addition to diagnosis of an alcohol use disorder, were associated with individual differences in the extent of recovery over 6 weeks. The lack of difference in cognitive recovery in different substance use disorders may be associated with the relatively small size of the drug-using group or that this group included users of a variety of drugs of abuse. The question of differential recovery linked to specific drug abuse deserves further attention in a larger treatment sample that can be more stringently differentiated on the basis of the profile of substances used.
In addition, although age was strongly associated with all four latent abilities at treatment entry, it did not directly predict differential short-term recovery of any cognitive ability when other risk factors were taken into account. A large body of literature supports the finding that older alcoholics display poorer neuropsychological abilities than younger alcoholics (for review, see Rourke and Loberg, 1996); however, the effect of age on recovery is less clear (Brandt et al., 1983; Ellenberg et al., 1980; Goldman et al., 1983; Munro et al., 2000). That in the present study age did not predict recovery argues that individuals with substance use disorders of all ages are capable of some degree of short-term cognitive recovery and that extent of recovery may be more linked to a combination of risk factors rather than to any one.
Abstinence had a modest effect on recovery of Executive functioning only. However, in this sample, the majority of clients reported abstinence (75%), thus diminishing its predictive utility at 6 weeks. Nonetheless, this finding was not surprising in light of the mixed reports regarding the role of abstinence on recovery (Brandt et al., 1983; Donovan et al., 1984; Medina et al., 2004; Muuronen et al., 1989). It may be that the effect of age and abstinence on the rate of recovery becomes evident only as time elapses (Adams et al., 1980; Reed and Grant, 1990) and greater variability in substance use ensues.
Overall, risk factors that significantly predicted recovery at 6 weeks had small, unique effect sizes, suggesting that individual client characteristics were of modest practical utility in that, independently, they are unlikely to be useful as markers for recovery potential. However, when considered in combination, risk factors did explain between 7 and 35% of the true variance in recovery of the four abilities across 6 weeks. Although beyond the scope of this article to review, large bodies of literature assess the impact of individual traits (e.g., abstinence, antisocial personality disorder) on cognitive impairment and recovery in individuals with substance use disorders. The contribution of the current study is that it measures the impact of multiple client features and the relative extent of both clinical and statistical significance, concurrently in the same individuals. The pattern of results seen in the present study suggests that development of a multidimensional risk profile may be of greater utility to treatment providers in estimating the extent of cognitive recovery that might be expected than consideration of client characteristics individually.
It is important to consider that the present study was limited by the moderate size of the sample and attrition at follow-up. Consideration of the size of effects in addition to their statistical significance, however, suggests that a larger sample would not have yielded substantively different results. The dichotomous variable of abstinence was limited in not distinguishing between frequent and infrequent, high- and low-quantity alcohol and drug use. In this respect, a larger sample would have allowed for a sufficiently sized number of participants who varied in substance use early in treatment to more fully examine this question. In the present sample, the majority (83.25%) of clients presented with an alcohol use disorder, whereas only a minority of clients exhibited specific drug use disorders alone or in addition to their alcohol-related problems. These drug use disorders included cocaine, opiates, marijuana, and, to a lesser degree, sedatives, hallucinogens, and stimulants. Each of these substances have specific and distinct targets within the brain and may have an impact on cognitive ability and recovery in differing ways (Carlin and O’Malley, 1996; Fals-Stewart et al., 1994; Rogers and Robbins, 2001). In the present study, it was not possible to assess further the role of individual drugs of abuse (e.g., cocaine) on the recovery of latent abilities because of the limited sample size. Finally, it should be noted that drug abstinence was ascertained from reports of substance use by the patients and a knowledgeable collateral. Future research might include urinalysis and drug screening to further validate self-reports of drug use.
