Abstract
Medulloblastoma encompasses a collection of clinically and molecularly diverse tumor subtypes that together comprise the most common malignant childhood brain tumor1–4. These tumors are thought to arise within the cerebellum, with approximately 25% originating from granule neuron precursor cells (GNPCs) following aberrant activation of the Sonic Hedgehog pathway (hereafter, SHH-subtype)3–8. The pathological processes that drive heterogeneity among the other medulloblastoma subtypes are not known, hindering the development of much needed new therapies. Here, we provide evidence that a discrete subtype of medulloblastoma that contains activating mutations in the WNT pathway effector CTNNB1 (hereafter, WNT-subtype)1,3,4, arises outside the cerebellum from cells of the dorsal brainstem. We found that genes marking human WNT-subtype medulloblastomas are more frequently expressed in the lower rhombic lip (LRL) and embryonic dorsal brainstem than in the upper rhombic lip (URL) and developing cerebellum. Magnetic resonance imaging (MRI) and intra-operative reports showed that human WNT-subtype tumors infiltrate the dorsal brainstem, while SHH-subtype tumors are located within the cerebellar hemispheres. Activating mutations in Ctnnb1 had little impact on progenitor cell populations in the cerebellum, but caused the abnormal accumulation of cells on the embryonic dorsal brainstem that included aberrantly proliferating Zic1+ precursor cells. These lesions persisted in all mutant adult mice and in 15% of cases in which Tp53 was concurrently deleted, progressed to form medulloblastomas that recapitulated the anatomy and gene expression profiles of human WNT-subtype medulloblastoma. We provide the first evidence that subtypes of medulloblastoma have distinct cellular origins. Our data provide an explanation for the marked molecular and clinical differences between SHH and WNT-subtype medulloblastomas and have profound implications for future research and treatment of this important childhood cancer.
SHH-subtype medulloblastoma is characterized by aberrant SHH signaling that is often driven by inactivating mutations in PTCH13,4. These medulloblastomas tend to arise in very young children, display a ‘large cell-anaplastic’ or ‘desmoplastic’ histology and have a relatively poor prognosis2–4. WNT-subtype medulloblastomas are strikingly different. Arising in much older children, these highly curable tumors have ‘classic’ morphology and activating mutations in CTNNB11–4. Mouse models have shown that SHH-subtype medulloblastomas arise from committed GNPCs of the cerebellum7,8 and enabled the development of new therapies that suppress the oncogenic SHH-signal9,10. It has been suggested that the other medulloblastoma subtypes might have a different cellular origin5,11,12, but little is known about their biology and there are no mouse models of these tumors.
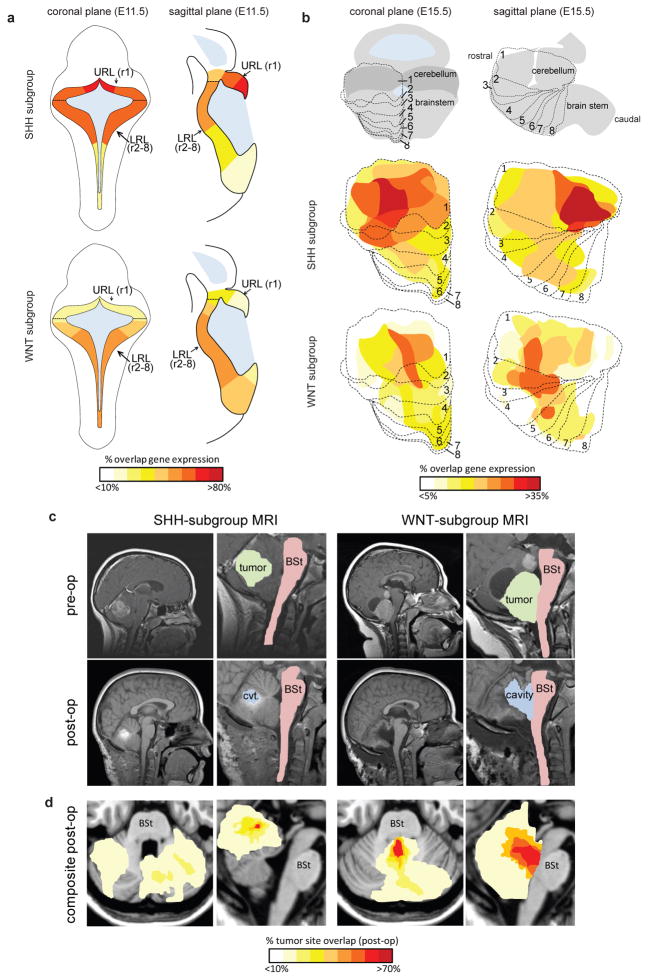
Recently, we showed that subtypes of the brain tumor ependymoma arise from discrete populations of neural progenitor cells with which they share similar gene expression profiles13. Therefore, to determine if medulloblastoma subtypes also arise from discrete cell populations, we first used four online gene expression databases to chart the regional expression of 110 genes that mark human SHH or WNT-subtype medulloblastomas3. Twenty-four WNT-subtype and 25 SHH-subtype medulloblastoma signature genes are contained within ‘Brain Explorer 2’ that generates 3-dimensional gene expression maps across the mouse brain (www.brain-map.org, Supplemental Methods, Supplemental Dataset 