Abstract
Background
A key underlying process that may contribute to attention-deficit/hyperactivity disorder (ADHD) involves alterations in reward evaluation, including assessing the relative value of immediate over delayed rewards. This study examines whether children with ADHD discount the value of delayed rewards to a greater degree than typically developing children using a delay discounting task.
Methods
Children aged 7–9 years diagnosed with ADHD and controls completed a task in which they chose between a hypothetical $10 available after a delay (7, 30, 90 and 180 days) versus various amounts available immediately.
Results
ADHD participants discounted more steeply than controls. However, this effect did not survive covarying of IQ.
Conclusions
ADHD is associated with a steeper delay gradient when contemplating hypothetical later rewards, but not independently of IQ. The interplay of cognitive processing and IQ with reward evaluation in ADHD requires further exploration.
Keywords: impulsivity, delay discounting, ADHD
Introduction
Understanding the psychological mechanisms underlying attention-deficit/hyperactivity disorder (ADHD) continues to be essential to inform better assessment, conceptualization, and eventual intervention (Epstein et al., 2010). ADHD has a multifactorial etiology, with substantial heritability as well as many suspected or demonstrated environmental influences on its development. The multifactorial nature of its etiology and difficulty in identifying strong major gene or environment causes has mandated examination of pathophysiology and of psychological and neural mechanisms that might help clarify causal process. In this regard, one major focus has been on cognitive processes related to ADHD and particularly to the symptom domain of inattention, such as executive functioning (Barkley, 1997; Nigg, 2001; Pennington & Ozonoff, 1996). Another focus has been on various processes related to reward, motivation, and learning in ADHD, which may be more closely related to impulsivity and/or hyperactivity. For example, Sagvolden and colleagues (1992; 2005; Johansen, Sagvolden, & Kvande, 2005) have articulated a theory of disturbed reinforcement learning that may be related to a steep discounting gradient. Others have suggested abnormalities in the valuation of reward (Tripp & Wickens, 2008) perhaps related to difficulty in being motivated by distal rewards. A central prediction in these theories is that ADHD is associated with a steepened discounting function when anticipating future rewards or reinforcers.
While a reward can be seen as a desired object that motivates approach, a reinforcer is an outcome contingent on behavior that increases the likelihood that the behavior will reoccur (Rachlin, 1976). Not all rewards function as reinforcers, and not all reinforcers are rewards. Strictly speaking, our study focuses on the evaluation of putative rewards, as our task does not permit the outcome of each individual’s choices to influence subsequent behavior. However, the expectation is that the dysfunction of reinforcement processes that result in steeper discounting gradients observed in experiential procedures used with rats (Johansen, Sagvolden, & Kvande, 2005) will be captured using procedures assessing delay discounting in humans (Madden & Johnson, 2010).
For prospective rewards to influence behavior, an individual must assign them value prior to their occurrence, i.e., when they are not immediately available. Such valuation, in turn, depends on the reward magnitude, as well as the certainty/uncertainty of obtaining the reward and the temporal delay before receiving it (see Green & Myerson, 2004 for review). Sagvolden et al. (2005) most clearly predicted that ADHD is associated with a steeper decline in subjective impact of reinforcers, which in our view should extend to the subjective value of delayed rewards if those rewards are to function as reinforcers (i.e., steeper delay discounting). As we noted, related theoretical predictions have been offered by others (Luman, Tripp, & Scheres, 2010; Tripp & Wickens, 2008), all of which predict a steepened discounting function for future valued outcomes. Thus, the evaluation of reward-delay gradients in ADHD has become central to testing recent theoretical predictions about the disorder.
Empirical examination of delay discounting among children with ADHD has not kept pace with theoretical claims; indeed, agreed-upon methods for evaluating reward-delay gradients in ADHD have not emerged. Responding to historical interest in reward and ADHD, numerous studies have used single-delay task designs (repeated choices between a small reward now and a large reward later) to show that children with ADHD typically prefer immediate rewards more than non-ADHD individuals (Antrop, et al., 2006, Bitsakou, Psychogiou, Thompson, & Sonuga-Barke, 2009; Marco, et al., 2009; Paloyelis, Asherson, & Kuntsi, 2009; Solanto, et al., 2001; Tripp & Alsop, 2001). Additionally, Paloyelis and colleagues (2009) found that preference for immediate rewards using a single-delay task design was positively related to ratings of inattention in children (average 8.8 years old). However, because these procedures measure relative preference at only a single delay, the slope of the discounting function is unspecified and the relative contribution of delay aversion (Sonuga-Barke, 2005) and reward devaluation is unclear.
To address delay gradients per se, the literature has suggested multiple methods, not all of which have been much used with ADHD. A key question is whether to offer real or hypothetical rewards. To preserve ecological validity it seems preferable to use real rewards and require individuals to experience delays before receiving them. That approach is helpful when one is interested in very short delay gradients (on the order of seconds or minutes). On the other hand, instantiating real-world delays for many typical rewards (i.e., hours, days, or weeks) is infeasible within such a task. To capture these longer delay periods, the use of hypothetical rewards is usually necessary. Some results have suggested similar discounting patterns in the two paradigms as long as reward magnitude is held constant (e.g., Madden et al., 2004). On the other hand, the two approaches may activate cognitive and affective responses in different ways. Balancing both approaches may ultimately be advisable, but the present study focused on the development of a laboratory-based method with hypothetical scenarios to examine longer delays than is feasible otherwise, because development of that approach has been neglected in the ADHD literature and may enhance understanding of how delay gradients function in real world waiting periods.