CONCLUSIONS AND CLINICAL IMPLICATIONS
The current study revealed increases in cognitive functioning in a sample of individuals with alcohol and other drug use disorders between treatment entry and follow-up 6 weeks later. The magnitude of increases in the latent ability means, however, suggested recovery of modest clinical significance in executive functioning, verbal processing speed, and verbal ability domains, with a medium effect size recovery in the memory domain. With 50% or more of clients presenting for treatment with some extent of neuropsychological impairment (Loberg and Miller, 1986; Meek et al., 1989; Morgenstern and Bates, 1999; O’Malley et al., 1992) and the observation that spontaneous, “time-dependent” cognitive recovery from alcohol and other drug use disorders may be limited in the short term, the question remains as to whether more complex topics and interventions that require abstraction abilities or rapid information processing should be introduced as treatment progresses across the first 1 to 2 months. This is an important question in light of the current trend of shorter treatment durations.
The present findings have treatment implications in two areas. First, although the average level of recovery was modest, there was considerable variability in extent of improvement. Developing a profile of risk factors that predict individual differences in spontaneous cognitive recovery could assist treatment providers in identifying clients who may benefit from therapeutic techniques that are designed to compensate for or rehabilitate neuropsychological impairments. Risk profiles provide a potentially inexpensive alternative to costly and time-consuming neuropsychological testing of entire treatment populations by allowing treatment providers to identify more easily those who are at greatest risk for neurocognitive impairments. The present findings, however, are only a preliminary step toward understanding the utility of developing a client profile. Future research should extend this risk profile to include a more comprehensive set of risk factors (e.g., the effects of individual drugs, head injury, and social support to reinforce abstinence).
Second, although typically not a strong, direct predictor of addiction treatment outcomes, cognitive impairment may influence outcome via indirect and moderated pathways (Bates et al., 2002a). There is evidence that cognitive impairment adversely affects treatment retention of clients with alcohol (Pawlak et al., 2004), cocaine (Aharonovich et al., 2003), and other drug (Fals-Stewart and Schafer, 1992) use disorders and that treatment retention is a strong predictor of outcome (e.g., Grella et al., 1999; Simpson et al., 1997). Cognitive deficits may lead to lack of motivation and treatment engagement, which are often interpreted as negative client attributes by treatment providers (Fals-Stewart et al., 1995; Goldman, 1995; Weinstein and Shaffer, 1993). However, when informed of a patient’s cognitive abilities, therapists rate participation and therapeutic alliance higher and patients stay in treatment longer (Grohman and Fals- Stewart, 2004). Corrigan et al. (2005) also observed that reducing logistical barriers to treatment attendance and providing financial incentives can be used to improve rates of treatment engagement in drug abusers with cognitive impairment caused by traumatic brain injury. Such techniques may potentially enhance engagement of clients who have alcohol- and drug-related cognitive impairment of mixed causes. Alternatively, directly improving the cognitive functioning of clients in addiction treatment [e.g., promoting “experience-dependent” recovery (Roehrich and Goldman, 1993)] may be feasible through cognitive rehabilitation techniques (Allen et al., 1997; Fals-Stewart et al., 1994) such as practice with neuropsychological testing materials (Goldman, 1995), computer-assisted programs (Grohman and Fals-Stewart, 2003), and goal-setting (Scheurich et al., 2004). Individuals who received computer-assisted cognitive rehabilitation stayed in residential treatment longer and had more positive discharge status (Grohman and Fals-Stewart, 2003). Moreover, this rehabilitation seemed to accelerate recovery even in clients who do not evidence clinically significant levels of impairment. Further addiction treatment research should address the utility of including cognitive compensation and/or rehabilitation for impaired clients to promote clinically meaningful gains in neurocognitive functioning early in treatment and also should address the impact of rehabilitation on treatment retention and the processes that are thought to support positive treatment outcomes.
Acknowledgments
This study was supported by grants P50 AA 08747, R01 AA 11594, and K02 AA 00325 from the National Institute of Alcohol Abuse and Alcoholism. Preliminary results were presented at the 109th Annual Meeting of the American Psychological Association, San Francisco, CA, August, 2001.