1). As expected14, these data revealed the URL at embryonic day (E) 11.5 and the E15.5 cerebellum to be the most common sites of SHH-subtype signature gene expression (Figure 1a,b and Supplemental Dataset 1). In contrast, WNT-subtype medulloblastoma signature genes were predominantly expressed within the E11.5 LRL (rhombomeres [r]2-r8) and the E15.5 dorsal brainstem. Expression of an additional 61 medulloblastoma signature genes, reported by three other online databases, confirmed this differential pattern (Supplemental Figure 1, Supplemental Table 1). These data suggest that SHH and WNT-subtype medulloblastomas arise from distinct regions of the hindbrain and identify the dorsal brainstem as a potential source of WNT-subtype tumors.
Figure 1. WNT and SHH-subtypes of medulloblastoma are anatomically distinct.
(a) Expression distribution in (a) E11.5 and (b) E15.5 mouse hindbrain of orthologs that distinguish human WNT and SHH-subtype medulloblastoma (Supplemental Dataset 1). Cartoons in (b) denote the position of rhombomeres relative to the cerebellum and brainstem. (c) Top=pre and bottom=post-operative MRI scans of exemplary SHH and WNT-subtype medulloblastomas. Right panels show close up views of left. Brainstem (BSt), post-operative tumor cavity (cvt.). (d) Frequency and site of post-operative surgical cavities of SHH (n=6) and WNT (n=6)-subtype medulloblastomas. Axial (left) and sagittal (right) views are shown.
If SHH and WNT-subtype medulloblastomas have different origins then we reasoned that these tumors should demonstrate anatomical differences at diagnosis. Remarkably, all validated WNT-subtype medulloblastomas examined (n=6/6, Supplemental Figure 2) were located within the IV ventricle and infiltrated the dorsal surface of the brainstem; while all SHH-subtype tumors (n=6/6) were distributed away from the brainstem within the cerebellar hemispheres (Figure 1c,d, Supplemental Figure 3, exact Mann-Whitney P<0.005). Five of the six WNT-subtype, but no SHH-subtype tumors, were adherent to the dorsal brainstem at surgery (Fisher’ s Exact Test, P<0.005). Thus, WNT-subtype medulloblastomas are anatomically distinct from SHH-tumors and are intimately related to the IV ventricle and dorsal brainstem.
We noted various cell types surrounding the IV ventricle that could give rise to WNT-subtype medulloblastomas, including dorsal brainstem progenitors of cochlear, mossy fiber (MF) and climbing fiber (CF) neurons (Figure 1a,b; Supplemental Figure 4)15. But it remained possible that cerebellar ventricular zone (VZ) radial glia12,16 or GNPCs generate WNT-subtype medulloblastomas. To identify hindbrain cells that are susceptible to transformation by Ctnnb1, we generated mice carrying a cre-dependent mutant allele of Ctnnb1 (Ctnnb1lox(ex3))17 and the Blbp-Cre transgene18. Blbp-Cre induces efficient recombination in progenitor cell populations across the hindbrain including the cerebellar VZ, GNPCs of the external germinal layer (EGL) and Olig3+ progenitor cells in the LRL19 (Supplemental Figure 5). We also generated Blbp-Cre+/− ; Ctnnb1+/lox(ex3) (hereon, Ctnnb1-mutant) mice that were homozygous for a cre-dependent mutant allele of Tp53 (Tp53flx)20 since loss of this tumor suppressor accelerates medulloblastoma formation in Ptch1+/− mice21. As expected, Ctnnb1-mutant embryos expressed mutant nuclear-Ctnnb1 in all hindbrain germinal zones, regardless of Tp53 status (Supplemental Figures 5k and 6). Surprisingly, mutation of Ctnnb1 did not affect significantly the proliferation or apoptosis of VZ cells or GNPCs in the cerebellum (Figure 2a and Supplemental Figure 7).
