Abstract
Background
Mast cells and basophils are key effector cells of IgE-mediated anaphylactic reactions. The Chinese herbal formula, FAHF-2, protects against peanut anaphylaxis in mice. However, the mechanisms underlying this effect are not fully elucidated.
Objective
To investigate whether FAHF-2 inhibits mast cell/basophil numbers and IgE mediated activation.
Methods
Peanut allergic mice (PNA mice) were treated with FAHF-2 intragastrically (i.g.) for 7 weeks and challenged (i.g.) with peanut 1 day and 4 weeks post treatment. Peripheral blood basophil numbers and peritoneal mast cell numbers and FcεRI expression were determined. Direct effects of FAHF-2 on murine mast cell line MC/9, and effects of four fractions and three compounds isolated from FAHF-2 on rat basophilic leukemia cells (RBL-2H3) and human skin mast cells degranulation, and on the IgE mediated Syk signaling pathway were determined.
Results
While all sham-treated PNA mice developed anaphylaxis, FAHF-2-treated PNA mice were protected against anaphylaxis following peanut challenge at 1 day and 4 weeks post therapy. Reduction of peripheral blood basophils began after 1 week of treatment and continued for at least 4 weeks post therapy. The number and FcεRI expression of peritoneal mast cells were also significantly decreased 4 weeks post therapy. FAHF-2 treated MC/9 cells showed significantly reduced IgE-induced FcεRI expression, FcεRI γ mRNA subunit expression, proliferation, and histamine release upon challenge. Fraction 2 (F2) from FAHF-2 inhibited RBL-2H3 cell and human mast cell degranulation. Three compounds from F2- berberine, palmatine and jatrorrhizine inhibited of RBL-2H3 cell degranulation via suppressing Syk phosphorylation.
Conclusion
FAHF-2 reduction of basophils and mast cell numbers as well as suppression of IgE-mediated mast cell activation may contribute to FAHF-2 persistent protection against peanut anaphylaxis.
Clinical Implications
FAHF-2 blocked peanut anaphylaxis. This effect was associated with reduction of mast cells/basophils activation and proliferation. FAHF-2 may be a novel anti-peanut anaphylaxis therapy.
Keywords: peanut anaphylaxis, Chinese herbal medicine, mast cells/basophils, FcεRI
INTRODUCTION
Anaphylaxis is a potentially life-threatening allergic reaction of rapid-onset. Food allergy reactions account for one-third to one-half of anaphylaxis cases in emergency departments.(1) Food-induced anaphylaxis is an IgE-mediated Type I hypersensitivity reaction, in which mast cells and basophils are key effector cells. Mast cells, released from the bone marrow as undifferentiated precursor cells mature in tissues, where they reside. In contrast, basophils complete their differentiation in the bone marrow and subsequently circulate in the blood stream.(2) Both cells express high affinity IgE receptors (FcεRI), which bind to specific IgE molecules. Upon exposure, specific allergen cross-links the surface IgE molecules, which stimulate mast cells and basophils to release potent preformed mediators such as histamine. Reduction of FcεRI expression is an active research area with the goal of preventing or treating allergic disorders.(3)Animal models are important tools for testing potential therapeutic modalities for food allergy and exploring novel pharmacological agents. Using a well-established murine model of peanut (PN) anaphylaxis, we found that the food allergy herbal formula-2 (FAHF-2) abrogated anaphylactic reactions in mice and this protection persisted for at least 36 weeks after therapy was discontinued. (4–6) The increased IFN-γ production by CD8+ T cells, as shown previously, may be an important mechanism underlying FAHF-2 modulated potent and long-term protection.(4;6) However, neutralization of IFN-γ or depletion of CD8+ T cells blocked the suppression of IgE and Th2 cytokine production induced by FAHF-2, while protection from anaphylaxis still existed up to 4 weeks post therapy.(6) We therefore hypothesized that FAHF-2 may also directly inhibit mast cells/basophils.
In this communication, we investigated the effect of FAHF-2 on the number of peripheral blood basophils and peritoneal mast cells, as well as mast cell FcεRI expression in PN allergic mice. We further investigated FAHF-2 in vitro effects on murine mast cell (MC/9 cells) proliferation, histamine release, and FcεRI subunits expression in response to IgE. Using preparative High Performance Liquid Chromatography (prep-HPLC), we identified and tested the effect of four fractions (F) and 3 major alkaloid compounds on rat mast cells (RBL-2H3) and human skin mast cells. In addition, we determined the mechanism by which the isolated compounds inhibit mast cell degranulation.
METHODS
Mice and reagents
C3H/HeJ mice (female, 5 week-old) purchased from the Jackson Laboratory (Bar Harbor, ME) were maintained on PN-free chow under specific pathogen-free conditions according to standard guidelines for the care and use of animals. Freshly ground, whole roasted PN were prepared as previously described.(7;8) Cholera toxin (CT) was purchased from List Biological Laboratories, Inc (Campbell, CA). MC/9 cells (mouse mast cell line) and RBL-2H3 cells (rat basophilic leukemia cell line) were obtained from American Type Culture Collection (Manassas, Virginia). FAHF-2 is dried powder of aqueous extract of 9 herbs. The formula and process of preparation of the final product are the same as described in previous publications(6;9) and included in repository (Methods E1). To identify active compounds, FAHF-2 was first extracted with butanol, then dried, and then further divided into 4 fractions (F) based on polarity by prep-HPLC. Compounds were identified by liquid chromatography mass spectrometry (LC MS) with standard compounds as described in details in the repository (Methods E2).
Antibodies
Phycoerythrin (PE) –conjugated mAbs specific for mouse FcεRI mAb (MAR-1), allophycocyanin (APC)-conjugated anti- mouse Gr-1 (RB6-8C5), anti-c-kit (2B8), fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgE (23G3) and FITC-conjugated control hamster IgG were obtained from eBioscience (San Diego, CA). FITC-conjugated anti-mouse CCR3 mAb (83101) was purchased from R&D Systems (Minneapolis, MN). PE-conjugated anti-mouse CD3e(145-2C11), PE-conjugated anti- mouse B220(RA3-6B2), APC-conjugated anti-mouse CD49b(DX5), FITC conjugated anti-mouse I-Ak (AbK, 10-3.6) and purified anti-mouse CD16/32 mAb (2.4G2) were purchased from BD Biosciences (San Diego, CA). Mouse anti-dinitrophenol (DNP) IgE was purchased from Sigma-Aldrich (St. Louis, MO).
Protocol for PN sensitization/challenge, FAHF-2 treatment and assessment of anaphylaxis
Mice were sensitized intragastrically (i.g.) with ground PN (10 mg/mouse) weekly for 5 wks, and boosted at weeks 6 and 8 (50 mg/mouse) (Figure 1A), as previously described.(4) FAHF-2 was administered 24h after the wk 8 boost, at which time PN hypersensitivity was established.(4) FAHF-2 was administered i.g. at 32 mg in 0.5 ml water, twice daily for 7 wks. The daily dose of FAHF-2 used in this study was calculated by normalizing the daily human dose to mouse body weight as previously described. (4;5)An additional group of PNA mice received equal volume of water (sham). Both groups exhibited equal levels of PN specific IgE prior to treatment. Naïve mice served as normal controls. All mice were PN challenged i.g. (200 mg/mouse) 1 day (wk 14) and 4 weeks post treatment (wk 18). CT as mucosal adjuvant at 20μg/mouse was co-administered with PN at all times except at final challenge. Anaphylactic symptoms were evaluated approximately 30 minutes after the challenge dose utilizing a previously described scoring system(4;5) (Repository Methods E3) in a blinded manner. Rectal temperatures were measured approximately 30 minutes following challenge using a thermal probe (Harvard Apparatus, Newark, NJ). Plasma histamine levels were determined 30 minutes after the second challenge and analyzed using an enzyme immunoassay kit (ImmunoTECH Inc., Marseille, France) as described by the manufacturer.