Footnotes
Two exceptions were that (1) performance on visual motor tests was not supported by a unique latent factor but rather co-varied strongly with executive performance, as has previously been found (Hill et al., 2000; Parkin and Java, 1999); and (2) the two syntactic information processing speed measures did not vary with the other verbal ability tests but rather were indicators of a unique receptive verbal speed factor.
Although the χ2 values derived from the unconstrained and constrained measurement models differed significantly (p < 0.05), no further adjustments to the model were made because assessment of other model fit statistics and the modification indices indicated that further freeing of parameters would enhance “statistical” fit but make no conceptually meaningful difference in the model. That is, modification indices for individual parameters were small, and all fit indices, other than the χ2, suggested close fit of the constrained model to the data (Hu and Bentler, 1999; Yu and Muthén, 2002), with the entire 90% confidence level for the RMSEA remaining <0.055.
Risk factors that are associated with latent ability at treatment entry have been reported previously (Bates et al., 2002b). Education had a large effect on Verbal ability, a medium effect on Executive ability, and small effects on Memory and Speed. Age had a large effect on Executive ability, medium effect sizes on Memory and Speed, and a small effect on Verbal ability. All other risk factors that significantly predicted baseline ability had small, unique effect sizes. These included diagnosis of an anxiety disorder (on Speed), a drug use disorder (on Speed and Verbal), abnormal medical test results (on Executive, Speed and Verbal), and positive family history of alcohol use disorders (on Verbal).
References
- Adams KM, Grant I. Influence of premorbid risk factors on neuropsychological performance in alcoholics. J Clin Exp Neuropsychol. 1986;8:362–70. doi: 10.1080/01688638608401327. [DOI] [PubMed] [Google Scholar]
- Adams KM, Grant I, Reed R. Neuropsychology in alcoholic men in their late thirties: one-year follow-up. Am J Psychiatry. 1980;137(8):928–931. doi: 10.1176/ajp.137.8.928. [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71(2):207–211. doi: 10.1016/s0376-8716(03)00092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DN, Goldstein G, Seaton BE. Cognitive rehabilitation of chronic alcohol abusers. Neuropsychol Rev. 1997;7:21–39. doi: 10.1007/BF02876971. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Endicott J, Spitzer RL, Winokur G. The family history method using diagnostic criteria: Reliability and validity. Arch Gen Psychiatry. 1977;34(10):1229–1235. doi: 10.1001/archpsyc.1977.01770220111013. [DOI] [PubMed] [Google Scholar]
- Bates ME, Barry D, Labouvie E, Fals-Stewart W, Voelbel GT, Buckman JF. Risk factors and neuropsychological recovery in alcohol use disordered clients exposed to different treatments. J Consult Clin Psychol. 2004 doi: 10.1037/0022-006X.72.6.1073. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Exp Clin Psychopharmacol. 2002a;10(3):193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- Bates ME, Convit A. Neuropsychology and neuroimaging of alcohol and illicit drug abuse. In: Calev A, editor. The Assessment of Neuropsychological Functions in Psychiatric Disorders. American Psychiatric Press; Washington, DC: 1999. pp. 373–445. [Google Scholar]
- Bates ME, Labouvie EW, Voelbel GT. Individual differences in latent neuropsychological abilities at addictions treatment entry. Psychol Addict Behav. 2002b;16(1):35–46. doi: 10.1037//0893-164x.16.1.35. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K. Multilingual aphasia examination. University of Iowa; Iowa City: 1976. [Google Scholar]