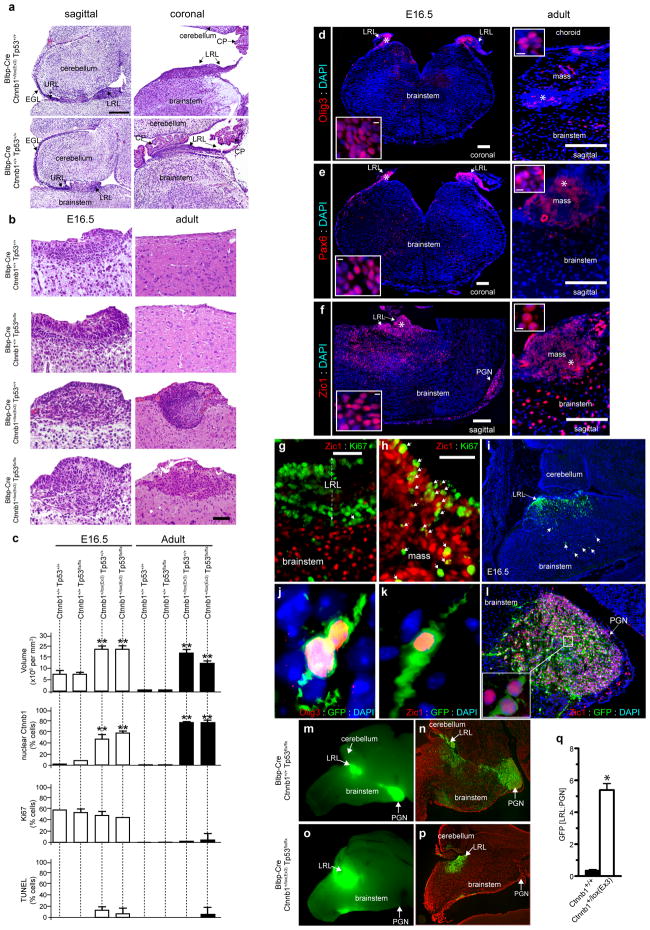
Figure 2. Mutant-Ctnnb1 causes aberrant accumulation of LRL cells.
(a) Low (scale=180 μm) and (b) high (scale=50 μm) power views of LRL/dorsal brainstem in Ctnnb1 mutant and wild-type E16.5 embryos. (b) Includes the corresponding adult brainstem region. (c) Volume and indicated immunoreactivity differences between Ctnnb1-mutant and wild-type LRL (n≥3 mice per group, bars=mean ±S.D). Immunofluorescence of Olig3 (d), Pax6 (e), Zic1 (f) in Ctnnb1-mutant E16.5 LRL (left) and aberrant adult dorsal brainstem masses (right, scale=180 μm). Inset=high-power views of ‘*’ (scale=5 μm). (g) Postmitotic MF precursor neurons (Zic1+/Ki67-) exit the proliferating E16.5 control LRL. (h) Ctnnb1-mutant LRL contains aberrant proliferating Zic1+ precursors (arrows, scale=50 μm). (i) GFP-electroporated wild-type LRL marks Olig3+ cells (j) and migrating precursors (arrows in i) that include Zic1+ MF neurons (k) that form the PGN (l). GFP-fluorescence of whole (m,o) and sectioned (n,p) Ctnnb1-mutant and wild-type P0 hindbrains electroporated at E12.5. (q) Mean ± SD of LRL:PGN GFP-fluorescence in whole hindbrains of three BlBp-Cre ; Ctnnb1+/+ and five Blbp-Cre ; Ctnnb1+/lox(Ex3) mice (graphs, *≤0.05, **≤0.005, Exact Mann-Whitney P).