To our knowledge, only four studies have examined the relationship between ADHD diagnosis or symptoms and discounting gradients by systematically varying the size of rewards and delays (whether real or hypothetical). Barkley, Edwards, Laneri, Fletcher, and Metevia (2001) compared adolescents (aged 12 to 19 years) with ADHD and comorbid oppositional defiant disorder (ODD) to controls; the ADHD group displayed steeper discounting of delayed hypothetical rewards of $100 but not $1000 when delays were between one month and 10 years. Scheres, Lee, and Sumiya (2008) found an association between ADHD hyperactivity/impulsivity symptoms, but not inattention symptoms, and steepness of the discounting gradient among college students when rewards were real, but not when they were hypothetical. That study did not examine individuals with an ADHD diagnosis. In contrast, Scheres and colleagues (2006) found no difference in delay discounting in children and adolescents with ADHD and matched controls using an experiential task with actual rewards up to $0.10 using very short delays (up to 30 seconds). However, Scheres and colleagues (2010) used three delayed reward amounts and delays up to one minute, again in a sample of 6–17 year olds with and without ADHD. They found steeper delay discounting among participants with ADHD-combined type across all tasks, but no difference between participants with ADHD-inattentive type and controls. Steeper discounting was specifically related to hyperactivity/impulsivity symptoms in that study.
Aside from Barkley et al (2001), no studies have examined subjective value across longer delays in ADHD. Further, the few studies of discounting slope have generally not considered the role of comorbid externalizing behaviors, IQ, or gender, and no studies have examined whether the hypothesized effects hold in samples exclusively prior to adolescence. IQ is particularly important as it is known to correlate moderately with discounting (de Wit, Flory, Acheson, McCloskey, & Manuck, 2007) and is expected to be somewhat lower among children with ADHD (Jepsen & Mortensen, 2009).
The present study extends previous research by addressing these gaps, using a paradigm involving hypothetical choices, more trials for each delayed amount, finer variations in the immediate amount, and longer delay intervals. The current study thus had two aims. First, to determine whether a child version of a delay-discounting task previously used in adults would yield reliable data in samples of young children with and without ADHD. As noted by Scheres and colleagues (2010), developing appropriate methods is a key goal of the field. The second aim was to evaluate the reward discounting gradient in children with ADHD, a critical step if we are to evaluate the hypotheses proposed by Sagvolden et al (2005) and others. Accordingly, we hypothesized that children with ADHD would exhibit steeper discounting gradients than children without ADHD, but that gradients would be equally well described by a hyperbolic discounting function for both groups of children. As a secondary analysis, we also examined decision times of decision-making differences in ADHD. Finally, in exploratory analysis we investigated whether delay gradient was related specifically with symptoms of hyperactivity-impulsivity rather than inattention-disorganization; we hypothesized it would be related to hyperactivity-impulsivity.
Methods
Participants
Participants were 70 children aged 7 to 9 years (mean age=7.93 years), of whom 35 met DSM-IV criteria for ADHD (12 predominantly inattentive, 3 predominantly hyperactive/impulsive, and 18 combined subtype), and 35 were typically-developing comparison children. Final diagnosis was established from a semi-structured clinical interview, as well as parent and teacher ratings, described below. Children had to produce a pattern of systematic choice behavior on the discounting task to be included, described below. In total 58 participants (27 ADHD and 31 controls) met these criteria.
The local Institutional Review Board approved the study, and participants were treated according to the “Ethical Principles of Psychologists and Code of Conduct” (American Psychological Association, 2002). Parents provided written informed consent and children provided assent.
Procedure
Recruitment and Diagnostic Identification
Families were recruited through public advertisements and mass mailings to commercial mailing lists targeted at parents of 7–9 year old children. When they called to volunteer, they were passed through a multi-gate screening process to assess eligibility and identify cases. At stage one, parents of potential participants completed a phone interview to rule out the use of psychotropic medications, neurological impairments, prior diagnoses of intellectual disabilities or pervasive developmental disorders, history of seizures, traumatic brain injury, or other medical conditions. At stage two, a parent and a teacher of the remaining eligible youth completed the ADHD Rating Scale (DuPaul, Power, Anastopolous, & Reid, 1998), the Strengths and Difficulties Questionnaire (SDQ; Goodman, 2001), and Conners (2008) Rating Scale-3rd Edition. A parent completed the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-E; Puig-Antich & Ryan, 1986) for DSM-IV with a master’s-level clinician. Interviews were videotaped and monitored for fidelity. Inter-interviewer reliability was satisfactory (k > 0.80). Children completed an IQ screening assessment consisting of three-subtests (Block Design, Vocabulary, and Information) of the Wechsler Intelligence Scales for Children, 4th edition (WISC-IV; Wechsler, 2003). Children also completed three subscales (Word Reading, Math Reasoning, and PseudoWord) from the Wechsler Individual Achievement Test, second edition (WIAT-II; Wechsler, 2005) to assist in evaluation of learning disabilities.