- Brandt J, Butters N, Ryan C, Bayog R. Cognitive loss and recovery in long-term alcohol abusers. Arch Gen Psychiatry. 1983;40(4):435–42. doi: 10.1001/archpsyc.1983.01790040089012. [DOI] [PubMed] [Google Scholar]
- Carlen PL, Wortzman G, Holgate RC, Wilkinson DA, Rankin JC. Reversible cerebral atrophy in recently abstinent chronic alcoholics measured by computed tomography scans. Science. 1978;200(4345):1076–8. doi: 10.1126/science.653357. [DOI] [PubMed] [Google Scholar]
- Carlin AS, O’Malley S. Neuropsychological consequences of drug abuse. In: Grant I, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric Disorders. Oxford University Press; New York: 1996. pp. 486–503. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- Corrigan J, Bogner J, Lamb-Hart G, Heinemann AW, Moore D. Increasing substance abuse treatment compliance for persons with traumatic brain injury. Psychol Addict Behav. 2005 doi: 10.1037/0893-164X.19.2.131. in press. [DOI] [PubMed] [Google Scholar]
- De Fillippis N, McCampbell E. The Booklet Category Test: Research and Clinical Form Manual. Psychological Assessment Resources; Odesa, FL: 1991. [Google Scholar]
- Delis D, Kramer JH, Kaplan E, Ober B. California Verbal Learning Test: Adult Version Manual. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Dennis M, Kohn B. Comprehension of syntax in infantile hemiplegics after cerebral hemidecortication: Left-hemisphere superiority. Brain Language. 1975;2(4):472–482. doi: 10.1016/s0093-934x(75)80084-8. [DOI] [PubMed] [Google Scholar]
- Dennis M, Kohn B. The Active-Passive Test: An age-referenced clinical test of syntactic discrimination. Dev Neuropsychol. 1985;1(2):113–137. [Google Scholar]
- Donovan DM, Kivlahan DR, Walker RD. Clinical limitations of neuropsychological testing in predicting treatment outcome among alcoholics. Alcohol Clin Exp Res. 1984;8(5):470–475. doi: 10.1111/j.1530-0277.1984.tb05704.x. [DOI] [PubMed] [Google Scholar]
- Drake AI, Butters N, Shear PK, Smith TL, Bondi M, Irwin M, Schuckit MA. Cognitive recovery with abstinence and its relationship to family history for alcoholism. J Stud Alcohol. 1995;56(1):104–9. doi: 10.15288/jsa.1995.56.104. [DOI] [PubMed] [Google Scholar]
- Ellenberg L, Rosenbaum G, Goldman MS, Whitman RD. Recoverability of psychological functioning following alcohol abuse: Lateralization effects. J Consult Clin Psychol. 1980;48(4):503–510. doi: 10.1037//0022-006x.48.4.503. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, Schafer J. The relationship between length of stay in drug-free therapeutic communities and neurocognitive functioning. J Clin Psychol. 1992;48(4):539–543. doi: 10.1002/1097-4679(199207)48:4<539::aid-jclp2270480416>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, Schafer J, Lucente S, Rustine T, Brown L. Neurobehavioral consequences of prolonged alcohol and substance abuse: A review of findings and treatment implications. Clin Psychol Rev. 1994;14:755–778. [Google Scholar]
- Fals-Stewart W, Shanahan T, Brown L. Treating alcoholism and substance abuse: A neuropsychiatric perspective. Psychother Priv Prac. 1995;14:1–21. [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. West J Med. 1990;152(5):531–7. [PMC free article] [PubMed] [Google Scholar]
- Finney JW, Hahn AC, Moos RH. The effectiveness of inpatient and outpatient treatment for alcohol abuse: the need to focus on mediators and moderators of setting effects. Addiction. 1996;91(12):1773–96. [PubMed] [Google Scholar]
- Forsberg LK, Goldman MS. Experience-dependent recovery of visuospatial functioning in older alcoholic persons. J Abnorm Psychol. 1985;94(4):519–529. doi: 10.1037//0021-843x.94.4.519. [DOI] [PubMed] [Google Scholar]
