Abstract
Objectives
To evaluate UDP-glucuronyltransferase (UGT) activity and the pharmacokinetics of a single oral dose of acetaminophen (APAP) in children with non-alcoholic fatty liver disease (NAFLD).
Methods
Twelve boys 10–17 years old with biopsy-proven NAFLD and 12 age and gender-matched controls without NAFLD were recruited. Following administration of a single oral dose of APAP (5mg/kg, maximum 325mg), APAP and its glucuronide metabolite (APAP-G) were measured in plasma, urine, and sputum at various intervals up to 24 hours. The activity of UGT was estimated by the plasma ratio of APAP-G to APAP at 4 hours.
Results
Following administration of APAP, children with NAFLD had significantly higher concentrations of APAP-G in serum (p=.0071) and urine (p=.0210) compared to controls. No significant differences in APAP pharmacokinetics parameters were observed between the two groups.
Conclusions
APAP glucuronidation is altered in children with fatty liver disease. Despite the altered disposition of this metabolite, the pharmacokinetics of a single 5 mg/kg dose of APAP is the same in children with NAFLD as in children with normal liver function.
Keywords: nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, acetaminophen, UDP-glucuronyltransferase, pharmacokinetics, pediatrics
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of liver disease in children. NAFLD is a clinico-pathological diagnosis characterized by the accumulation of macrovesicular fat in hepatocytes in the absence of alcohol consumption. NAFLD encompasses a spectrum of of histopathological features ranging from simple steatosis to steatosis with inflammation, ballooning degeneration and pericellular fibrosis (nonalcoholic steatohepatitis, NASH) to cirrhosis. The existing population-based prevalence studies suggest that NAFLD is a global problem with reports published in North and South America, Europe, Australia and Asia (1). However, since NAFLD is diagnosed by liver biopsy, it is hard to estimate the prevalence in children in a population-based study. In a community representative autopsy study which was based on liver histology conducted from 1993 to 2003, the standardized prevalence of fatty liver disease in children aged 2 to 19 years was estimated at 9.6% (2).
The detailed effects of intra-hepatocellular lipid accumulation on hepatic function are poorly understood. In particular, knowledge of the potential consequences of steatosis on the drug-metabolizing capability of the human liver is limited. Several studies have reported on cytochrome P450-mediated drug metabolism in animal models of fatty liver disease; however, there are few studies evaluating the activity of UDP glucuronyltransferases (UGT) and other phase II enzymes in NAFLD. Microsomal cytochrome P450 dysregulation has been well documented in animal models of steatosis, obesity, and steatohepatitis (3, 4). Drug clearance may also be impaired in NAFLD. In obesity, which is highly associated with NAFLD, changes in pharmacokinetics are frequently observed as a result of altered drug distribution and biotransformation (5). Lipid accumulation of drugs in peripheral adipose tissue is known to increase the volume of drug distribution and decrease drug clearance. It is conceivable that drugs may also accumulate in the lipid-filled subcellular compartments of the liver in NAFLD and similarly limit the rates of drug elimination. With diminished ability to metabolize and eliminate commonly used medications and dietary supplements, children with fatty liver disease may be at increased risk for drug-induced hepatotoxicity. In children with diagnosed NAFLD, lower metabolic activity could represent a hazard as drugs given in conventional doses may accumulate and produce toxicity. In obese subjects with undiagnosed NAFLD, treatment with potentially hepatotoxic medications metabolized through these pathways may cause idiopathic drug-induced liver injury.
Acetaminophen (APAP) is the most common cause of acute liver failure in the pediatric population (6). Although safe at therapeutic doses, overdoses can lead to severe hepatoxicity, especially in individuals with preexisting liver disease (7). In APAP overdose, a highly reactive metabolite is formed and covalently binds to macromolecules to cause cellular damage (6). Recent studies suggest that steatohepatitis sensitizes the liver to APAP toxicity in mice (8). The purpose of the current study was to evaluate UGT activity and APAP pharmacokinetics in children with fatty liver disease, with the hypothesis that potentially disordered hepatic metabolism may drug disposition and potentially predispose to idiosyncratic hepatic toxicity.