Final ADHD and Other Diagnoses
Results from the interview, rating scales, child IQ and achievement tests, and interviewer and tester notes and observations were presented to a clinical diagnostic team consisting of a board certified child psychiatrist and a licensed clinical neuropsychologist. Each team member arrived independently at a “best estimate” diagnosis for ADHD, as well as comorbid conditions, using DSM-IV criteria. Clinician diagnostic agreement ratings for ADHD and comorbid disorders were acceptable (k > 0.80). Disagreements were resolved through consensus. If consensus was not readily achieved, the child was excluded.
To be assigned a diagnosis of ADHD, the child’s symptoms could not be better accounted for by another disorder, evidence of impairment had to be apparent (e.g., on the SDQ impairment ratings, in the parent/teacher comments, or in the school record), and cross-situational presentation were required (i.e., some elevation in both parent and teacher reports). If these conditions were met, symptoms were counted as present if endorsed by the parent on the KSADS-E or by the teacher on the ADHD rating scale, and this symptom count (the OR Algorithm) was used in subsequent analyses of symptom profile and discounting. An estimated Full Scale IQ > 75 was required for inclusion. Exclusion criteria included current major depression or learning disability, or history of mania, psychosis, or autism spectrum disorder.
Medication washout
Children who were prescribed stimulant medication underwent a 24–48 hour washout dependent on the preparation prescribed. Eight ADHD children (and no control children) were prescribed stimulants (1 Adderall; 1 Adderall XR; 1 Focalin Tablets; 1 Ritalin HCL; 3 Concerta; 1 Concerta with Methaphenidate). Covarying of medication status did not change results reported later.
Delay Discounting Task
The computerized task, based on the task described by Mitchell (1999), presented a series of 91 questions. Specific answers had no effect on the overall duration of the task; however, children’s response time affected task length because the next question was not presented until the previous question was answered. For each question, children chose between (1) a varying amount of hypothetical money now or (2) a hypothetical $10.00 after a varying delay. Participants indicated whether they preferred the immediate or delayed alternative using the computer mouse. The immediate money varied from $0.00–$10.50 in $0.50 increments. The delayed money (always $10.00) was available after one of four delays (7, 30, 90, or 180 days). A delayed and an immediate item were selected to form each question so that each delayed alternative was paired with each immediate alternative, and presented in a random sequence. Three additional questions were used to assess attention to the task ($10 in 0 days vs. $10.50, $9.50, and $9.00 now). The task took approximately 12 minutes.
Dependent variables
Two main dependent variables were derived from the task. The first was the point at which there was no preference between the immediate item and the delayed $10.00 at each delay (i.e., the indifference point). This was defined as midway between the smallest value of the immediate alternative consistently accepted and the largest value consistently rejected for each delay (see Mitchell, 1999).
The other dependent variable was the discounting gradient, or rate at which the delayed outcome was discounted. A hyperbolic equation was fitted to each participant’s indifference points (Mazur, 1987) using the Solver subroutine in Microsoft Excel 2007 as follows:
| Equ 1 |
V represents the subjective value of the delayed item, as indexed by the indifference point. M represents the objective value of the delayed item ($10). D represents the delay length associated with receiving $10. The free parameter, k, represents the gradient of the discounting function. Larger values indicate a steeper gradient and lower indifference points (i.e., a greater preference for immediate rewards). k values were not normally distributed (as is typical) and were natural log-transformed, so we refer to ln(k) in results. For three individuals (two with ADHD), the log-transformation could not be performed because k values were negative; their discounting data were excluded from all analyses on ln(k). However, these children were included in analyses of the indifference points.
Identification of unsystematic data
Non-systematic patterns occur for many reasons, but indicate the action of processes other than reward/delay evaluation, including changing response strategy during the task or specific time points being associated with a need for money in the child’s real context, e.g., to purchase a present for an upcoming birthday. R2 values (range: 0.00 – 1.00) are computed to assess the goodness-of-fit to each participant’s indifference points. However, R2 values tend to be positively correlated with the degree of discounting and may not be an appropriate measure when attempting to evaluate systematicity of discounting data (Johnson & Bickel, 2008). Instead, they proposed a model-free method of identifying non-systematic delay discounting data, which we used here, based on the assumption that indifference points should get smaller as delays increase.
Under their criterion, data are determined to be non-systematic if any indifference point is larger than the indifference point associated with the adjacent (i.e., shorter) delay by a magnitude greater than 20% of the delayed reward (i.e. 20% of $10). For example, the adjacent, shorter delay to 30 days would be 7 days. Assume the indifference point at 7 days is $7; adding 20% of $10 to $7 yields $9.00. Therefore, in this example, individuals with an indifference point of $7 at 7 days but more than $9 at 30 days would have unsystematic discounting data.