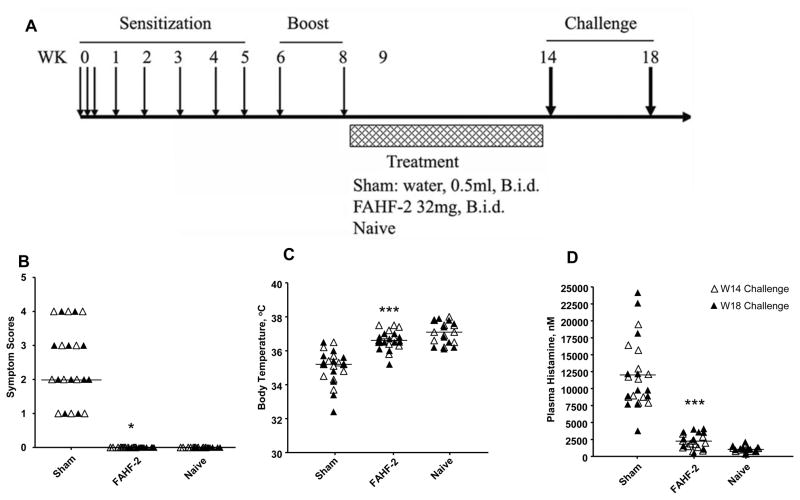
Figure 1.
A. Experimental protocol. Female C3H/HeJ mice were sensitized intragastrically with freshly ground roasted peanut together with cholera toxin as indicated and treated with FAHF-2 (n = 10), or water (Sham, n = 10). All mice were orally challenged with peanut at wk14 and 18. Naïve mice were served as controls (n = 10). B. Anaphylactic symptom scores. C. Post-challenge body temperatures. D. Post-challenge plasma histamine levels. Bars in B indicates median of scores of mice from each group with combined data at wk 14 (open triangles) and wk 18 challenges (solid triangles). (Sham, n=20; FAHF-2, n=20; and naïve, n=20). Bars in C and D are means of each group of mice from combined data at wk 14 (open circles) and wk 18 (open triangles) challenges. *, p< 0.05; ***, p<0.001 vs sham.
Flow cytometry measurement of basophil number, peritoneal mast cell number and Fcε RI expression
Peripheral blood leukocytes (PBLCs) were obtained before FAHF-2 treatment, then weekly during treatment and 4 weeks post therapy from all mice, as shown in figure 1A. Approximately 150μl of EDTA anti-coagulated blood samples per mouse were pooled and subjected to red cell lysis using Pharmlyse(BD Biosciences, CA). PBLCs (1×106) were incubated in 100μl of staining buffer (HBSS containing 0.02%NaN3 and 2 mM EDTA, 0.1%BSA, 2% FBS, 1% normal mouse serum, 1% normal rat serum) and 20 μg/mlof purified anti-mouse CD16/32mAb (2.4G2) as Fcγ receptor–blocking mAb for 30 minutes at 4°C. PE-conjugated anti-mouse FcεRI, FITC-conjugated anti-mouse I-Ak, APC conjugated Gr 1 were then added to the cell suspension in the presence of Fcγ receptor blocking mAb on ice for 30 minutes. After washing, cells were acquired on an LSR-II flow cytometer (BD Bioscience, CA) and data was analyzed using Flowjo software (Tree Star, Inc. Ashland, OR). FcεRI +CCR3−Gr− is defined as mouse blood basophils. (10–12)
Two days after the final challenge at wk 18, mice were sacrificed, and peritoneal cavity cells were lavaged using 10 ml of cold HBSS. Peritoneal cavity cells from every 3–4 mice in each group were combined in a total three subsets. 5×105 cells were incubated in 100 μl of HBSS staining buffer containing 20 μg/mlof purified anti-mouse CD16/32mAb (2.4G2) as Fcγ receptor–blocking mAb for 30 minutes at 4°C. They were then incubated with the following antibodies for 30 min: PE conjugated anti-mouse FcεRI, FITC conjugated anti-mouse I-Ak and APC conjugated anti-mouse c-kit. After washing, cell acquisition and data analysis were performed as described above.
Flow cytometric determination of FAHF-2 effects on murine MC/9 mast cell FcεRI expression in vitro
MC/9 cells cultured at 5×105/ml in Dulbecco’s Modified Eagle Medium(DMEM) containing 10% FBS were incubated with 2μg/ml of mouse anti-DNP IgE (Sigma-Aldrich, St. Louis, MO) in the presence or absence of different dose of FAHF-2 (at 10 or 20 μg/ml) for 48 hours. After washing, cells were then incubated in 100 μl of HBSS buffer containing 20 μg/mlof purified anti-mouse CD16/32mAb (2.4G2) as Fcγ receptor blocking mAb for 30minutes at 4°C as previous described.(13) Purified mouse anti-DNP IgE (2μg/ml) was then added to the cell suspension in the presence of Fcγ receptor blocking antibody and incubated on ice for another 30 minutes to saturate any FcεRI expressed by these cells as previously described.(13) After washing twice, 1μg of FITC conjugated rat anti-mouse IgE was added to 100μl of cell suspension in HBSS containing 0.1% BSA and incubated for 30minutes at 4°C. FITC conjugated rat IgG1 was used as isotype control. After washing twice, cell acquisition and data analysis were performed as described above.
Determination of the effect of FAHF-2 on mast cell proliferation and degranulation in vitro
To examine the effect of FAHF-2 on mast cell growth, 1×105 MC/9 cells were cultured in 200μl of DMEM containing 10% FBS in the presence or absence of 2μg/ml mouse anti-DNP IgE with or without 20μg/ml FAHF-2. Cell viability was assessed 24h, 48h and 72h after treatment by trypan blue exclusion. (14) The fold of cell growth was calculated (number of viable cells at 24h, 48h and 72h divided by the number of cells on day 0). The percent of trypan blue positive cells was used to assess toxicity.
Mast cell degranulation was determined by measuring histamine release as previously described.(15;16) Briefly, 1×105 of MC/9 cells were cultured in 200μl of DMEM containing 10% FBS in the presence of 2μg/ml of mouse anti-DNP IgE. Cells were co-cultured with or without FAHF-2 at 20μg/ml for 48 hrs. This concentration was used based on the above in vitro study showing FAHF-2 markedly reduced Fcε RI expression by MC/9 mast cells at 20μg/ml. The cells were then collected, washed with DMEM and resuspended in 100 μl of Tyrode’s solution containing 1mg/ml BSA and 1mM CaCl2. FcεRI cross-linking was performed by stimulation with specific antigen, 100ng/ml of DNP conjugated with human serum albumin (HSA) (Sigma-Aldrich, St. Louis, MO) for 45 minutes at 37°C, as previously described. (15;16) After centrifugation at 300 g for 5 min supernatant was collected and cell pellets were lysed with 100μl of distilled water, followed by freeze-thaw cycle twice. Histamine levels in the supernatants and cell lysates were measured using ELISA as described above. Additional control cultures included MC/9 cells cultured in the media alone, with anti-DNP IgE but without DNP stimulation, or with irrelevant antigen, conalbumin (CA, 100ng/ml) stimulation.