MATERIALS AND METHODS
Participants
Inclusion criteria for cases were boys age 10 to 17 years with histological evidence of NAFLD based on liver biopsy obtained between Jan 2008 and Nov 2009 at the University of California San Diego. Given that this study is a pilot, only male subjects were chosen to minimize other potentially confounding variables associated with gender. NAFLD is more common in boys, even when matched for other variables. At UCSD 80% of children with NAFLD are male. Table 1 summarizes the basic characteristics of the study subjects. Inclusion criteria for controls was serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) less than 30 U/L and MR spectroscopy with less than 5% calculated liver fat who were otherwise matched to the children with NAFLD for age and gender. MR spectroscopy was done on a 3T MR scanner at the Dept. of Radiology at the University of California, San Diego by an MR physicist with 10 years experience in liver spectroscopy.
Table 1.
Demographic characteristics of study groups
| Control | NAFLD | |
|---|---|---|
| n=12 | n=12 | |
| Age (y, mean ±SD) | 14.4±4.5 | 14.8±1.8 |
| BMI (mean±SD) | 26.22±10.95 | 34.00±6.14 * |
| BMI z-score (mean±SD) | 1.21±1.42 | 2.30±0.43 * |
| ALT (mean±SD) | 20±8 | 100±73 ** |
| AST (mean±SD) | 22±9 | 55±31 ** |
| NAS Score 1 (median, range) | - | 4 (2–7) |
indicates significant difference from control group (p<0.05) by two-tailed t-test
indicates significant difference from control group (p<.005) by two-tailed t-test
NAS Score: nonalcoholic fatty liver disease activity score (1–7), tabulated by summing scores for steatosis (0–3), lobular inflammation (0–3), and ballooning degeneration (0–2)
Participants were excluded from study consideration if any of the following criteria were present: (1) other causes of chronic hepatitis including hepatitis B, hepatitis C, alpha-1 antitrypsin deficiency, autoimmune hepatitis, Wilson's disease, drug toxicity, total parenteral nutrition, and chronic alcohol intake; (2) history of significant renal, gastrointestinal (other than NAFLD) or cardiac disease; (3) taking medications which induce or inhibit drug metabolizing enzyme activity including smoking tobacco products; (4) inability to swallow medication; (5) allergy or intolerance to acetaminophen.
Study Protocol
Following an overnight fast and a 3-day drug free baseline period, each subject received a single oral dose of acetaminophen 5mg/kg (up to 325 mg). In addition, subjects received simultaneous administration of a single oral dose of dextromethorphan 0.3mg/kg (up to 15mg) and 8oz Diet Coke (Coca-Cola Company, Atlanta, GA, USA) as in vivo probes to study the enzymes CYP2D6, CYP1A2, and CYP3A (reported elsewhere). Food was withheld and subjects remained in the upright position for 2 hours following drug administration. Baseline urine and saliva samples were obtained prior to drug administration. Total voided urine was collected 0–4 h and 4–24 h following drug administration and non-stimulated saliva samples were collected at intervals for up to 4 h (15, 30, 45, 60, 120, AND 240 min). A single blood sample was collected 4 h after drug administration. All samples (except the 4–24 h urine samples that were collected at home) were collected in the University of California, San Diego General Clinical Research Center. The harvested plasma, urine and saliva aliquots were stored frozen at −70° C until analyzed. The study was approved by the University of California San Diego Institutional Review Board (#080430). Written informed consent was obtained from subjects’ parents and assent from subjects.
Analysis of APAP and Metabolites
APAP and acetaminophen-glucuronide (APAP-G) metabolites were measured by HPLC. Orthophosphoric acid 85%, perchloric acid 70%, acetonitrile HPLC grade, and HPLC grade water were purchased from Fisher Scientific (Fair Lawn, NJ, USA). Potassium hydroxide 50% was purchased from RICCA Chemical Co (Arlington, TX, USA). Formic acid 88%, APAP and AG were purchased from Sigma Aldrich (St. Louis, MO, USA). Normal human EDTA plasma and normal human saliva were purchased from Biochemed (Winchester, VA, USA). Normal human urine was obtained from a normal donor. The HPLC system consisted of an autosampler (SpectraSYSTEM AS 3000), HPLC pump (SpectraSYSTEM P 4000), a detector (Spectra Focus Forward Optical Scanner), a data integrator (ChromQuest version 4.0, Thermo Electron, San Jose, CA, USA) and a C-18 reversed phase HPLC column (MAC-MOD ACE 5, 4.6 × 150 mm). All assays were conducted with the analytical column at ambient temperature.
Standard curves were prepared using standard solutions of APAP and APAP-G with normal human urine, plasma or saliva. The stock solution was serially diluted with normal human urine, plasma or saliva to produce the required range of concentrations. Calibration standards were evaluated using a least-squares linear regression algorithm to plot the peak height versus concentration with 1/response weighting. Standard curves were automatically generated by ChromQuest software (San Jose, CA, USA).