Data from 12 children (17%; 8 ADHD and 4 non-ADHD) were deemed unsystematic. Children with unsystematic responses did not differ according to ADHD status or sex (χ2 tests; all ps > 0.20) or on the subscales of the WISC-IV or WIAT-II or age (two-tailed independent sample t-tests, ps > 0.05). Children whose data were unsystematic were omitted from further analyses. However, results were qualitatively similar when these individuals were included, suggesting results were not attributable to removal of children with unsystematic data.
Reaction times
ADHD may be characterized by faster decision-making, with ADHD children spending less time than controls to evaluate task questions prior to selecting an alternative. This effect might be exaggerated for questions closer to the indifference point, because those decisions should be more difficult than questions for which difference between the subjective value of alternatives are very large. To test this hypothesis, each question was categorized as “hard” (close to the indifference point) or “easy” (far from the indifference point)1. Average response times for hard and easy questions were determined for each participant for each of the non-zero delays. Response times longer than 60 seconds were omitted as outliers; they accounted for < 1% of responses.
Results
Demographics
Table 1 summarizes participant characteristics. Groups did not differ in age or gender distribution. Children with ADHD had lower estimated IQs than controls and, as expected, scored higher than control children on rating scales of inattention and hyperactivity/impulsivity.
Table 1.
Mean (SD) Participant Characteristics of Each Group
| ADHD | Control | p-valued | |
|---|---|---|---|
| N (Male:Female) | 27 (17:10) | 31 (12:19) | 0.07 |
| Age (years) | 8.00 (0.62) | 7.97 (0.75) | 0.86 |
| Subtype - N (%) | |||
| Inattentive | 11 (41%) | N/A | N/A |
| Hyperactive/Impulsive | 2 (7%) | N/A | N/A |
| Combined | 14 (52%) | N/A | N/A |
| Comorbid ODD – N (%) | 7 (26%) | 0 (0%) | 0.002 |
| WISC-IVa | |||
| Full Scale IQ | 104.67 (12.49) | 117.84 (11.72) | 0.000 |
| WIAT-IIb | |||
| Reading | 104.52 (11.50) | 116.84 (12.86) | 0.004 |
| Math | 101.67 (11.49) | 119.87 (14.23) | 0.000 |
| Diagnostic team OR-Algorithmc | |||
| Inattention Symptoms | 8.19 (0.23) | 1.60 (0.30) | 0.000 |
| Hyperactivity/Impulsivity Symptoms | 6.80 (0.44) | 1.05 (0.21) | 0.000 |
Notes.
WISC-IV: Wechsler Intelligence Scales for Children
WIAT-II: Wechsler Individual Achievement Test
Number of symptoms
χ2 test for gender, Fisher’s Exact Test for ODD, two-tailed independent sample t-tests for all others.
Indifference points on the delay-discounting task
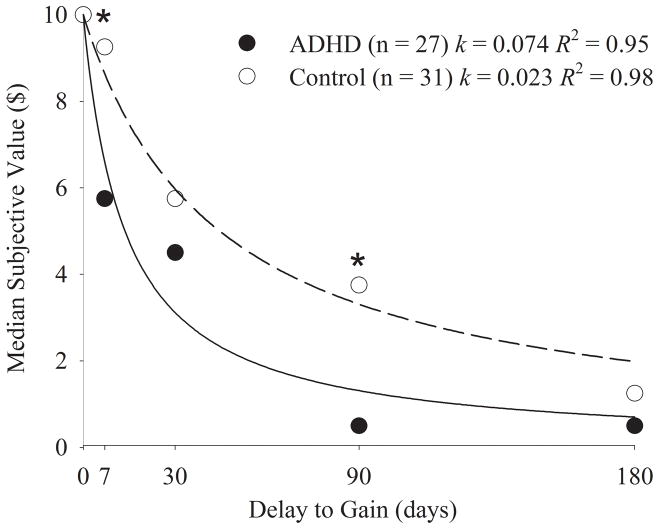
Figure 1 shows the indifference points and the delay discounting functions, and associated R2 values, for each group of children after exclusion of the 12 children with invalid data. Analyses of variance (ANOVA), with Huyn-Feldt correction, indicated that, as expected, indifference points decreased systematically as the delay increased for both groups of children. That is, as the delay to receipt of the $10 reward increased, the amount of immediate money selected over the delayed $10 decreased (ADHD: F[3.20, 95.93] = 42.74, p<.001, Controls: F[2.49, 64.72] = 45.70, p<.001). At each delay (except 0-days), the ADHD group exhibited a lower median indifference point than the control group, and while indifference points for the two groups differed significantly only at the 7- and 90-day delays, the interaction between group and delay was not significant (F[2.91, 163.15] = 2.065, p=0.109).
Figure 1.
Median indifference points for the ADHD and control children on the delay discounting task. Lines represent the hyperbolic functions fitted to these data points for each group with the fitted k and R2 values reported.
*p ≤ 0.05 using a Mann-Whitney U test to test for differences in indifference points between groups at each delay.