Quantifying FcεRI subunit mRNA levels by real-time PCR (qPCR)
Real-time reverse transcription quantitative PCR (qPCR) was performed using a SYBR green protocol and an ABI7900 HT machine (Applied Biosystems, Foster City, CA, USA) as previously described. (17) (Repository Methods E6)
Testing isolated fractions and identified compounds from FAHF-2 on RBL-2H3 and human skin mast cells
RBL-2H3 were plated at 2.5× 105 cells/well in 24 well plates for experiments described below. For β-hexosaminidase assays, RBL-2H3 cells were pretreated with FAHF-2 fraction 1 or 4 (18, 36, 72μg/ml), faction 2 or 3 (9, 18, 36μg/ml) and three compounds from fraction 2, berberine (1.25, 2.5,5, 10μg/ml), palmatine or jatrorrhizine (1.25, 2.5, 5, 10, 20, 40μg/ml) for 24 hours. The doses used were based on our preliminary studies. Cells were then sensitized with anti-DNP IgE (72 ng/ml) and challenged with DNP-BSA(150μg/ml). β-hexosaminidase was assayed according to a previously published method. (18)Cell viability was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium bromide (MTT) assay following the method of previous publications. (19)
Human skin-derived mast cells were prepared as previously described. (20)Mast cells (1 × 105) were treated with FAHF-2 fraction 2 at 200 μg/ml for 24 hours. The cells were then washed and stimulated with FcεRIα specific mAb 22E7 (1 μg/ml) or compliment pathway activator C5a(100ng/ml). Degranulation was assessed by measuring β-hexosaminidase in culture supernatants and cell lysates as previously described. (20)
Western blot
IgE-sensitized RBL-2H3 cells (7 ×106) were cultured with or without B+P+J for 24 hours and stimulated with DNP (150μg/ml) for 5 minutes. The cells were then lysed and western blot analysis for determination of early FcεRI-mediated signal events was performed using anti–Syk or anti-phosphorylated Syk antibodies (Cell Signaling, Danvers, MA)as described previously. (21) (Repository Methods E7).
Statistical analysis
Data were analyzed using the SigmaStat statistical software package (Systat, Chicago, IL). For normally distributed data, differences between multiple groups were analyzed by one-way ANOVA followed by the Bonferroni T test for all pairwise comparisons. For any data failed normality test, one-way ANOVA on ranks followed by all pairwise comparison was employed. The differences between two groups were analyzed by T test and Mann-Whitney Rank Sum Test for normally distributed data and non-normally distributed data, respectively. P- values<0.05 were considered significant.
RESULTS
FAHF-2 prevented PN induced anaphylactic reactions in the chronic PN allergic murine model
To investigate the mechanisms by which FAHF-2 abrogates PN-induced anaphylaxis, we used the previously described model (Figure 1A).(4) As expected, all sham-treated mice developed anaphylactic reactions following the first oral PN challenge at week 14 (median symptom score = 3, Figure 1B). Concurrently, body temperatures of sham-treated mice decreased significantly compared to those of naïve mice, a sign of systemic anaphylaxis (p<0.001, Figure 1C). Plasma histamine was also markedly elevated in all sham treated mice (p<0.001, Figure 1D). In addition all sham treated mice responded to PN re-challenge 4 weeks later at wk 18 (Figure 1C–D, p<0.001). In contrast, FAHF-2 treated mice showed no anaphylactic symptoms immediately following treatment (at week 14), or 4weeks post therapy (at wk 18) maintained normal body temperatures, and showed suppressed histamine levels (Figure 1B–D).
FAHF-2 reduced the number of peripheral blood basophils
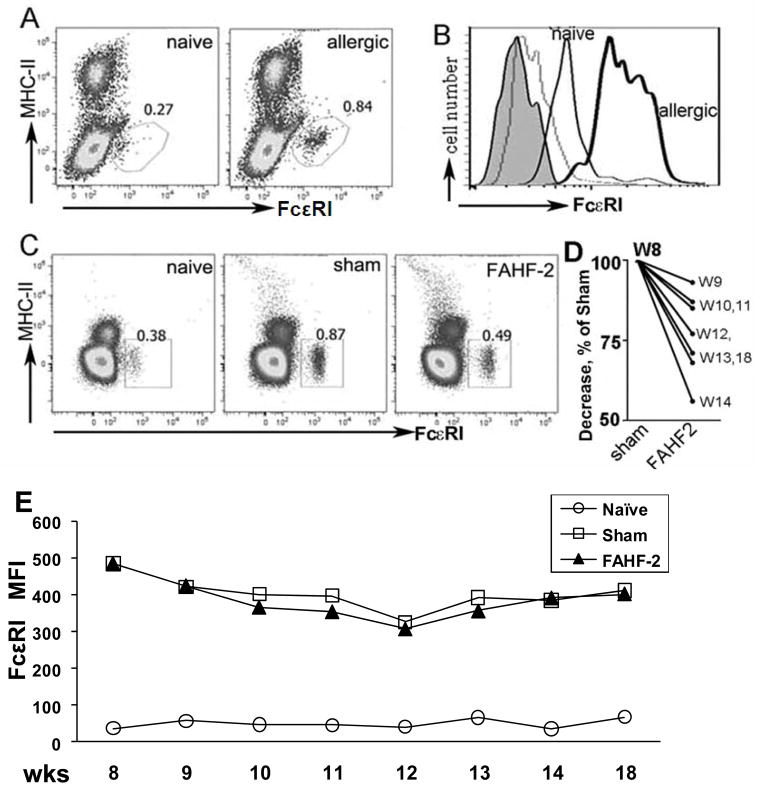
Using the model described above, we used flow cytometry to determine the number of peripheral blood basophils before and after FAHF-2 treatment. As shown in figure 2A, the FcεRI positive cells were MHC-II negative, thereby excluding B cells, monocytes and dendritic cells; and CCR3 negative, excluding eosinophils. This phenotype is characteristic of blood basophils.(10–12) Blood from PNA mice contained significantly more FcεRI positive peripheral blood cells (0.84%) than naïve mice (0.27%) when treatment was initiated at wk 8 (Figure 2A). Mean fluorescent intensity (MFI) was also increased in PNA mice (Figure 2B), reflecting increased FcεRI expression. However, basophil numbers in FAHF-2 treated mice were significantly reduced when compared to sham treated mice immediately after completing treatment (wk 14, 0.49% and 0.87% respectively (Figure 2C). In fact, they were essentially the same as naïve mice (0.38%). Figure 2D shows the reduction of basophils between weeks 9–14 and post FAHF-2 therapy (wk 18). This reduction persisted until 4 wks post therapy. However, FAHF-2 treatment did not affect the density of FcεRI expression (Figure 2E). We also employed the CD49b+FcεRI+ basophil criteria to detect the difference between FAHF-2 treated PNA mice and PNA sham mice and the basophil results were similar to what was observed using FcεR+CCR3−Gr − as markers (see the result and figure E1 in Online Repository).
Figure 2. FAHF-2 reduced the number of peripheral blood basophils.
A. Dot plots show an increased percent of basophils in PNA mice, at wk 8 following the last boost, as compared to naïve mice. B. Histogram shows labeled FcεRI expression on cells from allergic (bold line) and naïve mice (thin line). Shaded = unstained cells; gray = isotype controls. C. Percent of basophils in peripheral blood from each group at wk 14 immediately after treatment. D. Reduction of basophil numbers as determined weekly at different time points as indicated. Numbers were normalized to and expressed as % of sham. E. FcεRI MFI of blood basophils from each group over time as in D.