Data Analysis
The activity of UGT was estimated by the plasma ratio of APAP-G to APAP at 4 hours. APAP elimination rate constant (kel·hr−1) over the linear range was determined by linear regression of the logarithm of the concentration against time. It involved at least 5 sampling points. The half-life was obtained from the elimination rate constant (t1/2 = ln 2/kel). A one compartment model was used to describe APAP elimination10. The area under the concentration/time curves from 0 to 4h were calculated using PRISM 5.0 software (GraphPad Software Inc., La Jolla, CA, USA). The clearance of APAP was calculated by dividing the initial APAP dose by the area under the concentration/time curve for each patient and expressed per kg of body weight.
Statistical Analysis
Data are expressed as means ±S.D. Comparison between NAFLD and control at a single time point was made using the Wilcoxon rank-sum test. The comparison between different time points for each group was made by two-way ANOVA followed by the Bonferroni post-hoc test. Statistical analyses were performed using PRISM 5.0 (GraphPad Software Inc., La Jolla, CA, USA). Two-sided p values less than 0.05 were considered statistically significant.
RESULTS
Subject Characteristics
Twenty-four children were included in this study, 12 children with NAFLD and 12 children without NAFLD. There was no significant difference among the groups with respect to age. BMI was significantly higher in subjects with NAFLD (34.00±6.14) as compared to control (26.22±10.95, p=.043). As expected, plasma aminotransferases were substantially and significantly elevated in subjects with NAFLD as compared to control (p<.0018). Serum ALT was most dramatically elevated in subjects with NAFLD (100±73) as compared to control (20±8, p=.001). Of the patients with NAFLD, 42% (n=5) had a fibrosis stage of 2 or greater on liver biopsy.