Since GNPCs generate SHH-subtype medulloblastomas7,8 we sought additional evidence that these cells are not impacted by mutant Ctnnb1. First, we generated Atoh1-Cre+/− ; Ctnnb1+/lox(ex3) mice since Atoh1-Cre drives efficient recombination in GNPCs, generating medulloblastomas in conditional Ptch1 mice (see Supplemental Figure 8a–j and Ref. 7). We also used the Atoh1 enhancer element present in the Atoh1-Cre allele, to drive expression of a constitutively active Ctnnb1-green fluorescence fusion protein in GNPCs (Atoh1-Ctnnb1ΔN90GFP, Supplemental Figure 8k–o)22. Neither Atoh1-Cre+/− ; Ctnnb1+/lox(ex3) nor Atoh1-Ctnnb1ΔN90GFP mice (>20 mice examined each) developed hyperplasia or masses within the URL or EGL. Concordantly, aberrant Ctnnb1 signaling did not impact the proliferation of GNPCs ex vivo (Supplemental Figure 8p). Thus, in contrast to aberrant Shh signaling, mutant Ctnnb1 does not appear to disrupt cell cycle or differentiation control in GNPCs.
In stark contrast to the cerebellum, by E16.5 all Ctnnb1-mutant mice developed aberrant cell collections in the dorsal brainstem that persisted into adulthood (exact Mann-Whitney P<0.005, Figure 2a–f). These cells were marked by Olig3 and Pax6 suggesting they may be derived from progenitor cells within the LRL19,23 (Figure 2d,e). This abnormality was independent of Tp53 status and did not involve the floor plate that is not targeted by Blbp-Cre (Supplemental Figure 9). Progenitors within the embryonic dorsal brainstem proliferate to produce daughter cells that express specific marker proteins and follow complex migration streams to their respective nuclei in the developing brainstem (Supplemental Figure 4)15. We observed no significant differences in the overall proliferation (Ki-67 labeling), apoptosis (TUNEL labeling) or cell cycle duration (5-bromo-2-deoxyuridine pulse-chase) of progenitors in the dorsal brainstem of Ctnnb1-mutant versus control mice (Figure 2c, data not shown). However, a significant fraction of proliferating cells within Ctnnb1-mutant dorsal brainstems expressed Zic1 (37% Zic1+/Ki67+=122/322; Figure 2c,f–h). This expression is aberrant since Zic1 normally marks postmitotic MF neuron precursors as they exit the dorsal brainstem to form nuclei in the ventral brainstem23,24 (Figure 2g). Thus, mutant Ctnnb1 might stall the dorso-ventral migration of brainstem neuron precursors, resulting in aberrant dorsal cell collections25. To test this, we used in utero GFP-electroporation to track the fate of embryonic dorsal brainstem precursors (Figure 2i–q; Supplemental Figures 10–11). GFP-labeled Zic1+ MF neuron precursors underwent normal migration from the dorsal brainstem to the PGN and other brainstem nuclei in control mice (Figure 2k–n; Supplemental Figure 11). In contrast, mutation of Ctnnb1 markedly reduced the numbers of precursors transiting from the dorsal brainstem to the PGN (Figure 2o–q; exact Mann-Whitney, P<0.05). Together, these data demonstrate that mutant Ctnnb1 disrupts the normal differentiation and migration of progenitor cells on the dorsal brainstem, resulting in the accumulation of aberrant cell collections. These cells may include stalled MF neuron precursors, but further work is required to determine their precise lineage.