- Forsberg LK, Goldman MS. Experience-dependent recovery of cognitive deficits in alcoholics: Extended transfer of training. J Abnorm Psychol. 1987;96(4):345–353. doi: 10.1037//0021-843x.96.4.345. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Stoelting Company; Chicago: 1978. [Google Scholar]
- Goldman MS. Cognitive impairment in chronic alcoholics: Some cause for optimism. Amer Psychol. 1983;38:1045–1054. doi: 10.1037//0003-066x.38.10.1045. [DOI] [PubMed] [Google Scholar]
- Goldman MS. Recovery of cognitive functioning in alcoholics - the relationship to treatment. Alcohol Health Res World. 1995;19(2):148–154. [PMC free article] [PubMed] [Google Scholar]
- Goldman MS, Williams DL, Klisz DK. Recoverability of psychological functioning following alcohol abuse: Prolonged visual-spatial dysfunction in older alcoholics. J Consult Clin Psychol. 1983;51(3):370–378. doi: 10.1037//0022-006x.51.3.370. [DOI] [PubMed] [Google Scholar]
- Grant I, Adams KM, Carlin AS, Rennick PM, Judd LL, Schooff K, Reed R. Organic impairment in polydrug users: Risk factors. Am J Psychiatry. 1978;135(2):178–184. doi: 10.1176/ajp.135.2.178. [DOI] [PubMed] [Google Scholar]
- Grella CE, Hser Y-I, Joshi V, Anglin MD. Patient histories, retention and outcome models for younger and older adults in DATOS. Drug Alcohol Depend. 1999;57:151–166. doi: 10.1016/s0376-8716(99)00082-4. [DOI] [PubMed] [Google Scholar]
- Grohman K, Fals-Stewart W. Computer-assisted cognitive rehabilitation with substance-abusing patients: effects on treatment response. J Cognit Rehabil. 2003;21:10–17. [Google Scholar]
- Grohman K, Fals-Stewart W. Sharing substance-abusing patients’ neuropsychological assessment results: Effects on treatment response. Poster presented at the Annual Convention of the American Psychological Association; Honolulu, HI. 2004. [Google Scholar]
- Hill KG, White HR, Chung I-J, Hawkins JD, Catalano RF. Early adult outcomes of adolescent binge drinking: Person- and variable-centered analyses of binge drinking trajectories. Alcohol Clin Exp Res. 2000;24(6):892–901. [PMC free article] [PubMed] [Google Scholar]
- Hu L-t, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. [Google Scholar]
- Jacobson NS, Roberts LJ, Berns SB, McGlinchey JB. Methods for defining and determining the clinical significance of treatment effects: description, application, and alternatives. J Consult Clin Psychol. 1999;67(3):300–7. doi: 10.1037//0022-006x.67.3.300. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Fein D, Morris R, Delis D. WAIS-R as a neuropsychological instrument. The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Kline RB. Principles and practices of structural equation modeling. Guilford Press; New York: 1998. [Google Scholar]
- Knight RG, Longmore BE. Clinical Neuropsychology of Alcoholism. Erlbaum; Hillsdale, NJ: 1994. [Google Scholar]
- Lezak MD. Neuropsychological assessment. 3. Oxford University Press; New York: 1995. [Google Scholar]
- Little R, Rubin D. Statistical Analysis with Missing Data. Wiley; New York: 1987. [Google Scholar]
- Loberg T, Miller WR. Personality, cognitive, and neuropsychological correlates of harmful alcohol consumption: a cross-national comparison of clinical samples. Ann NY Acad Sci. 1986;472:75–97. doi: 10.1111/j.1749-6632.1986.tb29612.x. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychol Methods. 1996;1(2):130–149. [Google Scholar]
- Medina KL, Shear PK, Schafer J, Armstrong TG, Dyer P. Cognitive functioning and length of abstinence in polysubstance dependent men. Arch Clin Neuropsychol. 2004;19(2):245–58. doi: 10.1016/S0887-6177(03)00043-X. [DOI] [PubMed] [Google Scholar]