APAP Pharmacokinetics
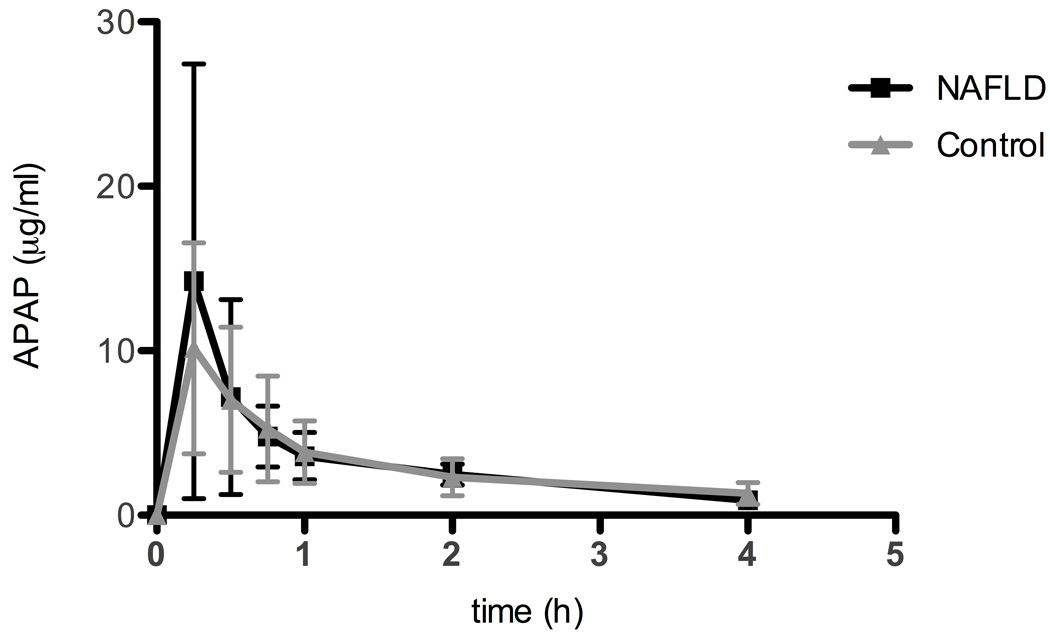
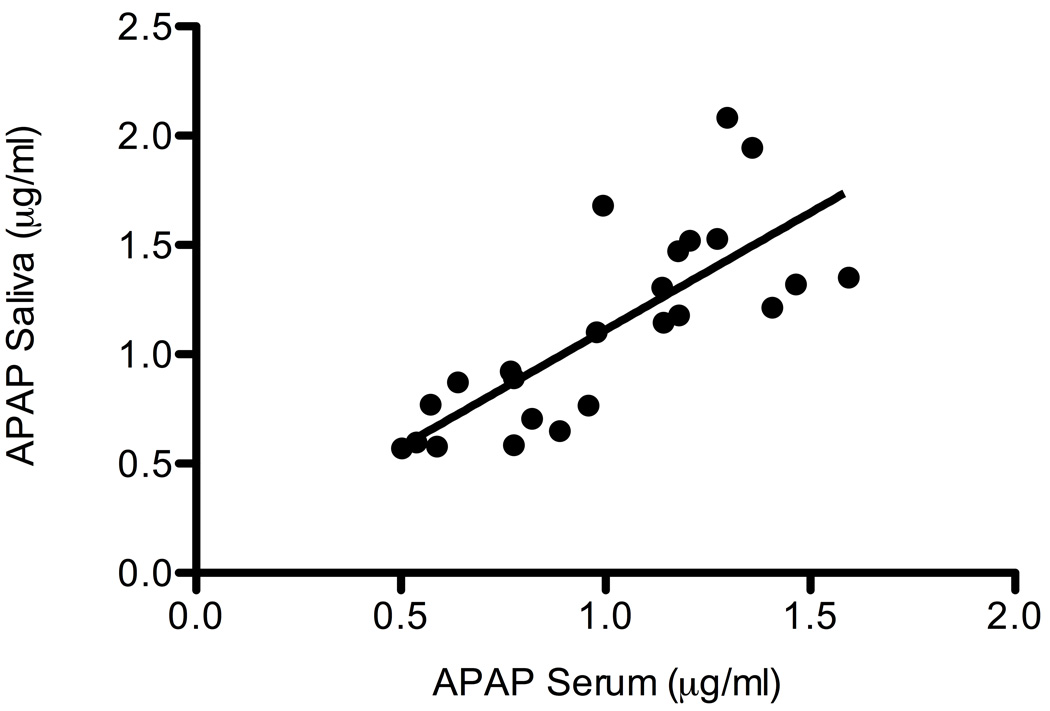
The mean dose of APAP administered to children with NAFLD was 3.6 mg/kg (STD 0.8) and the mean dose administered to children in the control group was 3.8 mg/kg (STD 0.9). Salivary concentrations were used to define the pharmacokinetic profile of APAP in children with and without fatty liver disease (9). Figure 1 shows the concentration of APAP in saliva over 4 h in boys with NAFLD compared to control. No APAP metabolites were detectable in the saliva of either group. Figure 2 shows the correlation between plasma and salivary APAP concentrations in all subjects at 4 h. A regression of the concentration of APAP in saliva (y) versus the plasma concentration (x) showed a significant linear relationship (r2 = 0.595, p<.0001), which could be described by the equation y=1.07x + 0.413. Table 2 summarizes selected APAP pharmacokinetic parameters. Non-parametric analyses failed to show a significant difference in any of the pharmacokinetic parameters (clearance, half-life, AUC, and peak APAP concentration) in children with NAFLD compared to control.
Figure 1.
Acetaminophen (APAP) concentrations in saliva at various time points after administration of a single dose APAP in children with nonalcoholic fatty liver disease (NAFLD) compared to control. No APAP metabolites were measured in saliva. Values are the mean ± SD.
Figure 2.
Scatterplot depicting correlation between saliva and plasma concentrations of acetaminophen in all 24 children at 4h. Linear regression showed a significant linear relationship (r2 = 0.595, p<.0001). Each point on the graph represents one study participant.
Table 2.