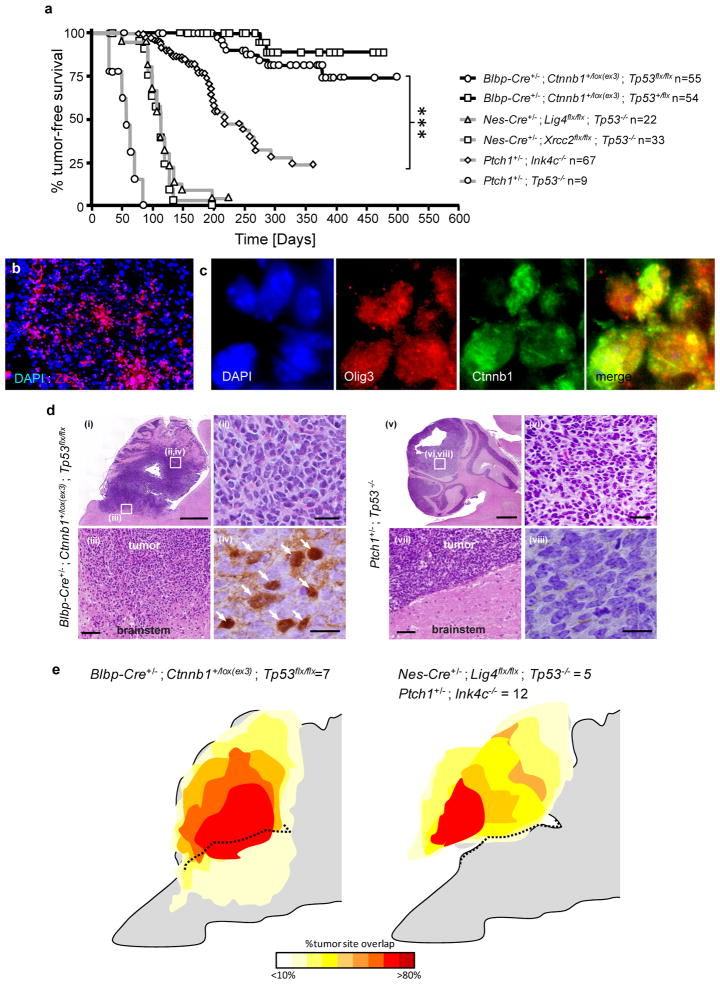
Aberrant cell collections in the dorsal brainstem of Ctnnb1-mutant mice are reminiscent of the EGL hyperplasia that precedes formation of SHH-subtype medulloblastoma in the cerebellum of Ptch1 deficient mice26. Therefore, we aged Ctnnb1-mutant mice harboring Tp53+/+, Tp53+/flx or Tp53flx/flx alleles to test if WNT-subtype medulloblastomas might arise from the dorsal brainstem (n>54 mice per genotype). Aberrant cell collections persisted throughout adulthood on the dorsal brainstem of all Ctnnb1-mutant ; Tp53+/+ mice but these animals did not develop medulloblastoma or tumors in any part of the hindbrain (median follow up 365 days). In contrast, 2 of 10 Ctnnb1-mutant ; Tp53flx/flx mice aged <6 months harbored asymptomatic tumors that were confined to the dorsal brainstem (Supplemental Figure 12). When aged for longer periods, 15% (n=8/55) of Ctnnb1-mutant ; Tp53flx/flx and 4% (n=2/54) Ctnnb1-mutant ; Tp53+/flx mice developed ‘classic’ medulloblastomas that were Zic1+ and contained populations of nuclear-Ctnnb1+/Olig3+ cells (median follow up 290 and 287 days, respectively; Figure 3a–d). These mouse medulloblastomas displayed an immunoprofile similar to human WNT-subtype tumors and were invariably connected with the brainstem (Figure 3d–e; Supplemental Figure 13). In contrast, mouse models of human SHH-subtype medulloblastoma21,27,28 are nuclear-Ctnnb1 negative, arise within the cerebellum and do not invade the brainstem (Figure 3d,e). Together, these data support the hypothesis that progenitor cells within the dorsal brainstem are susceptible to transformation by concurrent mutation in Ctnnb1 and Tp53, resulting in the formation of tumors that mimic the anatomical features of human WNT-subtype medulloblastoma. Deletion of Tp53 is presumably required to permit key second mutations during transformation of the LRL in Ctnnb1-mutant mice. Notably, we have observed two cases of TP53-mutant human WNT-subtype medulloblastoma, suggesting this gene also suppresses these tumors in humans (Supplemental Figure 14).
Figure 3. Mutant-Ctnnb1 and SHH-subtype mouse medulloblastomas are anatomically distinct.
(a) Tumor free survival of SHH-subtype medulloblastoma mouse models (Nes-Cre+/−; Lig4flx/flx ; Tp53−/−, Nes-Cre+/−; Xrcc2flx/flx ; Tp53−/−, Ptch1+/−; Ink4c−/−, Ptch1+/−; Tp53−/− data from Refs.14,27,28) and Ctnnb1-mutant ; Tp53flx/flx and Ctnnb1-mutant ; Tp53+/flx mice. ***=Log Rank P<0.0001. Immunoflourescence of (b) Zic1 and (c) Olig3 and Ctnnb1 expression in a Ctnnb1-mutant ; Tp53flx/flx medulloblastoma. (d) Hematoxylin and eosin stained low (i, v; scale=800 μm) and high (ii, vi; scale=25 μm) power views of mouse medulloblastomas and tumor-brainstem interface (iii, vii; scale bar=50μm). Ctnnb1 immunostaining (iv, viii; scale=10 μm, arrows indicate nuclear immunoreactivity). Boxes indicate location of high power views. (e) Frequency and anatomical site of mouse medulloblastomas.