FAHF-2 reduced the number and FcεRI expression of peritoneal mast cells
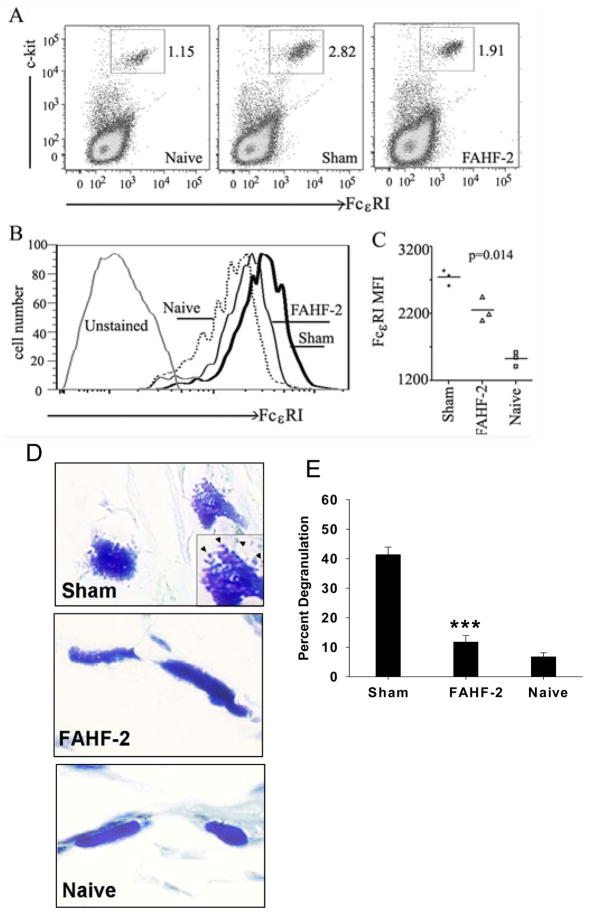
To determine the effect of FAHF-2 on mast cell numbers and FcεRI expression, mice were sacrificed after the final challenge (wk 18) and peritoneal cavity cells were lavaged from naïve, sham-treated and FAHF-2 treated mice. The number of mast cells (c-kit+ cells) and their FcεRI expression were determined. As shown in figure 3A, the percent of mast cells in peritoneal cavities of sham-treated mice was increased compared with naïve mice (2.68± 0.27% in sham treated vs. 0.98± 0.14% in naïve mice). Peritoneal mast cells in FAHF-2 treated mice were 1.9± 0.16%, significantly lower than in sham treated mice (p=0.014, Figure 3A). FcεRI expression was also reduced (Figure 3B,C).
Figure 3. FAHF-2 reduced the number of and FcεRI expression of peritoneal cavity mast cells.
A. Data show one representative result with mast cells within the gated area. Numbers indicate percent. B. Mast cells (c-kit and FcεRI double-positive) were gated to show FcεRI levels in each group as indicated. C. FcεRI MFI. Each dot indicates one set of pooled cells from each group. Bars indicate the mean values of each group. D. Representative images of cutaneous mast cells in skin tissue of each group of mice. E. Percent of degranulated cutaneous mast cells in skin samples post challenge. Data is shown as Mean ± SEM (n=5). ***p<0.001 vs naïve.
In addition, we determined mast cell numbers and extent of degranulation in skin and intestinal tissues histologically (Repository Methods E4). Unlike peritoneal mast cells there were no differences between in numbers of intestinal or cutaneous mast cells in FAHF-2 treated and sham treated groups (data not shown). Consistent with our previous findings,(8;22) the percent of degranulated mast cells in tissues from FAHF-2 treated mice was significantly lower than in sham treated (11.7±2.1% vs 41.3±2.5%, p<0.001, Figure 3D and E). We also found a trend of reduction of % degranulation of intestinal mast cells (FAHF-2 vs sham, p=0.053, data not shown).
FAHF-2 inhibited IgE mediated MC/9 mast cell proliferation and degranulation
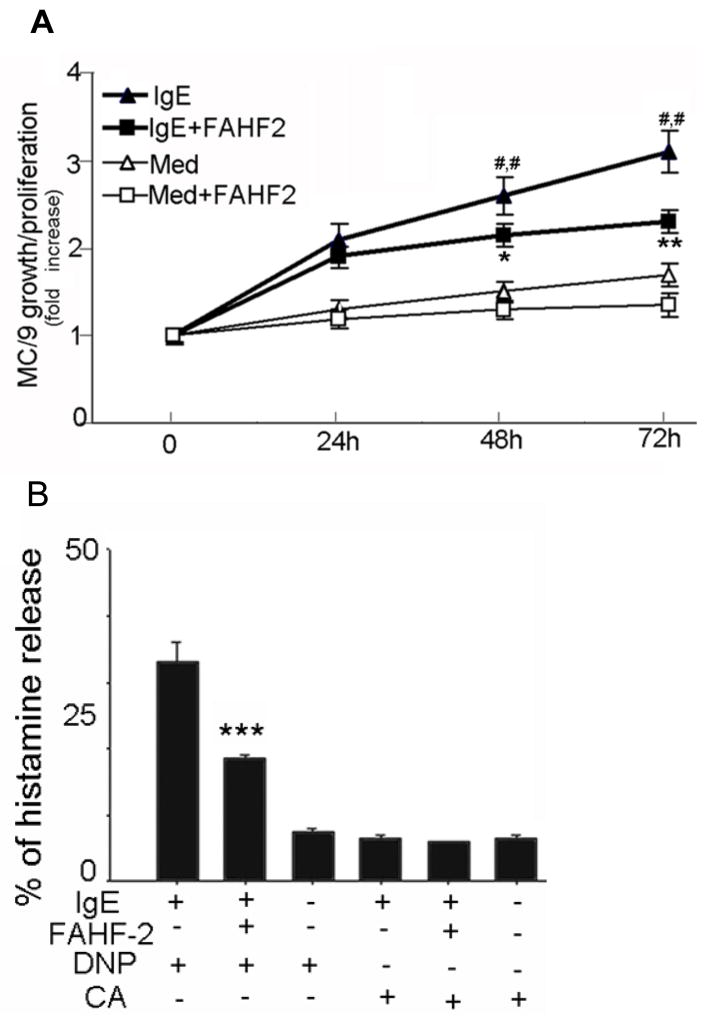
Binding of monomeric IgE to its high-affinity receptor (FcεRI), promotes mouse mast cell survival and growth.(13;14;23) We hypothesized that FAHF-2 may inhibit mast cell proliferation. As found in our preliminary study, FAHF-2 at 20 μg/ml was not cytotoxic to MC/9 cells. Therefore, this concentration was used in the following experiments. MC/9 cells were incubated with FAHF-2 for 24, 48, and 72 h, and viable cells were counted at each time point. As shown in figure 4A, the number of mast cells increased at each time point, demonstrating proliferation. Addition of FAHF-2 caused no statistically significant decrease of proliferation. Addition of anti-DNP IgE significantly increased mast cell proliferation at both 48h and 72h as compared with medium alone (p<0.01, Figure 4A). Co-culture with FAHF-2 significantly reduced anti-DNP IgE induced proliferation at 72h (p<0.01, Figure 4A). These results indicate that FAHF-2 specifically inhibited IgE stimulated mast cell proliferation. No cytotoxicity was detected using trypan blue exclusion assay (data not shown).