- Meek PS, Clark HW, Solana VL. Neurocognitive impairment: the unrecognized component of dual diagnosis in substance abuse treatment. J Psychoactive Drugs. 1989;21(2):153–60. doi: 10.1080/02791072.1989.10472155. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos B. Life Stressors and Social Resources Inventory - Adult Form Manual. Center for Health Care Evaluation; Palo Alto, CA: 1992. [Google Scholar]
- Morgenstern J, Bates ME. Effects of executive function impairment on change processes and substance use outcomes in 12-step treatment. J Stud Alcohol. 1999;60(6):846–55. doi: 10.15288/jsa.1999.60.846. [DOI] [PubMed] [Google Scholar]
- Munro CA, Saxton J, Butters MA. The neuropsychological consequences of abstinence among older alcoholics: A cross-sectional study. Alcohol Clin Exp Res. 2000;24(10):1510–1516. [PubMed] [Google Scholar]
- Murphy KR, Myors B. Statistical power analysis. 2. Erlbaum; Mahwah, NJ: 2004. [Google Scholar]
- Muthén L, Muthén B. Mplus: The comprehensive modeling program for applied researchers: User’s guide. Muthén & Muthén; Los Angeles, CA: 1998. [Google Scholar]
- Muuronen A, Bergman H, Hindmarsh T, Telakivi T. Influence of improved drinking habits on brain atrophy and cognitive performance in alcoholic patients: a 5-year follow-up study. Alcohol Clin Exp Res. 1989;13(1):137–41. doi: 10.1111/j.1530-0277.1989.tb00298.x. [DOI] [PubMed] [Google Scholar]
- Nelson HE. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12:313–324. doi: 10.1016/s0010-9452(76)80035-4. [DOI] [PubMed] [Google Scholar]
- Nixon SJ. Assessing cognitive impairment. Alcohol Health Res World. 1995;19:97–103. [PMC free article] [PubMed] [Google Scholar]
- O’Malley S, Adamse M, Heaton RK, Gawin FH. Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse. 1992;18(2):131–44. doi: 10.3109/00952999208992826. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Barrett KA. Measures of effect size in the reporting of rehabilitation research. Am J Phys Med Rehabil. 1989;68(2):52–8. doi: 10.1097/00002060-198904000-00002. [DOI] [PubMed] [Google Scholar]
- Page RD, Linden JD. “Reversible” organic brain syndrome in alcoholics. A psychometric evaluation. Q J Stud Alcohol. 1974;35(1):98–107. [PubMed] [Google Scholar]
- Parkin AJ, Java RI. Deterioration of frontal lobe function in normal aging: Influences of fluid intelligence versus perceptual speed. Neuropsychol. 1999;13:539–545. doi: 10.1037//0894-4105.13.4.539. [DOI] [PubMed] [Google Scholar]
- Parsons OA, Schaeffer KW, Glenn SW. Does neuropsychological test performance predict resumption of drinking in posttreatment alcoholics? Addict Behav. 1990;15(3):297–307. doi: 10.1016/0306-4603(90)90073-7. [DOI] [PubMed] [Google Scholar]
- Pawlak A, Bates ME, Labouvie E, Tonigan JS, Fals-Stewart W. Indirect effects of neuropsychological ability on addiction treatment outcome. Poster presented at the International Neuropsychological Society; Baltimore, MD. 2004. [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19(5):1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pople LE, Cybulski KA, Epstein E, Rotgers R, McCrady BS, Morgenstern J. Treatment expectancies and consequences of use: New measures for the substance abuse field. Paper presented at the 102nd Annual Convention of the American Psychological Association; Los Angeles, CA. 1994. [Google Scholar]
- Reed RJ, Grant I. The long-term neurobehavioral consequences of substance abuse: conceptual and methodological challenges for future research. NIDA Res Monogr. 1990;101:10–56. [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological battery: Theory and clinical implications. Neuropsychology Press; Tucson, AZ: 1985. [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–7. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Roehrich L, Goldman MS. Experience-dependent neuropsychological recovery and the treatment of alcoholism. J Consult Clin Psychol. 1993;61(5):812–21. doi: 10.1037//0022-006x.61.5.812. [DOI] [PubMed] [Google Scholar]