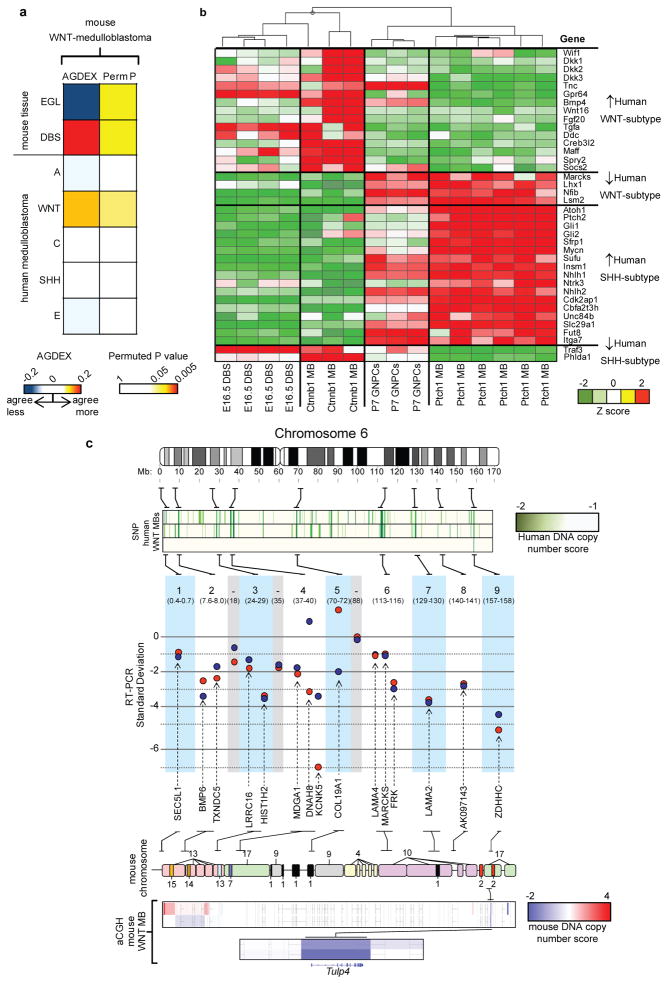
To test further the fidelity of Ctnnb1-mutant mouse medulloblastoma as a model of human WNT-subtype disease, we compared the tumor transcriptomes in the two species using an algorithm we have developed for cross-species genomic comparisons13. Remarkably, the transcriptome (n=11,049 orthologs) of Ctnnb1-mutant ; Tp53flx/flx medulloblastomas matched only human WNT-subtype medulloblastoma and the cells of the embryonic dorsal brainstem (both permuted P<0.05), validating it as a model of this human tumor subtype and further pinpointing the brainstem as the source of WNT-subtype medulloblastomas (Figure 4a,b). Finally, since human WNT-subtype medulloblastomas selectively delete chromosome 6 (Ref. 3), we looked in Ctnnb1-mutant mouse medulloblastomas to see if syntenic regions of this chromosome are deleted (Figure 4c). DNA microarray analysis identified a single common deletion of mouse chromosome 17 3.2 cM/human 6q25.3 in tumors in the two species. This locus encodes a single gene, TULP4, that is a distant member of the tubby-gene family implicated in regulating neuronal cell apoptosis29. Thus, Ctnnb1-mutant ; Tp53flx/flx mouse medulloblastomas accurately model the molecular characteristics of human WNT-subtype tumors and pinpoint TULP4 as a novel candidate suppressor gene of these tumors. By demonstrating that subtypes of medulloblastoma have distinct cellular origins our data should significantly accelerate the hunt for curative treatments of these diseases which must now account for the different developmental origins of these tumors.
Figure 4. Mutant-Ctnnb1 mouse medulloblastomas recapitulate the molecular characteristics of human WNT-subtype disease.
(a) AGDEX comparison of Ctnnb1-mutant ; Tp53flx/flx mouse medulloblastoma, and mouse EGL, E16.5 dorsal brainstem (DBS) and human medulloblastoma subgroups. (b) Unsupervised clustering of human WNT and SHH-subtype medulloblastoma signature ortholog expression in E16.5 DBS, Ctnnb1-mutant ; Tp53flx/flx mouse medulloblastoma (Ctnnb1 MB), P7 GNPCs and Ptch1+/−; Tp53−/− medulloblastoma (Ptch1 MB). (c) Top-bottom: Nine SNP-inferred homozygous deletions in three human WNT-subtype medulloblastomas. Real-Time PCR validation of deletions in the human tumors (SD below the mean diploid copy-number). Mouse chromosomal regions syntenic for human chromosome 6. ArrayCGH-inferred copy number in Ctnnb1-mutant ; Tp53flx/flx mouse medulloblastomas identifies common syntenic deletion of TULP4.