Acetaminophen pharmacokinetic data calculated from saliva samples (reported as median, range values)
| Control | NAFLD | |
|---|---|---|
| Cmax 1 (mg/L) | 10.1 (3.23–24.7) | 10.0 (5.46–49.45) |
| Elimination rate constant kel (h−1) | 0.198 (0.071–0.310) | 0.236 (0.130–0.406) |
| Elimination half life (h) | 3.52 (2.236–9.763) | 2.94 (1.707–5.332) |
| AUC 2 (mg/h·L) | 10.2 (5.89–23.0) | 12.6 (6.58–28.2) |
| Clearance (L/kg·h) | 0.31 (0.15–0.53) | 0.31 (0.11–0.59) |
Cmax: peak concentration after drug administration
AUC: area under the curve
Plasma concentration APAP and APAP-G
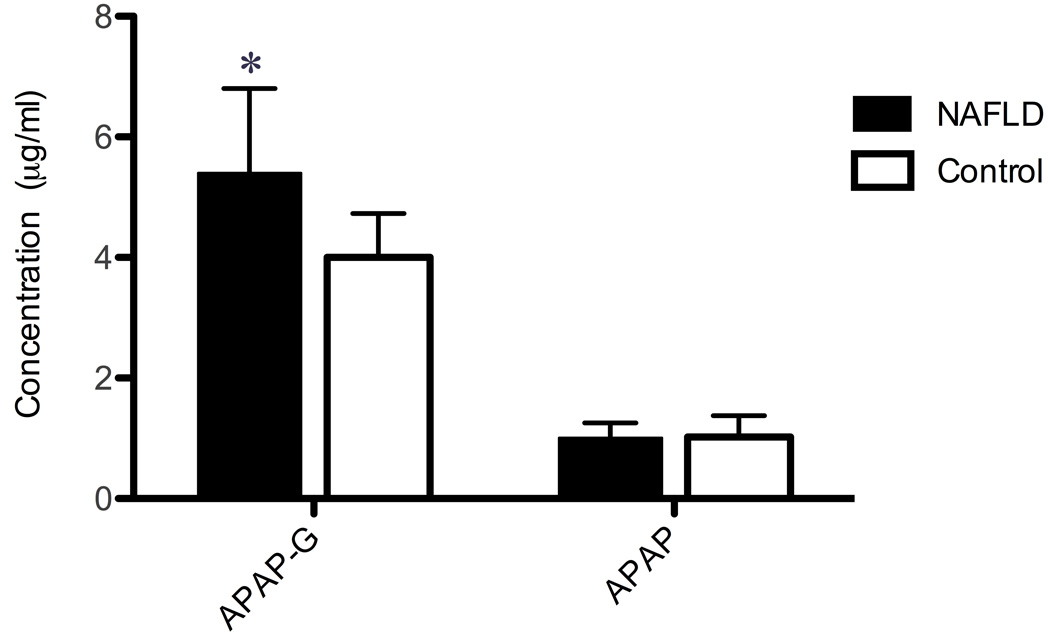
Figure 3 shows the concentration of APAP and APAP-G in plasma at 4 h. The concentration of APAP-G was 35% higher in children with NAFLD compared to controls (p=.0071). There was a statistically significant increase in the ratio of APAP-G to APAP (p=0.0277) in children with NAFLD.
Figure 3.
Acetaminophen (APAP) and acetaminophen-glucuronide (APAP-G) concentrations in serum 4 hours after a single dose of APAP in children with NAFLD compared with control. Values are the mean ± SD. *, significant difference from the control group (p<0.05).
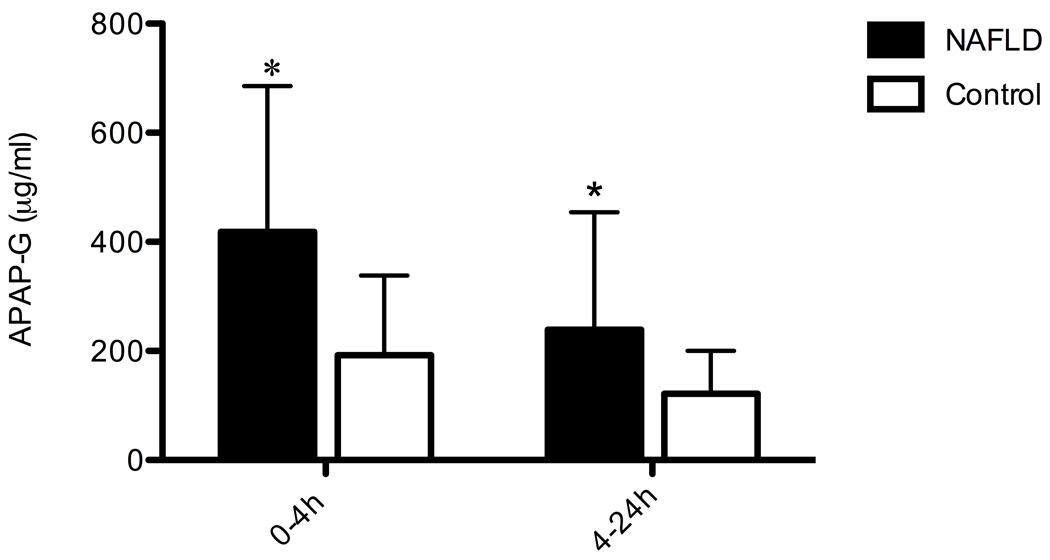
Excretion APAP and APAP-G in urine
Figure 4 shows the cumulative concentration of APAP-G excreted in urine samples collected over 24 h. The pooled concentration of APAP-G in urine collected 4 h after APAP administration was 2.2-fold higher in children with NAFLD compared to children without liver disease (p=.0151). The pooled concentration of APAP-G in urine collected between 4 and 24 h after APAP administration was 2.0-fold higher in children with NAFLD (p=.0210).