- Rourke SB, Loberg T. The neurobehavioral correlates of alcoholism. In: Grant I, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric Disorders. Oxford University Press; New York: 1996. pp. 423–485. [Google Scholar]
- Russell EW, Levy M. Revision of the Halstead Category Test. J Consult Clin Psychol. 1987;55(6):898–901. doi: 10.1037//0022-006x.55.6.898. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Loh C. Effect sizes and statistical testing in the determination of clinical significance in behavioral medicine research. Ann Behav Med. 2004;27(2):138–45. doi: 10.1207/s15324796abm2702_9. [DOI] [PubMed] [Google Scholar]
- Sanchez-Craig M, Walker K. Teaching coping skills to chronic alcoholics in a coeducational halfway house: I. Assessment of programme effects. Br J Addict. 1982;77:35–50. doi: 10.1111/j.1360-0443.1982.tb03248.x. [DOI] [PubMed] [Google Scholar]
- Schafer K, Butters N, Smith T, Irwin M, Brown S, Hanger P, Grant I, Schuckit M. Cognitive performance of alcoholics: A longitudinal evaluation of the role of drinking history, depression, liver function, nutrition, and family history. Alcohol Clin Exp Res. 1991;15(4):653–660. doi: 10.1111/j.1530-0277.1991.tb00574.x. [DOI] [PubMed] [Google Scholar]
- Scheurich A, Muller MJ, Szegedi A, Anghelescu I, Klawe C, Lorch B, Kappis B, Bialonski H-G, Haas S, Hautzinger M. Neuropsychological status of alcohol-dependent patients: increased performance through goal-setting instructions. Alcohol Alcohol. 2004;39:119–25. doi: 10.1093/alcalc/agh026. [DOI] [PubMed] [Google Scholar]
- Simon HA. The functional equivalence of problem solving skills. Cognit Psychol. 1975;7(2):268–288. [Google Scholar]
- Simpson DD, Joe GW, Rowan-Szal GA. Drug abuse treatment retention and process effects on follow-up outcomes. Drug & Alcohol Dependence. 1997;47(3):227–235. doi: 10.1016/s0376-8716(97)00099-9. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ: 1992. pp. 41–69. [Google Scholar]
- Spitzer R, Williams J, Gibbon M, First M. Structured Clinical Interview for DSM-III-R, Patient Edition (Version 1.0) American Psychiatric Press; Washington, DC: 1990. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms and commentary. 2. Oxford University Press; New York: 1998. [Google Scholar]
- Sussman S, Rychtarik RG, Mueser K, Glynn S, Prue DM. Ecological relevance of memory tests and the prediction of relapse in alcoholics. J Stud Alcohol. 1986;47(4):305–10. doi: 10.15288/jsa.1986.47.305. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Edwards KL. Brief and comprehensive neuropsychological assessment of alcohol and substance abuse. In: Hartlage LC, Asken MJ, Hornsby JL, editors. Essentials of Neuropsychological Assessment. Springer; New York: 1987. pp. 138–158. [Google Scholar]
- Tarter RE, McBride H, Buonpane N, Schneider DU. Differentiation of alcoholics: Childhood history of minimal brain dysfunction, family history, and drinking pattern. Arch Gen Psychiatry. 1977;34(7):761–768. doi: 10.1001/archpsyc.1977.01770190023002. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. Positron emission tomography and single-photon emission computed tomography in substance abuse research. Semin Nucl Med. 2003;33(2):114–28. doi: 10.1053/snuc.2003.127300. [DOI] [PubMed] [Google Scholar]
- Weinstein CS, Shaffer HJ. Neurocognitive aspects of substance abuse treatment: A psychotherapist’s primer. Psychotherapy. 1993;30:317–333. [Google Scholar]
- Yu C-Y, Muthén B. Evaluation of model fit indices for latent variable models with categorical and continuous variables. Unpublished manuscript 2002 [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale: Revised Manual. Western Psychological Services; Los Angeles: 1986. [Google Scholar]