This relationship was well described by a hyperbolic function for individual children in both groups. When the hyperbolic discounting function was fit to individual ADHD subjects and controls the median R2 values were 0.93 and 0.82, respectively, and these values did not differ significantly (medians are reported because of skewness of R2 values; Mann-Whitney U=328.00, p=0.158). The slightly (but not significantly) smaller R2 values observed in control children were not unexpected, as R2 values tend to be positively correlated with degree of discounting (r=0.66, n=55, p<0.001 collapsed across ADHD and Control groups; Johnson & Bickel, 2008).
Delay discounting gradient
The effects of ADHD status, age, and gender on discounting rate, ln(k), were examined with a three-way ANOVA (Group [ADHD, control] × Age [7, 8, or 9 years] × Gender [male, female]). Neither the main effects of age nor gender nor any of their interaction effects were significant (all ps>0.14). They were removed from the model for simplicity and are not discussed further. As hypothesized, children with ADHD discounted significantly more steeply than controls (Mean ln(k)[SD], ADHD: −2.21[3.17]; Control: −3.96[2.47]; F[1.00, 53.00] = 5.31, p=0.025).
WISC-IV estimated IQ scores were negatively correlated with ln(k) (r = −0.47, n = 55, p<0.001). This negative association held within both groups, although it was only significant within the ADHD group (See Figure 2; ADHD: r = −0.55, n = 25, p = 0.004; Control: r = −0.20, n = 30, p = 0.287). The main effect of ADHD status was no longer significant when IQ was controlled (F[1.00, 52.00] = 0.611, p=0.438).
Figure 2.

WISC-IVFull Scale IQ as a function of ln(k). Lines represent the linear regression fitted to these data points for each group.
Times required for decision-making
The effects of ADHD status, age, and gender on average reaction time were examined with a three-way ANOVA (Group [ADHD, control] × Age [7, 8, or 9 years] × Gender [male, female]). As expected, children with ADHD tended to respond more quickly than control children (F[1.00, 46.00] = 3.85, p=0.056; Mean[SD]-ADHD: 5.33[2.38]; Control: 6.06[2.71]). However, there was a significant interaction between age and ADHD status (F[2.00, 46.00] = 1.43, p=0.022). Analysis of simple effects indicated that only 7-old participants with ADHD responded significantly faster than 7-year old controls (t[12] = 2.59, p=0.024). No other main effects or interactions were significant (all ps>0.248), implying that the ADHD slope difference was not explainable by differences in decision-making speed.
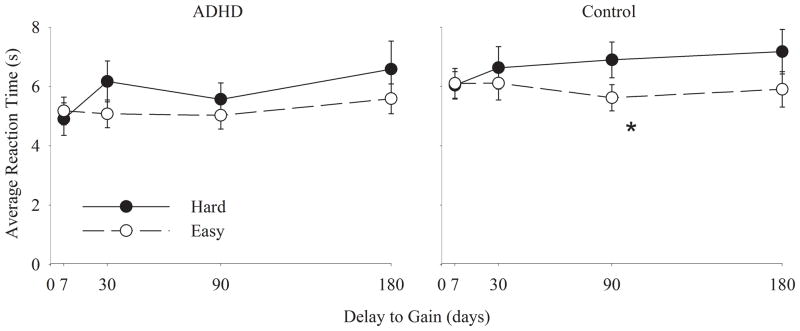
As expected, hard questions took longer to answer than easy questions (see Figure 3): F(1.00, 56.00) = 6.69, p=0.012. However, ADHD and control participants exhibited similar reaction time differences between the two types of questions.
Figure 3.
Mean response times as a function of delay for questions that were “hard” (subjective value of both alternatives was almost equivalent by virtue of being close to the indifference point) or “easy” (subjective value of each alternative was different). *p < .05 using a paired samples t-test to compare response times for different question types at the same delay.
Attention to task requirements
To assess attention to the task, the three questions for which both alternatives were immediate money were analyzed. Control children answered significantly more of these questions correctly (choosing the larger amount) than children with ADHD (Mann-Whitney U = 229.50, p=0.001; Median correct: ADHD = 2; Control = 3), suggesting that control children may have been more attentive to the task than ADHD children. Indeed, the main effect of ADHD status on delay discounting was no longer significant when the number of correct answers was controlled (F[1.00, 52.00] = 0.775, p=0.383). However, the number of questions answered accurately was not related to whether the data was systematic (Fisher’s exact test, p=0.822). Further, the number of questions answered correctly was not dependent on age, gender, or IQ (ps>0.05).
Reaction times for these questions did not differ between the ADHD and Control groups (t[56] = 0.50, p=0.623; Mean[SD] ADHD: 7.05[3.59]; Control: 7.48[3.06]). However, longer reaction times were associated with more correct responses (Multinomial Regression, χ2[3] = 10.29 p=0.016), suggesting that the number of correct responses may be a valid measure of task attention.