Figure 4.
Cumulative concentrations of acetaminophen-glucuronide (APAP-G) in the urine at 0–4 h and 4–24 h after acetaminophen (APAP) administration. Values are the mean ± SD. *, significant difference from the control group (p<0.05).
DISCUSSION
This is the first study to assess drug metabolism in children with NAFLD. We show elevated serum and urine concentrations of APAP-G in children with NAFLD. However, salivary time-concentration profiles after a single dose of APAP in children with NAFLD appear to be the same in children with fatty liver disease as children without liver disease. The peak serum APAP concentrations, APAP half-life and APAP clearance were nearly identical in these groups. The altered metabolism of APAP in children with NAFLD does not appear to affect its rate of elimination. These data suggest that a similar dosage schedule should apply for children with NAFLD as for normal children.
The safety of APAP in children with NAFLD is more difficult to assess. Hepatotoxicity is dependent on the balance between the rate of the toxic metabolite (N-acetyl-p-benzoquinone imine, NAPQI) formation through oxidative pathways, the capacity of the safe elimination pathways of sulfate and glucuronide conjugation, and the rate of hepatic glutathione synthesis. These processes are largely undefined in children with NAFLD.
In normal subjects, 90% of APAP is metabolized to APAP-G and acetaminophen-sulfate (APAP-S) in the liver by UGT and sulfotransferases and subsequently excreted in urine (6). Sulfate conjugation is the dominant metabolic pathway in neonates and young children, but glucuronide metabolism increases with age (10–12). Less than 10% of APAP is oxidized by the cytochrome P450 pathway (particularly CYP 2E1) to NAPQI, which is immediately neutralized by conjugation with glutathione and excreted in the urine as cysteine and mercapturic acid conjugates (APAP-C and APAP-M) (6). However, this pathway assumes greater significance in conditions where glucuronidation is reduced or CYP 2E1 activity is increased (6).
CYP 2E1 activity is known to be upregulated in clinical settings associated with NASH, particularly diabetes and obesity (13–15), and has been shown in adults with NASH (15–17) and animal models of steatohepatitis (18). In fact, enhanced CYP 2E1 activity is associated with hepatic microsomal lipid peroxidation and oxidative stress in NASH and is believed to play a role in the progression from simple steatosis to NASH (4, 19). Enhanced CYP 2E1 activity in NASH could predispose the liver to APAP-induced hepatotoxicity. Recently, it was shown that steatohepatitis sensitizes the liver to APAP-induced hepatotoxicity and hepatic failure in mice (20). In the current study, the contribution of the CYP 2E1 oxidative pathway to APAP metabolism in children with NAFLD was not addressed. Urine APAP-C and APAP-M concentrations reflect the amount of APAP converted to the reactive intermediate NAPQI by cytochrome P450 oxidation (6). A significant elevation in these metabolites might suggest that children with NAFLD are at increased risk for APAP-induced hepatotoxicity. In this study, however, measurement of these toxic adducts was not feasible in light of a single sub-therapeutic dose of APAP.
Here we show a significant increase in the formation of APAP-G in children with fatty liver disease, which is likely due to UGT upregulation. While this may represent an overall increase in APAP metabolism, it is far more likely a reflection of decreased activity in other metabolic pathways not measured in this study. This hypothesis is supported by the normal pharmacokinetic parameters, particularly AUC and peak concentration, in the face of elevated APAP-G formation. We suspect that the higher rate of APAP-G formation is compensating for a deficiency in APAP-S formation in children with NAFLD. Impaired sulfate formation in NAFLD could present a significant problem for APAP metabolism in young children in whom conjugation to sulfate is the predominant elimination pathway. Because sulfate conjugation is the dominant metabolic pathway in normal children and potentially impaired in children with NAFLD, a limitation of this study was the inability of our assays to quantify APAP-S in urine. In a study of APAP metabolism in children aged 6 months to 7 years, chronic liver disease of varying etiologies (biliary atresia, Alagille’s syndrome, TPN cholestasis, α-1 antitrypsin deficiency, chronic active hepatitis and congenital hepatic fibrosis) was associated with an increased urinary APAP-G/APAP-S ratio (9, 21). Since the current study did not include a group of children with chronic liver diseases other than NAFLD, it is impossible to determine whether the increased formation of APAP-G we observed was specific to NAFLD as opposed to a more general effect of liver disease in children. We also cannot exclude the possibility of a reverse association between UGT activity and the development of NAFLD--it is conceivable that children who metabolize APAP toward APAP-G are more prone to develop NAFLD. Regardless of the nature of the metabolites formed, this study shows that the pharmacokinetics of APAP in children with NAFLD is the same as in children without liver disease.