Figure 4. FAHF-2 inhibited mast cell proliferation and degranulation.
A. Mast cell proliferation. MC/9 cells were cultured and viable cells were counted at indicated time points. Cell numbers are expressed as fold increase vs day 0. Data are shown as Means ± SEM of 3 individual experiments. * p<0.05, ** p<0.01 vs. IgE alone. ##, p<0.01 vs Medium alone (Med). B. Mast cell degranulation. MC/9 cells were cultured and then challenged at different conditions as indicated. Supernatant and total cell histamine levels were determined. Data expressed as percent of histamine release (Means ± SEM of 3 individual experiments). ***p<0.001vs IgE + DNP without FAHF-2 (1st bar).
We next determined the effect of FAHF-2 on IgE receptor cross-linking induced mast cell degranulation. MC/9 cells were sensitized by incubation with FAHF-2 in the presence of mouse anti-DNP IgE. Cells were then activated by addition of 100ng/ml of anti-DNP IgE. Histamine levels were determined by ELISA. As shown in figure 4B, anti-DNP IgE (1st bar) following DNP-challenge induced significant release of histamine. FAHF-2 (2nd bar) significantly decreased specific antigen-mediated histamine release (14.5 ±1.2% vs. 35.8 ± 2.9%, p<0.001). Spontaneous release following DNP only (3rd bar), non specific antigen conalbumin challenge (CA, 4th bar), unspecific antigen CA plus FAHF-2 (5th bar) and, CA only (6th bar) were all less than 5%.
FAHF-2 blocked IgE-induced up-regulation of FcεRI expression by MC/9 cells
IgE up-regulates mast cell FcεRI expression in vitro and in vivo. (13;24) We hypothesized that FAHF-2 might directly suppress IgE-mediated up-regulation of FcεRI expression. To address this possibility we employed the well-characterized murine mast cell line MC/9 treated with DNP specific IgE. As expected, IgE augmented FcεRI expression by MC/9 cells (Figure 5A). Addition of FAHF-2 to the cultures attenuated this augmentation. FcεRI MFI was reduced 37% by 10μg/ml and 53% by 20μg/ml FAHF-2 as compared to controls (Figure 5A).
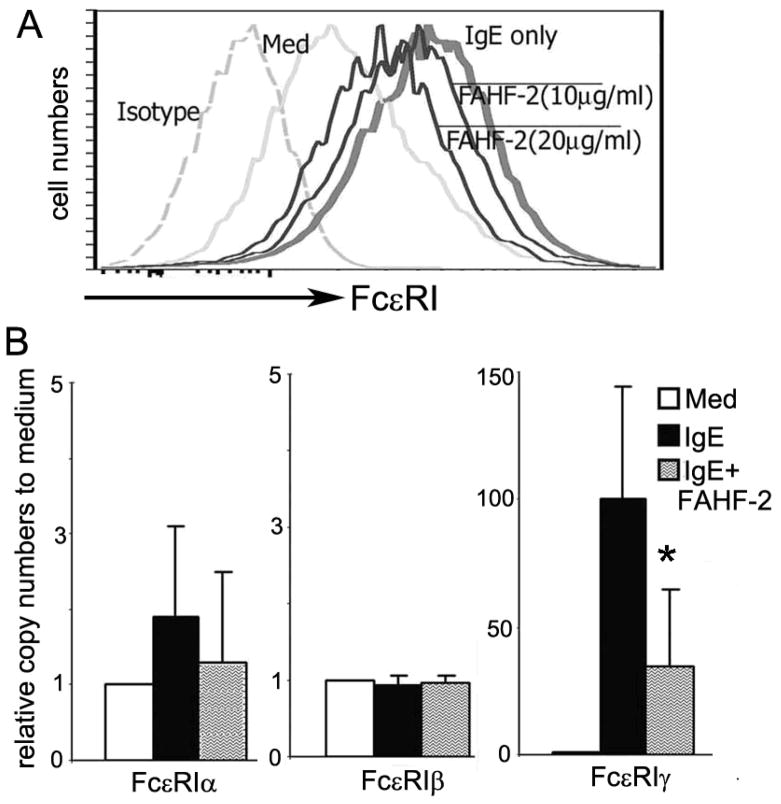
Figure 5. FAHF-2 treatment in vitro reduced the expression of surface FcεRI and mRNA levels of FcεRI γ subunit by the mast cell line MC/9.
A. Flow-cytometry detection of mast cell surface FcεRI expression. Dashed line, isotype control; Grey line, medium alone (Med); bold line, 2μg/ml of IgE (IgE only); thin lines, IgE plus 10μg/ml and 20μg/ml of FAHF-2. B. Messenger levels of FcεRI subunits of MC/9 cells in the presence or absence of anti-DNP IgE with or without FAHF-2. Data are expressed as means ± SD of 3 individual experiments (*p<0.05 vs IgE alone, # # # vs Med alone).
We next determined Fcε RI subunit mRNA expression by real-time quantitative PCR. Receptor surface expression could not be measured due to the lack of a commercially available antibody against murine Fcε RI subunits. As shown in figure 5B, mRNA levels of the FcεRI γ subunit were significantly enhanced by anti- DNP IgE as compared to the medium alone group (p<0.001). IgE-mediated up-regulation of the γ subunit was significantly attenuated by FAHF-2 (p<0.05). FcεRIα, and β subunit mRNA expression was not affected by the presence of IgE with or without FAHF-2 treatment.
FAHF-2 fraction 2 inhibited β-hexosaminidase release by RBL-2H3 and human skin mast cells
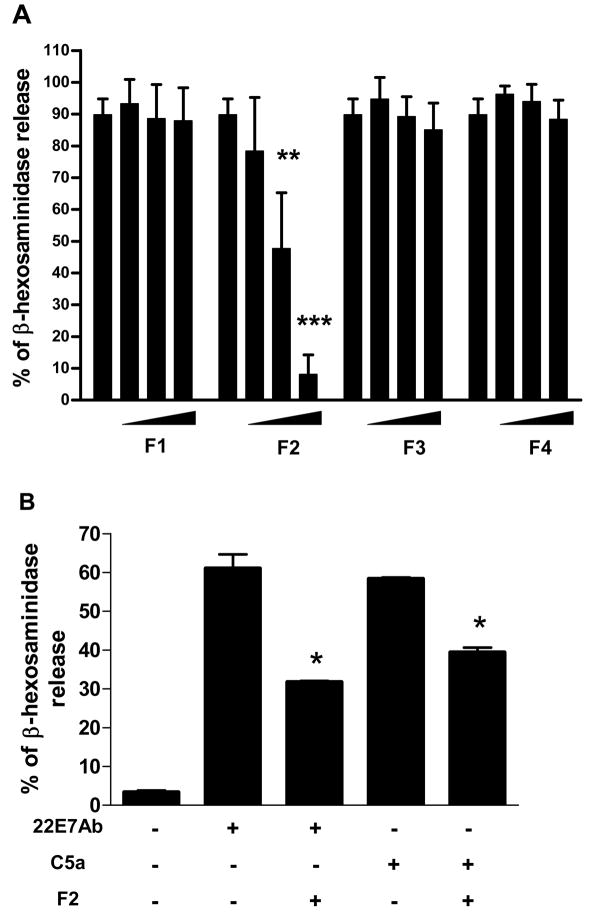
To identify active compounds, FAHF-2 was first extracted with butanol, then further divided into 4 fractions (F) based on polarity by prep-HPLC. F1 contains water soluble compounds, F2 mainly alkaloids compounds, F3 and 4 mainly flavonoids and terpenoids (method E2). The RBL-2H3 cell line has been widely used as a mast cell model for IgE-mediated degranualtion studies, but may be more similar to basophils than other histamine-releasing cell types.(18) The β-hexosaminidase assay has been widely used to monitor RBL-2H3 mast cell degranulation. We therefore employed the RBL-2H3 cell line as an in vitro model and the β-hexosaminidase assay for testing effects of isolated fractions and identified compounds from FAHF-2 on degranulation. RBL-2H3 cells were treated with varying doses of FAHF-2 fractions (F 1, 2, 3 and 4) for 24 hours prior to sensitization by anti-DNP IgE. Figure 6A shows that, F2 at 18μg/ml and 36μg/ml (3rd and 4th bars) significantly reduced β-hexosaminidase release compared to untreated control (1st bar) (p < 0.01-p<0.001, Figure 6A), in a non toxic manner (data not shown). F 1, 3 and 4 did not result in significant inhibition (Figure 6A). In view of the inhibitory effects of fraction 2 on RBL-2H3 cells, we examined its effects on human skin mast cells. F 2 also inhibited human skin mast cell degranulation triggered by IgE cross-linking and C5a (p < 0.05 for both, Figure 6B).