Task performance relationship to ADHD symptom dimensions
While ADHD combined type subjects tended to discount delayed rewards less steeply than ADHD inattentive type subjects, this difference was not significant (t[21] = 1.20, p=0.243: Mean[SD] – ADHD combined: −2.77[3.21]; ADHD inattentive: −1.10[3.34]). However, because group sizes were too small for the analysis to have sufficient power and questions related to temporal stability of subtypes persist (Lahey, Pelham, Loney, Lee, & Willcutt, 2005), we examined the number of symptoms of inattention and hyperactivity/impulsivity, possibly a more robust measure of ADHD subtype (Lahey & Willcutt, in press). We used the symptom scores as determined by the diagnostic team’s OR Algorithm (described above). Inattention, but not hyperactivity/impulsivity scores were significantly associated with ln(k) alone (Inattention: β = 0.244, p=0.025, R2 = 0.091; Hyperactivity/impulsivity: β = 0.118, p=0.319, R2 = 0.019). Inattention remained significant in the presence of the hyperactivity/impulsivity scores (Inattention & Hyperactivity/impulsivity: β I = 0.476, p=0.013; β H/i = −0.299, p=0.134, R2 =0.130), but not when IQ was added to the model (Inattention, Hyperactivity/impulsivity, & IQ: βI = 0.328, p = 0.071; βH/i = −0.302, p=0.104, βIQ = −0.089, p=0.003, R2 = 0.267).
Discussion
Heightened delay discounting is hypothesized to be a core deficit in ADHD by theories focusing on reward dysfunction or on reinforcement learning in ADHD (e.g., Tripp & Wickens, 2008; Sagvolden et al., 2005). To examine this hypothesis, we evaluated whether a discounting function can be estimated in young children using hypothetical rewards. Rates of non-systematic responding in children were only slightly higher than previously reported in adults (17% vs. 8%) and non-systematicity was not associated with ADHD. Discounting functions were otherwise lawful, and the hyperbolic function used in adult studies described the data well. Therefore, the method introduced here looks promising for use in subsequent, larger studies to evaluate discounting in children and in ADHD.
Our second aim was to evaluate the delay gradient in children with ADHD. Supporting the cited theories, children with ADHD had steeper gradients than children without ADHD, regardless of sex, age, ADHD subtype (although power was too low for reliable subtype comparisons), medication status, or comorbid diagnosis of ODD. That finding extends data from Scheres and colleagues (2010), who used short intervals and actual rewards, as well as Barkley and colleagues (2001) who used hypothetical rewards albeit without a formal assessment of discounting rate. Our result is consistent with studies in adults that have suggested that impulsivity is related to steeper discounting (see Yi, Mitchell, & Bickel, 2009). Furthermore, this sample was younger than in prior studies of the discounting gradient and ADHD, which is important, as it is theorized that abnormal discounting is an early deficit from which other ADHD-related symptoms develop (Sagvolden et al., 2005).
However, the interpretation of the steeper gradient in ADHD children is qualified by two other findings. First, the gradient was moderately related to IQ, and the diagnostic group difference in gradient was removed when IQ was treated as a covariate. Barkley and colleagues (2001) did not covary IQ but treated it as a factor without altering results. Although Scheres and colleagues (2010) did not covary IQ, the group effects observed in that study remained when IQ was treated as a covariate (A. Scheres, personal communication, June, 2010). The other two studies examining gradient in relation to ADHD did not assess IQ (Scheres et al., 2008) or did not treat it as a covariate (Scheres et al 2006). Thus, it remains unclear whether ADHD is associated with a steeper discounting function independently of IQ.
Two comments are in order. First, that IQ is related to discounting gradient is well established. Shamosh and Gray (2008) conducted a meta-analysis that concluded an aggregate correlation of −0.23 across 24 studies. While only five of these studies used hypothetical rewards, they found no difference in effect sizes between studies that used real or hypothetical rewards. Although the association seen in our study (r = −0.47) was larger than that estimate, a similar association magnitude has been reported in some studies of discounting in children (e.g., Cuskelly, Zhang, & Hayes, 2003) though not in all (e.g., Kendall, Zupan, & Braswell, 1981). Thus, it is possible that over many studies of ADHD, IQ may account for the observed results. This influence of cognitive factors in delay gradients may be more apparent when delays are lengthy or rewards are hypothetical, when decisions rely more on executive functioning, than with real rewards or very short delays (Scheres et al., 2010) that may utilize more emotional processing. Second, the functional relationship between ADHD, delay discounting, and IQ remains to be understood. The importance of this is underlined by the IQ-discounting relationship being more pronounced in the ADHD children than the controls (Figure 2). Steep delay discounting may interfere with the ability to perform on IQ tests, and vice versa. Thus, the core deficit in ADHD (i.e., a putative steeper delay gradient) may also lead to impairment in IQ. If so, covarying IQ is inappropriate, as we would be covarying an element of the ADHD syndrome (for discussions of misuse of covariance in this instance, see Dennis et al., 2009; Miller & Chapman, 2001). Another possibility is that lower IQ might be associated with a lack of economic knowledge about the value or purchasing power of money, and that lack of knowledge results in a lowering of the subjective value assigned to monetary rewards. Given that it is established that rates of discounting are steeper for smaller rewards (Green, Myerson, & McFadden, 2004; Estle, Green, Myerson, & Holt, 2006), this economic explanation may contribute to the relationship between IQ and the discounting gradient. Future studies could assess children’s experience with purchasing items within the range of the monetary rewards as a way to determine the extent of intersubject variability and its relationship to IQ and discounting.