Additionally, we demonstrate salivary sampling as a viable alternative to plasma sampling for defining APAP pharmacokinetic parameters in children. No measurable levels of APAP conjugates were detectable in the saliva of our patients. However, a highly significant relationship between plasma and salivary concentrations of APAP was observed, and the estimated pharmacokinetic parameters were consistent with published values (9, 21). The use of saliva for drug monitoring and future pharmacokinetic studies in children would offer a significant advantage as repetitive venipuncture can be associated with anxiety and trauma in children.
Of note, the children in this study were given a cocktail of APAP, dextromethorphan, and caffeine for the simultaneous measurement of the activity of multiple drug-metabolizing enzymes (CYP2D6, CYP1A2, CYP3A4, and UGT). The use of such cocktails have been validated in several studies, which indicate no pharmacokinetic or pharmacodynamic interactions between the probe drugs (22,23).
In conclusion, our data suggests that APAP metabolism is altered in children with fatty liver disease. These findings support the hypothesis that NAFLD has a significant effect on drug metabolism and underscore the importance of evaluating in detail the potential influence of hepatic steatosis on the metabolism, safety and efficacy of potentially hepatotoxic drugs. Here we show that regardless of the metabolites formed, the pharmacokinetics of a single 5 mg/kg dose of APAP is the same in children with NAFLD as in children with normal liver function. These findings suggest that the pharmacotherapeutic dosing regimen for APAP in children with NAFLD should be the same as for children without liver disease. Future studies measuring APAP-C and APAP-M formation following exposure to the recommended doses of APAP are necessary to determine whether children with NAFLD are at increased risk for APAP-induced hepatic injury.
Acknowledgments
Financial Support: This work was funded in part by NIH grants R21DK71486, P60 MD00220, and M01RR000827 and the National Institute of Child Health and Human Development.
The authors wish to thank Lisa Clark, PhD for coordinating subject visits and Steven Rossi and Rowena Espina for performing drug assays.
List of Abbreviations
- NAFLD
Nonalcoholic fatty liver disease
- UGT
UDP glucuronyltransferase
- APAP
acetaminophen
- APAP-G
acetaminophen-glucuronide
- NASH
nonalcoholic steatohepatitis
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- AUC
area under the curve
- NAPQI
N-acetyl-p-benzoquinone imine
- APAP-S
acetaminophen-sulfate
- APAP-C
acetaminophen-cysteine
- APAP-A
acetaminophen-mercapturic acid
- NAS
nonalcoholic fatty liver activity score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Barshop NJ, Sirlin CB, Schwimmer JB, et al. Review article: epidemiology, pathogenesis and potential treatments of paediatric non-alcoholic fatty liver disease. Alimentary Pharmacology & Therapeutics. 2008;28:13–24. doi: 10.1111/j.1365-2036.2008.03703.x. [DOI] [PubMed] [Google Scholar]
- 2.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 3.Leclercq I, Horsmans Y, Desager JP, et al. Reduction in hepatic cytochrome P-450 is correlated to the degree of liver fat content in animal models of steatosis in the absence of inflammation. Journal of hepatology. 1998;28:410–416. doi: 10.1016/s0168-8278(98)80314-0. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Lechon MJ, Jover R, Donato MT. Cytochrome P450 and Steatosis. Current Drug Metabolism. 2009 doi: 10.2174/138920009789895543. [DOI] [PubMed] [Google Scholar]
- 5.Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39:215–231. doi: 10.2165/00003088-200039030-00004. [DOI] [PubMed] [Google Scholar]
- 6.Chun LJ, Tong MJ, Busuttil RW, et al. Acetaminophen hepatotoxicity and acute liver failure. Journal of clinical gastroenterology. 2009;43:342–349. doi: 10.1097/MCG.0b013e31818a3854. [DOI] [PubMed] [Google Scholar]