Methods Summary
MRI analysis
MRI images of patients were spatially normalized into a standard stereotaxic space for quantitative comparison of tumor location (SPM5; www.fil.ion.ucl.ac.uk/spm). Radiologists masked to patient subtype determined the 3-dimensional location of the tumor or surgical cavity relative to predefined anatomical landmarks.
Expression mapping
The expression of mouse orthologs of key signature genes of human WNT and SHH-subtype medulloblastoma (Supplemental Dataset 1 and Supplemental Table) were mapped in the developing mouse hindbrain using four publically accessible datasets (see Supplemental Methods).
Mouse studies
Blbp-Cre, Ctnnb1lox(ex3)/lox(ex3), Atoh1-Cre and Tp53flx/flx mice were bred to generate appropriate genotypes and subject to clinical surveillance for signs of tumor development. RosaYFP and RosaLacz reporter strains traced the lineage of Cre-recombined cells. Mouse tumors comprised ≥85% tumor cells. Atoh1-Ctnnb1ΔN90 transgenic mice were generated by pro-nuclear injection. In utero electroporation and cell tracking were performed by anesthetizing pregnant mice of the appropriate genotype. The uterus was externalized and the dorsal brainstem of E12.5 embryos electroporated with CMV-eGFP plasmid DNA. GNPCs for culture studies were isolated from postnatal day 7 Atoh1-GFP transgenic mice. 2 × 105 GFP+ cells/well were cultured in poly-D-lysine-coated 96-well plates and challenged with mutant-Ctnnb1-GFP, control GFP virus, Wnt1 protein (50ng/ml) or Shh supernatant (3ug/ml) prior to pulsing with [methyl-3H]thymidine and scintillation counting.
Histology, mRNA and DNA microarray profiling
Immunohistochemistry was performed using routine techniques and primary antibodies of the appropriate tissues as described (Supplemental Methods). Cells undergoing apoptosis were detected with the Apoptag kit (Millipore, S7100). mRNA expression (GEO accession number GSE24628) and DNA copy number profiles (available at http://stjuderesearch.org/site/authors/gilbertson) were generated from mouse and human tissues using appropriate microarray platforms as detailed (Supplemental Methods). Reverse transcriptase Real Time-PCR analysis and gene re-sequencing of human medulloblastomas were performed as described previously3. mRNA expression and DNA microarray profiles of human and mouse medulloblastomas were integrated using established and novel bioinformatic and statistical approaches.
Supplementary Material
Acknowledgments
R.J.G. holds the Howard C. Schott Research Chair from the Malia's Cord Foundation, and is supported by grants from the National Institutes of Health (R01CA129541, P01CA96832 and P30CA021765), the Collaborative Ependymoma Research Network (CERN) and by the American Lebanese Syrian Associated Charities (ALSAC). We are grateful to Anjen Chenn, Jane Johnson and Carmen Birchmeier for their generous gifts of reagents and the staff of the Hartwell Center for Bioinformatics and Biotechnology and ARC at St Jude Children’ s Research Hospital for technical assistance.
Footnotes
Author contributions. R.J.G. conceived the research and planned experiments. P.G., also planned and conducted the great majority of the experiments. Y.T., G.R., D.S.C., M.C.T., T.H., H.P., J.M., J.C.L., Y.L., F.Z., C.E., S.C.C., M.F.R., P.J.M., and R.W-R., conducted experiments. D.F., and S.P., provided bioinformatic expertise. A.G., F.A.B., and R.A.S., provided clinical advice and tumor samples. D.H.G., provided the Blbp-Cre mouse and data. M.M.T., provided the Ctnnb1lox(ex3)/lox(ex3) mouse. Z.P., and R.O., reviewed and analyzed the human MRI scans. D.W.E., provided pathology review. All authors contributed to the writing of the manuscript.
Competing interests.
None.
Supplemental Information is available online.