In addition to the complexities associated with IQ, a second complication associated with interpreting the steeper discounting gradients observed for the ADHD children is that, although the steeper delay gradient did not appear to be explainable by rapid decision times, the ADHD-control difference was abolished when levels of attention-to-task were controlled. This raises the question of the extent to which the discounting slope is a consequence of failure to fully process decision parameters, i.e., secondary to other cognitive deficits associated with ADHD. Few other studies have examined this possibility.
It was notable, along these lines, that we also found that discounting was associated with symptoms of inattention/disorganization but not with symptoms of hyperactivity/impulsivity. This symptom-related finding was contrary to our hypothesis and to the results reported by Scheres et al (2008; 2010), who reported discounting to be positively associated with hyperactivity/impulsivity symptoms but not inattention symptoms. It is not entirely clear which of the key procedural and sample differences might account for this result difference. However, the usage of very different sets of delays might be critical. The shorter delays in those studies (wait times in the order of seconds and minutes) may be highly sensitive to the degree of hyperactivity/impulsivity exhibited by the ADHD group. In contrast, the longer delays reported here, may be dependent on cognitive capacities related to attention symptoms. This possibility is intriguing for theories of ADHD etiology.
It could be that the influence of reward and reinforcement discounting on ADHD varies with temporal context. Again, future studies can begin to explore whether temporal context and hypothetical versus real reward tasks place different kinds of cognitive demands in such a way that they highlight different aspects of ADHD.
Overall, the present study provides a feasible method of examining discounting gradients in a sample of young children with ADHD. Like other studies of this topic, there was a relatively small sample size, which limited our ability to examine subtype differences. However, these results provide support for a possible amplified discounting gradient in ADHD, and raise questions about the nature of the effect that should encourage future research.
Key Points.
A key underlying process that may contribute to attention-deficit/hyperactivity disorder (ADHD) involves alterations in evaluation of rewards, including the relative value of immediate over delayed rewards.
ADHD is associated with a steeper delay gradient when contemplating hypothetical later rewards, but not independently of IQ.
The interplay of cognitive processing and IQ with reward evaluation in ADHD requires further evaluation.
Acknowledgments
This study was supported by MH59105 (Nigg) and DA024195 (Mitchell).
Footnotes
“Hard” questions (those close to the indifference point) were those that included the lowest value of the immediate option for which a participant consistently preferred that alternative, the highest value of the immediate option for which they consistently preferred the delayed item, and any questions that fell between these two values. All other questions were “easy” questions.
References
- American Psychological Association. Ethical principles of psychologists and code of conduct. American Psychologist. 2002;57:1060–1073. [PubMed] [Google Scholar]
- Antrop I, Stock P, Verte S, Wiersema JR, Baeyens D, Roeyers H. ADHD and delay aversion: The influence of non-temporal stimulation on choice for delayed rewards. Journal of Child Psychology and Psychiatry. 2006;47:1152–1158. doi: 10.1111/j.1469-7610.2006.01619.x. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit/hyperactivity disorder (AD/HD) and oppositional defiant disorder (ODD) Journal of Abnormal Child Psychology. 2001;29:541–546. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- Bitsakou P, Psychogiou L, Thompson M, Sonuga-Barke EJ. Delay aversion in attention-deficit/hyperactivity disorder: An empirical investigation of the broader phenotype. Neuropsychologia. 2009;47:446–456. doi: 10.1016/j.neuropsychologia.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Conners KC. Conners. 3. New York: Multi-Health Systems Inc; 2008. [Google Scholar]
- Cuskelly M, Zhang A, Hayes A. A mental age-matched comparison study of delay of gratification in children with down syndrome. International Journal of Disability, Development, and Education. 2003;50(3):239–251. [Google Scholar]
- de Wit H, Flory JD, Acheson A, McCloskey M, Manuck SB. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Personality and Individual Differences. 2007;42:111–121. [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopolous AD, Reid R. ADHD Rating Scale—IV: Checklists, Norms, & Clinical Interpretation. New York: Guilford; 1998. [Google Scholar]
- Epstein JN, Langberg JM, Lichtenstein PK, Altaye M, Brinkman WB, House K, Stark LJ. Attention-deficit/hyperactivity disorder outcomes for children treated in community-based pediatric settings. Archives of Pediatrics and Adolescent Medicine. 2010;164:160–165. doi: 10.1001/archpediatrics.2009.263. [DOI] [PubMed] [Google Scholar]
- Estle SJ, Green L, Myerson J, Holt DD. Differential effects of amount on temporal and probability discounting of gains and losses. Memory & Cognition. 2006;34:914–28. doi: 10.3758/bf03193437. [DOI] [PubMed] [Google Scholar]
- Goodman R. Psychometric properties of the Strengths and Difficulties Questionnaire (SDQ) Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1337–1345. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, McFadden E. Rate of temporal discounting decreases with amount of reward. Memory & Cognition. 1997;25:715–723. doi: 10.3758/bf03211314. [DOI] [PubMed] [Google Scholar]