Figure 6. Effect of active fractions of FAHF-2 on RBL-2H3 cells and human skin mast cells.
A. Effect of fractions at different doses fraction 1(0, 18, 36,72μg/ml), fraction 2 and 3(0, 9,18, 36μg/ml, ) and fraction 4(0,18, 36,72μg/ml) of FAHF-2 on β-hexosaminidase released by RBL-2H3. B. Effect of fraction 2 on human skin mast cells. β-hexosaminidase release was triggered by mAb 22E7 or C5a. Data are mean±SD of 3 experiments. *p<0.05, **p<0.01,***p<0.001vs untreated control.
Berberine, palmatine and jatrorrhizine effects on β-hexosaminidase release, Syk signaling pathway and IgE binding in RBL-2H3 cells
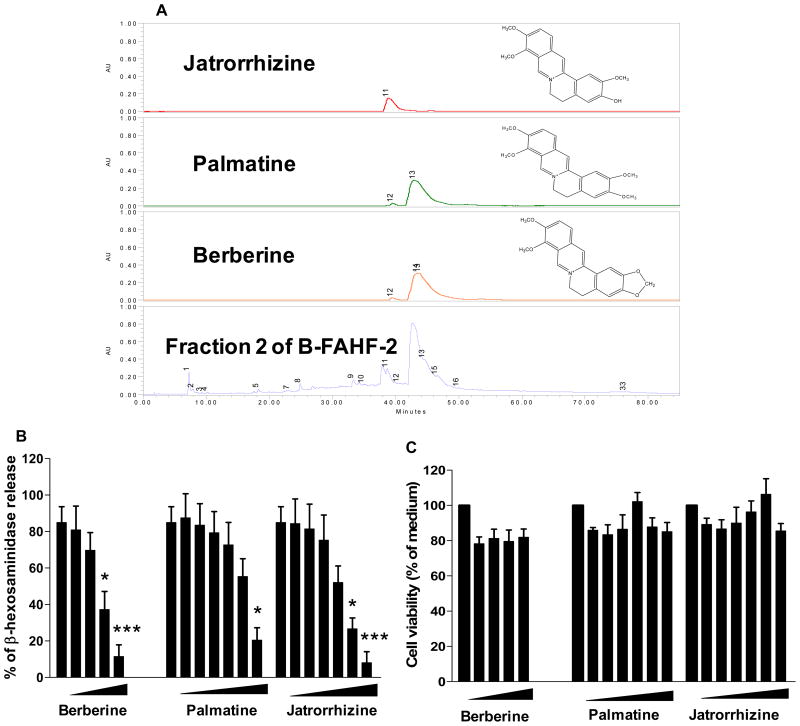
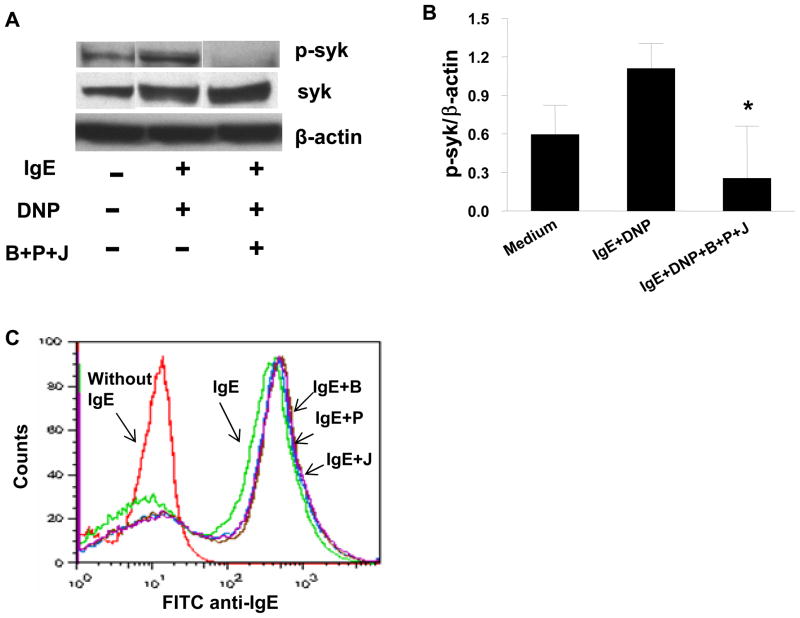
Using LC MS we identified 3 major alkaloid compounds peaks (11, 13 and 15) that corresponded to jatrorrhizine, palmatine and berberine (Figure 7A) in fraction 2. We then tested effects of these compounds on RBL-2H3 cells. Figure 7B shows that β-hexosaminidase release in the presence of berberine at 5μg/ml and 10μg/ml (4th and 5thbars), palmatine at 40μg/ml (7th bar) and jatrorrhizine at 20μg/ml and 40μg/ml (6th and 7th bars) was significantly inhibited when compared with untreated cells (1st bars) (Figure 7B, p<0.05-p<0.001 ), in a non toxic dose-dependent manner (Figure 7C). Furthermore, we determined effects of combination of BPJ on Syk, an IgE-mediated FcεRI early signaling event, focusing on Syk, which is required for mast cell degranulation. Phosphorylation of Syk in antigen-stimulated RBL-2H3 cells was significantly inhibited by the combination of these compounds (Figure 8A and 8B). To further clarify the mechanisms of inhibition of RBL-2H3 cell degranulation by berberine, palmatine and jatrorrhizine, we examined the effect of these compounds on IgE binding to RBL-2H3 cells using the method as previous established(25) and described in Repository Methods E8. Co-culture of cells with mouse IgE in the presence of berberine, palmatine and jatrorrhizine (Figure 8C, purple, brown and blue lines) showed no difference in IgE binding to mast cells from cells cultured with mouse IgE alone (Figure 8C, green line), demonstrating that berberine, palmatine and jatrorrhizine did not inhibit IgE binding to the mast cells.
Figure 7. Major alkaloid compounds in fraction 2 inhibited mast cell degranulation in RBL-2H3 Cells.