- 7.Benson GD, Koff RS, Tolman KG. The therapeutic use of acetaminophen in patients with liver disease. Am J Ther. 2005;12:133–141. doi: 10.1097/01.mjt.0000140216.40700.95. [DOI] [PubMed] [Google Scholar]
- 8.Osabe M, Sugatani J, Fukuyama T, et al. Expression of hepatic UDP-glucuronosyltransferase 1A1 and 1A6 correlated with increased expression of the nuclear constitutive androstane receptor and peroxisome proliferator-activated receptor alpha in male rats fed a high-fat and high-sucrose diet. Drug metabolism and disposition: the biological fate of chemicals. 2008;36:294–302. doi: 10.1124/dmd.107.017731. [DOI] [PubMed] [Google Scholar]
- 9.Al-Obaidy SS, Li Wan Po A, McKiernan PJ, et al. Assay of paracetamol and its metabolites in urine, plasma and saliva of children with chronic liver disease. Journal of pharmaceutical and biomedical analysis. 1995;13:1033–1039. doi: 10.1016/0731-7085(95)01303-3. [DOI] [PubMed] [Google Scholar]
- 10.Alam SN, Roberts RJ, Fischer LJ. Age-related differences in salicylamide and acetaminophen conjugation in man. The Journal of pediatrics. 1977;90:130–135. doi: 10.1016/s0022-3476(77)80787-7. [DOI] [PubMed] [Google Scholar]
- 11.Cummings AJ, King ML, Martin BK. A kinetic study of drug elimination: the excretion of paracetamol and its metabolites in man. British journal of pharmacology and chemotherapy. 1967;29:150–157. doi: 10.1111/j.1476-5381.1967.tb01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Marel CD, Anderson BJ, van Lingen RA, et al. Paracetamol and metabolite pharmacokinetics in infants. European journal of clinical pharmacology. 2003;59:243–251. doi: 10.1007/s00228-003-0608-0. [DOI] [PubMed] [Google Scholar]
- 13.Raucy JL, Lasker JM, Kraner JC, et al. Induction of cytochrome P450IIE1 in the obese overfed rat. Molecular pharmacology. 1991;39:275–280. [PubMed] [Google Scholar]
- 14.Dong ZG, Hong JY, Ma QA, et al. Mechanism of induction of cytochrome P-450ac (P-450j) in chemically induced and spontaneously diabetic rats. Archives of Biochemistry and Biophysics. 1988;263:29–35. doi: 10.1016/0003-9861(88)90610-8. [DOI] [PubMed] [Google Scholar]
- 15.Emery MG, Fisher JM, Chien JY, et al. CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.) 2003;38:428–435. doi: 10.1053/jhep.2003.50342. [DOI] [PubMed] [Google Scholar]
- 16.Chalasani N, Gorski JC, Asghar MS, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md.) 2003;37:544–550. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 17.Weltman MD, Farrell GC, Hall P, et al. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology (Baltimore, Md.) 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 18.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 19.Villanova N, Moscatiello S, Ramilli S, et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology (Baltimore, Md.) 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- 20.Donthamsetty S, Bhave VS, Mitra MS, et al. Nonalcoholic steatohepatitic (NASH) mice are protected from higher hepatotoxicity of acetaminophen upon induction of PPARalpha with clofibrate. Toxicology and applied pharmacology. 2008;230:327–337. doi: 10.1016/j.taap.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Al-Obaidy SS, McKiernan PJ, Li Wan Po A, et al. Metabolism of paracetamol in children with chronic liver disease. European journal of clinical pharmacology. 1996;50:69–76. doi: 10.1007/s002280050071. [DOI] [PubMed] [Google Scholar]
- 22.Evans WE, Relling MV, Petros WP, et al. Dextromethorphan and caffeine as probes for simultaneous determination of debrisoquin-oxidation and N-acetylation phenotypes in children. Clin Pharmacol Ther. 1989 May;45(5):568–573. doi: 10.1038/clpt.1989.74. [DOI] [PubMed] [Google Scholar]
- 23.Streetman DS, Bleakley JF, Kim JS, et al. Combined phenotypic assessment of CYP1A2, CYP2C19, CYP2D6, CYP3A, N-acetyltransferase-2, and xanthine oxidase with the "Cooperstown cocktail". Clin Pharmacol Ther. 2000 Oct;68(4):375–383. doi: 10.1067/mcp.2000.109519. [DOI] [PubMed] [Google Scholar]