- Jepsen JRM, Mortensen EL. Do attention deficits influence IQ assessment in children and adolescents with ADHD? Journal of Attention Disorders. 2009;12:551–562. doi: 10.1177/1087054708322996. [DOI] [PubMed] [Google Scholar]
- Johansen EB, Sagvolden T, Kvande G. Effects of delayed reinforcers on the behavior of an animal model of attention-deficit/hyperactivity disorder (ADHD) Behavioural Brain Research. 2005;162:47–61. doi: 10.1016/j.bbr.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. An algorithm for identifying nonsystematic data in delay discounting research. Experimental and Clinical Psychopharmacology. 2008;16:264–274. doi: 10.1037/1064-1297.16.3.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall PC, Zupan BA, Braswell L. Self-control in children: Further analyses of the self-control rating scale. Behavior Therapy. 1981;12:667–681. [Google Scholar]
- Lahey BB, Pelham WE, Loney J, Lee SS, Willcutt E. Instability of the DSM-IV Subtypes of ADHD from preschool through elementary school. Archives of General Psychiatry. 2005;62:896–902. doi: 10.1001/archpsyc.62.8.896. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Willcutt E. Predictive validity of a continuous alternative to nominal subtypes of attention-deficit hyperactivity disorder in DSM-IV. Journal of Clinical Child and Adolescent Psychology. doi: 10.1080/15374416.2010.517173. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: A review and research agenda. Neuroscience & Biobehavioral Reviews. 2010;34:744–54. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Raiff BR, Lagorio CH, Begotka AM, Mueller AM, Hehli DJ, Wegener AA. Delay discounting of potentially real and hypothetical rewards: II. Between- and within-subject comparisons. Experimental & Clinical Psychopharmacology. 2004;12:251–261. doi: 10.1037/1064-1297.12.4.251. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Johnson PS. A Delay-discounting primer. In: Madden GJ, Bickel WK, Critchfield TS, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2009. pp. 11–37. [Google Scholar]
- Marco R, Miranda A, Schlotz W, Melia A, Mulligan A, Muller U. Delay and reward choice in ADHD: An experimental test of the role of delay aversion. Neuropsychology. 2009;23:367–380. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. The effect of delay and of intervening event on reinforcement value. Quantitative analyses of behavior. Vol. 5. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc; 1987. pp. 55–73. [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Is ADHD a disinhibitory disorder? Psychological Bulletin. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Kuntsi J. Are ADHD symptoms associated with delay aversion or choice impulsivity? A general population study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:837–846. doi: 10.1097/CHI.0b013e3181ab8c97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Massetti GM, Wilson T, Kipp H, Myers D, Newman Standly BB, Billheimer S, Waschbusch DA. Implementation of a comprehensive schoolwide behavioral intervention: The ABC program. Journal of Attention Disorders. 2005;9:248–260. doi: 10.1177/1087054705281596. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Clinical Child Psychology and Psychiatry. 1996;37:57–78. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Puig-Antich J, Ryan N. Kiddie Schedule for Affective Disorders and Schizophrenia. Pittsburgh, PA: Western Psychiatric Institute; 1986. [Google Scholar]
- Rachlin H. Behavior and Learning. San Francisco CA: Freeman; 1976. [Google Scholar]
- Sagvolden T, Metzger MA, Schiørbeck HK, Rugland AL, Spinnangr I, Sagvolden G. The spontaneously hypertensive rat (SHR) as an animal model of childhood hyperactivity (ADHD): changed reactivity to reinforcers and to psychomotor stimulants. Behavior and Neural Biology. 1992;58:103–112. doi: 10.1016/0163-1047(92)90315-u. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behavioral and Brain Sciences. 2005;28:397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, Casellanos FX. Temporal and probabilistic discounting of rewards in children and adolescents: Effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Scheres A, Lee A, Sumiya M. Temporal reward discounting and ADHD: Task and symptom specific effects. Journal of Neural Transmission. 2008;115:221–226. doi: 10.1007/s00702-007-0813-6. [DOI] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Thoeny AL, Kaczkurkin A. Temporal reward discounting in attention-deficit/hyperactivity disorder: The contribution of symptom domains, reward magnitude, and session length. Biological Psychiatry. 2010;67:641–648. doi: 10.1016/j.biopsych.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, Gray JR. Delay discounting and intelligence: A meta-analysis. Intelligence. 2008;36:289–305. [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke EJ, Schachar R, Logan GD, Wigal T. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: A supplement to the NIMH multimodal treatment study of AD/HD. Journal of Abnormal Child Psychology. 2001;29:215–228. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS. Causal models of attention deficit-hyperactivity disorder: From common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward delay in children with attention deficit hyperactivity disorder. Journal of Clinical Child Psychology and Psychiatry. 2001;42:691–698. [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Dopamine transferase deficit: A neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of Child Psychology and Psychiatry. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children, Fourth Edition: Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Wechsler D. Examiner’s Manual. 2. San Antonio: Psychological Corporation; 2005. Weschler Individual Achievement Test. [Google Scholar]
- Yi R, Mitchell SH, Bickel WK. Delay discounting and substance abuse. In: Madden GJ, Bickel WK, Critchfield TS, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2009. pp. 191–211. [Google Scholar]