A. Identification and chemical structures of berberine, palmatine and jatrorrhizine in F2 of butanol FAHF-2 (B-FAHF-2). B. Dose dependent responses to berberine, palmatine and jatrorrhizine by RBL-2H3 cells. C. Cell viability. Berberine, palmatine and jatrorrhizine toxic effects on RBL-2H3 cells using MTT assay. *p<0.05, ***p<0.001vs untreated control.
Figure 8. Berberine, palmatine and jatrorrhizine effects on Syk signaling pathway and IgE binding in RBL-2H3 cells.
A. Down-regulation of Syk-phosphorylation (p-syk) in IgE-triggered RBL-2H3 cells by berberine, palmatine and jatrorrhizine combination (B+P+J). Bands are representative of three individual western blot experiments. B. Quantitative assay by densitometry of p-syk/β-actin ratio. All data are Mean ± SD of 3 individual experiments.*p<0.05, DNP+IgE+B+P+J vs DNP+IgE. C. Effects of B, P and J on IgE binding to RBL-2H3 cells. Red line=cells only without IgE, green=cells with IgE, purple, brown, blue= B, P, J +IgE.
DISCUSSION
PNA is a type I hypersensitivity. During the sensitization phase, peanut allergen presented by antigen presenting cells to T cells stimulates B cells to produce specific IgE that binds to effector cells such as basophils and mast cells. During the activation phase, following antigen challenge, effector cells degranulate, and release mediators such as histamine that lead to clinical symptoms. In this study, we demonstrated that FAHF-2 protected PNA mice against oral PN challenge-induced anaphylaxis, and that this effect is persistent, as previously reported.(4) FAHF-2 treated animals exhibited normal body temperature, no allergy symptoms, or no increased histamine release. In this study, FAHF-2 was administered at week 8, at which time PN hypersensitivity was established.(4) This simulates a potential human treatment regimen to prevent food-induced anaphylaxis. We further demonstrated that FAHF-2 treatment reduced the number of peripheral blood basophils and peritoneal mast cells, and cutaneous mast cell degranulation. Basophil numbers began to decrease after 1 wk of treatment, reached nadir at the end of treatment (wk 14), and remained lower for at least 4 weeks post treatment (wk 18). At that time, the number of peritoneal mast cells was also significantly reduced as was their expression of FcεRI.
In our previous publications, and in this study (data not shown) we found a significant reduction in PN-specific IgE levels after four weeks of treatment.(4–6) Previous publications reported that binding of monomeric IgE to FcεRI promotes mouse mast cell/basophil survival and function.(3;14;23) Therefore, decreased peanut specific IgE levels could play a role in decreased mast cell and basophil levels after 7 weeks of treatment with FAHF-2 in this model. However, the finding that peripheral blood basophil numbers reduced as early as 1 week of treatment, prior to IgE reduction, suggests that additional mechanisms may co-exist. To test the possibility that FAHF-2 may also directly suppress basophil and mast cell activities, we employed the MC/9 mast cell line as an in vitro model and showed that MC/9 cells treated with FAHF-2 showed significantly suppressed both anti-DNP IgE induced cell proliferation, and histamine release upon DNP challenge in a non cytotoxic manner. FAHF-2 also significantly suppressed anti-DNP IgE induced FcεRI expression. These results suggested that FAHF-2 suppression of mast cell and basophil numbers and activation contributes to FAHF-2 persistent protection against peanut anaphylaxis.
Mast cell activation is triggered by binding of IgE to the high affinity receptor, FcεRI. Rodent FcεRI is a tetrameric receptor that consists of one α-chain, unique to this receptor, one β-chain and two disulfide-linked γ-chains. Our in vitro study showed that FAHF-2 treatment suppressed γ subunit FcεRI expression by MC/9 cells. These data together with the in vivo findings that peritoneal mast cells exhibited significantly reduced FcεRI expression suggest that FAHF-2 suppression of mast cells growth and activation may be through suppression of mast cells high affinity receptors and specific suppression of the signaling γ chain. In this study, we found FAHF-2 reduced mast cell FcεRI expression, but peripheral blood basophil FcεRI expression was unaffected although basophil numbers were significantly reduced. The mechanisms of basophil reduction were not determined. One possibility is FAHF-2 suppression of IL-3 and/or IL-33 receptors, because activation of these receptors are important for basophil maturation, survival and migration. (26) Further investigation is required.
FAHF-2 is a water extract of 9 herbs. Previously, we began to determine how FAHF-2 acts on the allergic immune response in the murine model of peanut allergy at a single component herb level.(9) While FAHF-2 blocked histamine release, significantly reduced peanut-specific serum IgE and IL-4, IL-5 and increased IgG2a and IFN-γ levels, no individual herb affected all these aspects. These results suggested that component herbs of FAHF-2 may work synergistically to produce the therapeutic effects produced by the whole formula. In this study, we focused on identification of active compounds in FAHF-2 that affect mast cells using rat RBL-2H3 cells, widely used in previous studies. (18;19)We found that the alkaloid rich fraction 2 inhibited RBL-2H3 cell degranulation and human skin mast cell degranulation activated by FcεRIα specific mAb 22E7 as well as the compliment pathway activator C5a. We for the first time demonstrated that 3 major alkaloid compounds (berberine, palmatine and jatrorrhizine) in FAHF-2 showed dose depend responses, berberine being most effective. Interestingly, the mixture of 3 compounds showed a synergistic action (data not shown), which was associated with suppression of the phosphorylation of Syk in antigen-stimulated RBL-2H3 cells. A previous study also showed honeybee-collected pollen inhibition of mast cell degranulation associated with inhibition of IgE binding to mast cells, but no effect on FcεRI expression.(25) However, our study showed that FAHF-2 compounds did not affect IgE binding to mast cells. This finding suggests that FAHF-2 inhibition of IgE-mediated mast cell degranulation is less likely via “a steric hindrance” or “competes off the IgE” phenomena. (27–28)
In summary, we demonstrated for the first time that FAHF-2 reduces basophil and mast cell numbers in a murine model of PNA. FAHF-2 also suppressed FcεRI expression in vivo and directly suppressed IgE induced mast cell growth, and activation in vitro, which may be due to suppression of FcεRI γ subunit expression. The 3 major alkaloid compounds may contribute to FAHF-2 inhibition of mast cells/basophils activation.
Supplementary Material
Acknowledgments
We would like to acknowledge Dr. HA Sampson for his support for this study.
Supported by the Dugan Family Foundation, the Food Allergy Initiative, and National Institutes of Health/National Center for Complementary and Alternative Medicine grant no.1R01AT001495-01A1, no. 2 R01 AT001495-05A1.
Abbreviations
- PN
peanut (s)
- PNA
Peanut allergy (peanut allergic)
- FAHF-2
Food allergy herbal formula -2
- i.g
intragastrically
- CT
cholera toxin
- wk (s)
week (s)
- B.i.d
Twice daily
- PBLCs
peripheral blood leukocytes
- MFI
Mean fluorescent intensity
- RBL-2H3
rat basophilic leukemia cell-2H3
- B+P+J
berberine, palmatine and jatrorrhizine
- Syk
spleen tyrosine kinase
- HPLC
high performance liquid chromatography
- LC MS
liquid chromatography mass spectrometry
- BF2
butanol FAHF-2
Footnotes
Conflict of interest: X-M. Li receives grant support from the Food Allergy Initiative and the NIH, is a consultant for the FAI; has shares of US Patent PCT/US 05/08600 on FAHF-2, and Herbal Springs LLC. P. Busse is a consultant for ViroPharma, receives research support from the AAAAI and the NIH, and has provided legal consultation services/expert witness testimony in cases related to hereditary angioedema. W. Zhao receives research support from the NIH. The other authors have declared that they have no conflict of interest.