References
- 1.Ellison DW, et al. beta-Catenin status predicts a favorable outcome in childhood medulloblastoma: the United Kingdom Children's Cancer Study Group Brain Tumour Committee. J Clin Oncol. 2005;23:7951–7957. doi: 10.1200/JCO.2005.01.5479. [DOI] [PubMed] [Google Scholar]
- 2.Gajjar A, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 3.Thompson MC, et al. Genomics identifies medulloblastoma subgroups that are enriched for specific genetic alterations. J Clin Oncol. 2006;24:1924–1931. doi: 10.1200/JCO.2005.04.4974. Epub 2006 Mar 1927. [DOI] [PubMed] [Google Scholar]
- 4.Kool M, et al. Integrated genomics identifies five medulloblastoma subtypes with distinct genetic profiles, pathway signatures and clinicopathological features. PLoS One. 2008;3:e3088. doi: 10.1371/journal.pone.0003088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbertson RJ, Ellison DW. The Origins of Medulloblastoma Subtypes. Annu Rev Pathol. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- 6.Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 7.Schuller U, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–134. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang ZJ, et al. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells. Cancer Cell. 2008;14:135–145. doi: 10.1016/j.ccr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romer JT, et al. Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−)p53(−/−) mice. Cancer Cell. 2004;6:229–240. doi: 10.1016/j.ccr.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Rudin CM, et al. Treatment of Medulloblastoma with Hedgehog Pathway Inhibitor GDC-0449. N Engl J Med. 2009;2:2. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Louis D, Ohgaki H, Wiestler O, Cavenee W, editors. World Health Organization Classification of Tumours of the Central Nervous System. IARC; Lyon: 2007. [Google Scholar]
- 12.Huang X, et al. Transventricular delivery of Sonic hedgehog is essential to cerebellar ventricular zone development. Proc Natl Acad Sci U S A. 2010;107:8422–8427. doi: 10.1073/pnas.0911838107. Epub 2010 Apr 8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson RA, et al. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466:632–636. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, et al. A molecular fingerprint for medulloblastoma. Cancer Res. 2003;63:5428–5437. [PubMed] [Google Scholar]
- 15.Ray RS, Dymecki SM. Rautenlippe Redux -- toward a unified view of the precerebellar rhombic lip. Curr Opin Cell Biol. 2009;21:741–747. doi: 10.1016/j.ceb.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morales D, Hatten ME. Molecular markers of neuronal progenitors in the embryonic cerebellar anlage. J Neurosci. 2006;26:12226–12236. doi: 10.1523/JNEUROSCI.3493-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. Embo J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegedus B, et al. Neurofibromatosis-1 Regulates Neuronal and Glial Cell Differentiation from Neuroglial Progenitors In Vivo by Both cAMP- and Ras-Dependent Mechanisms. Cell Stem Cell. 2007;1:443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Storm R, et al. The bHLH transcription factor Olig3 marks the dorsal neuroepithelium of the hindbrain and is essential for the development of brainstem nuclei. Development. 2009;136:295–305. doi: 10.1242/dev.027193. Epub 2008 Dec 2015. [DOI] [PubMed] [Google Scholar]
- 20.Jonkers J, et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 21.Wetmore C, Eberhart DE, Curran T. Loss of p53 but not ARF accelerates medulloblastoma in mice heterozygous for patched. Cancer Res. 2001;61:513–516. [PubMed] [Google Scholar]
- 22.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 23.Landsberg RL, et al. Hindbrain rhombic lip is comprised of discrete progenitor cell populations allocated by Pax6. Neuron. 2005;48:933–947. doi: 10.1016/j.neuron.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 24.DiPietrantonio HJ, Dymecki SM. Zic1 levels regulate mossy fiber neuron position and axon laterality choice in the ventral brain stem. Neuroscience. 2009;162:560–573. doi: 10.1016/j.neuroscience.2009.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farago AF, Awatramani RB, Dymecki SM. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50:205–218. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Oliver TG, et al. Loss of patched and disruption of granule cell development in a pre-neoplastic stage of medulloblastoma. Development. 2005;132:2425–2439. doi: 10.1242/dev.01793. Epub 2005 Apr 2420. [DOI] [PubMed] [Google Scholar]
- 27.Uziel T, et al. The tumor suppressors Ink4c and p53 collaborate independently with Patched to suppress medulloblastoma formation. Genes Dev. 2005;19:2656–2667. doi: 10.1101/gad.1368605. Epub 2005 Oct 2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frappart PO, et al. Recurrent genomic alterations characterize medulloblastoma arising from DNA double-strand break repair deficiency. Proc Natl Acad Sci U S A. 2009;106:1880–1885. doi: 10.1073/pnas.0806882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda A, Ikeda S, Gridley T, Nishina PM, Naggert JK. Neural tube defects and neuroepithelial cell death in Tulp3 knockout mice. Hum Mol Genet. 2001;10:1325–1334. doi: 10.1093/hmg/10.12.1325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.