References
- 1.Wang J, Sampson HA. Food anaphylaxis. Clin Exp Allergy. 2007;37(5):651–60. doi: 10.1111/j.1365-2222.2007.02682.x. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ. Mast cells and basophils. Curr Opin Hematol. 2000;7(1):32–9. doi: 10.1097/00062752-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002;2(10):773–86. doi: 10.1038/nri914. [DOI] [PubMed] [Google Scholar]
- 4.Qu C, Srivastava K, Ko J, Zhang TF, Sampson HA, Li XM. Induction of tolerance after establishment of peanut allergy by the food allergy herbal formula-2 is associated with up-regulation of interferon-gamma. Clin Exp Allergy. 2007;37(6):846–55. doi: 10.1111/j.1365-2222.2007.02718.x. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava KD, Kattan JD, Zou ZM, Li JH, Zhang L, Wallenstein S, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115(1):171–8. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol. 2009;123(2):443–51. doi: 10.1016/j.jaci.2008.12.1107. [DOI] [PubMed] [Google Scholar]
- 7.Beyer K, Morrow E, Li XM, Bardina L, Bannon GA, Burks AW, et al. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001;107(6):1077–81. doi: 10.1067/mai.2001.115480. [DOI] [PubMed] [Google Scholar]
- 8.Li XM, Serebrisky D, Lee SY, Huang CK, Bardina L, Schofield BH, et al. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J Allergy Clin Immunol. 2000;106(1 Pt 1):150–8. doi: 10.1067/mai.2000.107395. [DOI] [PubMed] [Google Scholar]
- 9.Kattan JD, Srivastava KD, Zou ZM, Goldfarb J, Sampson HA, Li XM. Pharmacological and immunological effects of individual herbs in the Food Allergy Herbal Formula-2 (FAHF-2) on peanut allergy. Phytother Res. 2008;22(5):651–9. doi: 10.1002/ptr.2357. [DOI] [PubMed] [Google Scholar]
- 10.Luccioli S, Brody DT, Hasan S, Keane-Myers A, Prussin C, Metcalfe DD. IgE(+), Kit(−), I-A/I-E(−) myeloid cells are the initial source of Il-4 after antigen challenge in a mouse model of allergic pulmonary inflammation. J Allergy Clin Immunol. 2002;110(1):117–24. doi: 10.1067/mai.2002.125828. [DOI] [PubMed] [Google Scholar]
- 11.Dvorak AM. The mouse basophil, a rare and rarely recognized granulocyte. Blood. 2000;96(4):1616–7. [PubMed] [Google Scholar]
- 12.Lantz CS, Yamaguchi M, Oettgen HC, Katona IM, Miyajima I, Kinet JP, et al. IgE regulates mouse basophil Fc epsilon RI expression in vivo. J Immunol. 1997;158(6):2517–21. [PubMed] [Google Scholar]
- 13.Yamaguchi M, Lantz CS, Oettgen HC, Katona IM, Fleming T, Miyajima I, et al. IgE enhances mouse mast cell Fc(epsilon)RI expression in vitro and in vivo: evidence for a novel amplification mechanism in IgE-dependent reactions. J Exp Med. 1997;185(4):663–72. doi: 10.1084/jem.185.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asai K, Kitaura J, Kawakami Y, Yamagata N, Tsai M, Carbone DP, et al. Regulation of mast cell survival by IgE. Immunity. 2001;14(6):791–800. doi: 10.1016/s1074-7613(01)00157-1. [DOI] [PubMed] [Google Scholar]
- 15.Kitaura J, Xiao W, Maeda-Yamamoto M, Kawakami Y, Lowell CA, Kawakami T. Early divergence of Fc epsilon receptor I signals for receptor up-regulation and internalization from degranulation, cytokine production, and survival. J Immunol. 2004;173(7):4317–23. doi: 10.4049/jimmunol.173.7.4317. [DOI] [PubMed] [Google Scholar]
- 16.Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proc Natl Acad Sci U S A. 2003;100(22):12911–6. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busse PJ, Zhang TF, Srivastava K, Schofield B, Li XM. Effect of ageing on pulmonary inflammation, airway hyperresponsiveness and T and B cell responses in antigen-sensitized and -challenged mice. Clin Exp Allergy. 2007;37(9):1392–403. doi: 10.1111/j.1365-2222.2007.02775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passante E, Ehrhardt C, Sheridan H, Frankish N. RBL-2H3 cells are an imprecise model for mast cell mediator release. Inflamm Res. 2009;58(9):611–8. doi: 10.1007/s00011-009-0028-4. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Lee SH, Le QT, Kim MM, Kim SK. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and Fc epsilonRI. J Agric Food Chem. 2008;56(24):12073–80. doi: 10.1021/jf802732n. [DOI] [PubMed] [Google Scholar]
- 20.Zhao W, Oskeritzian CA, Pozez AL, Schwartz LB. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J Immunol. 2005;175(4):2635–42. doi: 10.4049/jimmunol.175.4.2635. [DOI] [PubMed] [Google Scholar]
- 21.Han EH, Park JH, Kim JY, Jeong HG. Houttuynia cordata water extract suppresses anaphylactic reaction and IgE-mediated allergic response by inhibiting multiple steps of FcepsilonRI signaling in mast cells. Food Chem Toxicol. 2009;47(7):1659–66. doi: 10.1016/j.fct.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 22.Li XM, Zhang TF, Huang CK, Srivastava K, Teper AA, Zhang L, et al. Food Allergy Herbal Formula-1 (FAHF-1) blocks peanut-induced anaphylaxis in a murine model. J Allergy Clin Immunol. 2001;108(4):639–46. doi: 10.1067/mai.2001.118787. [DOI] [PubMed] [Google Scholar]
- 23.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, et al. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14(6):801–11. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 24.Chen XJ, Enerback L. Regulation of IgE-receptor expression, IgE occupancy and secretory capacity of mast cells. APMIS. 2000;108(10):633–41. doi: 10.1034/j.1600-0463.2000.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa Y, Tokura T, Nakano N, Hara M, Niyonsaba F, Ushio H, et al. Inhibitory effect of honeybee-collected pollen on mast cell degranulation in vivo and in vitro. J Med Food. 2008;11(1):14–20. doi: 10.1089/jmf.2006.163. [DOI] [PubMed] [Google Scholar]
- 26.Valent P, Dahinden CA. Role of interleukins in the regulation of basophil development and secretion. Curr Opin Hematol. 2010;17(1):60–6. doi: 10.1097/MOH.0b013e328331fae9. [DOI] [PubMed] [Google Scholar]
- 27.Casale TB. Experience with monoclonal antibodies in allergic mediated disease: seasonal allergic rhinitis. J Allergy Clin Immunol. 2001;108(2 Suppl):S84–S88. doi: 10.1067/mai.2001.116433. [DOI] [PubMed] [Google Scholar]
- 28.Qian W, Zhang X, Li B, Zhang D, Tong Q, Chen L, et al. Development and characterization of a novel anti-IgE monoclonal antibody. Biochem Biophys Res Commun. 2010;395(4):547–52. doi: 10.1016/j.bbrc.2010.04.063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.